Evaluation of the Nutritional Quality of Different Soybean and Pea Varieties: Their Use in Balanced Diets for Different Pathologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Analysis of Soybean Lipids
2.3. Protein and Amino Acid Analysis of Soybeans and Pea Beans
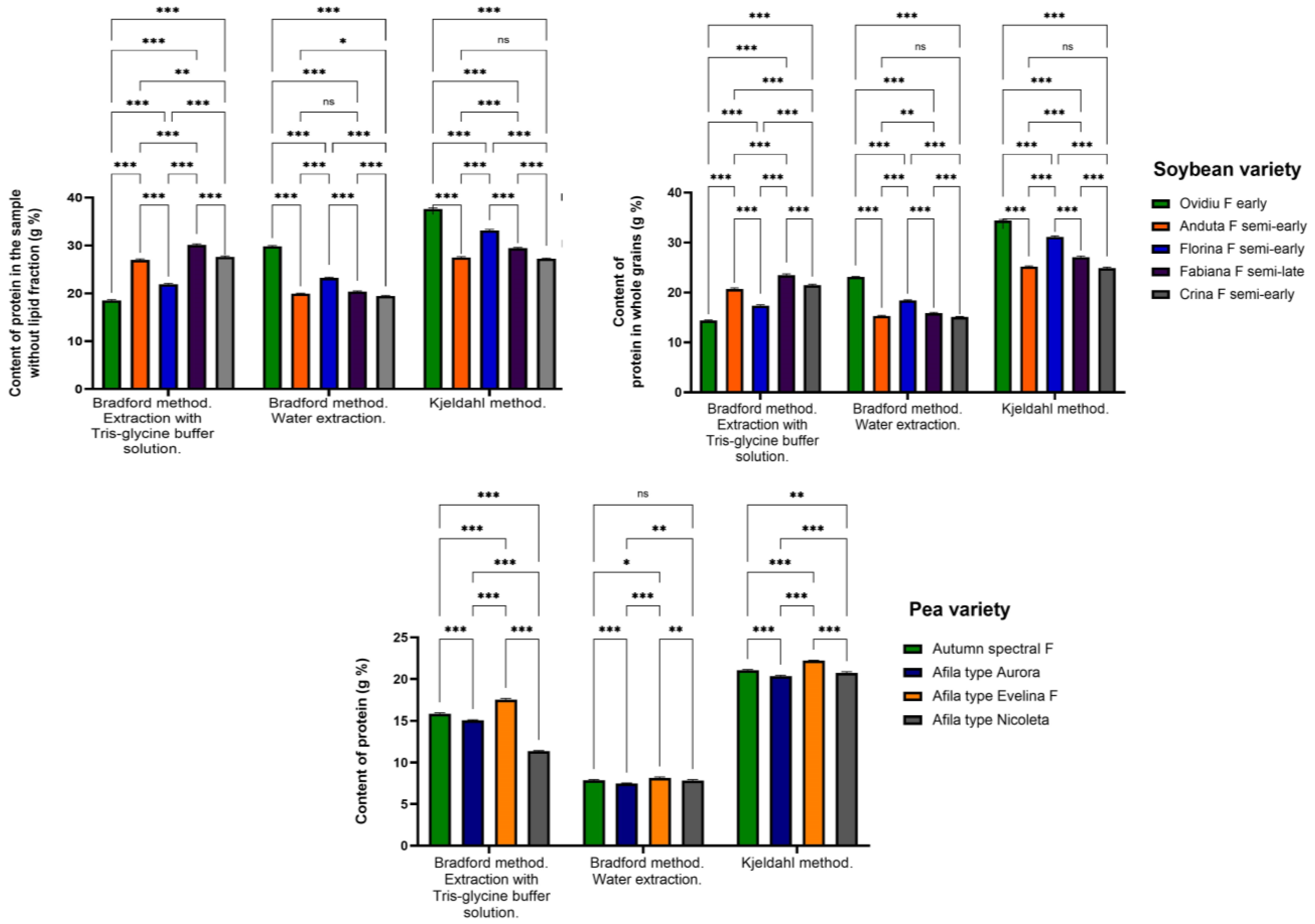
2.3.1. Protein Dosage by the Bradford Method
Protein Determination Using the Kjeldahl Method
2.3.2. Amino Acids Determination by Gas Chromatography
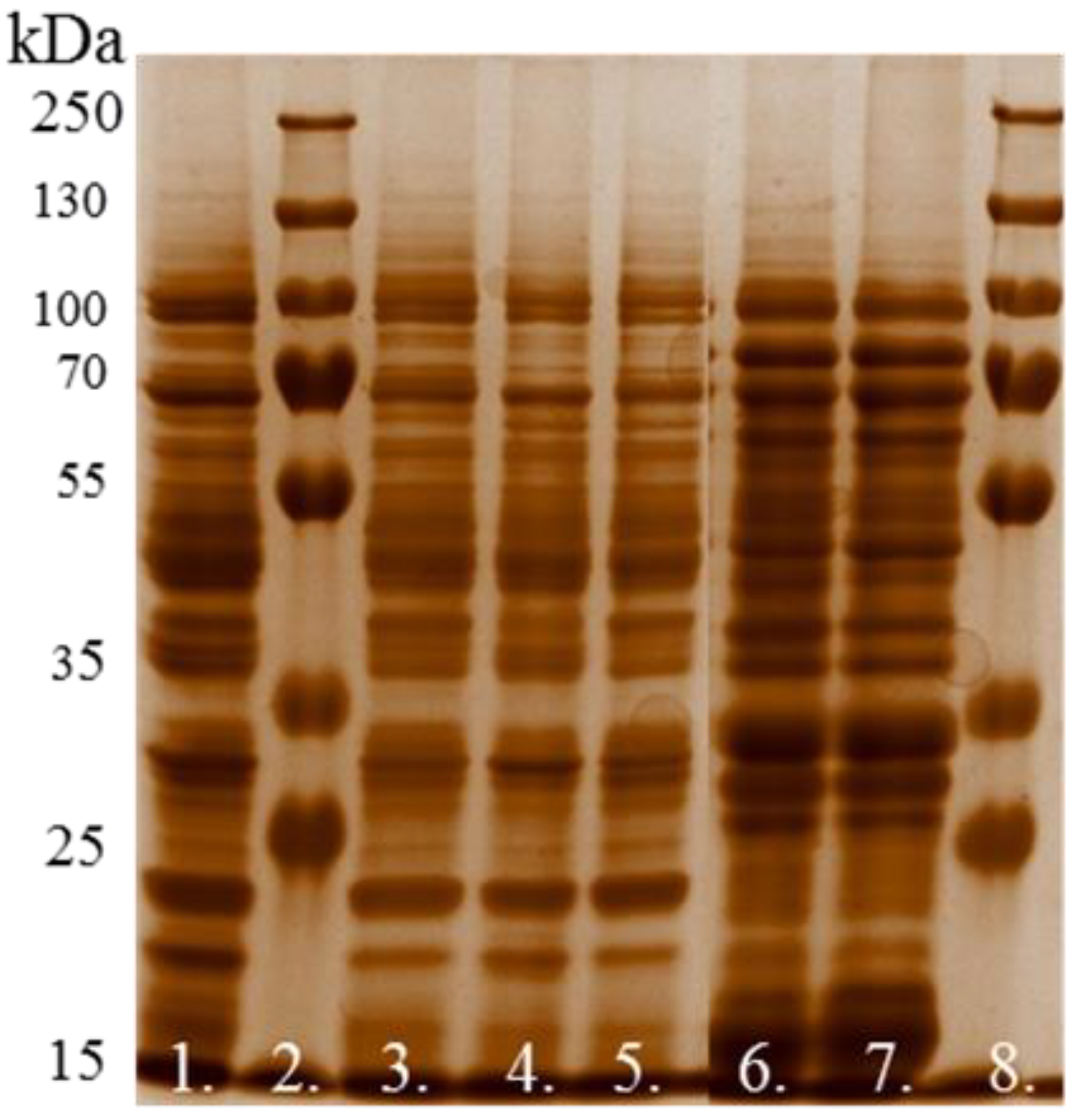
2.3.3. Protein Electrophoresis
- (i).
- The 30% acrylamide–bisacrylamide solution was prepared as follows: 29.2000 g of acrylamide and 0.8000 g of N,N′-methylenebisacrylamide were weighed and were introduced into a 100 mL volumetric flask. They were dissolved in Milli-Q® ultrapure water and made up to volume with the same solvent. Then, they were filtered and stored in brown glass vials in the refrigerator for 30 days.
- (ii).
- Preparation of the 10% sodium dodecyl sulfate (SDS) solution: 10 g of sodium dodecyl sulfate was weighed and placed in a 100 mL volumetric flask. It was dissolved in Milli-Q® ultrapure water and made up to volume with the same solvent. The solution was stored at room temperature.
- (iii).
- Preparation of gel buffer for separation: 18.1500 g of Trizma base® (tromethamine) was weighed and placed in a Berzelius beaker. Then, it was dissolved into Milli-Q® ultrapure water, and the pH was adjusted to 8.80 using the Mettler Toledo FiveEasy pH F20 pH meter with 6 N HCl solution. The content was transferred to a 100 mL volumetric flask and brought to the mark with the same solvent. It was stored in the refrigerator. The final concentration of Tris was 1.5 M.
- (iv).
- Preparation of the gel buffer for concentration: 6.0000 g of Trizma base® was weighed and placed into a Berzelius beaker. Then, it was dissolved in Milli-Q® ultrapure water, and the pH was adjusted to 6.80 using the Mettler Toledo FiveEasy pH F20 pH meter with 6 N HCl solution. The contents were transferred into a 100 mL volumetric flask and made up to the mark with the same solvent. It was stored in the refrigerator. The final concentration of Tris was 0.5 M.
- (v).
- Preparation of 10% ammonium persulfate (APS) solution: 0.1000 g of ammonium persulfate was weighed using the analytical balance and dissolved in 1 mL of Milli-Q® ultrapure water.
- (vi).
- Preparation of migration buffer pH 8.3:3.0300 g of Trizma base® and 14.4000 g of glycine were weighed on the analytical balance. They were placed in a 1000 mL volumetric flask. It was dissolved in ultrapure Milli-Q® water. Then, 10 mL of 10% SDS solution was added and made up to the mark with the same solvent. No pH adjustment was made.
- (vii).
- Preparation of fixing solution: 12.0000 g of trichloroacetic acid was weighed and placed in a 100 mL volumetric flask. It was dissolved in Milli-Q® ultrapure water and made up to a volume with the same solvent.
- (viii).
- Preparation of Coomassie staining solution: 0.1800 g of Coomassie Brilliant Blue G-250® was dissolved in 45 mL of methanol. In total, 10 mL glacial acetic acid and 45 mL Milli-Q® ultrapure water were added.
- (ix).
- Preparation of the bleaching solution: the solution was prepared with a volumetric methanol/acetic acid/ultrapure water ratio of 40:10:50.
- (x).
- Preparation of anti-fade solution: a solution of 2% acetic acid in ultrapure water was prepared.
- (xi).
- Tris-glycine pH 8.3 buffer was prepared according to the procedure presented for protein extraction.
- (a)
- The formation of gels
- (b)
- Staining and interpretation of bands
- (a)
- The gels were washed twice with ultrapure Milli-Q® water for 5 min each. Then, the water was removed.
- (b)
- They were fixed with ethanol/acetic acid/ultrapure water 3:1:6 v/v/v solution twice for 15 min. The solution was removed.
- (c)
- They were washed with 10% ethanol solution in water twice, 5 min each.
- (d)
- The gels were immersed in a solution consisting of 25 mL Milli-Q® ultrapure water and 50 μL Silver Stain Sensitizer reagent for exactly 1 min.
- (e)
- They were washed with ultrapure water twice for exactly 1 min, after which the water was removed.
- (f)
- They were immersed in a solution consisting of 25 mL Milli-Q® ultrapure water and 250 μL Silver Stain Enhancer reagent for exactly 5 min.
- (g)
- They were washed with ultrapure water twice for exactly 20 s, after which the water was removed.
- (h)
- They were immersed in a mixture of 25 mL Silver Stain Developer and 250 μL Silver Stain Enhancer for approximately 30 s until bands became visible.
- (i)
- The reaction was quickly stopped with 5% acetic acid solution.
2.4. Statistical Analysis
3. Results
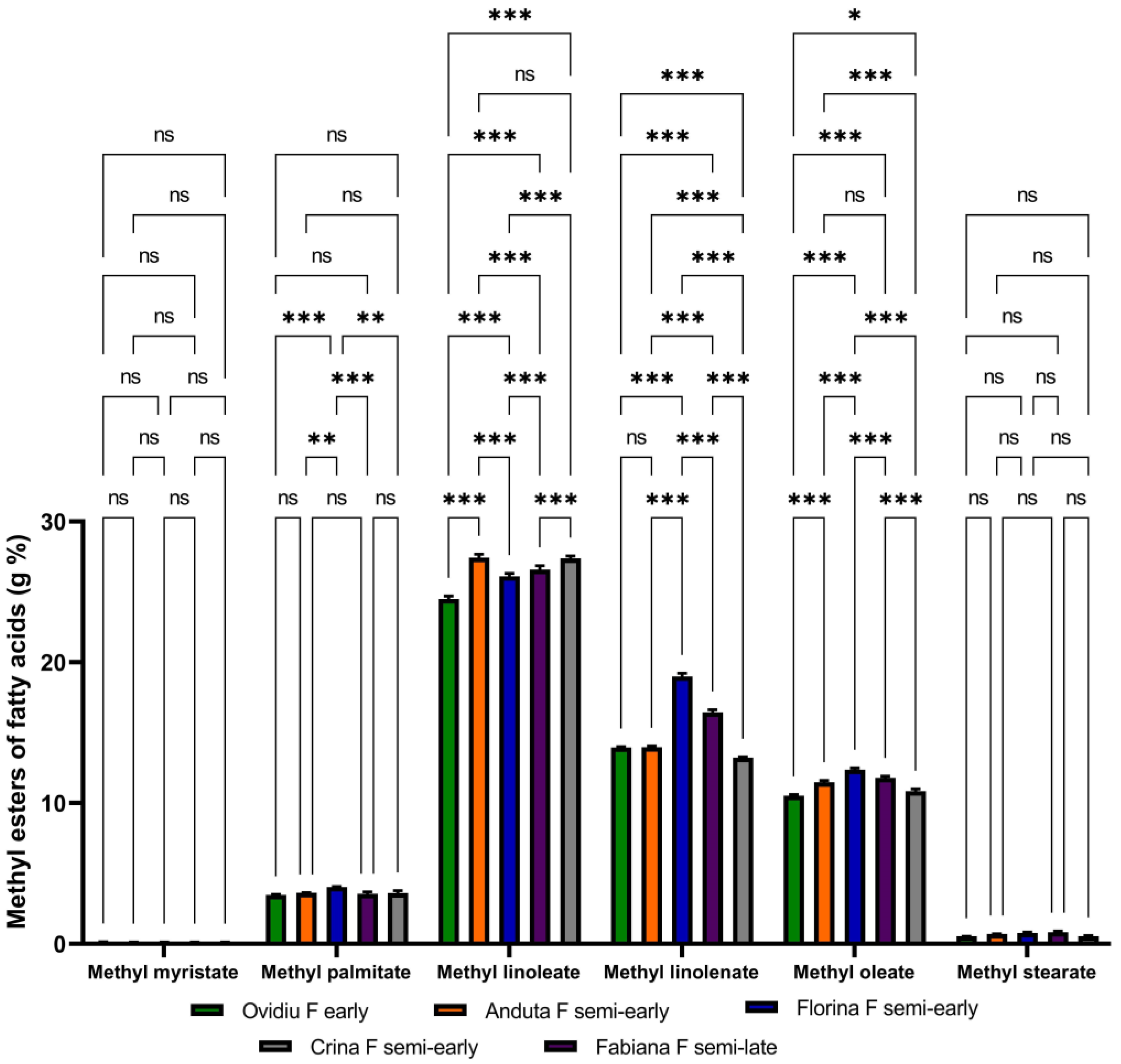
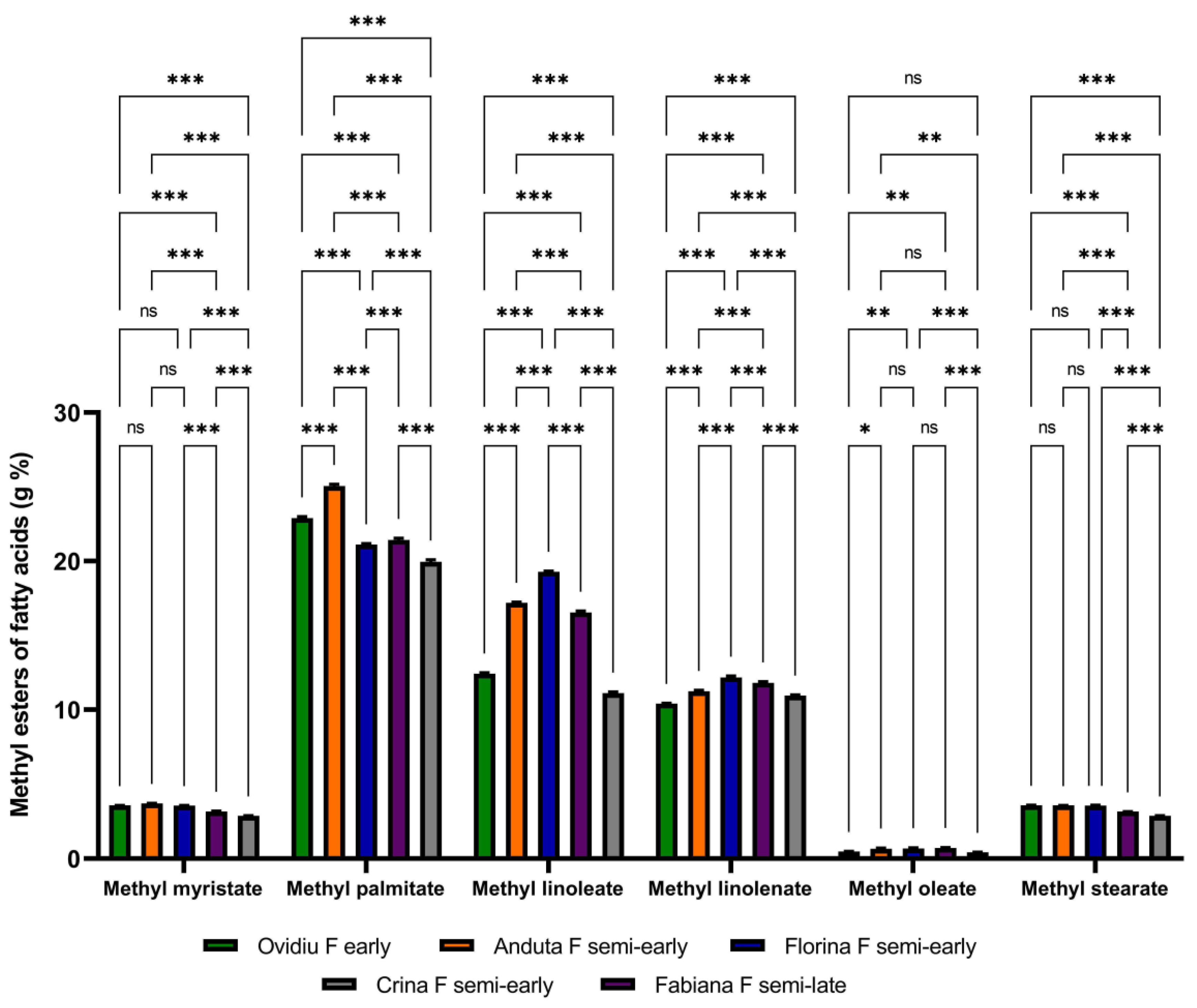
3.1. The Lipid Content of Soybeans
3.2. The Protein Content of Soybeans and Peas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willis, H. Growing Great Soybeans. Acres USA 1989, 1, 6–8. Available online: http://eap.mcgill.ca/CPSO_3.htm (accessed on 10 February 2023).
- Stacey, G. Genetics and Genomics of Soybean; Springer: New York, NY, USA, 2008; eBook; ISBN 978-0-387-72299-3. [Google Scholar] [CrossRef]
- Perkins, E.G. Composition of Soybeans and Soybean Products. In Practical Handbook of Soybean Processing and Utilization; Erickson, D.R., Ed.; AOCS Press: Champaign, IL, USA, 1995; pp. 9–28. [Google Scholar] [CrossRef]
- Navarro, D.M.D.L.; Abelilla, J.J.; Stein, H.H. Structures and characteristics of carbohydrates in diets fed to pigs: A review. J. Anim. Sci. Biotechnol. 2019, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Ioniță-Mîndrican, C.-B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.-E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Huang, Y.; Hua, Y. Isolation of glycinin (11S) from lipid-reduced soybean flour: Effect of processing conditions on yields and purity. Molecules 2012, 17, 2968–2979. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between Secondary Structure and Surface Hydrophobicity of Soybean Protein Isolate Subjected to Heat Treatment. J. Chem. 2014, 2014, 475389. [Google Scholar] [CrossRef]
- Silva, S.S.; Fernandes, E.; Pina, S.; Silva-Correia, J.; Vieira, S.; Oliveira, J.; Reis, R.L. 2.11 Polymers of Biological Origin. Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 228–252. ISBN 9780081006924. [Google Scholar] [CrossRef]
- Fukushima, D.; Proteins, S.; Phillips, G.O.; Williams, P.A. (Eds.) Handbook of Food Proteins; Woodhead Publishing: Cambridge, UK, 2011; pp. 210–232. [Google Scholar] [CrossRef]
- Iritani, N.; Hosomi, H.; Fukuda, H.; Tada, K.; Ikeda, H. Soybean protein suppresses hepatic lipogenic enzyme gene expression in Wistar fatty rats. J. Nutr. 1996, 126, 380–388. [Google Scholar] [CrossRef]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Doerge, D.R.; Sheehan, D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ. Health Perspect. 2002, 110, 349–353. [Google Scholar] [CrossRef]
- Ahnen, R.T.; Jonnalagadda, S.S.; Slavin, J.L. Role of plant protein in nutrition, wellness, and health. Nutr. Rev. 2019, 77, 735–747. [Google Scholar] [CrossRef]
- Ho, S.C.; Woo, J.L.; Leung, S.S.; Sham, A.L.; Lam, T.H.; Janus, E.D. Intake of soy products is associated with better plasma lipid profiles in the Hong Kong Chinese population. J. Nutr. 2000, 130, 2590–2593. [Google Scholar] [CrossRef]
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and Gut Microbiota: Interaction and Implication for Human Health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef]
- Rosell, M.S.; Appleby, P.N.; Spencer, E.A.; Key, T.J. Soy intake and blood cholesterol concentrations: A cross-sectional study of 1033 pre- and postmenopausal women in the Oxford arm of the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2004, 80, 1391–1396. [Google Scholar] [CrossRef]
- Tokede, O.A.; Onabanjo, T.A.; Yansane, A.; Gaziano, J.M.; Djoussé, L. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 831–843. [Google Scholar] [CrossRef]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Devi, M.K.A.; Gondi, M.; Sakthivelu, G.; Giridhar, P.; Rajasekaran, T.; Ravishankar, G.A. Functional attributes of soybean seeds and products, with reference to isoflavone content and antioxidant activity. Food Chem. 2009, 114, 771–776. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef]
- Nagata, C. Ecological study of the association between soy product intake and mortality from cancer and heart disease in Japan. Int. J. Epidemiol. 2000, 29, 832–836. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, J.H.; Nam, S.J.; Ryu, S.; Kong, G. Dietary intake of soy protein and tofu in association with breast cancer risk based on a case-control study. Nutr. Cancer 2008, 60, 568–576. [Google Scholar] [CrossRef]
- Andreani, G.; Sogari, G.; Marti, A.; Froldi, F.; Dagevos, H.; Martini, D. Plant-Based Meat Alternatives: Technological, Nutritional, Environmental, Market, and Social Challenges and Opportunities. Nutrients 2023, 15, 452. [Google Scholar] [CrossRef]
- Kumari, T.; Deka, S.C. Potential healthbenefits of garden pea seeds and pods: A review. Legume Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Ioniţă, A.C.; Ghica, M.; Moroşan, E.; Nicolescu, F.; Mititelu, M. In vitro effects of some synthesized aminoacetanilide N’-substituted on human leukocytes separated from peripheral blood. Farmacia 2019, 67, 684–690. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Philipp, C.; Emin, M.A.; Buckow, R.; Silcock, P.; Oey, I. Pea protein-fortified extruded snacks: Linking melt viscosity and glass transition temperature with expansion behaviour. J. Food Eng. 2018, 217, 93–100. [Google Scholar] [CrossRef]
- Barac, M.B.; Pesic, M.B.; Stanojevic, S.P.; Kostic, A.Z.; Cabrilo, S.B. Techno-functional properties of pea (Pisum sativum) protein isolates: A review. Acta Period. Technol. 2015, 46, 1–18. [Google Scholar] [CrossRef]
- Sedeek, K.E.M.; Mahas, A.; Mahfouz, M. Plant Genome Engineering for Targeted Improvement of Crop Traits. Front. Plant Sci. 2019, 10, 114. [Google Scholar] [CrossRef]
- Available online: https://www.incda-fundulea.ro/fise/fise.html (accessed on 28 May 2023).
- Mititelu, M.; Neacsu, S.M.; Oprea, E.; Dumitrescu, D.-E.; Nedelescu, M.; Drăgănescu, D.; Nicolescu, T.O.; Rosca, A.C.; Ghica, M. Black Sea Mussels Qualitative and Quantitative Chemical Analysis: Nutritional Benefits and Possible Risks through Consumption. Nutrients 2022, 14, 964. [Google Scholar] [CrossRef]
- Mititelu, M.; Udeanu, D.I.; Nedelescu, M.; Neacsu, S.M.; Nicoara, A.C.; Oprea, E.; Ghica, M. Quality Control of Different Types of Honey and Propolis Collected from Romanian Accredited Bee-Keepers and Consumer’s Risk Assessment. Crystals 2022, 12, 87. [Google Scholar] [CrossRef]
- Mehraj, S.S.; Kamili, A.N.; Nazir, R.; Haq, E.; Balkhi, H.M. Comparative evaluation of extraction methods for total proteins from Crocus sativus L. (Saffron). Saudi J. Biol. Sci. 2018, 25, 1603–1608. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tsugita, A.; Scheffler, J.-J. A Rapid Method for Acid Hydrolysis of Protein with a Mixture of Trifluoroacetic Acid and Hydrochloric acid. Eur. J. Biochem. 1982, 124, 585–588. [Google Scholar] [CrossRef]
- Csapó, J.; Csapó-Kiss, Z.; Wágner, L.; Tálos, T.; Martin, T.G.; Folestad, S.; Tivesten, A.; Némethy, S. Hydrolysis of proteins performed at high temperatures and for short times with reduced racemization, in order to determine the enantiomers of D- and L-amino acids. Anal. Chim. Acta 1997, 339, 99–107. [Google Scholar] [CrossRef]
- Fritsch, R.J.; Krause, I. Electrophoresis. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Trugo, L., Finglas, P.M., Eds.; Academic Press: New York, NY, USA, 2003; pp. 2055–2062. [Google Scholar]
- Gas, B. Electrophoresis Principles. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 363–370. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 10th ed.; EDQM, Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Morosan, E.; Secareanu, A.A.; Musuc, A.M.; Mititelu, M.; Ionită, A.C.; Ozon, E.A.; Raducan, I.D.; Rusu, A.I.; Dărăban, A.M.; Karampelas, O. Comparative Quality Assessment of Five Bread Wheat and Five Barley Cultivars Grown in Romania. Int. J. Environ. Res. Public Health 2022, 19, 11114. [Google Scholar] [CrossRef]
- De Maria, M.; Robinson, E.J.Z.; Kangile, J.R.; Kadigi, R.; Dreoni, I.; Couto, M.; Howai, N.; Peci, J.; Fiennes, S. Global Soybean Trade. The Geopolitics of a Bean; UK Research and Innovation Global Challenges Research Fund (UKRI GCRF) Trade, Development and the Environment Hub: London, UK, 2020. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A. Fatty Acids and Cardiovascular Risk. Evidence, Lack of Evidence, and Diligence. Nutrients 2020, 12, 3782. [Google Scholar] [CrossRef]
- Kim, I.S.; Kim, C.H.; Yang, W.S. Physiologically Active Molecules and Functional Properties of Soybeans in Human Health-A Current Perspective. Int. J. Mol. Sci. 2021, 22, 4054. [Google Scholar] [CrossRef]
- Viñado, A.; Castillejos, L.; Barroeta, A.C. Soybean Lecithin High in Free Fatty Acids for Broiler Chicken Diets: Impact on Performance, Fatty Acid Digestibility and Saturation Degree of Adipose Tissue. Animals 2019, 9, 802. [Google Scholar] [CrossRef]
- Messina, M. Soy and Health Update: Evaluation of the clinical and epidemiologic literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef]
- Paduraru, D.N.; Coman, F.; Ozon, E.A.; Gherghiceanu, F.; Andronic, O.; Ion, D.; Stanescu, M.; Bolocan, A. The use of nutritional supplement in romanian patients—Attitudes and beliefs. Farmacia 2019, 67, 1060–1065. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-Chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-Body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Mititelu, M.; Moroșan, E.; Nicoară, A.C.; Secăreanu, A.A.; Musuc, A.M.; Atkinson, I.; Cusu, J.P.; Nițulescu, G.M.; Ozon, E.A.; Sarbu, I.; et al. Development of immediate release tablets containing calcium lactate synthetized from Black Sea mussel shells. Mar. Drugs 2022, 20, 45. [Google Scholar] [CrossRef]
- Mititelu, M.; Stanciu, G.; Drăgănescu, D.; Ioniță, A.C.; Neacșu, S.M.; Dinu, M.; Stefanvan Staden, R.-I.; Moroșan, E. Mussel Shells, a Valuable Calcium Resource for the Pharmaceutical Industry. Mar. Drugs 2022, 20, 25. [Google Scholar] [CrossRef]
- Jassim Aziz, R.; Mihele, D.; Dogaru, E. Study Regarding the Influence of Vitis vinifera Fruit (Muscat of Hamburg Species) On Some Biochemical Parameters. Farmacia 2010, 58, 332–340. [Google Scholar]




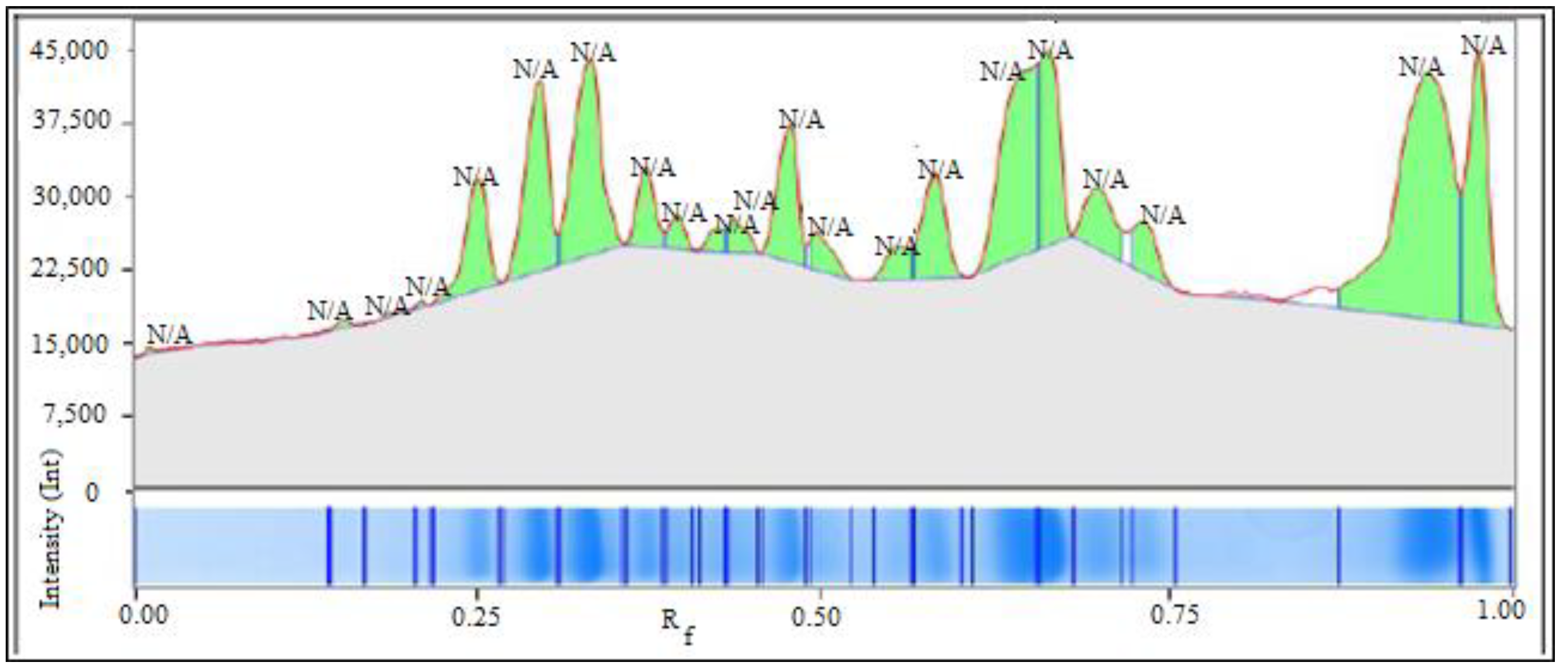
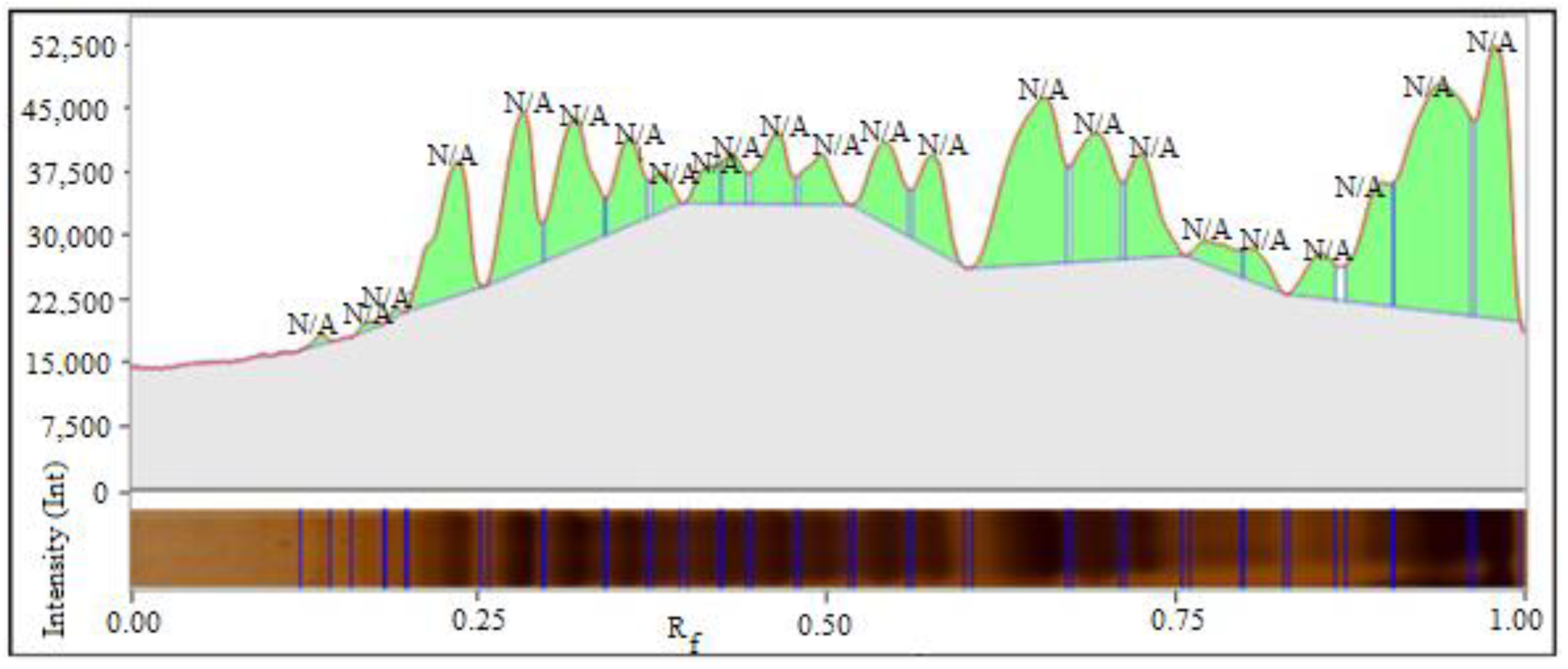
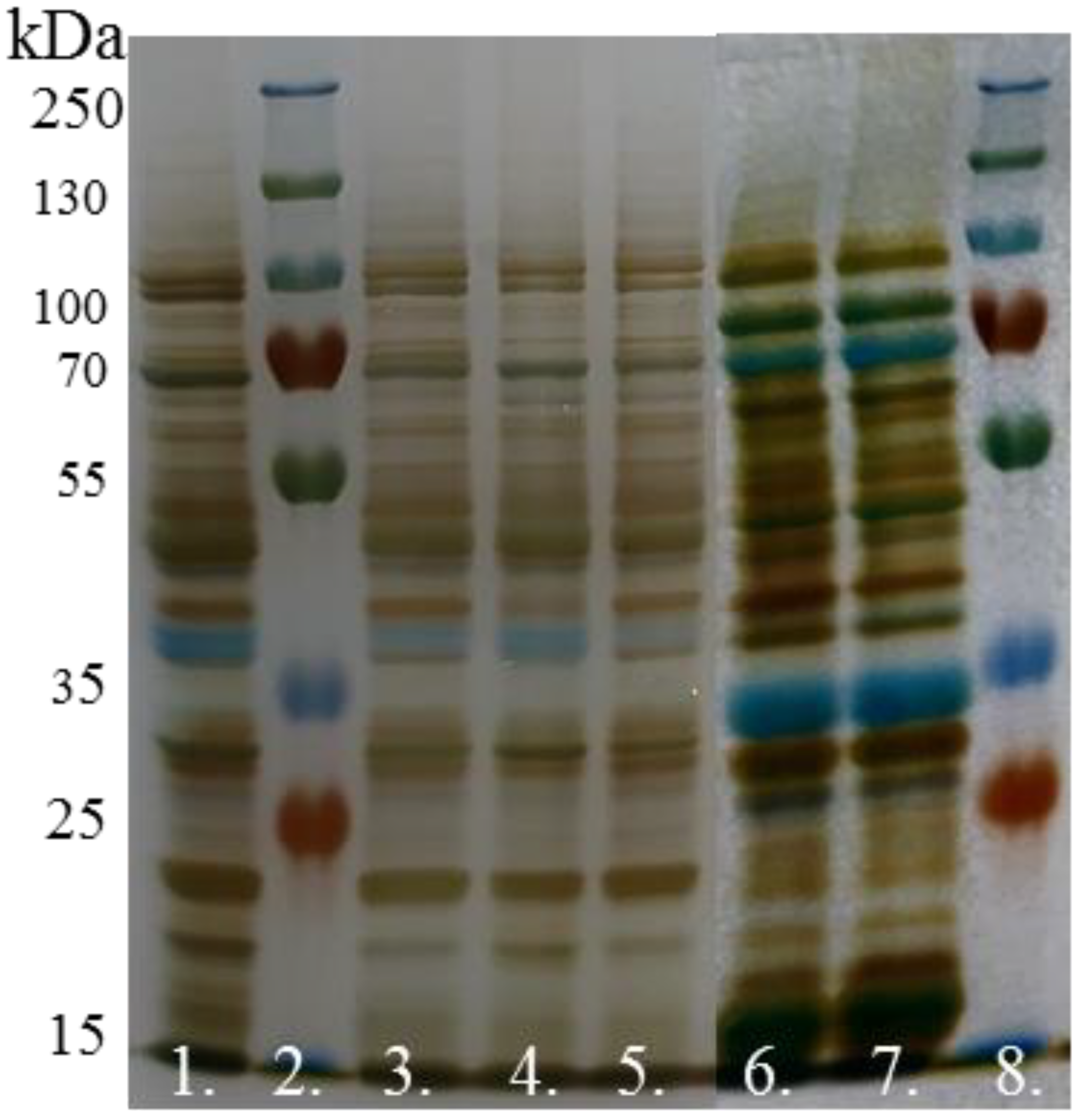
| Type of Varieties | Morphological Attributes | Physiological Characteristics | Quality |
|---|---|---|---|
| Soybeans | |||
| Ovidiu F early | Growth type: determined. Bush shape: semi-spreading. Pubescence: gray. Flower: white. Pod: dark brown. Plant height: 80–122 cm. Grain: yellow with yellow hilum. Mass: 100–160 g. Insertion height of the first pods: 12–20 cm. | Vegetation period: 100–110 days. Good drought and heat tolerance. Very good resistance to falling and shaking. Good resistance to soybean blight (Peronospora manshurica), bacterial burn (Pseudomonas glycinea) and Fusarium wilt (Fusarium oxysporum). | In terms of the non-irrigated period, the maximum production was 5765 kg/ha. The Ovidiu F variety has a high production capacity for the maturity stage to which it belongs (over 4600 kg/ha). |
| Anduța F semi-early | Growth type: determined. Bush shape: semi-spreading. Pubescence: gray. Flower: white. Pod: dark brown. Plant height: 90–110 cm. Grain: yellow with black hilum. Mass: 140–180 g. Insertion height of the first pods: 15–20 cm. | Vegetation period: 100–110 days. Good drought and heat tolerance. Very good resistance to falling and shaking. Good resistance to soybean blight (Peronospora manshurica), bacterial burn (Pseudomonas glycinea) and Fusarium wilt (Fusarium oxysporum). | Under the non-irrigated period conditions, the maximum production was 5395 kg/ha. The Anduţa F variety has a high production capacity for the maturity group to which it belongs (over 4500 kg/ha). |
| Florina F semi-early | Growth type: indeterminate. Bush shape: semi-spreading. Pubescence: gray. Flower: purple. Pod: dark brown. Plant height: 95–125 cm. The grain: yellow with a brown hilum. Mass: 120–140 g. Insertion height of the first pods: 15–17 cm. | Vegetation period: 100–110 days. Good drought and heat tolerance. Very good resistance to falling and shaking. Good resistance to soybean blight (Peronospora manshurica), bacterial burn (Pseudomonas glycinea) and Fusarium wilt (Fusarium oxysporum). | In terms of non-irrigated period, the maximum production was 5088 kg/ha. The Florina F variety has a high production capacity for the maturity group to which it belongs (over 4000 kg/ha). |
| Fabiana F semi-late | Growth type: indeterminate. Bush shape: compact. Pubescence: gray. Flower: purple. Pod: light brown. Plant height: 115–122 cm. The grain: yellow with a brown hilum. Mass: 120–140 g. Insertion height of the first pods: 15–17 cm. | Vegetation period: 118–130 days. Good drought and heat tolerance. Very good resistance to falling and shaking. Good resistance to soybean blight (Peronospora manshurica), bacterial burn (Pseudomonas glycinea) and Fusarium wilt (Fusarium oxysporum). | Maximum production in non-irrigated conditions was 5808 kg/ha. The Fabiana F variety has a capacity of high production for the maturity group from which belongs over 4600 kg/ha. |
| Crina F semi-early | Growth type: determinate. Compact bush shape. Pubescence: gray. Flower: white. Pod: gray. Plant height: 85–100 cm. Grain: yellow with a light brown hilum. Mass: 140–150 g. | Vegetation period: 114–123 days. Good drought and heat tolerance. Very good resistance to falling and shaking. Good resistance to bacterial blight (Peronospora manshurica) and manna (Pseudomonas glycinea). | Crina F has a high production capacity for the maturity group to which it belongs, between 2500 and 4500 kg/ha. The variety shows a superior stability of production in the conditions in the southern part of the country, as a result of an increased tolerances to atmospheric drought. |
| Peas | |||
| Spectral F autumn | It has good winter hardiness level. The vegetation period is 135–140 days. The color of the flowers is white. The grains are spherical, smooth and yellow pericarp. The plant height is of 150–200 cm. | ||
| Aurora-type afila | Plant height: 45–55 cm; Leaf: “Afila” type; Flowers: white, Pod: medium to small, green; Grain: yellow. | Vegetation period: 78–93 days. Good resistance to falling and shaking. The protein content varies from 25% to 27%. | Average production in the three years of testing, very different from the climatic point of view, it 2541 kg/ha. |
| Evelina F-type afila | Growth type: undetermined. Plant height: 65–90 cm. Leaf: “Afila” type, with leaflets transformed into tendrils strongly developed and branched with large stems in the middle. Flowers: white, grouped by two in a raceme. Pod: small to medium, green. Grain: smooth with yellow skin. Mass: 250–280 g. | Vegetation period: 75–90 days. Very good resistance to falling and shaking. Good resistance to pea powdery mildew (Erysiphe polygoni), anthracnose (Ascochya pisi) and viruses. The protein content varies from 24.5% to 26%. The percentage of shells is 7.5%. | Average production in the three years of testing, very different from the climatic point of view, was 3786 kg/ha. |
| Nicoleta-type afila | Growth type: undetermined. Plant height: 60–85 cm. Leaf: “Afila” type, with leaflets transformed into tendrils strongly developed and branched with large stems in the middle. Flowers: white, grouped by two in a raceme. Pod: small to medium, green. Grain: smooth with yellow skin. Mass: 250–280 g. | Vegetation period: 78–96 days. Very good resistance to falling and shaking. Good resistance to pea powdery mildew (Erysiphe polygoni), anthracnose (Ascochya pisi) and viruses. The protein content varies from 24.5% to 26%. The percentage of shells is 7.5%. | Production in the three years of testing, very different from the climatic point of view, was 3567 kg/ha. |
| tR—Retention Time (min) | Rate (°C/min) | Target Temperature (°C) | Stationary Time (min) |
|---|---|---|---|
| 0.000 | Run | ||
| 2.000 | 0.00 | 170.0 | 2.00 |
| 14.667 | 3.00 | 202.0 | 2.00 |
| 26.267 | 5.00 | 250.0 | 2.00 |
| Concentration of BSA (μg/mL) | Stock Solution Volume of BSA (μL) | Solvent Volume (μL) |
|---|---|---|
| 50 | 62.5 | 1187.5 |
| 100 | 125 | 1125 |
| 200 | 250 | 1000 |
| 400 | 500 | 750 |
| 600 | 750 | 500 |
| Retention Time (min) | Rate (°C/min) | Target Temperature (°C) | Stationary Time (min) |
|---|---|---|---|
| 0.000 | Run | ||
| 4.000 | 0.00 | 100.0 | 4.00 |
| 11.000 | 3.00 | 170.0 | 0.00 |
| 17.667 | 3.00 | 190.0 | 0.00 |
| 46.667 | 10.00 | 280.0 | 20.00 |
| Soybean Variety | Sample Table Taken in Work (g) | Total Lipid Extract (g) | Total Lipid Extract (% Dry Weight) |
|---|---|---|---|
| Ovidiu F early | 5.03 ± 0.01 | 1.13 ± 0.25 | 22.46 ± 0.12 |
| Anduta F semi-early | 5.03 ± 0.01 | 1.17 ± 0.33 | 23.28 ± 0.25 |
| Florina F semi-early | 5.01 ± 0.02 | 1.03 ± 0.55 | 20.67 ± 0.18 |
| Fabiana F semi-late | 5.01 ± 0.02 | 1.10 ± 0.66 | 22.00 ± 0.33 |
| Crina F semi-early | 5.00 ± 0.01 | 1.11 ± 0.42 | 22.29 ± 0.15 |
| Soybean Kernels | |||||
|---|---|---|---|---|---|
| Amino Acid | Ovidiu F Early (g%) | Anduta F Semi-Early (g%) | Florina F Semi-Early (g%) | Fabiana F Semi-Late (g%) | Crina F Semi-Early (g%) |
| L-Valine | 0.81 ± 0.02 | 0.80 ± 0.03 | 0.57 ± 0.01 | 1.07 ± 0.06 | 0.42 ± 0.01 |
| L-Alanine | 2.09 ± 0.08 | 0.95 ± 0.04 | 1.10 ± 0.03 | 1.33 ± 0.05 | 0.73 ± 0.04 |
| L-Leucine | 1.36 ± 0.08 | 1.31 ± 0.07 | 0.94 ± 0.06 | 1.85 ± 0.09 | 0.68 ± 0.08 |
| L-Proline | 1.00 ± 0.04 | 0.68 ± 0.02 | 0.75 ± 0.03 | 1.05 ± 0.04 | 0.90 ± 0.03 |
| L-Isoleucine | 1.12 ± 0.05 | 0.93 ± 0.02 | 0.68 ± 0.02 | 1.18 ± 0.04 | 0.29 ± 0.01 |
| L-Serine | 0.96 ± 0.02 | 0.86 ± 0.03 | 0.69 ± 0.02 | 1.27 ± 0.05 | 0.46 ± 0.02 |
| L-Threonine | 1.00 ± 0.03 | 0.71 ± 0.03 | 0.68 ± 0.02 | 1.04 ± 0.04 | 0.36 ± 0.01 |
| Glycine | 6.37 ± 0.05 | 1.53 ± 0.02 | 2.69 ± 0.01 | 1.32 ± 0.02 | 1.55 ± 0.02 |
| L-Aspartic acid | 2.54 ± 0.06 | 2.11 ± 0.04 | 1.74 ± 0.08 | 3.05 ± 0.08 | 1.11 ± 0.02 |
| L-Glutamic acid | 3.57 ± 0.02 | 2.80 ± 0.03 | 2.35 ± 0.02 | 4.49 ± 0.06 | 1.45 ± 0.02 |
| DL-Phenyl alanine | 0.72 ± 0.02 | 0.52 ± 0.02 | 0.49 ± 0.02 | 0.81 ± 0.03 | 0.23 ± 0.01 |
| N-Acetyl lysine | 5.96 ± 0.06 | 3.85 ± 0.02 | 3.12 ± 0.04 | 5.52 ± 0.02 | 1.66 ± 0.02 |
| Tyrosine | 0.19 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.28 ± 0.01 | 0.06 ± 0.01 |
| Pea Kernels | |||||
| Amino Acid | Autumn Spectral F (g%) | Afila-Type Aurora (g%) | Afila-Type Evelina F (g%) | Afila-Type Nicoleta (g%) | |
| L-Valine | 0.38 ± 0.02 | 0.28 ± 0.01 | 0.30 ± 0.02 | 0.48 ± 0.01 | |
| L-Alanine | 0.95 ± 0.06 | 0.54 ± 0.02 | 1.28 ± 0.06 | 1.00 ± 0.08 | |
| L-Leucine | 0.55 ± 0.02 | 0.46 ± 0.04 | 0.46 ± 0.02 | 0.72 ± 0.04 | |
| L-Proline | 0.57 ± 0.04 | 0.39 ± 0.02 | 0.59 ± 0.04 | 0.73 ± 0.04 | |
| L-Isoleucine | 0.35 ± 0.04 | 0.28 ± 0.02 | 0.16 ± 0.01 | 0.29 ± 0.01 | |
| L-Serine | 0.37 ± 0.01 | 0.27 ± 0.01 | 0.19 ± 0.01 | 0.48 ± 0.02 | |
| L-Threonine | 0.34 ± 0.02 | 0.26 ± 0.02 | 0.31 ± 0.02 | 0.51 ± 0.01 | |
| Glycine | 0.65 ± 0.04 | 1.20 ± 0.06 | 8.53 ± 0.14 | 1.25 ± 0.08 | |
| L-Aspartic acid | 0.95 ± 0.04 | 0.66 ± 0.04 | 0.90 ± 0.04 | 1.18 ± 0.08 | |
| L-Glutamic acid | 1.06 ± 0.06 | 0.72 ± 0.06 | 1.16 ± 0.06 | 1.49 ± 0.08 | |
| DL-Phenyl alanine | 0.21 ± 0.01 | 0.15 ± 0.01 | 0.20 ± 0.01 | 0.29 ± 0.01 | |
| N-Acetyl lysine | 2.22 ± 0.06 | 1.07 ± 0.08 | 2.73 ± 0.08 | 3.32 ± 0.10 | |
| Tyrosine | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroșan, E.; Lupu, C.E.; Mititelu, M.; Musuc, A.M.; Rusu, A.I.; Răducan, I.D.; Karampelas, O.; Voinicu, I.B.; Neacșu, S.M.; Licu, M.; et al. Evaluation of the Nutritional Quality of Different Soybean and Pea Varieties: Their Use in Balanced Diets for Different Pathologies. Appl. Sci. 2023, 13, 8724. https://doi.org/10.3390/app13158724
Moroșan E, Lupu CE, Mititelu M, Musuc AM, Rusu AI, Răducan ID, Karampelas O, Voinicu IB, Neacșu SM, Licu M, et al. Evaluation of the Nutritional Quality of Different Soybean and Pea Varieties: Their Use in Balanced Diets for Different Pathologies. Applied Sciences. 2023; 13(15):8724. https://doi.org/10.3390/app13158724
Chicago/Turabian StyleMoroșan, Elena, Carmen Elena Lupu, Magdalena Mititelu, Adina Magdalena Musuc, Andreea Ioana Rusu, Ionuț Daniel Răducan, Oana Karampelas, Ionuț Bogdan Voinicu, Sorinel Marius Neacșu, Monica Licu, and et al. 2023. "Evaluation of the Nutritional Quality of Different Soybean and Pea Varieties: Their Use in Balanced Diets for Different Pathologies" Applied Sciences 13, no. 15: 8724. https://doi.org/10.3390/app13158724
APA StyleMoroșan, E., Lupu, C. E., Mititelu, M., Musuc, A. M., Rusu, A. I., Răducan, I. D., Karampelas, O., Voinicu, I. B., Neacșu, S. M., Licu, M., Pogan, A. C., Cîrnațu, D., Ilie, E. I., & Dărăban, A. M. (2023). Evaluation of the Nutritional Quality of Different Soybean and Pea Varieties: Their Use in Balanced Diets for Different Pathologies. Applied Sciences, 13(15), 8724. https://doi.org/10.3390/app13158724








