Development and Characterization of N/S-Carbon Quantum Dots by Valorizing Greek Crayfish Food Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of N/S-Carbon QDs from Crayfish Waste
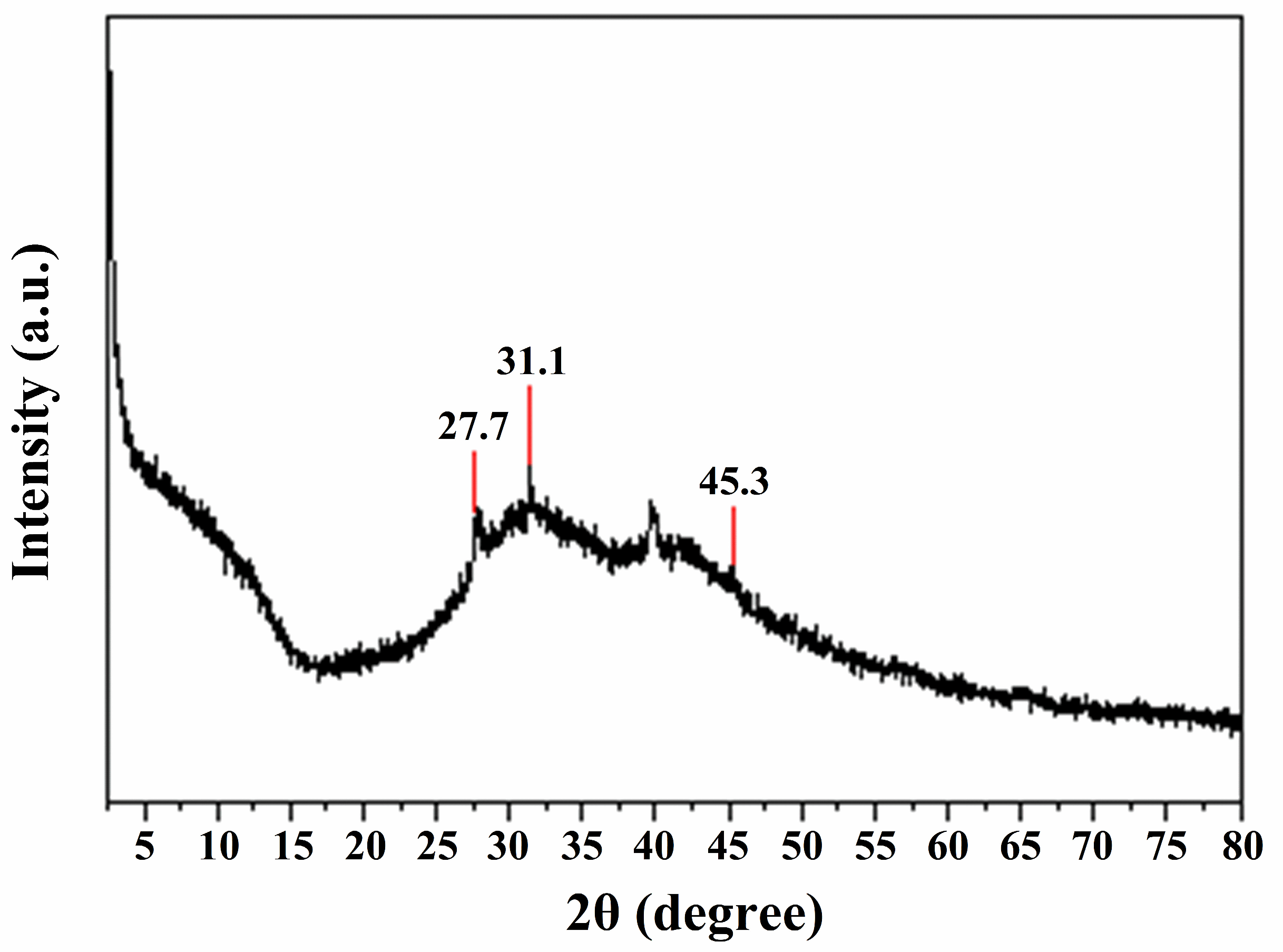
2.2. X-ray Powder Diffraction (XRD) Analysis
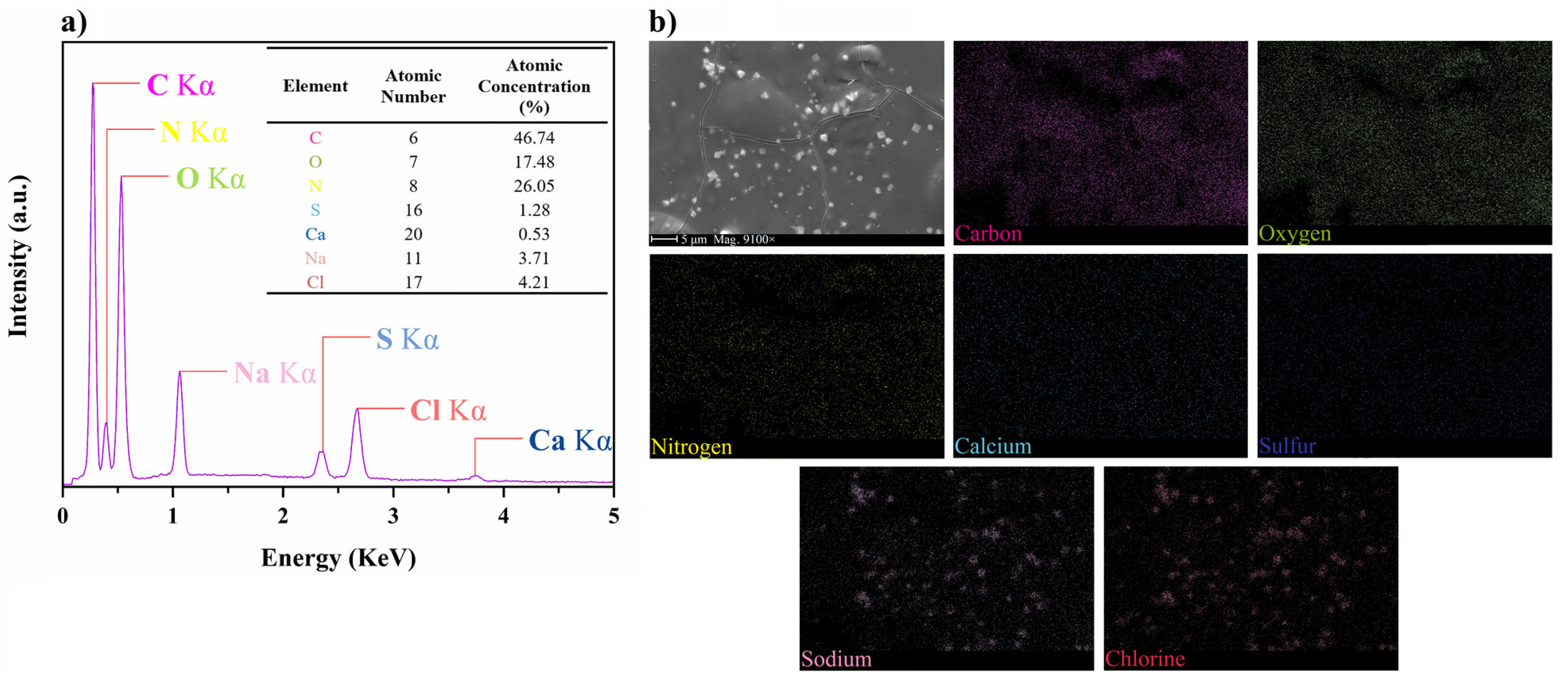
2.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.4. Atomic Force Microscopy (AFM) Analysis
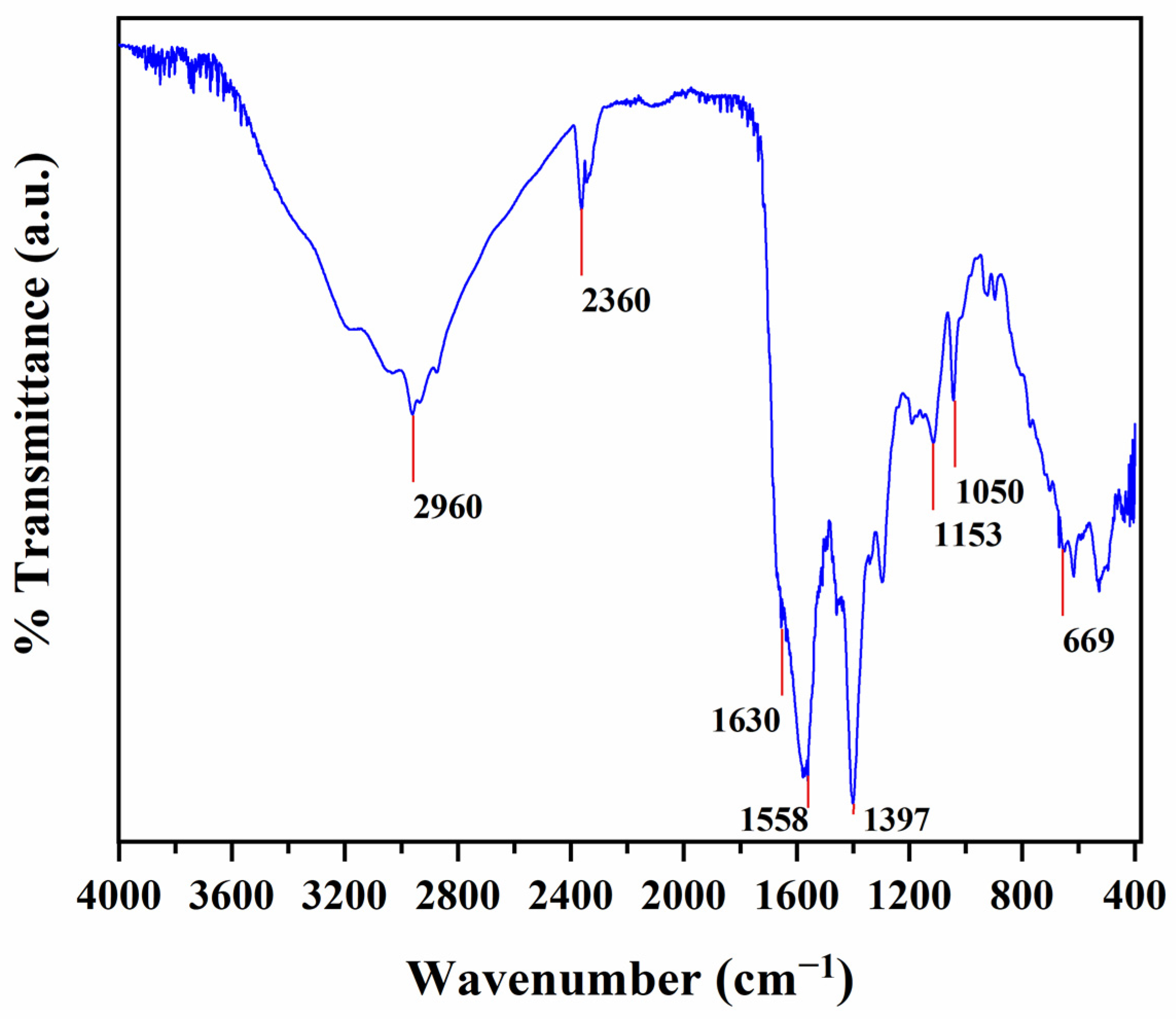
2.5. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FT-IR) Spectrometry
2.6. X-ray Photoelecton (XPS) Spectroscopy
2.7. Elemental Analysis
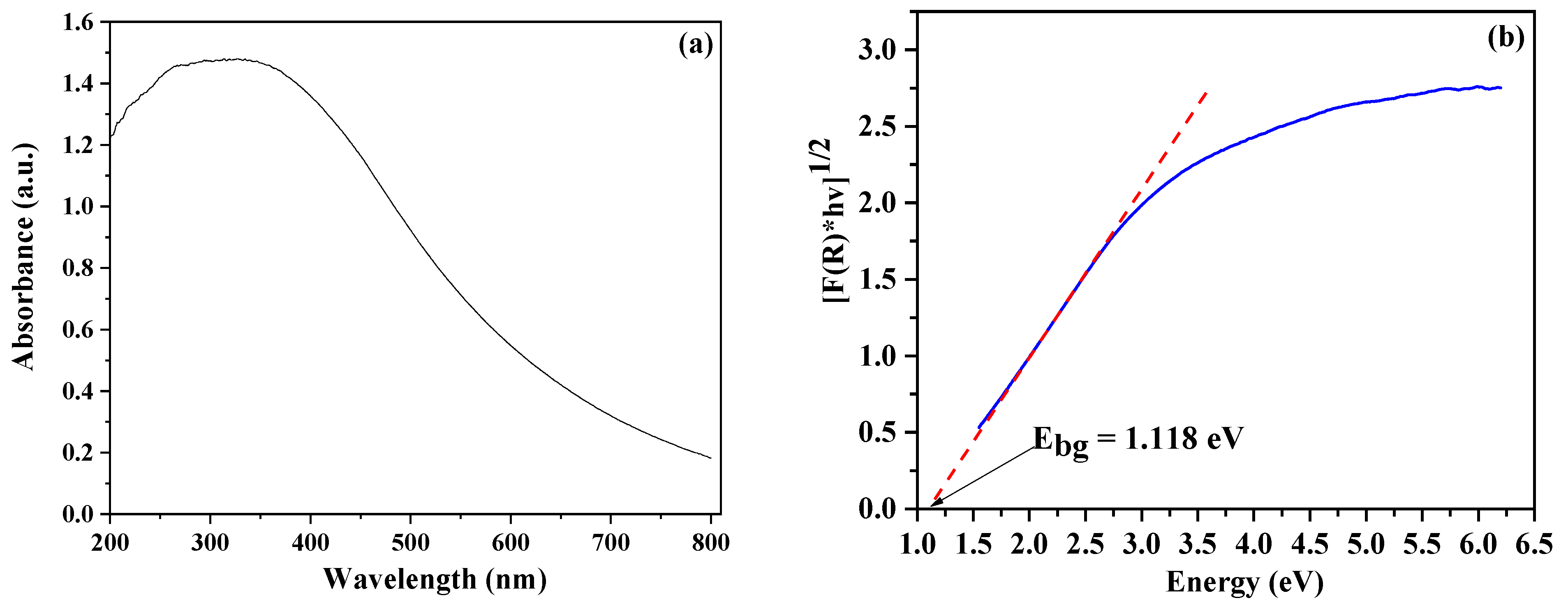
2.8. UV–Vis Diffuse Reflectance Spectroscopy (DRS)
2.9. Fluorescence Spectroscopy
2.10. Thermogravimetric Analysis
2.11. Ecotoxicity Assessment of N/S@Carbon QDs by Microtox Bioassay
2.12. In Vitro Determination of Antioxidant Activity
2.12.1. Preparation of DPPH Free Radical Standard Solution
2.12.2. Preparation of DPPH Free Radical Calibration Curve
2.12.3. Determination of the Effective Concentration (EC50) of N/S-Carbon QDs
2.13. Testing N/S-Carbon QDs in the Preservation of Fresh Strawberries
3. Results and Discussion
3.1. Material Characteriazation
3.1.1. Morphological and Structural Characteristics of N/S-Carbon QDs
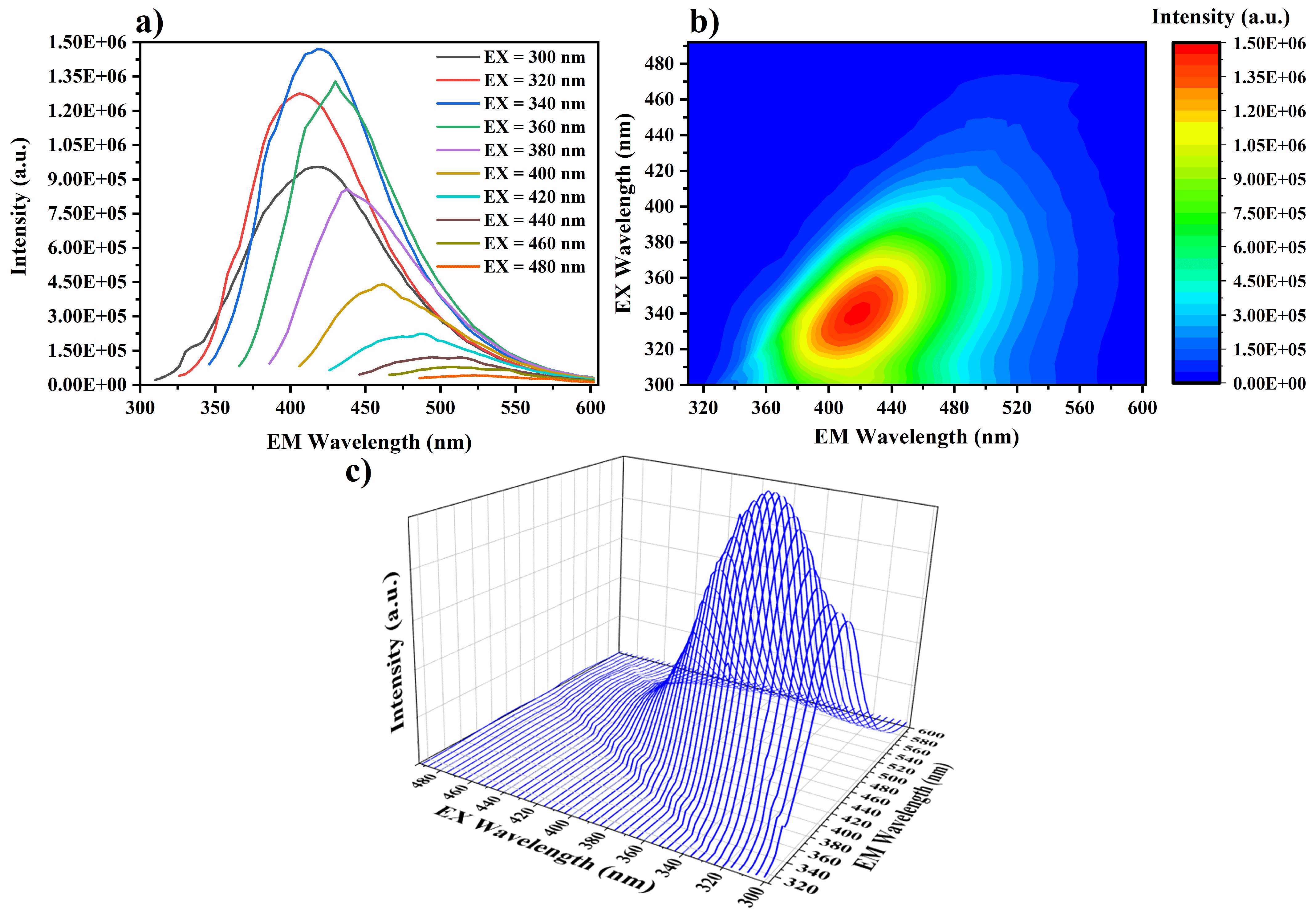
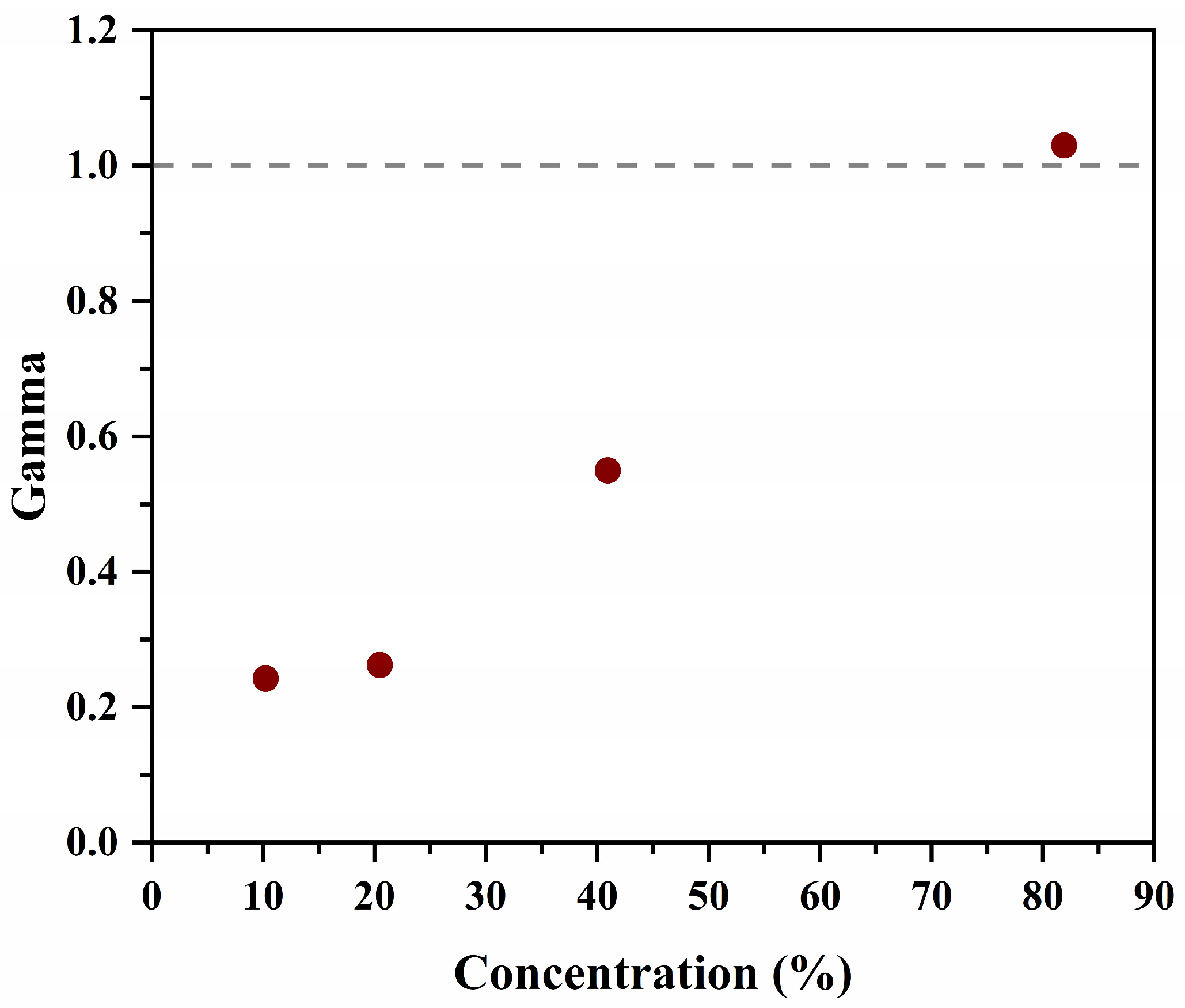
3.1.2. Optical Properties of N/S-Carbon QDs
3.1.3. Thermal Stability of N/S-Carbon QDs
3.1.4. Ecotoxicity Assessment of N/S-Carbon QDs
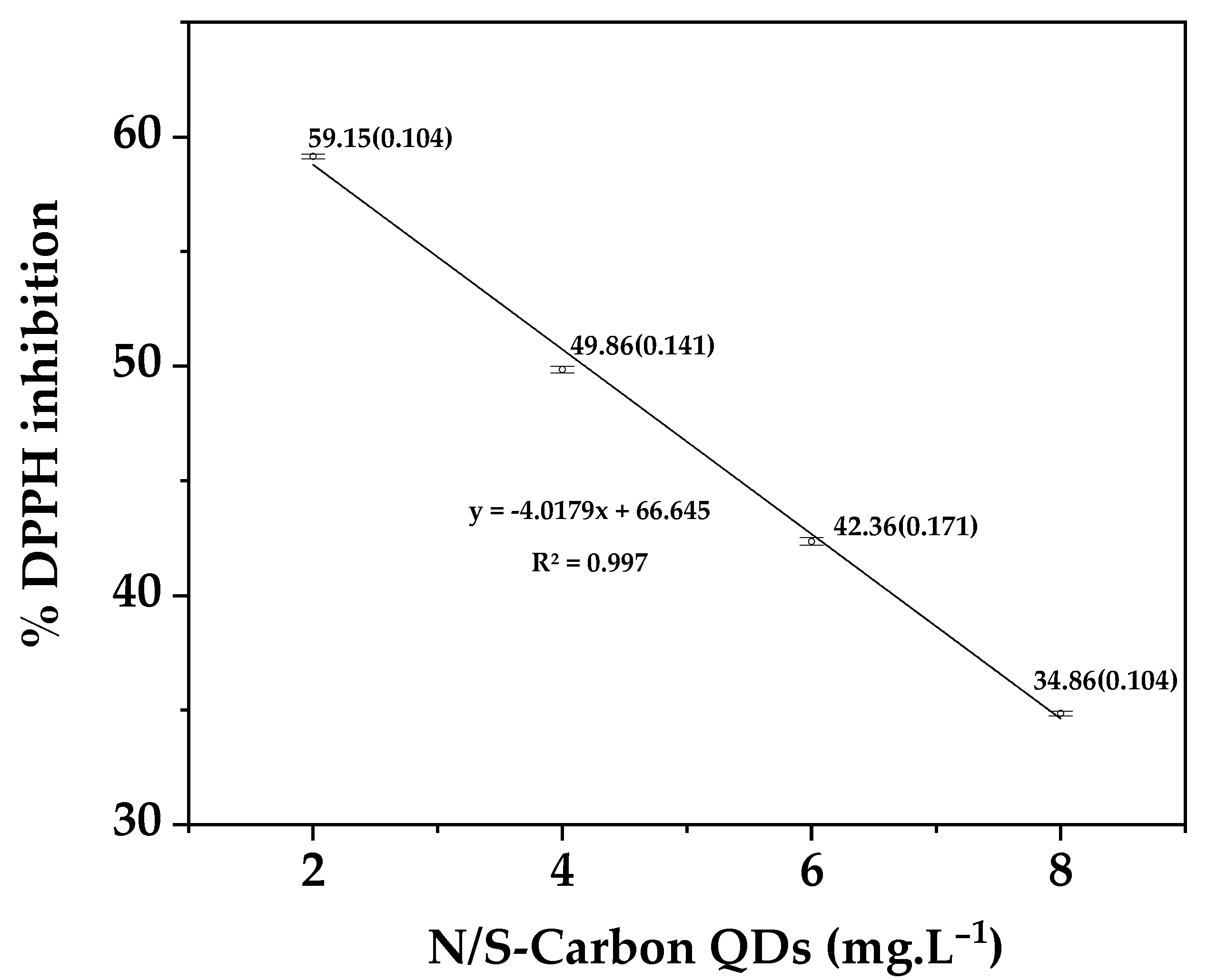
3.1.5. Antioxidant Activity of N/S-Carbon QDs
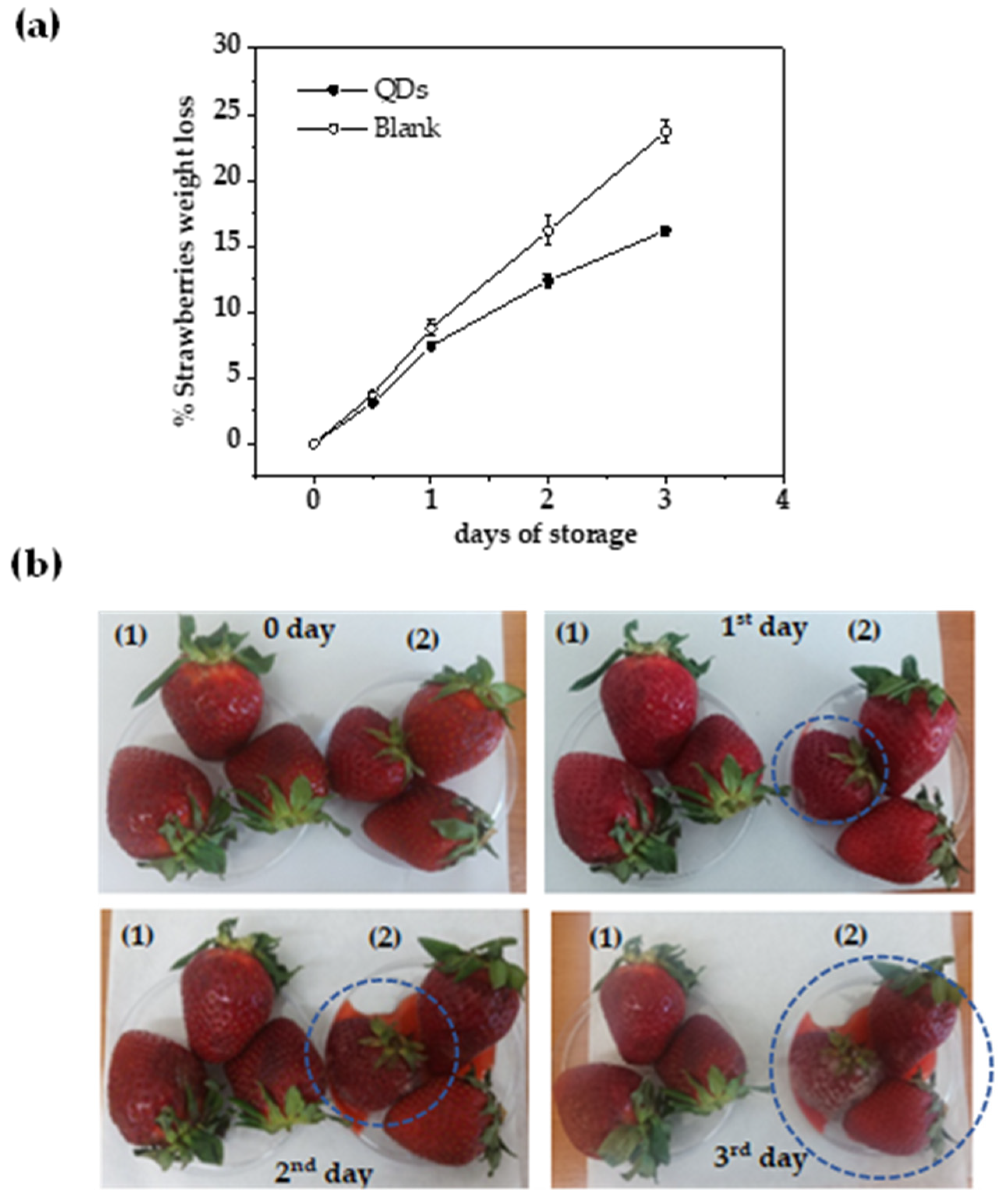
3.2. Testing N/S-Carbon QDs for the Preservation of Fresh Strawberries
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C. Food Waste as a Carbon Source in Carbon Quantum Dots Technology and Their Applications in Food Safety Detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.W.; Romainor, A.N.B.; Chin, S.F.; Ng, S.M. Carbon Dots Production via Pyrolysis of Sago Waste as Potential Probe for Metal Ions Sensing. J. Anal. Appl. Pyrolysis 2014, 105, 157–165. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Diao, H.; Chang, H.; Wang, H.; Li, T.; Wei, W. Green Synthesis of Carbon Dots from Rose-Heart Radish and Application for Fe3+ Detection and Cell Imaging. Sens. Actuators B Chem. 2017, 241, 190–198. [Google Scholar] [CrossRef]
- Janus, Ł.; Piątkowski, M.; Radwan-Pragłowska, J.; Bogdał, D.; Matysek, D. Chitosan-Based Carbon Quantum Dots for Biomedical Applications: Synthesis and Characterization. Nanomaterials 2019, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon Quantum Dots from Natural Resource: A Review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Khairol Anuar, N.K.; Tan, H.L.; Lim, Y.P.; So’aib, M.S.; Abu Bakar, N.F. A Review on Multifunctional Carbon-Dots Synthesized From Biomass Waste: Design/Fabrication, Characterization and Applications. Front. Energy Res. 2021, 9, 67. [Google Scholar]
- Chen, S.; Jiang, S.; Jiang, H. A Review on Conversion of Crayfish-Shell Derivatives to Functional Materials and Their Environmental Applications. J. Bioresour. Bioprod. 2020, 5, 238–247. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon Dots Prepared by Hydrothermal Treatment of Dopamine as an Effective Fluorescent Sensing Platform for the Label-Free Detection of Iron(III) Ions and Dopamine. Chem.—A Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef]
- Qian, Z.; Ma, J.; Shan, X.; Feng, H.; Shao, L.; Chen, J. Highly Luminescent N-Doped Carbon Quantum Dots as an Effective Multifunctional Fluorescence Sensing Platform. Chem.—A Eur. J. 2014, 20, 2254–2263. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal Treatment of Grass: A Low-Cost, Green Route to Nitrogen-Doped, Carbon-Rich, Photoluminescent Polymer Nanodots as an Effective Fluorescent Sensing Platform for Label-Free Detection of Cu(II) Ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Wang, H. Application of Carbon Dots in Food Preservation: A Critical Review for Packaging Enhancers and Food Preservatives. Crit. Rev. Food Sci. Nutr. 2022, 17, 1–19. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Kousheh, S.A.; Guimarães, J.T.; McClements, D.J. Carbon Dots Synthesized from Microorganisms and Food By-Products: Active and Smart Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 1943–1959. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Priyadarshi, R.; Roy, S.; Min, S.; Kim, Y.H.; Lee, S.-G.; Han, S. Preparation and Characterization of B, S, and N-Doped Glucose Carbon Dots: Antibacterial, Antifungal, and Antioxidant Activity. Sustain. Mater. Technol. 2022, 32, e00397. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Rezaei, Z. Carbon Quantum Dots-Based Antifungal Coating Film for Active Packaging Application of Avocado. Food Packag. Shelf Life 2022, 33, 100878. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Adhikari, B.; Wang, H. Preparation of a Novel Carbon Dot/Polyvinyl Alcohol Composite Film and Its Application in Food Preservation. ACS Appl Mater Interfaces 2022, 14, 37528–37539. [Google Scholar] [CrossRef]
- Khoshkalampour, A.; Ghorbani, M.; Ghasempour, Z. Cross-Linked Gelatin Film Enriched with Green Carbon Quantum Dots for Bioactive Food Packaging. Food Chem. 2023, 404, 134742. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Wang, P.; Yang, Y.; Wang, Y.; Xu, J.; Wang, Y.; Yu, W.W. Synthesis of Nitrogen and Sulfur Co-Doped Carbon Dots from Garlic for Selective Detection of Fe3+. Nanoscale Res. Lett. 2016, 11, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, H.; Xiao, Y.; Tang, J.; Liang, C.; Li, F.; Dong, H.; Xu, W. A Simple Approach for Synthesizing of Fluorescent Carbon Quantum Dots from Tofu Wastewater. Nanoscale Res. Lett. 2017, 12, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Li, W.; Wu, Q.; Liu, Y.; Liu, S. Hydrothermal Synthesis of Nitrogen-Doped Carbon Quantum Dots from Microcrystalline Cellulose for the Detection of Fe 3+ Ions in an Acidic Environment. RSC Adv. 2017, 7, 44144–44153. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Sheng, Z.; Han, H.; Zou, M.; Li, C. Facile Synthesis of Fluorescent Carbon Dots Using Watermelon Peel as a Carbon Source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Green Synthesis of Carbon Nanodots as an Effective Fluorescent Probe for Sensitive and Selective Detection of Mercury(II) Ions. J. Nanopart. Res. 2012, 15, 1344. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.S. Green Synthesis of Luminescent Nitrogen-Doped Carbon Dots from Milk and Its Imaging Application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef]
- Barati, A.; Shamsipur, M.; Arkan, E.; Hosseinzadeh, L.; Abdollahi, H. Synthesis of Biocompatible and Highly Photoluminescent Nitrogen Doped Carbon Dots from Lime: Analytical Applications and Optimization Using Response Surface Methodology. Mater. Sci. Eng. C 2015, 47, 325–332. [Google Scholar] [CrossRef]
- Chen, S.; Ouyang, W.; Zhu, Y.; He, L.; Zou, L.; Ao, X.; Liu, S.; Yang, Y.; Li, J. Facile Synthesis of N, S-Doped Carbon Quantum Dots from Food Waste as Fluorescent Probe for Sensitive Detection of Thiamphenicol and Its Analogues in Real Food Samples along with an Application in Bioimaging. Foods 2022, 11, 2414. [Google Scholar] [CrossRef]
- Ding, C.; Deng, Z.; Chen, J.; Jin, Y. One-Step Microwave Synthesis of N,S Co-Doped Carbon Dots from 1,6-Hexanediamine Dihydrochloride for Cell Imaging and Ion Detection. Colloids Surf. B Biointerfaces 2020, 189, 110838. [Google Scholar] [CrossRef]
- Smith, J.F.; Carlson, O.N.; Carlson, R.W. Allotropic Modifications of Calcium. J. Electrochem. Soc. 1956, 103, 409–413. [Google Scholar] [CrossRef]
- Elango, D.; Packialakshmi, J.S.; Manikandan, V.; Jayanthi, P. Sustainable Synthesis of Carbon Quantum Dots from Shrimp Shell and Its Emerging Applications. Mater. Lett. 2022, 312, 131667. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Arul, V.; Sethuraman, M.G. Ecofriendly Synthesis of Fluorescent Nitrogen-Doped Carbon Dots from Coccinia grandis and Its Efficient Catalytic Application in the Reduction of Methyl Orange. J. Fluoresc. 2020, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Weng, B.; Wang, B.; Li, C. Facile Synthesis of Nitrogen and Sulfur Co-Doped Carbon Dots and Application for Fe(III) Ions Detection and Cell Imaging. Sens. Actuators B Chem. 2016, 223, 689–696. [Google Scholar] [CrossRef]
- Liao, S.; Zhao, X.; Zhu, F.; Chen, M.; Wu, Z.; Song, X.; Yang, H.; Chen, X. Novel S, N-Doped Carbon Quantum Dot-Based “off-on” Fluorescent Sensor for Silver Ion and Cysteine. Talanta 2018, 180, 300–308. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Cai, C.; Lin, H. Conversion of Carbon Dots from Fluorescence to Ultralong Room-Temperature Phosphorescence by Heating for Security Applications. Adv Mater 2018, 30, e1800783. [Google Scholar] [CrossRef]
- Ren, S.; Liu, B.; Han, G.; Zhao, H.; Zhang, Y. Surface Chemistry in Calcium Capped Carbon Quantum Dots. Nanoscale 2021, 13, 12149–12156. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Mi, L.; Wang, P.-N. Improved Optical Response for N-Doped Anatase TiO2 Films Prepared by Pulsed Laser Deposition in N2/NH3/O2 Mixture. J. Cryst. Growth 2006, 289, 433–439. [Google Scholar] [CrossRef]
- Gomes, M.F.; Gomes, Y.F.; Lopes-Moriyama, A.; de Barros Neto, E.L.; de Souza, C.P. Design of Carbon Quantum Dots via Hydrothermal Carbonization Synthesis from Renewable Precursors. Biomass Convers. Bioref. 2019, 9, 689–694. [Google Scholar] [CrossRef]
- Tadesse, A.; Rama Devi, D.; Hagos, M.; Battu, G.; Basavaiah, K. Facile Green Synthesis of Fluorescent Carbon Quantum Dots from Citrus Lemon Juice for Live Cell Imaging. Asian J. Nanosci. Mater. 2018, 1, 36–46. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Soleimani Dinani, H.; Saeidi Tabar, F.; Khassi, K.; Janfaza, S.; Tasnim, N.; Hoorfar, M. Properties and Applications of Graphene and Its Derivatives in Biosensors for Cancer Detection: A Comprehensive Review. Biosensors 2022, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Li, P.; Zhang, Y.; Zhong, H.; Yan, H.; Su, W. Preparation, Characterization of Green Tea Carbon Quantum Dots/Curcumin Antioxidant and Antibacterial Nanocomposites. J. Mol. Struct. 2023, 1273, 134247. [Google Scholar] [CrossRef]
- Murru, C.; Badía-Laíño, R.; Díaz-García, M.E. Synthesis and Characterization of Green Carbon Dots for Scavenging Radical Oxygen Species in Aqueous and Oil Samples. Antioxidants 2020, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, T.; Sun, C.; Xia, J.; Jiao, Y.; Xu, H. Selenium-Doped Carbon Quantum Dots for Free-Radical Scavenging. Angew. Chem. Int. Ed. 2017, 56, 9910–9914. [Google Scholar] [CrossRef]
- Šafranko, S.; Stanković, A.; Hajra, S.; Kim, H.-J.; Strelec, I.; Dutour-Sikirić, M.; Weber, I.; Bosnar, M.H.; Grbčić, P.; Pavelić, S.K.; et al. Preparation of Multifunctional N-Doped Carbon Quantum Dots from Citrus Clementina Peel: Investigating Targeted Pharmacological Activities and the Potential Application for Fe3+ Sensing. Pharmaceuticals 2021, 14, 857. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| N% | C% | H% | S% | C/N |
|---|---|---|---|---|
| 10.3 | 53.6 | 7.1 | 6.3 | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kechagias, A.; Lykos, C.; Karabagias, V.K.; Georgopoulos, S.; Sakavitsi, V.; Leontiou, A.; Salmas, C.E.; Giannakas, A.E.; Konstantinou, I. Development and Characterization of N/S-Carbon Quantum Dots by Valorizing Greek Crayfish Food Waste. Appl. Sci. 2023, 13, 8730. https://doi.org/10.3390/app13158730
Kechagias A, Lykos C, Karabagias VK, Georgopoulos S, Sakavitsi V, Leontiou A, Salmas CE, Giannakas AE, Konstantinou I. Development and Characterization of N/S-Carbon Quantum Dots by Valorizing Greek Crayfish Food Waste. Applied Sciences. 2023; 13(15):8730. https://doi.org/10.3390/app13158730
Chicago/Turabian StyleKechagias, Achilleas, Christos Lykos, Vassilios K. Karabagias, Stavros Georgopoulos, Viktoria Sakavitsi, Areti Leontiou, Constantinos E. Salmas, Aris E. Giannakas, and Ioannis Konstantinou. 2023. "Development and Characterization of N/S-Carbon Quantum Dots by Valorizing Greek Crayfish Food Waste" Applied Sciences 13, no. 15: 8730. https://doi.org/10.3390/app13158730








