The Impact of Organic, Inorganic Fertilizers, and Biochar on Phytochemicals Content of Three Brassicaceae Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Turnips Field and Experimental Design
2.2. Arugula and Mustard Field Experimental Design
2.3. Glucosinolates (GSLs) Analysis
2.4. Vitamin C, Total Phenols, and Free Sugars Analysis
2.5. Soil Enzymes Analysis
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanate among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Merillon, J.M.; Ramawat, K.G. Glucosinolates; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Bednarek, P.; Pislewska-Bednarek, M.; Svatos, A.; Schneider, B.; Doubsky, J.; Mansurova, M.; Schulze-Lefert, P. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef]
- Beekwilder, J.; van Leeuwen, W.; van Dam, N.M.; Bertossi, M.; Grandi, V.; Mizzi, L.; Schipper, B. The impact of the absence of aliphatic glucosinolates on insect herbivory in Arabidopsis. PLoS ONE 2008, 3, e2068. [Google Scholar] [CrossRef]
- Andréasson, E.; Jørgensen, L.B. Localization of plant myrosinases and glucosinolates. In Integrative Phytochemistry: From Ethnobotany to Molecular Ecology: Recent Advances in Phytochemistry; Romeo, J.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 37, pp. 79–99. [Google Scholar]
- Cox, C. Metam sodium. J. Pesticide Reform. 2006, 26, 12–16. [Google Scholar]
- Vig, A.; Rampal, G.; Thinda, T.H.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Larkin, R.P.; Griffin, T.S. Control of soilborne potato diseases using Brassica green manure. Crop Protect. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.S.; Simoes, M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. J. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A.; Hansen, H.C.B. Extraction of glucosinolates from soil. J. Agric. Food Chem. 2005, 53, 9663–9667. [Google Scholar] [CrossRef]
- Borek, V.; Elberson, L.R.; McCaffery, J.P.; Morra, M.J. Toxicity of rapeseed meal and methyl isothiocyantate to larvae of the black vine weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 1997, 90, 109–112. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Control of soil-borne plant pests using glucosinolate-containing plants. Advan. Agron. 1997, 61, 167–231. [Google Scholar]
- Rosa, E.A.S.; Heaney, R.K.; Fenwick, G.R.; Portas, C.A.M. Glucosinolates in crop plants. Hort. Rev. 1997, 19, 99–215. [Google Scholar]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolate and isothiocyanate concentrations in soil following incorporation of Brassica biofumigants. Soil Biol. Biochem. 2006, 38, 2255–2264. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Antonious, A.G.; Trivette, T. Emerging technology for increasing glucosinolates in arugula and mustard greens. J. Environ. Sci. Health Part B 2017, 52, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.F. Glucosinolates in collard greens grown under three soil management practices. J. Environ. Sci. Health Part B 2015, 50, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.F. Emerging technology in glucosinolates biofumigation. In Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Science Publishers: New York, NY, USA, 2019; Chapter 6; Volume 25, pp. 183–211. [Google Scholar]
- Antonious, G.F.; Kasperbauer, M.J.; Byers, M.E. Light reflected from colored mulches to growing turnip leaves affects glucosinolates and sugars of edible roots. Photochem. Photobiol. 1996, 64, 605–610. [Google Scholar] [CrossRef]
- Renner, R. Rethinking biochar. Environ. Sci. Technol. 2007, 41, 5932–5933. [Google Scholar] [CrossRef]
- Shen, Z.; Som, A.M.; Wang, F.; Jin, F.; Mcmillan, O.; Al-Tabbaa, A. Long-term impact of biochar on the immobilization of nickel (II) and zinc (II) and the revegetation of a contaminated site. Sci. Total Enviro. 2016, 542, 771–776. [Google Scholar] [CrossRef]
- Rudolph, R.; Pfeufer, E.; Bessin, R.; Wright, S.; Strang, J. Vegetable Production Guide for Commercial Growers; University of Kentucky, College of Agriculture, Food and Environment Cooperative Extension Service: Lexington, KY, USA, 2020. [Google Scholar]
- McGrath, R.M.; Kaluza, W.Z.; Daiber, K.H.; Van der Riet, W.R.; Glennie, C.W. Polyphenols of sorghum grain, their change during malting and their inhibitory nature. J. Agric. Food Chem. 1982, 30, 450–456. [Google Scholar] [CrossRef]
- Antonious, G.F.; Kasperbauer, M.J. Color of light reflected to leaves modify nutrient content of carrot roots. Crop Sci. 2002, 42, 1211–1216. [Google Scholar] [CrossRef]
- Hashmi, M.H. Spectrophotometric determination with potassium ferricyanide. In Assay of Vitamins in Pharmaceutical Preparations; John Wiley and Sons: Hoboken, NJ, USA, 1973. [Google Scholar]
- VanEtten, C.H.; McGrew, C.E.; Doernbecher, M.E. Glucosinolate determination in Cruciferous seeds and meals by measurement of enzymatically related glucose. J. Agric. Food Chem. 1974, 22, 483–487. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT Guide, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Tabatabi, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Bagyaraj, D.J.; Rangaswami, G. Studies on the influence of foliar application of chemicals on the microflora and certain enzyme activities in the rhizosphere of Eleusine coracana Gaertn. Plant Soil 1970, 32, 198–206. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Hein, T. Not Your Grandpa’s Poultry Litter Stockpile; Manure Manager: Simcoe, ON, Canada, 2016; pp. 10–12. [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Antonious, G.F.; Perkins, E.; Cantor, A. Chicken manure increased concentration of organic sulfur compounds in field-grown onions. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2009, B44, 481–487. [Google Scholar] [CrossRef]
- Hell, R. Molecular physiology of plant sulfur metabolism. Planta 1997, 202, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Leustek, T.; Martin, M.N.; Bick, J.A.; Davis, J.P. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Schonhof, I.; Blankenburg, D.; Müller, S.; Krumbein, A. Sulfur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J. Plant Nutr. Soil Sci. 2007, 170, 65–72. [Google Scholar] [CrossRef]
- Li, S.; Schonhof, I.; Krumbein, A.; Li, L.; Stützel, H.; Schreiner, M. Glucosinolate Concentration in Turnip (Brassica rapa ssp. rapifera L.) Roots as Affected by Nitrogen and Sulfur Supply. J. Agric. Food Chem. 2007, 55, 8452–8457. [Google Scholar]
- Miller, C.V.; Hancock, T.C.; Denver, J.M. Environmental Fate and Transport of Arsenical Feed Amendments for Animal Agriculture. In Proceedings of the American Geophysical Union, 2000 Spring Meeting: Integrative Geoscience Solutions—A Start for the New Millennium, Washington, DC, USA, 30 May–3 June 2000. [Google Scholar]
- Kpomblekou, A.K.; Ankumah, R.O.; Ajwa, H.A. Trace and non-trace element con-tents of broiler litter. Commun. Soil Sci. Plant Anal. 2002, 33, 1799–1811. [Google Scholar] [CrossRef]
- Brown, B.L.; Slaughter, A.D.; Schreiber, M.E. Control of roxarosone transport in agricultural watersheds. Appl. Geochem. 2005, 20, 123–133. [Google Scholar] [CrossRef]
- Nigra, A.E.; Nachman, K.E.; Love, D.C.; Grau-Perez, M.; Navas-Acien, A. Poultry consumption and arsenic exposure in the U.S. population. Environ. Health Perspect. 2017, 125, 370–377. [Google Scholar] [CrossRef]
- Bellows, B.C. Arsenic in Poultry Litter: Organic Regulations. A Publication of the National Sustainable Agriculture Information Service. 2005. Available online: www.attra.ncat.org (accessed on 29 July 2023).
- Chastain, J.P.; Camberto, J.J.; Peterkewes, L. Poultry Manure Production and Nutrient Content. South Carolina Confined Animal Manure Managers Certification Program–Poultry; Clemson University: Clemson, SC, USA, 2002. [Google Scholar]
- Brinton, W. Compost Quality in America. Woods Hole Research Laboratory. Prepared for New York State Association of Recyclers. 2000. Available online: http://compost.css.cornell.edu/Brinton.pdf (accessed on 29 July 2023).
- Association for the Environmental Health of Soils (AEHS 2013). Study of State Soil Arsenic Regulations—RT Environmental. Amherst, MA 01002, PADEP › attachment3. Available online: http://www.rtenv.com (accessed on 29 July 2023).
- Fofaria, N.M.; Ranjan, A.; Kim, S.-H.; Srivastava, S.K. Mechanisms of the Anticancer Effects of Isothiocyanates. Enzymes 2015, 37, 111–137. [Google Scholar]
- Tang, L.; Zirpoli, G.R.; Guru, K.; Moysich, K.B.; Zhang, Y.; Ambrosone, C.B.; McCann, S.E. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Boreddy, S.R.; Srivastava, S.K. Pancreatic cancer chemoprevention by phytochemicals. Cancer Lett. 2013, 334, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Fowke, J.H.; Chung, F.L.; Jin, F.; Qi, D.; Cai, Q.; Conaway, C.; Cheng, J.R.; Shu, X.O.; Gao, Y.T.; Zheng, W. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003, 63, 3980–3986. [Google Scholar] [PubMed]
- Ambrosone, C.B.; McCann, E.B.; Freudenheim, J.L.; Marshall, J.R.; Zhang, Y.; Shields, B.G. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J. Nutr. 2004, 134, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Negri, E.; Franceschi, S.; Pelucchi, C.; Talamini, R.; Montella, M.; Conti, E.; La Vecchia, C. Diet and ovarian cancer risk: A case-control study in Italy. Int. J. Cancer. 2001, 93, 911–915. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Dawood, M.H. Duality of Biochar Impact on Soil Enzymes Activity. In Fruit and Vegetable Research Report; December 2020 Annual Research Report; University of Kentucky, College of Agriculture, Food and Environment: Lexington, KY, USA, 2020; pp. 28–31. [Google Scholar]
- Yang, Z.; Liu, S.; Zheng, D.; Feng, S. Effects of cadium, zinc and lead on soil enzyme activities. J. Environ. Sci. 2006, 18, 1135–1141. [Google Scholar] [CrossRef]
- Asad, S.A.; Young, S.; West, H. Effect of nickel and cadmium on glucosinolate production in Thlaspi caerulescens. Pak. J. Bot. 2013, 45, 495–500. [Google Scholar]
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard-Nielsen, H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012, 46, 73–79. [Google Scholar] [CrossRef]
- Galvez, A.; Sinicco, T.; Cayuela, M.L.; Mingorance, M.D.; Fornasier, F.; Mondini. C. Short term effects of bioenergy by-products on soil C and N dynamics, nutrient availability and biochemical properties. Agric. Ecosyst. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Devonshire, B.J.; Brookes, P.C. Microbial biomass growth, following incorporation of biochars produced at 350 °C or 700 °C, in a silty-clay loam soil of high and low pH. Soil Biol. Biochem. 2013, 57, 513–523. [Google Scholar] [CrossRef]
- Antonious, G.F. Biochar and animal manure impact on soil, crop yield and quality. In Agricultural Waste; Aladjadjiyan, A., Ed.; National Biomass Association, Bulgaria & Intech- Open Science Books: Rijeka, Croatia, 2018. [Google Scholar]
- Antonious, G.F. Soil amendments for agricultural production. In Organic Fertilizers: From Basic Concepts to Applied Outcomes; Larramendy, M.L., Soloneski, S., Eds.; Intech: Rijeka, Croatia, 2016; Chapter 7; pp. 157–187. [Google Scholar]
- Handisenim, M.; Zhou, X.-G.; Jo, Y.-K. Soil amended with Brassica juncea plant tissue reduces sclerotia formation, viability and aggressiveness of Rhizoctonia solani AG1-IA towards rice. Crop Prot. 2017, 100, 77–80. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. A novel approach for reliable activity determination of ascorbic acid depending myrosinases. J. Biochem. Biophys. Methods 2004, 59, 253–265. [Google Scholar] [CrossRef]
- Antonious, G.F.; Dawood, M.H.; Turley, E.T.; Paxton, R.B. Yield and quality of lettuce, pumpkin, and watermelon varieties grown under five soil management practices. Int. J. Appl. Agric. Sci. 2021, 7, 57–65. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Kochhar, T.S. Testing bioaccumulation of Cd, Pb, and Ni in Plants-grown in soil amended with municipal sewage sludge at three Kentucky locations. J. Environ. Sci. Ecol. 2017, 5, 1–10. [Google Scholar]
- Antonious, G.F. Reducing herbicides in agricultural runoff and seepage water. In Herbicides, Physiology of Action, and Safety; Andrew, P., Linda, S., Jessica, K., Eds.; Intech: Rijeka, Croatia, 2015; Chapter 1; pp. 3–34. [Google Scholar]
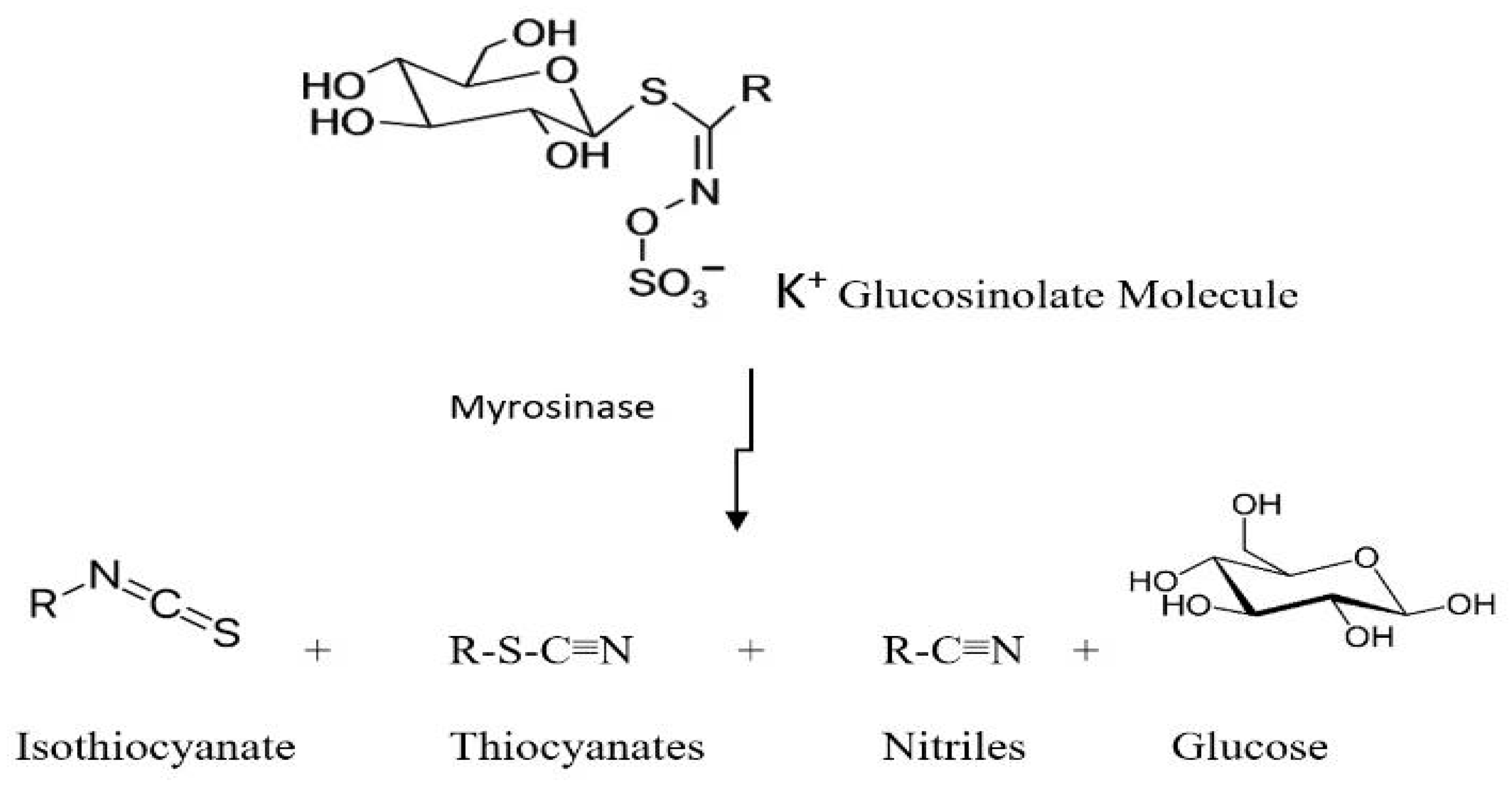
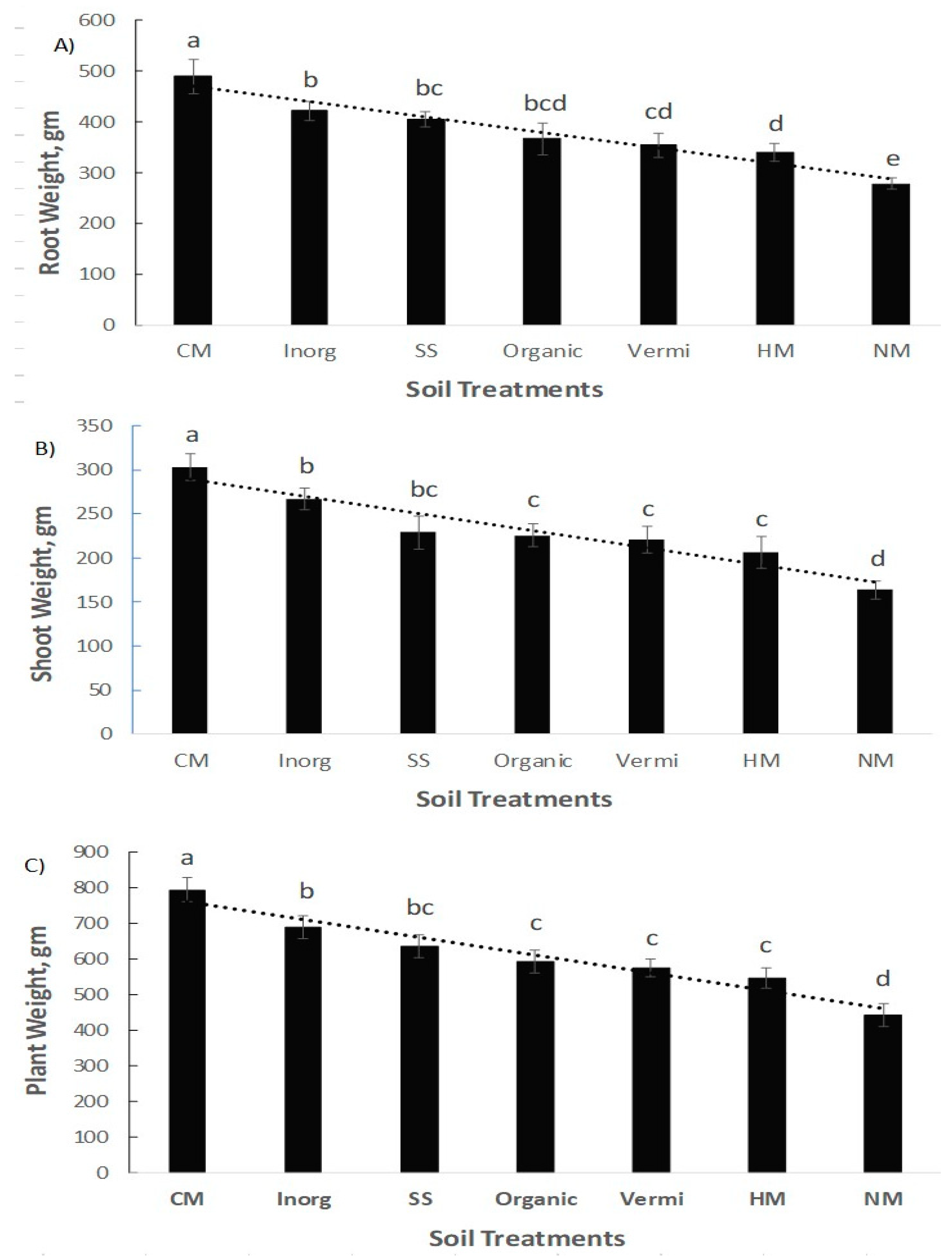
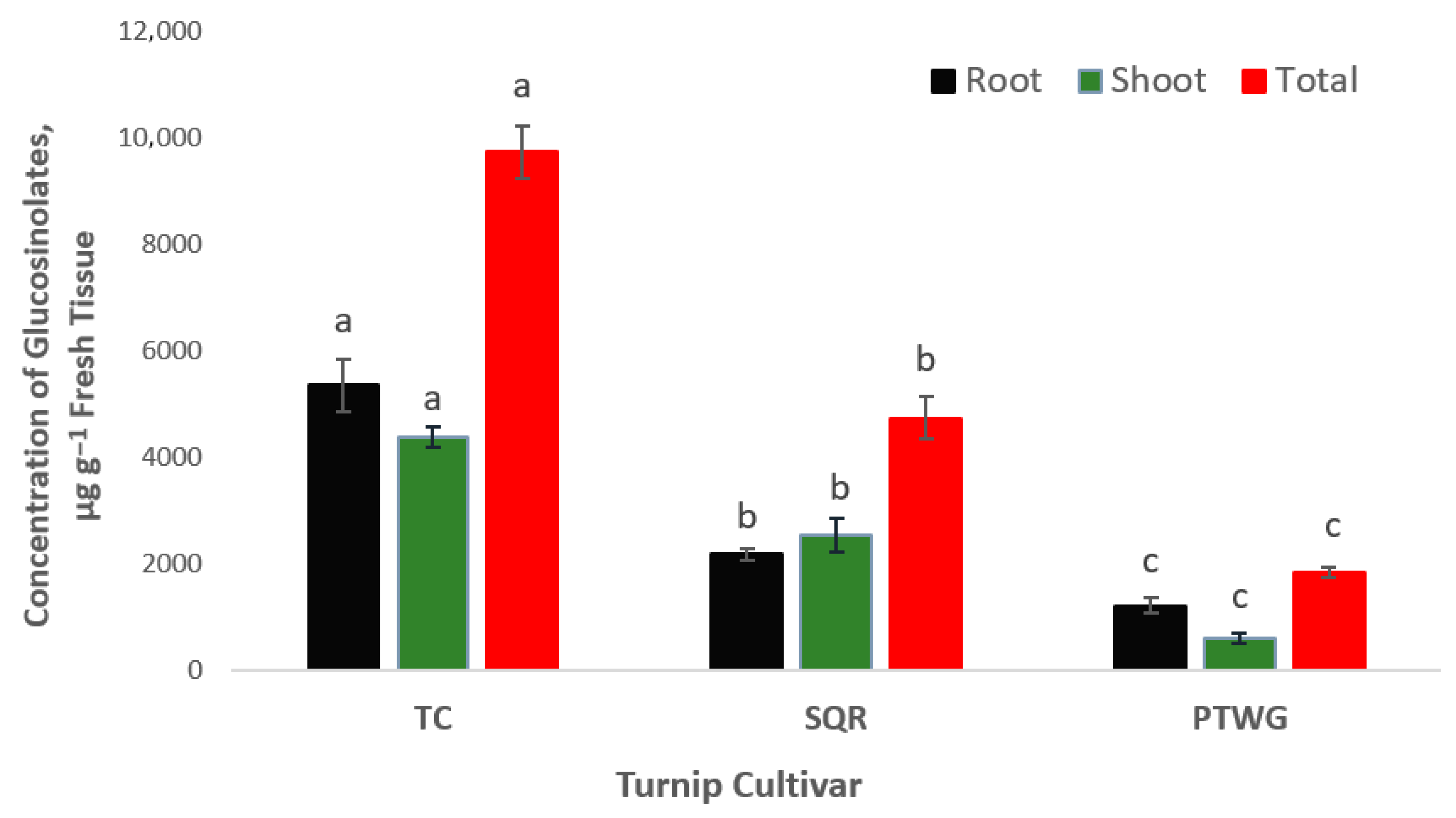
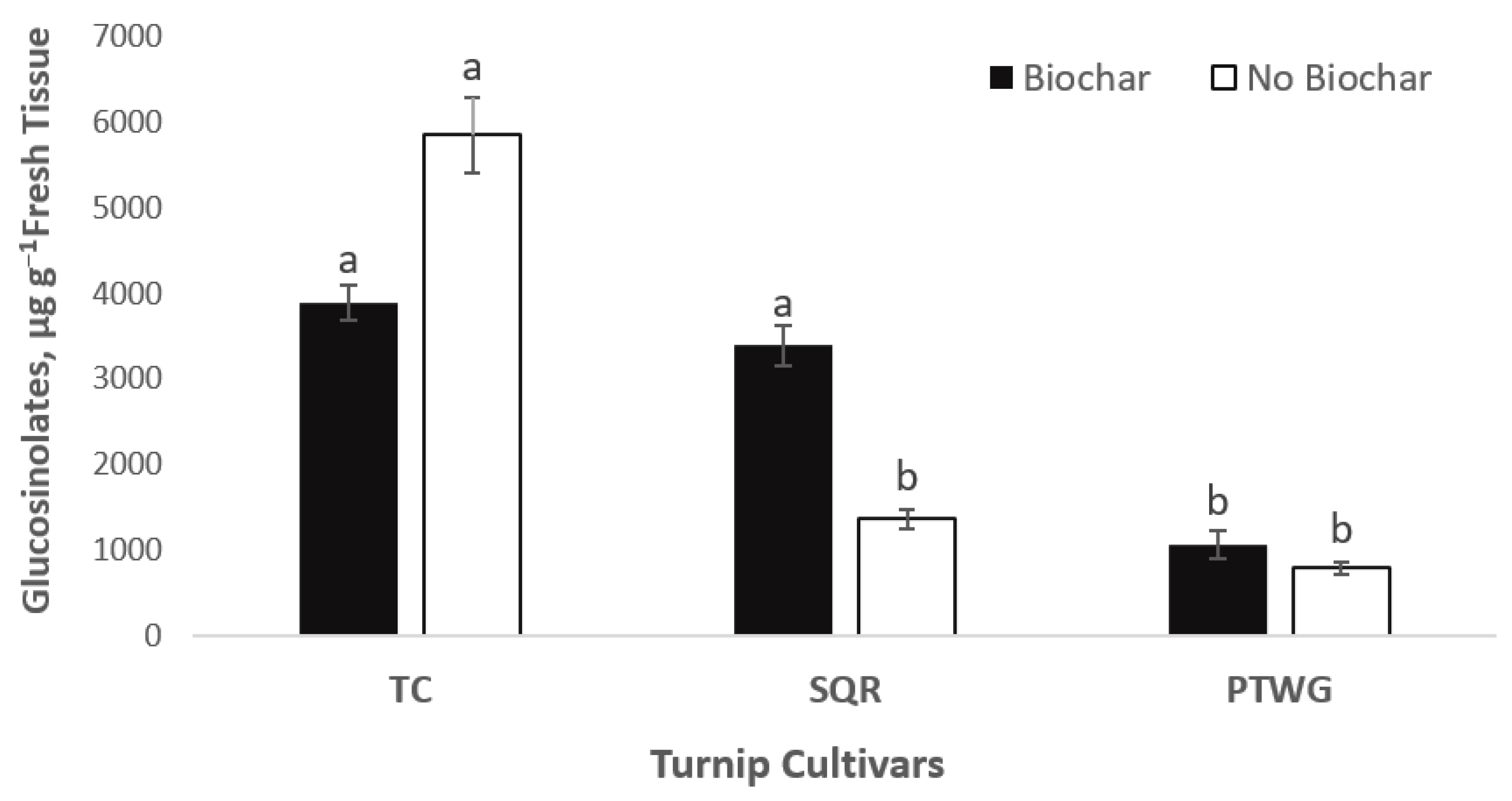
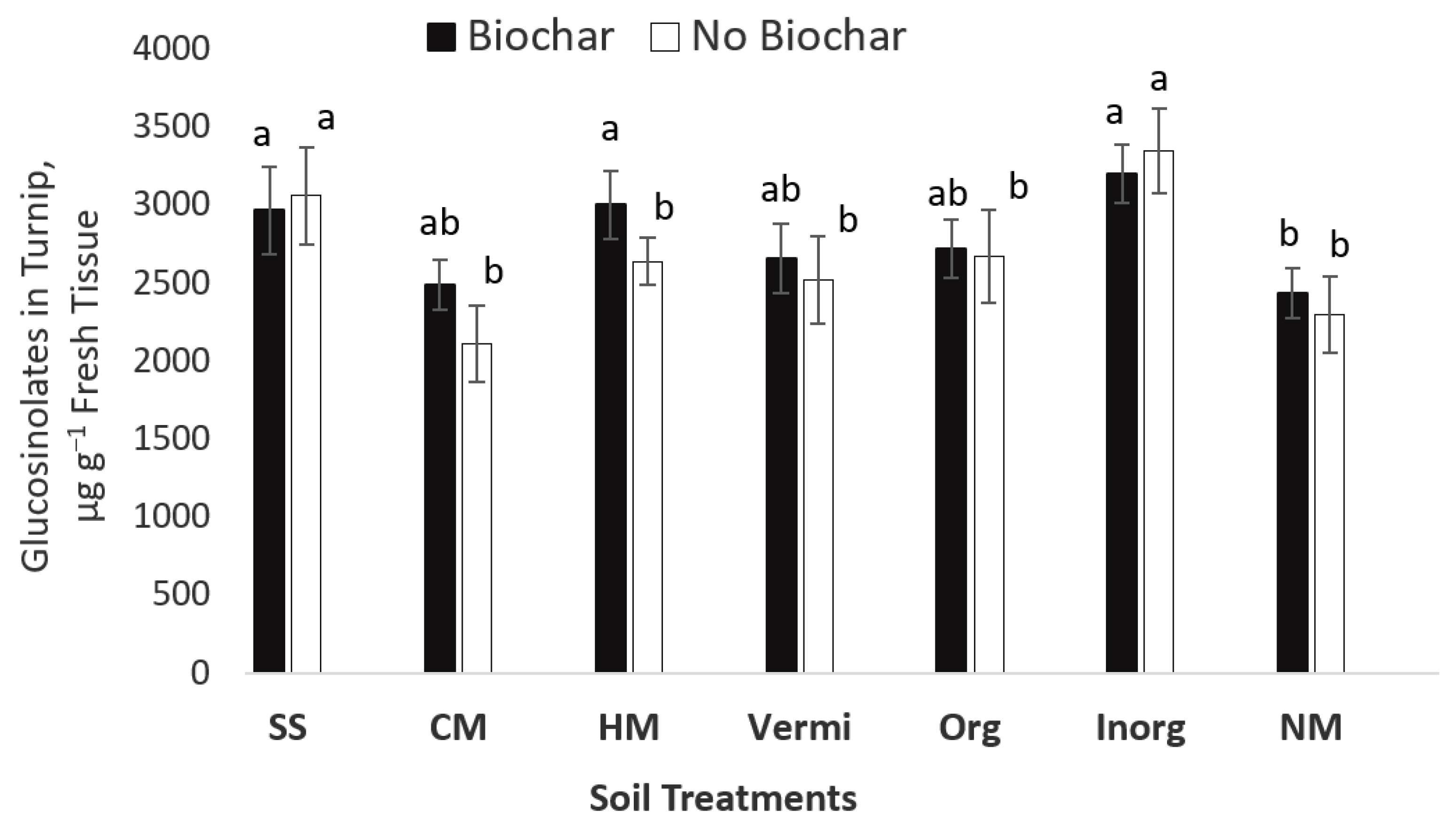
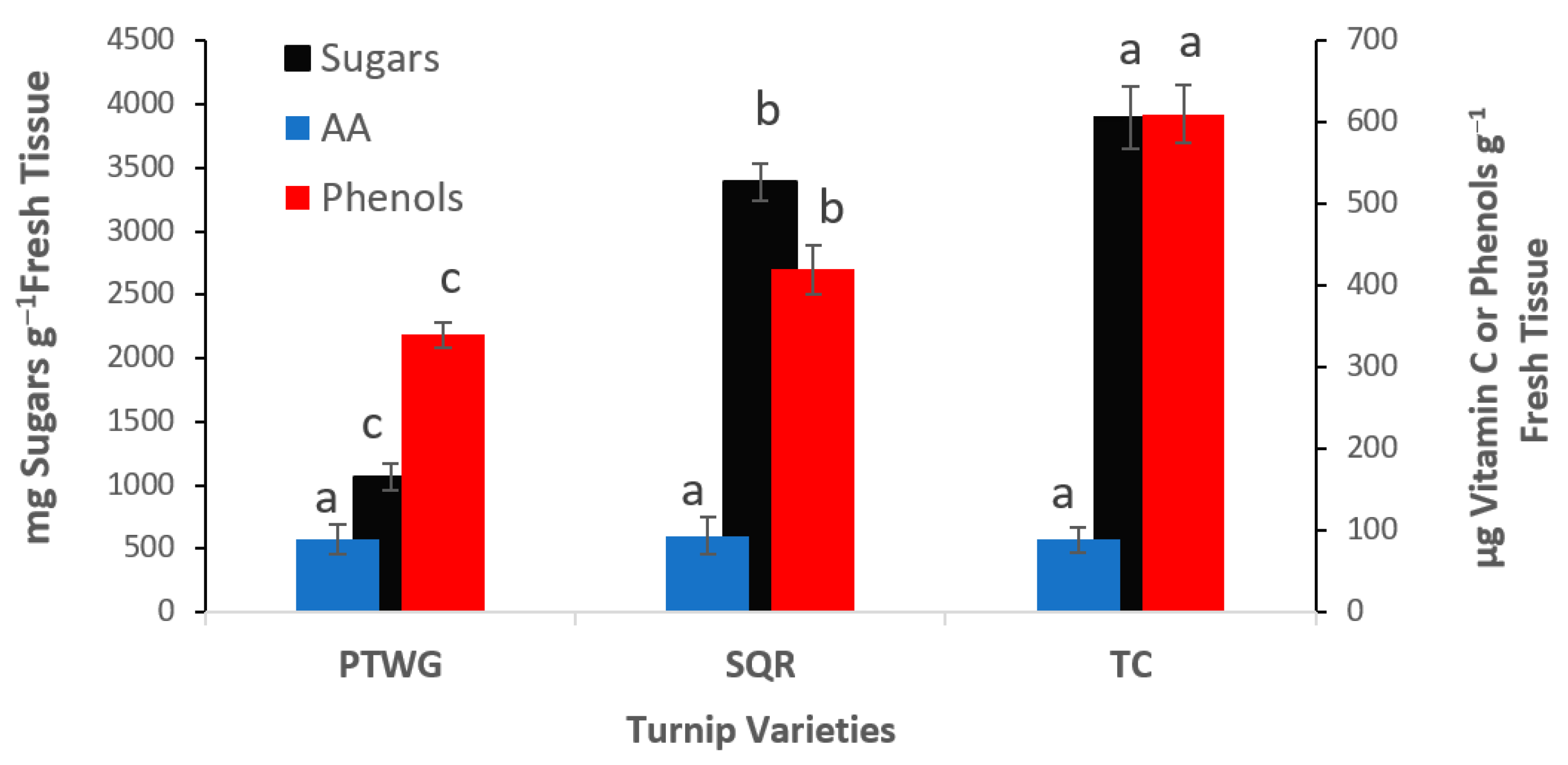
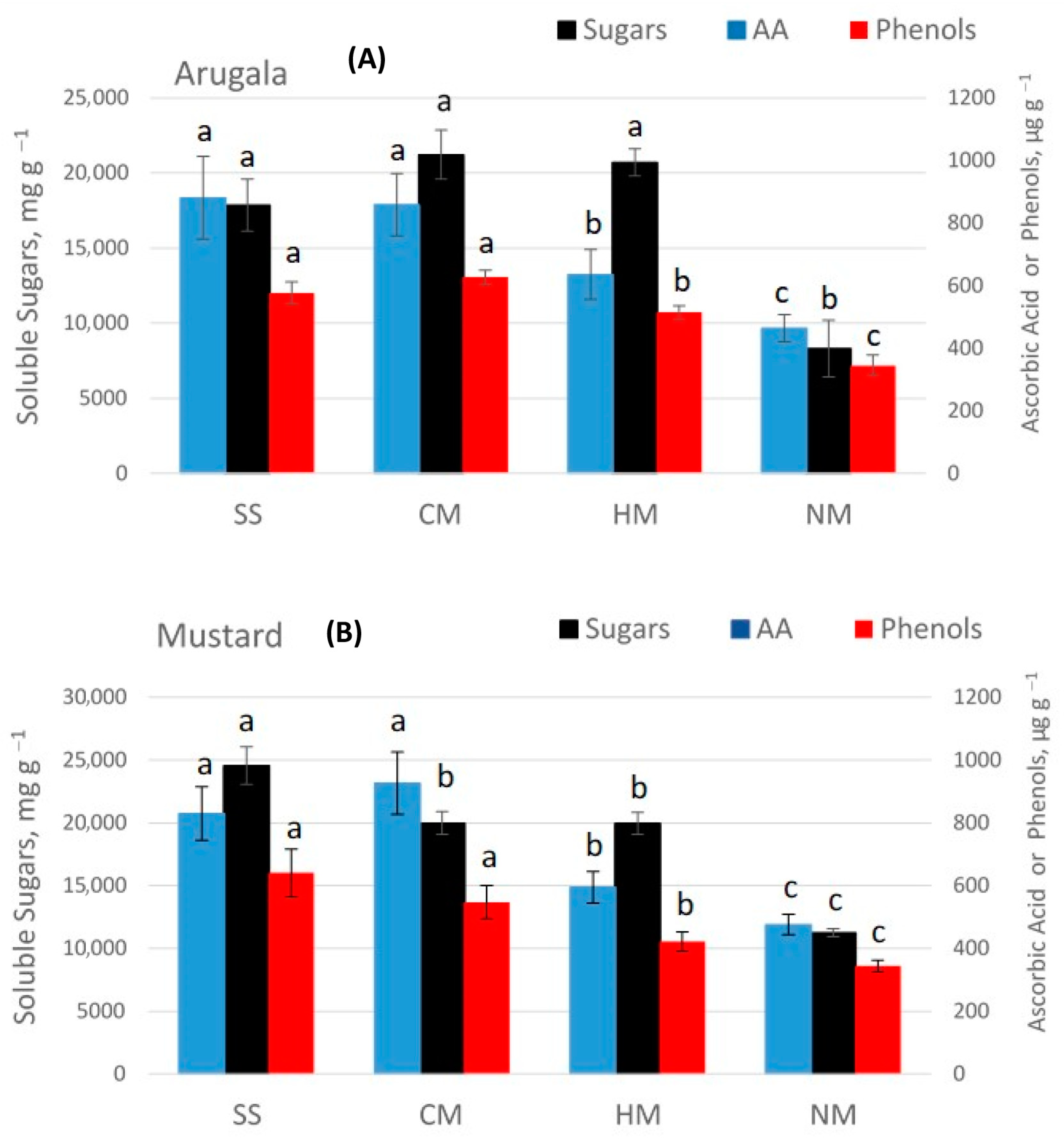


| Soil Properties | Sewage Sludge | Chicken Manure | Horse Manure | Vermi- Compost | Native Soil |
|---|---|---|---|---|---|
| Soil-Water pH | 5.67 b | 6.20 a | 5.64 b | 5.71 b | 6.1 a |
| N-NO3, mgkg−1 | 20.1 c | 18.3 d | 25.0 b | 37.3 a | 20.7 c |
| N-NH4, mgkg−1 | 29.7 b | 66.7 a | 3.33 c | 3.67 c | 5.67 c |
| P, mg kg−1 | 100.3 a | 89.3 a | 116.00 a | 87.67 a | 95.83 a |
| K, mg kg−1 | 327.5 d | 483.8 b | 365.5 cd | 557.3 a | 336.2 d |
| C, mg kg−1 | 1050.0 c | 1160.8 b | 1067.2 c | 1230.2 a | 1091.7 c |
| EC, µS cm−1 | 106.4 ab | 95.83 b | 89.3 b | 122.3 a | 94.4 b |
| Cd, mg kg−1 | 0.043 a | 0.043 a | 0.042 a | 0.040 a | 0.041 a |
| Cr, mg kg−1 | 0.04 a | 0.04 a | 0.04 a | 0.04 a | 0.04 a |
| Cu, mg kg−1 | 1.93 a | 2.01 a | 1.89 a | 2.0 a | 1.95 a |
| Zn, mg kg−1 | 2.27 a | 1.96 a | 2.04 a | 4.18 a | 1.98 a |
| Pb, mg kg−1 | 2.21 a | 2.18 a | 2.14 a | 2.11 a | 2.15 a |
| As, mg kg−1 | 8.62 a | 8.84 a | 8.22 a | 8.34 a | 8.35 a |
| Ni, mg kg−1 | 0.699 a | 0.675 a | 0.680 a | 0.683 a | 0.666 a |
| Urease 1 | 956.3 b | 993 b | 805.8 c | 1105. a | 911.8 b |
| Invertase 2 | 3696.9 a | 3736 a | 3610 a | 3970.3 a | 3234.2 b |
| Acid Phosphatase 3 | 1435.4 a | 1158.3 b | 1197.3 b | 1413.6 a | 1354.9 a |
| Alkaline Phosphatase 4 | 389.3 c | 524.43 a | 400.67 b | 309.4 c | 427.62 b |
| Soil Amendment | Nitrogen (% N) | Phosphorus (% P) | Potassium (% K) |
|---|---|---|---|
| Sewage Sludge | 5.00 | 3.00 | 0.00 |
| Chicken Manure | 1.10 | 0.80 | 0.50 |
| Horse Manure | 0.70 | 0.30 | 0.60 |
| Vermicompost | 1.50 | 0.75 | 1.50 |
| Organic Fertilizer | 10.00 | 2.00 | 8.00 |
| Inorganic Fertilizer | 20.00 | 20.00 | 20.00 |
| Amounts of Soil Amendments Added in kg/ha | |||
| Soil Amendment | Nitrogen (N) | Phosphorus (P) | Potassium (K) |
| Sewage Sludge | 2241.70 | 3736.17 | 0.00 |
| Chicken Manure | 10,189.62 | 14,010.64 | 22,417.02 |
| Horse Manure | 16,012.15 | 37,361.70 | 18,680.86 |
| Vermicompost | 7472.34 | 14,944.68 | 7472.34 |
| Organic Fertilizer | 1120.85 | 5604.26 | 1401.06 |
| Inorganic Fertilizer | 560.43 | 560.43 | 560.43 |
| (A) PTWG | Glucosinolates, µg g−1 Turnip Fresh Tissue | ||
|---|---|---|---|
| Soil Treatment | Shoot | Root | Total |
| Sewage Sludge | 1481 ± 17.1 a | 544 ± 179.3 a | 2024 ± 162.9 a |
| Chicken Manure | 903 ± 59.1 a | 609 ± 257.1 a | 1512 ± 190.0 ab |
| Horse Manure | 1163 ± 73.8 a | 454 ± 378.9 a | 1616 ± 391.9 ab |
| Vermicompost | 1024 ± 81.8 a | 435 ± 235.8 a | 1460 ± 160.9 ab |
| Organic Fertilizer | 1272 ± 55.1 a | 403 ± 382.9 a | 1674 ± 427.8 ab |
| Inorganic Fertilizer | 1260 ± 107.5 a | 476 ± 80.7 a | 1736 ± 65.9 a |
| No Amendment Native Soil | 759 ± 77.8 a | 160 ± 155.2 b | 919 ± 200.1 b |
| (B)SQR | |||
| Soil Treatment | Shoot | Root | Total |
| Sewage Sludge | 654.2 ± 141.3 a | 1782.9 ± 286.4 a | 2437.0 ± 145.7 ab |
| Chicken Manure | 1542.6 ± 679.4 a | 2240.4 ± 595.4 a | 3783.0 ± 1274.8 a |
| Horse Manure | 828.6 ± 36.6 a | 2037.2 ± 620.3 a | 2865.8 ± 613.4 ab |
| Vermicompost | 640.4 ± 159.8 a | 1662.4 ± 222.3 a | 2302.8 ± 321.0 ab |
| Organic Fertilizer | 743.8 ± 194.6 a | 2251.4 ± 332.1 a | 2995.2 ± 203.8 ab |
| Inorganic Fertilizer | 906.3 ± 351.3 a | 1468.0 ± 142.3 a | 2374.3 ± 411.5 ab |
| No Amendment Native Soil | 758.2 ± 117.4 a | 1480.9 ± 46.8 a | 1745.5 ± 424.6 b |
| (C) TC | |||
| Soil Treatment | Shoot | Root | Total |
| Sewage Sludge | 4274 ± 1072.5 a | 9637 ± 1365.9 ab | 13911 ± 338.5 ab |
| Chicken Manure | 3783 ± 648.6 a | 5134 ± 811.5 bc | 8917 ± 167.3 c |
| Horse Manure | 4361 ± 737.0 a | 7008 ± 1151.5 abc | 11369 ± 1882.9 abc |
| Vermicompost | 5074 ± 1090.2 a | 6295 ± 2552.3 bc | 11369 ± 1600.6 abc |
| Organic Fertilizer | 3699 ± 364.1 a | 7673 ± 1761.5 abc | 11373 ± 1405.6 abc |
| Inorganic Fertilizer | 4400 ± 1137.4 a | 11576 ± 972.7 a | 15976 ± 2102.7 a |
| No Amendment Native Soil | 4687 ± 345.1 a | 4399 ± 627.5 c | 9086 ± 411.2 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonious, G.F. The Impact of Organic, Inorganic Fertilizers, and Biochar on Phytochemicals Content of Three Brassicaceae Vegetables. Appl. Sci. 2023, 13, 8801. https://doi.org/10.3390/app13158801
Antonious GF. The Impact of Organic, Inorganic Fertilizers, and Biochar on Phytochemicals Content of Three Brassicaceae Vegetables. Applied Sciences. 2023; 13(15):8801. https://doi.org/10.3390/app13158801
Chicago/Turabian StyleAntonious, George Fouad. 2023. "The Impact of Organic, Inorganic Fertilizers, and Biochar on Phytochemicals Content of Three Brassicaceae Vegetables" Applied Sciences 13, no. 15: 8801. https://doi.org/10.3390/app13158801
APA StyleAntonious, G. F. (2023). The Impact of Organic, Inorganic Fertilizers, and Biochar on Phytochemicals Content of Three Brassicaceae Vegetables. Applied Sciences, 13(15), 8801. https://doi.org/10.3390/app13158801








