Microbial Composition of Natural, Agricultural, and Technogenic Soils of Both Forest and Forest-Tundra of the Russian North
Abstract
:1. Introduction
2. Materials and Methods
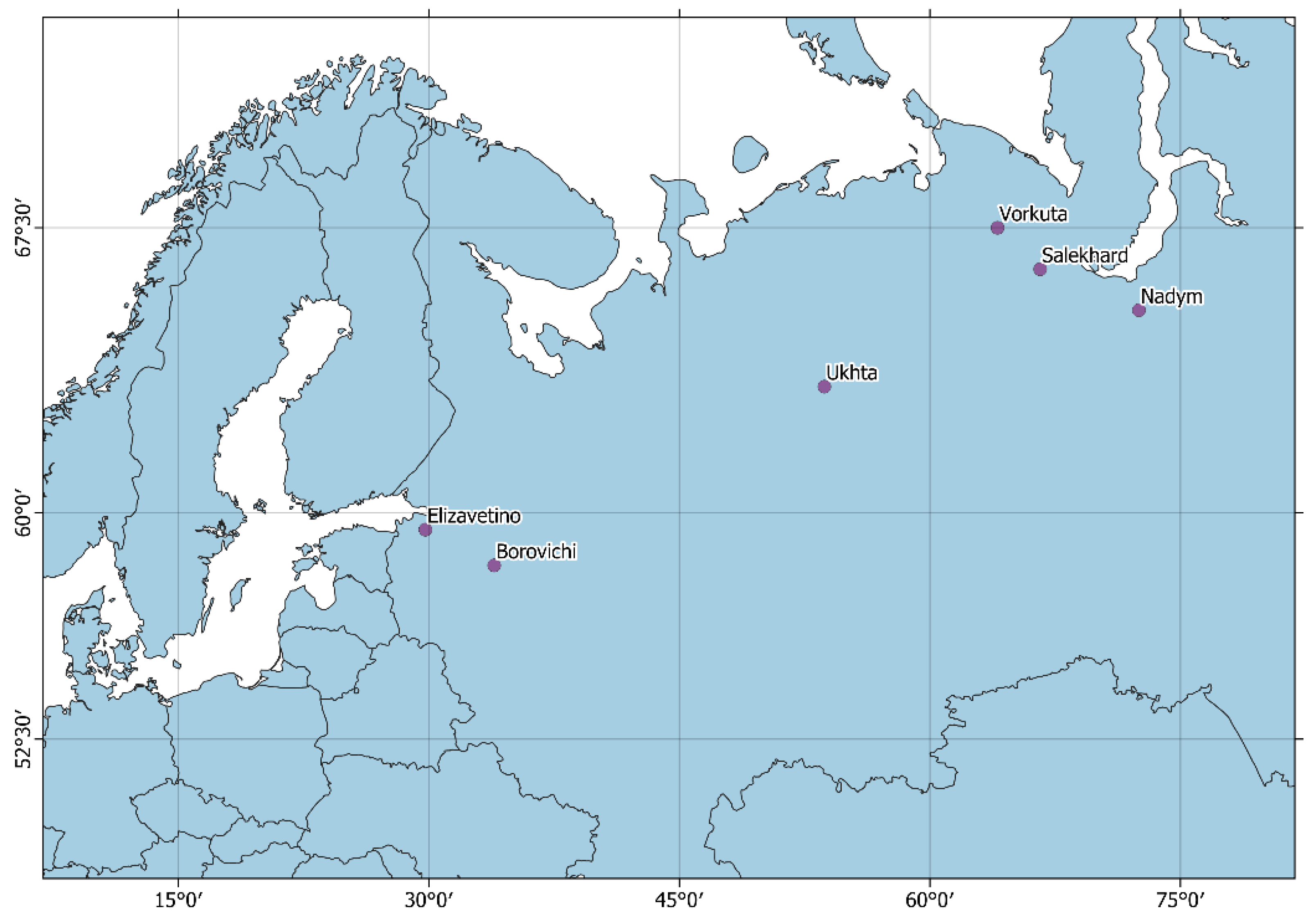
2.1. The Study Sites
2.2. Chemical and Microbiological Analysis
3. Results and Discussion
| Sample | SOC | pH | P | K | N-NO3 | N-NH4 |
|---|---|---|---|---|---|---|
| % | mg/kg | |||||
| Bmature | 2.50 | 7.40 | 15 | 156 | 1 | 32 |
| Bagro | 2.30 | 7.20 | 19 | 170 | 2 | 33 |
| Bmine | 0.45 | 5.60 | 10 | 100 | 1 | 21 |
| Emature | 2.15 | 7.40 | 25 | 120 | 1 | 12 |
| Eagro | 2.45 | 7.20 | 23 | 150 | 1 | 11 |
| Emine | 0.35 | 8.30 | 12 | 80 | 1 | 10 |
| Umature | 1.70 | 7.15 | 8 | 120 | 2 | 24 |
| Uagro | 1.90 | 7.00 | 12 | 125 | 2 | 27 |
| Umine | 0.50 | 7.10 | 8 | 45 | 2 | 12 |
| Vmature | 0.80 | 6.90 | 13 | 80 | 2 | 25 |
| Vagro | 0.70 | 6.70 | 12 | 67 | 2 | 29 |
| Vmine | 0.70 | 6.70 | 9 | 60 | 2 | 12 |
| Nmature | 1.20 | 5.20 | 5 | 20 | 2 | 12 |
| Nagro | 2.00 | 5.40 | 5 | 30 | 2 | 11 |
| Nmine | 0.30 | 5.00 | 2 | 12 | 2 | 9 |
| Smature | 2.16 | 6.70 | 5 | 55 | 3 | 13 |
| Sagro | 2.40 | 6.50 | 5 | 40 | 5 | 13 |
| Smine | 1.45 | 6.10 | 3 | 20 | 3 | 12 |
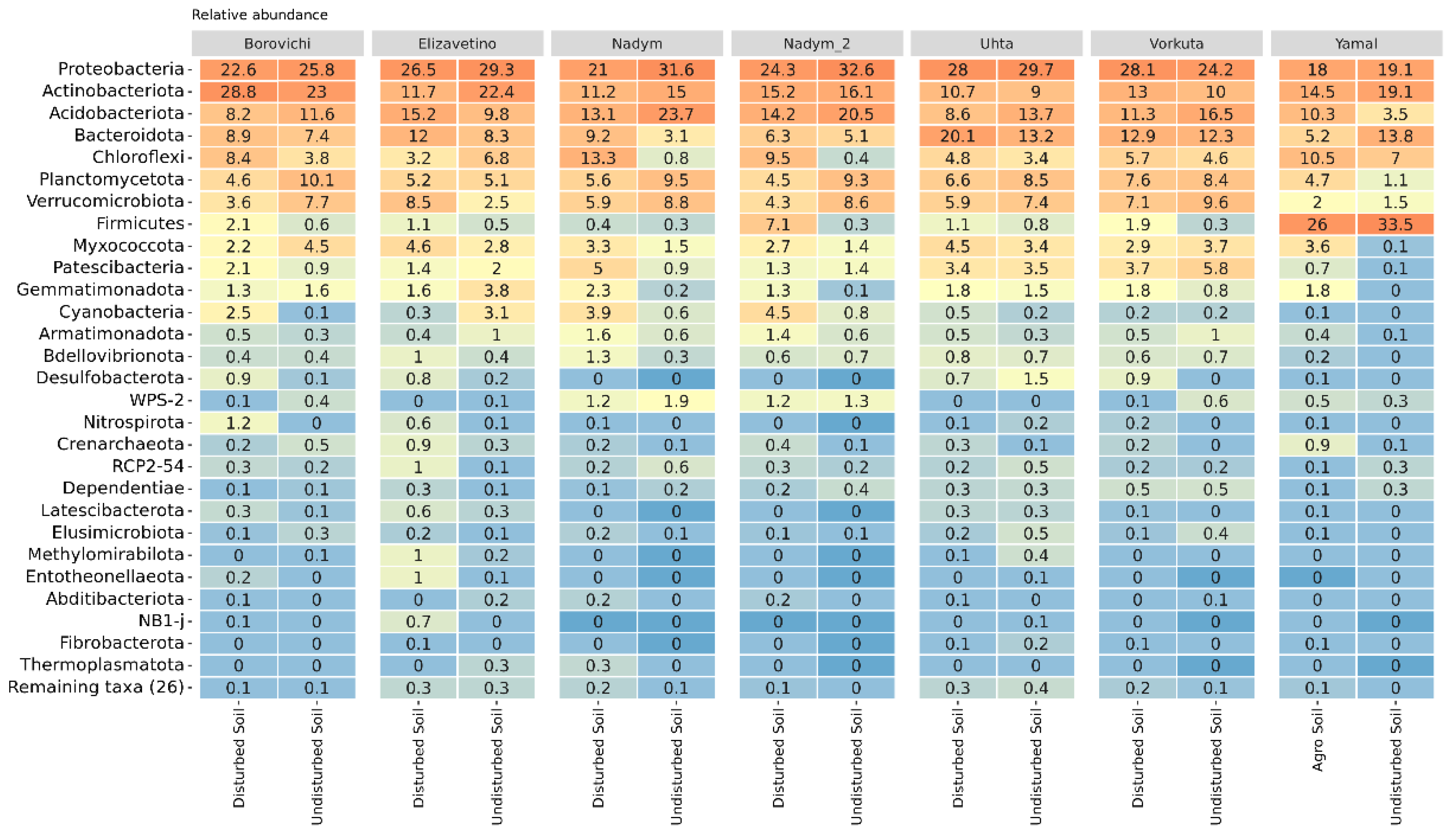
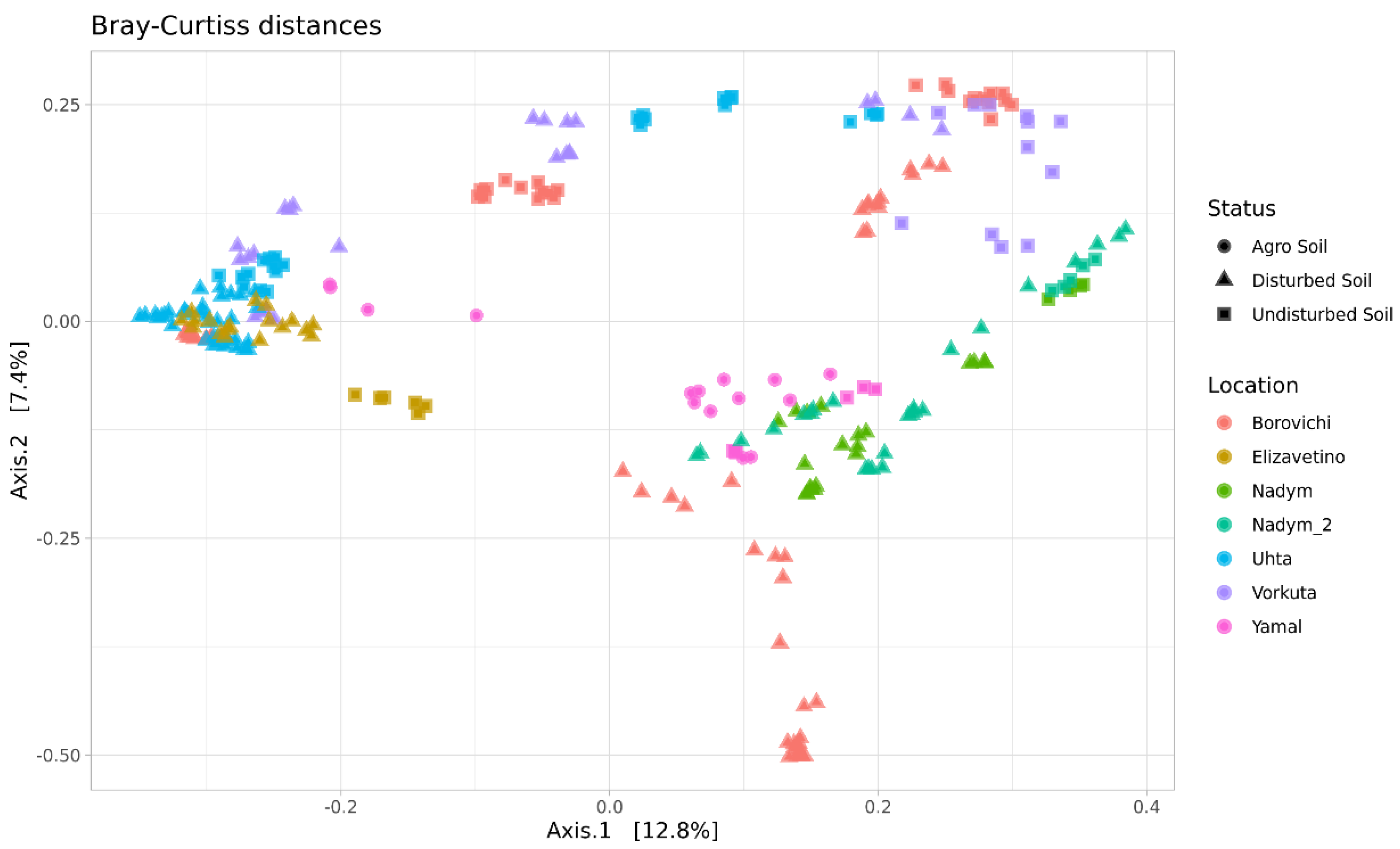
The Characteristics of the Soil Microbiome
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerra, C.A.; Delgado-Baquerizo, M.; Duarte, E.; Marigliano, O.; Görgen, C.; Maestre, F.T.; Eisenhauer, N. Global projections of the soil microbiome in the Anthropocene. Glob. Ecol. Biogeogr. A J. Macroecol. 2021, 30, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest microbiome and global change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef]
- Altdorff, D.; Borchard, N.; Young, E.H.; Galagedara, L.; Sorvali, J.; Quideau, S.; Unc, A. Agriculture in boreal and Arctic regions requires an integrated global approach for research and policy. Agron. Sustain. Dev. 2021, 41, 23. [Google Scholar] [CrossRef]
- Nizamutdinov, T.; Suleymanov, A.; Morgun, E.; Yakkonen, K.; Abakumov, E. Soils and olericultural practices in circumpolar region of Russia at present and in the past. Front. Sustain. Food Syst. 2022, 6, 1032058. [Google Scholar] [CrossRef]
- King, M.; Altdorff, D.; Li, P.; Galagedara, L.; Holden, J.; Unc, A. Northward shift of the agricultural climate zone under 21st-century global climate change. Sci. Rep. 2018, 8, 7904. [Google Scholar] [CrossRef] [Green Version]
- Uhlig, H.; Kreutzmann, H. Persistence and Change in High Mountain Agricultural Systems. Mt. Res. Dev. 1995, 15, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Abakumov, E.; Tembotov, R. Agriculture in the Baksan Gorge of the Central Caucasus, Kabardino-Balkaria, Russia. Foods Raw Mater. 2023, 11, 129–140. [Google Scholar] [CrossRef]
- Kravchenko, I.; Rayko, M.; Tikhonova, E.; Konopkin, A.; Abakumov, E.; Lapidus, A. Agricultural Crops Grown in Laboratory Conditions on Chernevaya Taiga Soil Demonstrate Unique Composition of the Rhizosphere Microbiota. Microorganisms 2022, 10, 2171. [Google Scholar] [CrossRef]
- Pershina, E.; Ivanova, E.; Korvigo, I.; Chirak, E.; Sergaliev, N.; Abakumov, E.; Provorov, N.; Andronov, E. Investigation of the core microbiome in main soil types from the East European plain. Sci. Total Environ. 2018, 631–632, 1421–1430. [Google Scholar] [CrossRef]
- Ivanova, E.; Pershina, E.; Karpova, D.; Rogova, O.; Abakumov, E.; Andronov, E. Soil Microbiome in Chronosequence of Spoil Heaps of Kursk Magnetic Anomaly. Bio. Comm. 2019, 64, 219–225. [Google Scholar] [CrossRef]
- Dmitrakova, Y.A.; Abakumov, E.; Pershina, E.; Ivanova, E.; Andronov, E. Dynamics of the plant community and microbiom of chrono-series of post-technological soil in limestone quarry in the conditions of recultivation. Agric. Biol. 2018, 53, 557–569. (In Russian) [Google Scholar] [CrossRef]
- Chernov, T.I.; Kholodov, V.A.; Kogut, B.M.; Ivanov, A.L. The method of microbiological soil investigations within the framework of the project “Microbiome of Russia”. Bull. VV Dokuchaev Soil Sci. Inst. 2017, 87, 100–113. [Google Scholar] [CrossRef]
- Andronov, E.E.; Ivanova, E.A.; Pershina, E.V.; Orlova, O.V.; Kruglov, Y.V.; Belimov, A.A.; Tikhonovich, I.A. Analysis of soil microbiome indicators in processes of soil formation, organic matter transformation and processes involved with fine regulation of vegetative processes. Dokuchaev Soil Bull. 2015, 80, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Hansel, C.M.; Fendorf, S.; Jardine, P.M.; Francis, C.A. Changes in Bacterial and Archaeal Community Structure and Functional Diversity along a Geochemically Variable Soil Profile. Appl. Environ. Microbiol. 2008, 74, 1620–1633. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, M.; Howes, C.G.; Vaninsberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Henrik Nilsson, R.; Hallam, S.J.; Mohn, W.W. Significant and Persistent Impact of Timber Harvesting on Soil Microbial Communities in Northern Coniferous Forests. ISME J. 2012, 6, 2199–2218. [Google Scholar] [CrossRef] [Green Version]
- Goberna, M.; García, C.; Insam, H.; Hernández, M.T.; Verdú, M. Burning Fire-Prone Mediterranean Shrublands: Immediate Changes in Soil Microbial Community Structure and Ecosystem Functions. Microb. Ecol. 2012, 64, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Santalahti, M.; Pumpanen, J.; Köster, K.; Berninger, F.; Raffaello, T.; Asiegbu, F.O.; Heinonsalo, J. Bacterial Community Structure and Function Shift across a Northern Boreal Forest Fire Chronosequence. Sci. Rep. 2016, 6, 32411. [Google Scholar] [CrossRef]
- Turner, S.; Mikutta, R.; Meyer-Stüve, S.; Guggenberger, G.; Schaarschmidt, F.; Lazar, C.S.; Dohrmann, R.; Schippers, A. Microbial Community Dynamics in Soil Depth Profiles over 120,000 Years of Ecosystem Development. Front. Microbiol. 2017, 8, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selenska-Pobell, S.; Kampf, G.; Flemming, K.; Radeva, G.; Satchanska, G. Bacterial Diversity in Soil Samples from Two Uranium Waste Piles as Determined by Rep-APD, RISA and 16S RDNA Retrieval. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2001, 79, 149–161. [Google Scholar] [CrossRef]
- Frouz, J.; Keplin, B.; Pižl, V.; Tajovský, K.; Starý, J.; Lukešová, A.; Nováková, A.; Balík, V.; Háněl, L.; Materna, J.; et al. Soil Biota and Upper Soil Layer Development in Two Contrasting Post-Mining Chronosequences. Ecol. Eng. 2001, 17, 275–284. [Google Scholar] [CrossRef]
- Escobar, I.E.C.; Santos, V.M.; da Silva, D.K.A.; Fernandes, M.F.; Cavalcante, U.M.T.; Maia, L.C. Changes in Microbial Community Structure and Soil Biological Properties in Mined Dune Areas During Re-Vegetation. Environ. Manag. 2015, 55, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.E.; White, J.R.; Wafula, D.; Athar, R.; Dickerson, T.; Williams, H.N.; Chauhan, A. Soil Functional Diversity Analysis of a Bauxite-Mined Restoration Chronosequence. Microb. Ecol. 2010, 59, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Aparin, B.F.; Sukhacheva, E.Y.; Kichko, A.A.; Andronov, E.E.; Valchenko, Y.V. Structure of Microbial Community in Forest and Anthropogenic Changed Soils of Megalopolis (St. Petersburg, Russia). In Processes and Phenomena on the Boundary between Biogenic and Abiogenic Nature; Lecture Notes in Earth System Sciences; Frank-Kamenetskaya, O., Vlasov, D., Panova, E., Lessovaia, S., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Korneykova, M.V.; Vasenev, V.I.; Nikitin, D.A.; Soshina, A.S.; Dolgikh, A.V.; Sotnikova, Y.L. Urbanization Affects Soil Microbiome Profile Distribution in the Russian Arctic Region. Int. J. Environ. Res. Public Health 2021, 18, 11665. [Google Scholar] [CrossRef]
- Zverev, A.O.; Gladkov, G.V.; Kimeklis, A.K.; Kichko, A.A.; Andronov, E.E.; Abakumov, E.V. Microbial Composition on Abandoned and Reclaimed Mining Sites in the Komi Republic (North Russia). Microorganisms 2023, 11, 720. [Google Scholar] [CrossRef]
- Zverev, A.; Petrov, A.; Kimeklis, A.; Kichko, A.; Andronov, E.; Petrov, A.; Abakumov, E. Microbiomes of the Initial Soils of Mining Areas of Yakutsk City (Eastern Siberia, Russia). Czech Polar Rep. 2020, 10, 69–82. [Google Scholar] [CrossRef]
- FAO. Standard Operating Procedure for Soil pH Determination; FAO: Rome, Italy, 2021. [Google Scholar]
- FAO. Standard Operating Procedure for Soil Organic Carbon: Tyurin Spectrophotometric Method; FAO: Rome, Italy, 2021. [Google Scholar]
- FAO. Standard Operating Procedure for Soil Available Phosphorus—Olsen Method; FAO: Rome, Italy, 2021. [Google Scholar]
- GOST R 54650–2011; Soils: Determination of Mobile Phosphorus and Potassium Compounds by Kirsanov Method Modified by ClNAO. Standartinform: Moscow, Russia, 2019; 8p.
- Caporaso, J.; Lauber, C.; Walters, W.; Berg-Lyons, D.; Lozupone, C.; Turnbaugh, P.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Callahan, B.; Mcmurdie, P.; Rosen, M.; Han, A.; Johnson, A.J.; Holmes, S. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Mcmurdie, P.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, B.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 22-1. 2015. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 15 July 2023).
- Frouz, J.; Nováková, A. Development of soil microbial properties in topsoil layer during spontaneous succession in heaps after brown coal mining in relation to humus microstructure development. Geoderma 2005, 129, 54–64. [Google Scholar] [CrossRef]
- Sokolov, D.; Androkhanov, V.; Kulizhskii, S.; Domozhakova, E.; Loiko, S. Morphogenetic diagnostics of soil formation on tailing dumps of coal quarries in Siberia. Eurasian Soil Sci. 2015, 48, 95–105. [Google Scholar] [CrossRef]
- Alexandrovskiy, A.L. Rates of soil–forming processes in three main models of pedogenesis. Rev. Mex. Cienc. Geo-lógicas 2007, 24, 283–292. [Google Scholar]
- Woś, B.; Nezhad, M.T.K.; Mustafa, A.; Pietrzykowski, M.; Frouz, J. Soil carbon storage in unreclaimed post mining sites estimated by a chronosequence approach and comparison with historical data. Catena 2023, 220, 106664. [Google Scholar] [CrossRef]
- Abakumov, E. Rendzinas of the Russian Northwest: Diversity, Genesis, and Ecosystem Functions: A Review. Geosciences 2023, 13, 216. [Google Scholar] [CrossRef]
- Radmanović, S.B.; Đorđević, A.R.; Nikolić, N.S. Humification degree of rendzina soil humic acids influenced by car-bonate leaching and land use. J. Agric. Sci. 2015, 60, 443–453. [Google Scholar]
- Bogdevitch, I.; Lomonos, O.; Tavrykina, O. Dynamics of acidity, calcium and magnesium supply in the arable and grassland soils of Belarus in the course of liming. Soil Sci. Agrochem. 2014, 1, 159–172. (In Russian) [Google Scholar]
- Ramos, L.R.; Vollú, R.E.; Jurelevicius, D.; Rosado, A.S.; Seldin, L. Firmicutes in different soils of Admiralty Bay, King George Island, Antarctica. Polar. Biol. 2019, 42, 2219–2226. [Google Scholar] [CrossRef]
- Gupta, P.; Sangwan, N.; Lal, R.; Vakhlu, J. Bacterial diversity of Drass, cold desert in Western Himalaya, and its comparison with Antarctic and Arctic. Arch. Microbiol. 2015, 197, 851–860. [Google Scholar] [CrossRef]
- Trubl, G.; Kimbrel, J.A.; Liquet-Gonzalez, J.; Nuccio, E.E.; Weber, P.K.; Pett-Ridge, J.; Jansson, J.K.; Waldrop, M.P.; Blazewicz, S.J. Active virus-host interactions at sub-freezing temperatures in Arctic peat soil. Microbiome 2021, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Xu, Y.; Zhang, J.; Hao, X.; Lu, Y. Core Microbiota in Agricultural Soils and Their Potential Associations with Nutrient Cycling. mSystems 2019, 4, e00313-18. [Google Scholar] [CrossRef] [Green Version]
- Abakumov, E.; Kimeklis, A.; Gladkov, G.; Andronov, E.; Morgun, E.; Nizamutdinov, T. Microbiome of abandoned soils of former agricultural cryogenic ecosystems of central part of Yamal region. Czech Polar Rep. 2022, 12, 232–245. [Google Scholar] [CrossRef]
- Abakumov, E.; Zverev, A.; Kichko, A.; Kimeklis, A.; Andronov, E. Soil microbiome of different-aged stages of self-restoration of ecosystems on the mining heaps of limestone quarry (Elizavetino, Leningrad region). Open Agric. 2021, 6, 57–66. [Google Scholar] [CrossRef]
- Abakumov, E.; Zverev, A.; Suleymanov, A.; Suleymanov, R. Microbiome of post-technogenic soils of quarries in the Republic of Bashkortostan (Russia). Open Agric. 2020, 5, 529–538. [Google Scholar] [CrossRef]
- Miralles, I.; Ortega, R.; Comeau, A.M. Functional and Taxonomic Effects of Organic Amendments on the Restoration of Semiarid Quarry Soils. Msystems 2021, 6, e00752-21. [Google Scholar] [CrossRef]
- Anderson, C.R.; Peterson, M.E.; Frampton, R.A.; Bulman, S.R.; Keenan, S.; Curtin, D. Rapid increases in soil pH solubilise organic matter, dramatically increase denitrification potential and strongly stimulate microorganisms from the Firmicutes phylum. PeerJ 2018, 6, e6090. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Grogan, P.; Sun, H.; Xiong, J.; Yang, Y.; Zhou, J.; Chu, H. Multi-scale variability analysis reveals the im-portance of spatial distance in shaping Arctic soil microbial functional communities. Soil Biol. Biochem. 2015, 86, 126–134. [Google Scholar] [CrossRef]
- Gladkov, G.; Kimeklis, A.; Zverev, A.; Pershina, E.; Ivanova, E.; Kichko, A.; Andronov, E.; Abakumov, E. Soil microbiome of the postmining areas in polar ecosystems in surroundings of Nadym, Western Siberia, Russia. Open Agric. 2019, 4, 684–696. [Google Scholar] [CrossRef] [Green Version]
- Ananyeva, N.D.; Ivashchenko, K.V.; Sushko, S.V. Microbial indicators of urban soils and their role in the assessment of ecosystem services: A review. Eurasian Soil Sci. 2021, 54, 1517–1531. [Google Scholar] [CrossRef]
- Burns, R.G.; De Forest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Korneykova, M.V.; Myazin, V.A.; Fokina, N.V.; Chaporgina, A.A.; Nikitin, D.A.; Dolgikh, A.V. Structure of Microbial Communities and Biological Activity in Tundra Soils of the Euro-Arctic Region (Rybachy Peninsula, Russia). Microorganisms 2023, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Yablokov, A.V.; Levchenko, V.F.; Kerzhentsev, A.S. The conception of controlled evolution as an alternative to the conception of sustainable development. Theor. Appl. Ecol. 2017, 2, 4–8. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abakumov, E.; Zverev, A.; Andronov, E.; Nizamutdinov, T. Microbial Composition of Natural, Agricultural, and Technogenic Soils of Both Forest and Forest-Tundra of the Russian North. Appl. Sci. 2023, 13, 8981. https://doi.org/10.3390/app13158981
Abakumov E, Zverev A, Andronov E, Nizamutdinov T. Microbial Composition of Natural, Agricultural, and Technogenic Soils of Both Forest and Forest-Tundra of the Russian North. Applied Sciences. 2023; 13(15):8981. https://doi.org/10.3390/app13158981
Chicago/Turabian StyleAbakumov, Evgeny, Aleksei Zverev, Evgeny Andronov, and Timur Nizamutdinov. 2023. "Microbial Composition of Natural, Agricultural, and Technogenic Soils of Both Forest and Forest-Tundra of the Russian North" Applied Sciences 13, no. 15: 8981. https://doi.org/10.3390/app13158981





