Use of Chlorella vulgaris and Ulva lactuca as Biostimulant on Lettuce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Biomass
2.2. Algal Extract Preparation
2.3. Seed Germination Assay
2.4. Greenhouse Assay
2.5. Algal Biomass and Lettuce Chemical Characterization
2.5.1. Moisture and Ashes Content
2.5.2. Crude Lipids
2.5.3. Total Nitrogen/Protein
2.5.4. Crude Fiber
2.5.5. Total Carbohydrates/Nitrogen-Free Extractives
2.5.6. Mineral and Trace Element Characterization
2.5.7. Caloric Value
2.6. Statistical Analysis
3. Results and Discussion
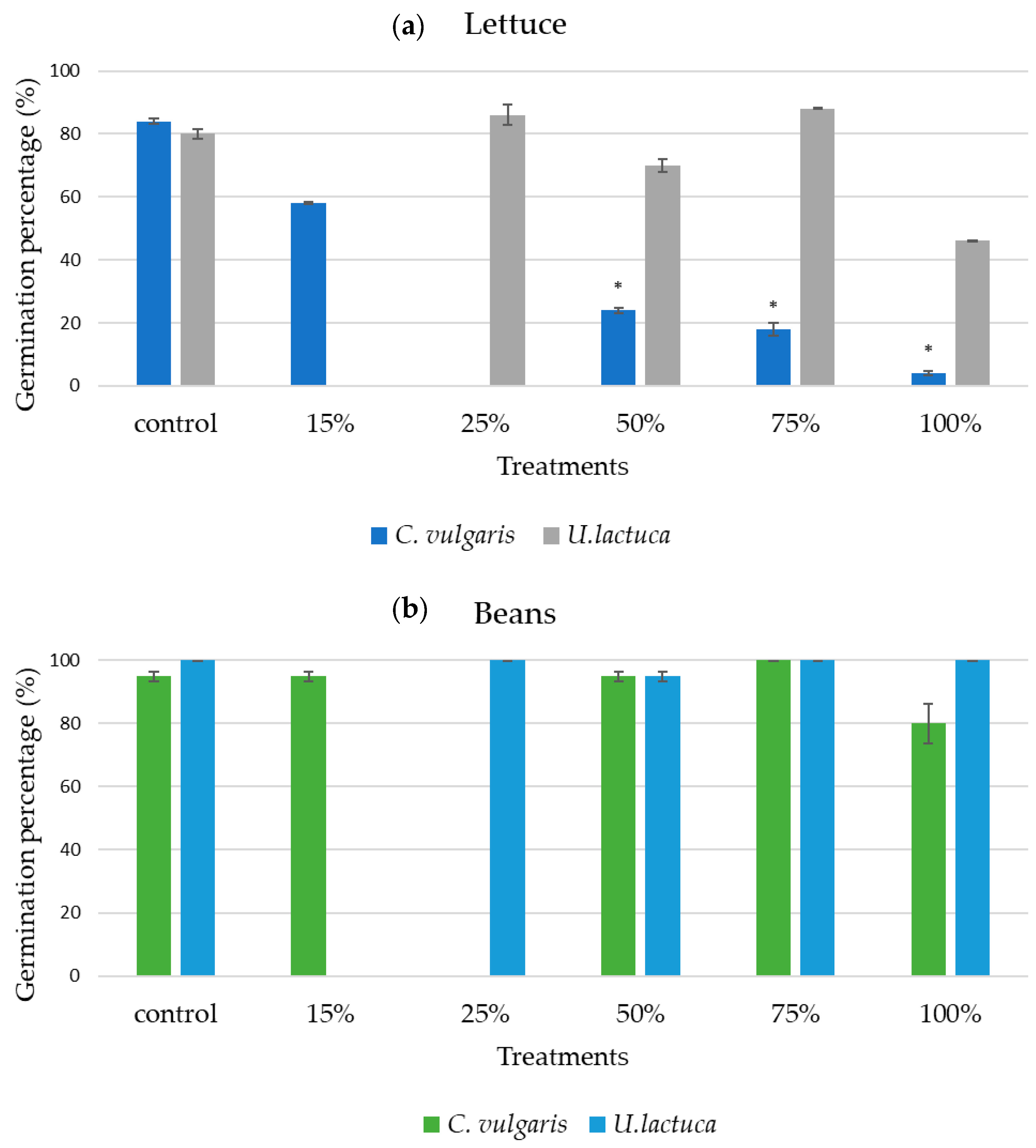
3.1. Algal Extract Dilutions Treatment on Seeds
3.2. Greenhouse Treatment
3.3. Chemical Composition
3.3.1. Algal Chemical Composition
3.3.2. Lettuce Chemical Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [Green Version]
- Savci, S. Investigation of effect of chemical fertilizers on environment. Apcbee Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic responses of maize shoots and roots elicited by combinatorial seed treatments with microbial and non-microbial biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Agenda 2030. Available online: https://sdgs.un.org/2030agenda (accessed on 24 January 2023).
- Dominguez, H.; Loret, E.P. Ulva lactuca, a source of troubles and potential riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, D. Seaweeds as Plant Health Promoters; University of Coimbra: Coimbra, Portugal, 2022. [Google Scholar]
- Sousa, T.; Nunes, J.P.; Lopes, J.; Cotas, J.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweed as Plant Biostimulants; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 183–200. [Google Scholar]
- Shatilov, M.; Razin, A.; Ivanova, M. Analysis of the world lettuce market. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Moscow, Russia, 27 May–6 June 2019; p. 012053. [Google Scholar]
- Boriss, H.; Brunke, H. Commodity Profile: Lettuce; University of California: San Diego, CA, USA, 2005. [Google Scholar]
- Available online: https://www.zipmec.com/la-produzione-di-insalata.html#:~:text=La%20Spagna%20possiede%20il%20primato,315%20milioni%20di%20tonnellate%20prodotte (accessed on 24 January 2023).
- Rayorath, P.; Jithesh, M.N.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J. Appl. Phycol. 2007, 20, 423–429. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Cunniff, P.; Washington, D. Official methods of analysis of aoac international. J. AOAC Int. 1997, 80, 127A. [Google Scholar]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- FAO. Food Energy—Methods of Analysis and Conversion Factors; FAO: Rome, Italy, 2002. [Google Scholar]
- Lucas, M.; Sequeira, E. Determinação do Cu, Zn, Mn, Fe, Ca, Mg, K, e Na totais das plantas por espectrofotometria de absorção atómica e fotometria de chama. Pedologia 1976, 11, 163–169. [Google Scholar]
- Ribas, M.; Veiga, M.; Curto, A.; Oliveira, E.; Barbeitos, M.; Ferreira, M.; Pacheco, C.; Peralta, M.; Duarte, M. Métodos de análise de material vegetal e terras. Pedologia 1988, 11, 163–169. [Google Scholar]
- FAO. Available online: https://www.fao.org/3/y5022e/y5022e04.htm (accessed on 24 January 2023).
- Hassan, S.; Ghareib, H. Bioactivity of Ulva lactuca L. acetone extract on germination and growth of lettuce and tomato plants. Afr. J. Biotechnol. 2009, 8, 3832–3838. [Google Scholar]
- Hajnal-Jafari, T.I.; Đurić, S.S.; Stamenov, D.R. Influence of green algae Chlorella vulgaris on initial growth of different agricultural crops. Zbornik Matice Srpske za Prirodne Nauke 2016, 2016, 29–33. [Google Scholar] [CrossRef]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and nutritional evaluation of Chlorella and Auxenochlorella biomasses relevant for food application. Front. Nutr. 2020, 7, 565996. [Google Scholar] [CrossRef] [PubMed]
- Tokuşoglu, Ö.; Üunal, M. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Rasyid, A. Evaluation of nutritional composition of the dried seaweed Ulva lactuca from Pameungpeuk waters, Indonesia. Trop. Life Sci. Res. 2017, 28, 119. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Khairy, H.M.; El-Shafay, S.M. Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia 2013, 55, 435–452. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Geraldson, C.M.; Tyler, K.B. Plant Analysis as an Aid in Fertilizing Vegetable Crops. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Hoboken, NJ, USA, 1990; Volume 3. [Google Scholar]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]

| Algal Extract Dilutions | pH | EC (mS) | |
|---|---|---|---|
| Chlorella | 15% | 5.69 | 0.54 |
| 50% | 5.91 | 1.67 | |
| 75% | 5.88 | 2.19 | |
| 100% | 5.90 | 2.33 | |
| Ulva | 25% | 6.79 | 1.27 |
| 50% | 7.04 | 2.70 | |
| 75% | 6.67 | 2.86 | |
| 100% | 6.97 | 3.23 | |
| Root Length | Root Weight | Aerial Part Weight | Aerial Part Diameter | FW/DW Ratio (Aerial Part) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | mm | se | g | se | se | se | mm | se | |
| control | 14.38 a | 0.88 | 22.84 b | 1.37 | 776.00 a | 39.09 | 20.88 a | 0.84 | 24.98 |
| C. vulgaris 15% | 14.25 a | 0.63 | 36.18 a | 4.61 | 738.74 a | 30.56 | 21.25 a | 1.00 | 25.29 |
| U. lactuca 25% | 15.63 a | 1.31 | 24.86 a,b | 2.15 | 624.93 a | 40.97 | 19.31 a | 0.28 | 24.14 |
| U. lactuca 75% | 15.38 a | 0.92 | 27.16 a,b | 1.45 | 615.45 a | 33.03 | 18.50 a | 0.25 | 23.61 |
| Dry Matter | ||
|---|---|---|
| C. vulgaris | U. lactuca | |
| MOISTURE (g/100 g) | 4.94 a | 11.16 a |
| ASHES (g/100 g) | 9.33 ± 0.04 a | 26.25 ± 0.08 b |
| FATS (g/100 g) | 1.36 ± 0.05 a | 0.99 ± 0.02 a |
| FIBER (g/100 g) | 1.09 ± 0.03 a | 7.51 ± 0.08 b |
| PROTEINS (g/100 g) | 37.11 ± 0.06 a | 13.58 ± 0.06 b |
| NON-NITROGEN EXTRACTIVES (g/100 g) | 51.11 ± 0.00 a | 51.67 ± 0.08 a |
| ENERGY (Kcal/100 g) | 365 ± 0.20 a | 270 ± 0.07 b |
| ENERGY (KJ/100 g) | 1529 ± 0.85 a | 1130 ± 0.31 b |
| N (%) | 5.94 ± 0.01 a | 2.17 ± 0.15 b |
| P (%) | 1.78 ± 0.03 a | 0.26 ± 0.01 b |
| Ca (%) | 1.13 ± 0.06 a | 0.64 ± 0.06 b |
| Mg (%) | 0.21 ± 0.01 a | 0.82 ± 0.10 b |
| K (%) | 0.75 ± 0.05 a | 1.57 ± 0.04 b |
| Na (%) | 0.13 ± 0.05 a | 0.20 ± 0.04 b |
| Cu (mg/Kg) | 25.75 ± 1.24 a | 12.75 ± 2.28 b |
| Zn (mg/Kg) | 30.51 ± 1.90 a | 23.64 ± 1.53 b |
| Fe (mg/Kg) | 38.24 ± 5.60 a | 210.70 ± 10.84 b |
| Mn (mg/Kg) | 93.21 ± 2.82 a | 33.53 ± 1.91 b |
| Dry Matter | ||||
|---|---|---|---|---|
| Control | 15% C. vulgaris | 25% U. lactuca | 75% U. lactuca | |
| ASHES (g/100 g) | 23.20 ± 0.26 a | 22.90 ± 0.09 a | 21.35 ± 0.73 a | 22.59 ± 0.09 a |
| FATS (g/100 g) | 3.37 ± 0.15 a | 2.98 ± 0.06 a,b | 2.75 ± 0.03 b | 3.13 ± 0.06 a |
| FIBER (g/100 g) | 14.42 ± 0.15 a,b | 14.58 ± 0.13 a | 14.18 ± 0.04 a,b | 14.03 ± 0.10 b |
| PROTEINS (g/100 g) | 24.07 ± 0.17 a | 20.51 ± 0.19 b | 21.10 ± 0.04 a,b | 21.71 ± 0.04 a,b |
| NON-NITROGEN EXTRACTIVES (g/100 g) | 34.94 ± 0.09 b | 39.02 ± 0.09 a,b | 40.62 ± 0.76 a | 38.54 ± 0.09 a,b |
| ENERGY (Kcal/100 g) | 266 ± 0.34 a,b | 265 ± 0.57 b | 272 ± 2.94 a | 269 ± 1.04 a,b |
| ENERGY (KJ/100 g) | 1115 ± 1.40 a,b | 1109 ± 2.37 b | 1137 ± 12.31 a | 1127 ± 4.37 a,b |
| N (%) | 4.24 ± 0.06 a | 3.61 ± 0.03 a | 3.63 ± 0.06 a | 3.82 ± 0.01 a |
| P (%) | 0.68 ± 0.01 a | 0.62 ± 0.01 a | 0.64 ± 0.01 a | 0.66 ± 0.02 a |
| Ca (%) | 0.74 ± 0.03 a | 0.62 ± 0.01 a | 0.67 ± 0.01 a | 0.67 ± 0.01 a |
| Mg (%) | 0.21 ± 0.01 a | 0.19 ± 0.01 a | 0.19 ± 0.01 a | 0.20 ± 0.01 a |
| K (%) | 7.39 ± 0.78 a | 7.13 ± 0.14 a | 7.20 ± 0.35 a | 8.01 ± 0.05 a |
| Na (%) | 0.17 ± 0.01 a | 0.16 ± 0.01 a | 0.12 ± 0.01 b | 0.15 ± 0.01 a |
| Cu (mg/Kg) | 14.94 ± 0.95 a | 13.14 ± 0.68 a | 13.54 ± 0.53 a | 13.32 ± 1.09 a |
| Zn (mg/Kg) | 42.06 ± 1.75 a | 31.23 ± 0.18 a | 40.06 ± 0.81 a | 531.80 ± 24.31 b |
| Fe (mg/Kg) | 20.28 ± 0.35 b | 29.44 ± 1.42 a | 25.30 ± 1.82 a,b | 23.21 ± 1.02 a,b |
| Mn (mg/Kg) | 25.23 ± 0.30 a | 22.00 ± 0.27 a | 22.05 ± 0.96 a | 22.19 ± 1.30 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammaturo, C.; Pacheco, D.; Cotas, J.; Formisano, L.; Ciriello, M.; Pereira, L.; Bahcevandziev, K. Use of Chlorella vulgaris and Ulva lactuca as Biostimulant on Lettuce. Appl. Sci. 2023, 13, 9046. https://doi.org/10.3390/app13169046
Ammaturo C, Pacheco D, Cotas J, Formisano L, Ciriello M, Pereira L, Bahcevandziev K. Use of Chlorella vulgaris and Ulva lactuca as Biostimulant on Lettuce. Applied Sciences. 2023; 13(16):9046. https://doi.org/10.3390/app13169046
Chicago/Turabian StyleAmmaturo, Chiara, Diana Pacheco, João Cotas, Luigi Formisano, Michele Ciriello, Leonel Pereira, and Kiril Bahcevandziev. 2023. "Use of Chlorella vulgaris and Ulva lactuca as Biostimulant on Lettuce" Applied Sciences 13, no. 16: 9046. https://doi.org/10.3390/app13169046
APA StyleAmmaturo, C., Pacheco, D., Cotas, J., Formisano, L., Ciriello, M., Pereira, L., & Bahcevandziev, K. (2023). Use of Chlorella vulgaris and Ulva lactuca as Biostimulant on Lettuce. Applied Sciences, 13(16), 9046. https://doi.org/10.3390/app13169046









