An Evaluation of the Physical and Chemical Parameters in Brassica Seedlings Grown on Various Organic Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Factors
- Broccoli (Brassica oleracea var. italica Plenck) cv. Cezar;
- White cabbage (Brassica oleracea var. capitata f. alba L.) cv. Zeli Bele;
- Cauliflower (Brassica oleracea var. botrytis L.) dv. Bola de Nieve X.
- Aura standard substrate for cabbage, cauliflower, and broccoli;
- PRO1 professional substrate containing 50% white milled peat and 50% black milled peat; particle size—0–10 mm;
- PRO2 professional substrate containing 50% white milled peat and 50% black milled peat; particle size—0–4 mm;
- PRO3 professional substrate containing 100% black milled peat; particle size—0–4 mm.
2.2. Experimental Design
2.3. Analysis of the Biometric Parameters of Seedlings
2.4. Analysis of the Mineral Composition of Leaves
2.5. Statistical Analysis
3. Results and Discussion
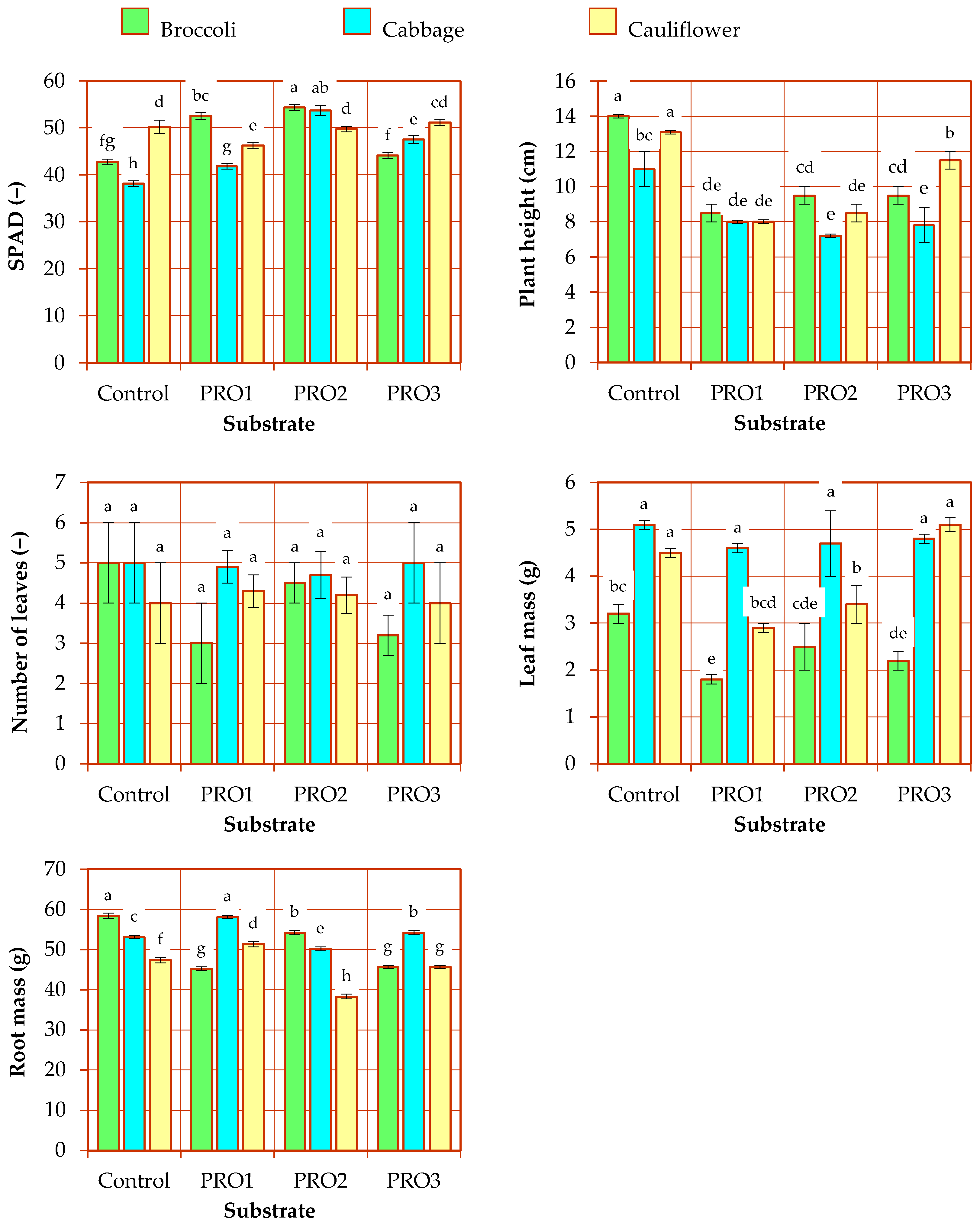
3.1. Biometric Parameters of Seedlings
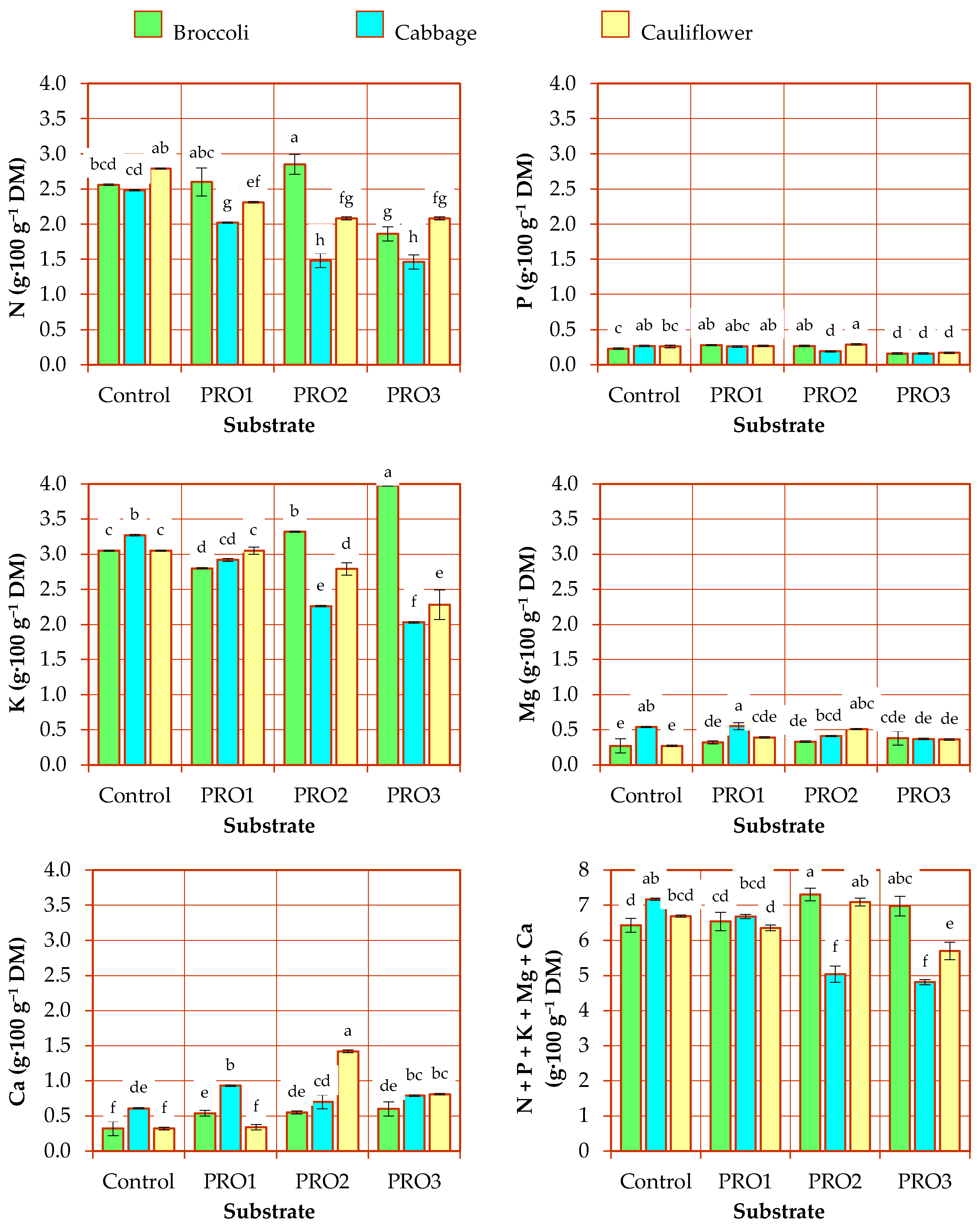
3.2. Chemical Composition of Plants
3.2.1. Macronutrient Content
3.2.2. Micronutrient Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascual, J.A.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic substrate for transplant production in organic nurseries. A review. Agron. Sustain. Dev. 2018, 38, 35. [Google Scholar] [CrossRef] [Green Version]
- Oda, M. Raising of vigorous and valuable seedlings. Regul. Plant Grow. Dev. 2007, 42, 176–182. [Google Scholar] [CrossRef]
- Raviv, M. Recent advances in soil-borne disease control using suppressive media. Acta Hortic. 2009, 819, 125–134. [Google Scholar] [CrossRef]
- Kubota, C.; Chia, P.; Yang, Z.; Li, Q. Applications of far-red light emitting diodes in plant production under controlled environments. Acta Hortic. 2012, 952, 59–66. [Google Scholar] [CrossRef]
- Unal, M. Effect of organic media on growth of vegetable seedlings. Pak. J. Agric. Sci. 2013, 50, 517–522. [Google Scholar]
- Restrepo, A.P.; Medina, E.; Perez-Espinosa, A.; Agulló, E.; Bustamante, M.A.; Mininni, C.; Bernal, M.P.; Moral, R. Substitution of peat in horticultural seedlings: Suitability of digestate-derived compost from cattle manure and maize silage codigestion. Commun. Soil Sci. Plant Anal. 2013, 44, 668–677. [Google Scholar] [CrossRef]
- Dobrzański, F.; Nawrocka, B. Integrated Cauliflower Production Methods, 3rd ed.; Plant Health and Seed Inspection Service PIORIN: Warsaw, Poland, 2020. (In Polish)
- Korbin, B.; Nawrocka, B. Integrated Cabbage Production Methods, 3rd ed.; Plant Health and Seed Inspection Service PIORIN: Warsaw, Poland, 2020. (In Polish)
- Sharifova, S.; Hasanov, S.; Babayev, A.; Guliyev, N. Some characteristics of the newly obtained constant sweet pepper (Capsicum annum L.) hybrids. Ratar. Povrt. 2012, 49, 122–125. [Google Scholar] [CrossRef]
- Medeiros, A.S.; Silva, E.G.; Luison, E.A.; Junior, R.A.; Andreani, D.I. Utilization of organic compost as substrate for vegetable seedling production. Rev. Agrar. 2010, 3, 261–266. (In Portuguese) [Google Scholar]
- de Mesquita, E.F.; Chaves, L.H.G.; Freitas, B.V.; Silva, G.A.; Sousa, M.V.R.; Andrade, R. Production of papaya seedlings using different substrates with cattle manure and recipients volumes. Bras. Cienc. Agrar. 2012, 7, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Monaco, P.A.V.L.; de Castro Vieira, J.; Colombo, J.N.; Krause, M.R.; Sousa Vieira, G.H.; Almeida, K.M. Use of agricultural waste material as an alternative substrate in cabbage seedling production and development. Emir. J. Food Agric. 2020, 32, 131–139. [Google Scholar] [CrossRef]
- Hirschler, O.; Osterburg, B.; Weimar, H.; Glasenapp, S.; Ohmes, M.-F. Peat Replacement in Horticultural Growing Media: Availability of Bio-Based Alternative Materials; Thünen Working Paper 190; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2022. [Google Scholar] [CrossRef]
- Strojny, J.; Nowak, J.S. Principles of substrate quality assessment in the EU countries. Zesz. Probl. Post. Nauk Roln. 2005, 504, 283–297. [Google Scholar]
- Zaller, J.G. Vermicompost as a substitute for peat in potting media: Effects on germination, biomass allocation, yields and fruit quality of three tomato varieties. Sci. Hortic. 2007, 112, 191–199. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance). Available online: https://www.legislation.gov.uk/eur/2019/1009/contents# (accessed on 19 March 2023).
- Łobanowski, G. Integrated Broccoli Production Methods, 2nd ed.; Plant Health and Seed Inspection Service PIORIN: Warsaw, Poland, 2020. (In Polish)
- Act of 8 March 2013 on Plant Protection Products. Journal of Laws of 2019, Item 1900, Art. 57 Sec. 2 Point 2. (In Polish). Available online: https://www.prawo.pl/akty/dz-u-2023-340-t-j,17976110.html (accessed on 19 March 2023).
- Hollas, Gardening Substrates. Available online: http://dabest.pl/attachments/article/106/Pod%C5%82o%C5%BCa%20ogrodnicze%20i%20inne%20produkty%20HOLLAS.pdf (accessed on 20 December 2022).
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Walters, D.; Walsh, D.; Newton, A.; Lyon, G. Induced resistance for plant disease control: Maximizing the efficacy of resistance elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venegas-Molina, J.; Proietti, S.; Pollier, J.; Orozco-Freire, V.; Ramirez-Villacis, D.; Leon-Reyes, A. Induced tolerance to abiotic and biotic stresses of broccoli and Arabidopsis after treatment with elicitor molecules. Sci. Rep. 2020, 10, 10319. [Google Scholar] [CrossRef]
- Kowalski, A. Rational Fertilization of Head Cabbage; InHort Instytut Ogrodnictwa: Skierniewice, Poland, 2020. (In Polish) [Google Scholar]
- Michelon, N.; Pennisi, G.; Myint, N.O.; Orsini, F.; Gianquinto, G. Optimization of substrate and nutrient solution strength for lettuce and Chinese cabbage seedling production in the semi-arid environment of central Myanmar. Horticulturae 2021, 7, 64. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Akinci, G.; Moustakas, K.; Tzortzakis, N. Printed paper waste as an alternative growing medium component to produce Brassica seedlings under nursery conditions. Sustainability 2020, 12, 5992. [Google Scholar] [CrossRef]
- Aquino, L.A.; Puiatti, M.; Pereira, P.R.G.; Pereira, F.H.F.; Ladeira, I.R.; Castro, M.R.S. Effect of spacing and doses of nitrogen on the qualitative characteristics of the yield of cabbage. Hortic. Bras. 2005, 23, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Filgueira, F.A.R. Novo Manual de Olericultura: Agrotecnologia Moderna na Producao e Comercializacao de Hortalicas, 3rd ed.; UFV Edicao: Viçosa, Brazil, 2008. (In Portuguese) [Google Scholar]
- Taiz, L.; Zeiger, E.; Santarem, E.R. Plant Physiology, 4th ed.; Artmed: Porto Alegre, Brazil, 2009. [Google Scholar]
- Babik, I. Effect of pot size and fertigation for the growth of vegetable seedlings and yield of plants in the field. Zesz. Probl. Post. Nauk Roln. 2002, 485, 15–28. [Google Scholar]
- Mitchell, E.; Frisbie, S.H.; Moral, M.T. Emergence and growth of cabbage seedlings in plastic, peat, paper, and newspaper containers. Cogent Food Agric. 2017, 3, 1326444. [Google Scholar] [CrossRef]
- Maselesele, D.; Ogola, J.B.O.; Murovhi, R.N. Nutrient uptake and yield of Chinese cabbage (Brassica rapa L. Chinensis) increased with application of macadamia husk compost. Horticulturae 2022, 8, 196. [Google Scholar] [CrossRef]
- Islam, M.M.; Karim, A.J.; Jahiruddin, M.; Majid, N.K.; Miah, M.G.; Ahmed, M.M.; Hakim, M.A. Effect of organic manure and chemical fertilizers on crops in radish-stem amaranth-Indian spinach cropping pattern in homestead area. Aust. J. Crop Sci. 2011, 5, 1370–1378. [Google Scholar]
- Liu, C.H.; Liu, Y.; Fan, C.; Kuang, S.Z. The effect of composted pineapple residue return on soil properties and the growth and yield of pineapple. J. Soil Sci. Plant Nutr. 2013, 13, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Kiran, M.; Jilani, M.S.; Waseem, K.; Sohail, M. Effect of organic manures and inorganic fertilizers on growth and yield of radish (Raphanus sativus L.). Pak. J. Agric. Res. 2016, 29, 363–372. [Google Scholar]
- Rady, M.M.; Semida, W.M.; Hemida, K.A.; Abdelhamid, M.T. The effect of compost on growth and yield of Phaseolus vulgaris plants grown under saline. Int. J. Recycl. Org. Waste Agric. 2016, 5, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Chimphango, S.B.M.; Ogola, J.B.O.; Maseko, S.; MacAlister, D.; Makonya, G.M. Balanced allocation of resources for acquisition of water and nutrients in field legumes. S. Afr. J. Bot. 2018, 115, 281–282. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K. Health promoting constituents of Brassica vegetables. Nowości Warzywnicze 2010, 51, 51–63. (In Polish) [Google Scholar]
- Antonkiewicz, J.; Wiśniowska-Kielian, B.; Baczek-Kwinta, R. Macroelements content in white cabbages cultivars cultivated in chemically polluted soils. Soil-Water J. 2013, 2, 785–792. [Google Scholar]
- Grabowska, A.; Sękara, A.; Kunicki, E.; Kalisz, A. Content of macroelements in index part of broccoli in relation to cultivation method and sparing. Acta Agroph. 2013, 20, 295–314. [Google Scholar]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Xu, D. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef]
- Pant, Y.; Lingwan, M.; Masakapalli, S.K. Metabolic, biochemical, mineral and fatty acid profiles of edible Brassicaceae microgreens establish them as promising functional food. bioRxiv 2023. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Xylia, P.; Petropoulos, S.; Tzortzakis, N. The use of spent coffee grounds in growing media for the production of Brassica seedlings in nurseries. Environ. Sci. Pollut. Res. 2021, 28, 24279–24290. [Google Scholar] [CrossRef] [PubMed]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Daničić, M.; Maksimović, I.; Putnik-Delić, M.; Ilin, Ž. The concentration of microelements in field-grown Brassica species under different fertilization treatments. Acta Hortic. 2021, 1320, 241–246. [Google Scholar] [CrossRef]
- Demir, H.; Polat, E. Effects of different growing media on seedling quality and nutrient contents in cabbage (Brassica oleraceae var. capitata L.). J. Food Agric. Environ. 2014, 12, 1378–1381. [Google Scholar]
- Godlewska, K.; Pacyga, P.; Michalak, I.; Biesiada, A.; Szumny, A.; Pachura, N.; Piszcz, U. Effect of botanical extracts on the growth and nutritional quality of field-grown white head cabbage (Brassica oleracea var. capitata). Molecules 2021, 26, 1992. [Google Scholar] [CrossRef]


| Substrate | pH-H2O | Conductivity | Salinity | N-NO3 | P | K | Mg | N-NH4 |
|---|---|---|---|---|---|---|---|---|
| (mS cm−1) | (g NaCl dm−3) | (mg dm−3) | (mg dm−3) | (mg dm−3) | (mg dm−3) | (mg dm−3) | ||
| Control | 6.55 | 0.94 | 1.43 | 121 | 105 | 235 | 1430 | 130 |
| PRO1 | 5.60 | 0.80 | 1.22 | 107 | 85 | 206 | 720 | 81 |
| PRO2 | 5.62 | 0.86 | 1.30 | 133 | 87 | 200 | 750 | 69 |
| PRO3 | 5.93 | 1.04 | 1.58 | 129 | 59 | 150 | 860 | 30 |
| Factor | SPAD | Plant Height | Number of Leaves | Leaf Mass | Root Mass |
|---|---|---|---|---|---|
| Substrate (A) | <0.001 | <0.001 | 0.422 | 0.159 | 0.156 |
| Species (B) | 0.117 | 0.056 | 0.017 | <0.001 | <0.001 |
| Interaction (A × B) | <0.001 | <0.001 | 0.107 | <0.001 | <0.001 |
| Factor | N | p | K | Mg | Ca |
|---|---|---|---|---|---|
| Substrate (A) | <0.001 | <0.001 | 0.454 | 0.475 | 0.004 |
| Species (B) | 0.002 | 0.426 | 0.003 | <0.001 | 0.080 |
| Interaction (A × B) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Factor | Cu | Fe | Mn | Zn | B |
|---|---|---|---|---|---|
| Substrate (A) | <0.001 | 0.297 | 0.003 | 0.145 | 0.065 |
| Species (B) | 0.007 | 0.026 | 0.114 | <0.001 | <0.001 |
| Interaction (A × B) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadwisieńczak, K.K.; Majkowska-Gadomska, J.; Francke, A.; Kaliniewicz, Z. An Evaluation of the Physical and Chemical Parameters in Brassica Seedlings Grown on Various Organic Substrates. Appl. Sci. 2023, 13, 9124. https://doi.org/10.3390/app13169124
Jadwisieńczak KK, Majkowska-Gadomska J, Francke A, Kaliniewicz Z. An Evaluation of the Physical and Chemical Parameters in Brassica Seedlings Grown on Various Organic Substrates. Applied Sciences. 2023; 13(16):9124. https://doi.org/10.3390/app13169124
Chicago/Turabian StyleJadwisieńczak, Krzysztof Konrad, Joanna Majkowska-Gadomska, Anna Francke, and Zdzisław Kaliniewicz. 2023. "An Evaluation of the Physical and Chemical Parameters in Brassica Seedlings Grown on Various Organic Substrates" Applied Sciences 13, no. 16: 9124. https://doi.org/10.3390/app13169124





