Output Force Density Saturation in COMSOL Simulations of Biomimetic Artificial Muscles
Abstract
:Featured Application
Abstract
1. Introduction
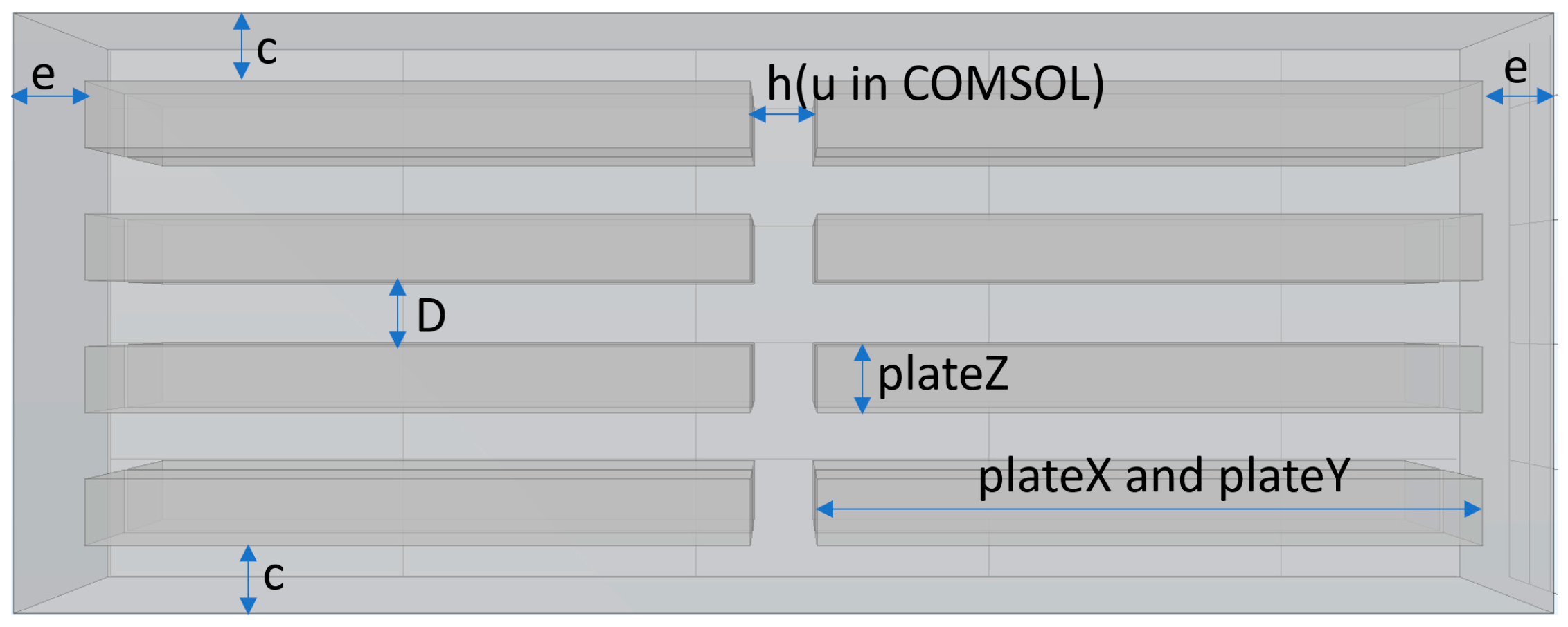
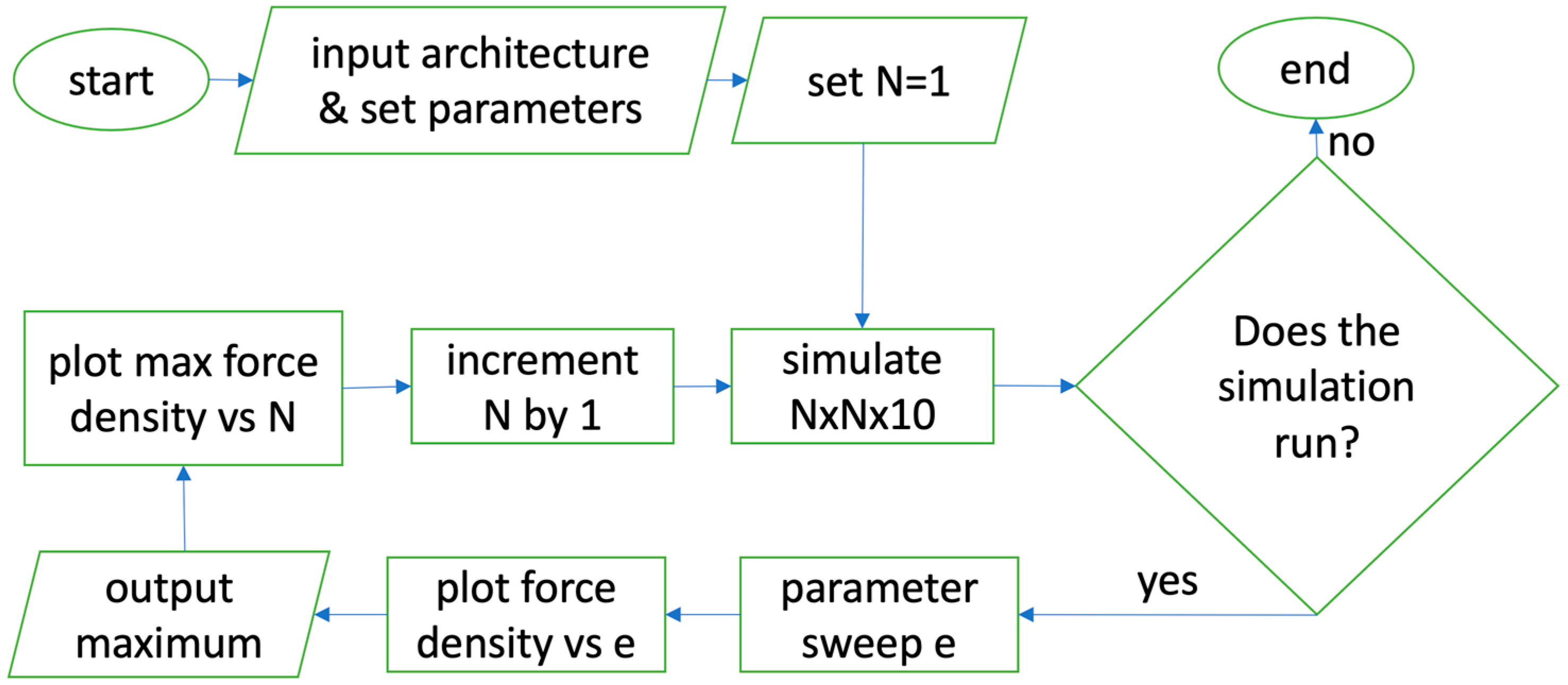
2. Materials and Methods
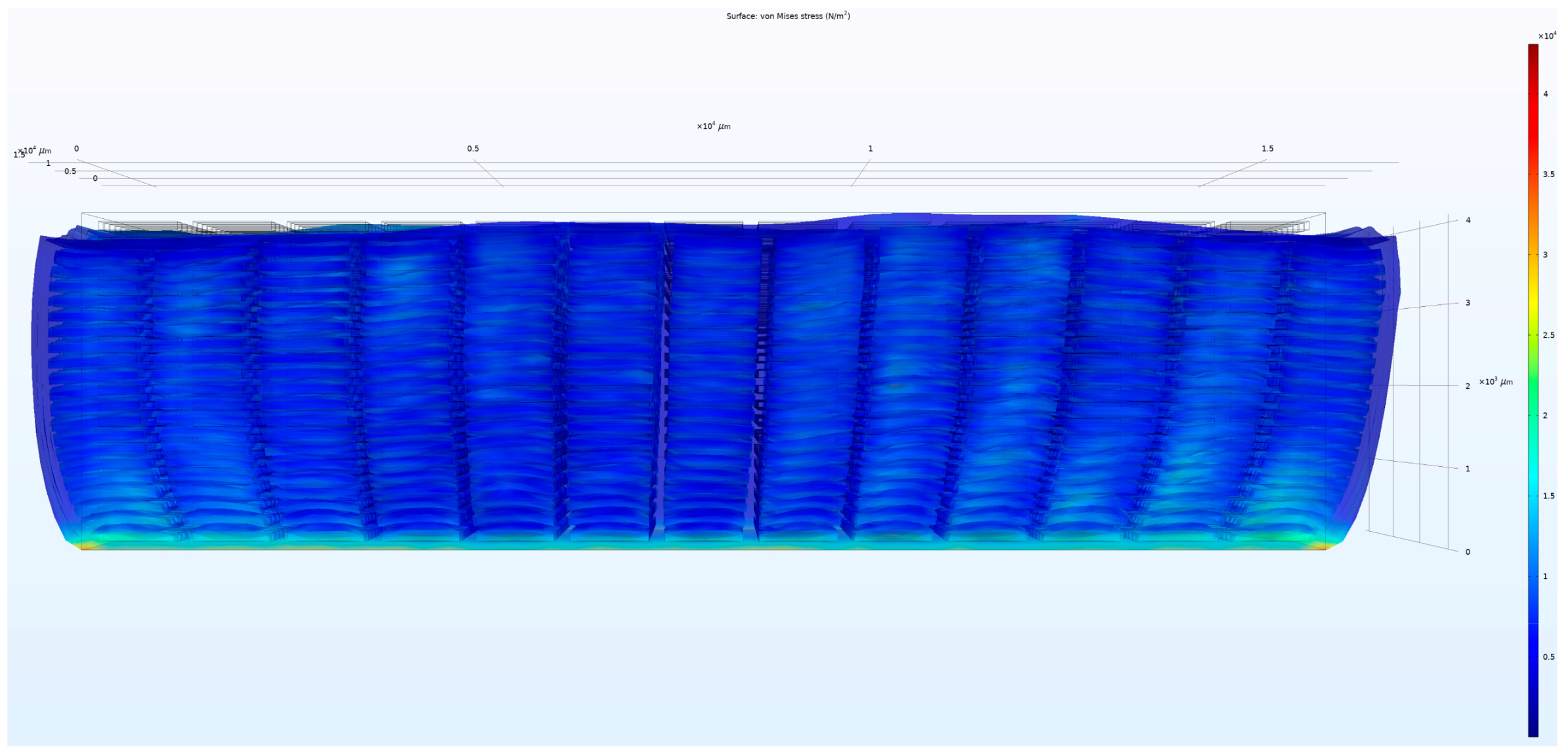
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipták, B.G. (Ed.) Instrument Engineers’ Handbook, 4th ed.; CRC Press: Boca Raton, FL, USA, 2003; p. 2464. [Google Scholar]
- Tavakoli, M.; Marques, L.; de Almeida, A.T. A comparison study on Pneumatic Muscles and electrical motors. In Proceedings of the 2008 IEEE International Conference on Robotics and Biomimetics [Internet], Bangkok, Thailand, 22–25 February 2009; pp. 1590–1594. Available online: http://ieeexplore.ieee.org/document/4913238/ (accessed on 13 August 2023).
- Richardson, A.; Rodgers, M. Vison-Based Semi-Autonomous Outdoor Robot System to Reduce Soldier Workload. In Proceedings of SPIE 4364, Unmanned Ground Vehicle Technology III; SPIE: Bellingham, WA, USA, 2001; Volume 4364, pp. 12–18. [Google Scholar] [CrossRef]
- Ali, H.I.; Noor, S.B.; Marhaban, M.H.; Bashi, S.M. Design of H-inf Controller with Tuning of Weights Using Particle Swarm Optimization. IAENG Int. J. Comput. Sci. 2011, 38, 103–112. [Google Scholar]
- Tiwari, R.; Meller, M.A.; Wajcs, K.B.; Moses, C.; Reveles, I.; Garcia, E. Hydraulic artificial muscles. J. Intell. Mater. Syst. Struct. 2012, 23, 301–312. [Google Scholar] [CrossRef]
- Zoss, A.; Kazerooni, H. Design of an electrically actuated lower extremity exoskeleton. Adv. Robot. 2006, 20, 967–988. [Google Scholar] [CrossRef]
- Mirvakili, S.M.; Hunter, I.W. Artificial Muscles: Mechanism, applications, and challenges. Adv. Mater. 2018, 30, 1704407. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, J.; O’Neill, C.T.; Walsh, C.J.; Wood, R.J.; Ryu, J.H.; Desai, J.P.; Yip, M.C. Robotic Artificial Muscles: Current Progress and Future Perspectives. IEEE Trans. Robot. 2019, 35, 761–781. [Google Scholar] [CrossRef]
- Bahramzadeh, Y.; Shahinpoor, M. A Review of Ionic Polymeric Soft Actuators and Sensors. Soft Robot. 2014, 1, 38–52. [Google Scholar] [CrossRef]
- Feng, C.; Rajapaksha, C.H.; Jákli, A. Ionic Elastomers for Electric Actuators and Sensors. Engineering 2021, 7, 581–602. [Google Scholar] [CrossRef]
- Akle, B.J.; Bennett, M.D.; Leo, D.J. High-strain ionomeric–ionic liquid electroactive actuators. Sens. Actuators A Phys. 2006, 126, 173–181. [Google Scholar] [CrossRef]
- Duduta, M.; Hajiesmaili, E.; Zhao, H.; Wood, R.J.; Clarke, D.R. Realizing the potential of dielectric elastomer artificial muscles. Proc. Natl. Acad. Sci. USA 2019, 116, 2476–2481. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.Y. Multi-Axis Soft Sensors Based on Dielectric Elastomer. Soft Robot. 2016, 3, 3–12. [Google Scholar] [CrossRef]
- Wang, H.; Totaro, M.; Beccai, L. Toward Perceptive Soft Robots: Progress and Challenges. Adv. Sci. 2018, 5, 1800541. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F.; Lee, K.-F.; Lee, M.Y. Development of a flexible PDMS capacitive pressure sensor for plantar pressure measurement. Microelectron. Eng. 2012, 99, 1–5. [Google Scholar] [CrossRef]
- Christians, C.; Goldberg, N.N.; Deheyn, D.D.; Cai, S.; Tolley, M.T. Translucent soft Robots driven by frameless fluid electrode dielectric elastomer actuators. Sci. Robot. 2018, 3, eaat1893. [Google Scholar] [CrossRef]
- Pelrine, R.E.; Kornbluh, R.D.; Joseph, J.P. Electrostriction of polymer dielectrics with compliant electrodes as a means of actuation. Sens. Actuators A Phys. 1998, 64, 77–85. [Google Scholar] [CrossRef]
- Shi, Y.; Askounis, E.; Plamthottam, R.; Libby, T.; Peng, Z.; Youssef, K.; Pu, J.; Pelrine, R.; Pei, Q. A processable, high-performance dielectric elastomer and multilayering process. Science 2022, 377, 228–232. [Google Scholar] [CrossRef]
- O’Neill, M.R.; Acome, E.; Bakarich, S.; Mitchell, S.K.; Timko, J.; Keplinger, C.; Shepherd, R.F. Rapid 3D Printing of Electrohydraulic (HASEL) Tentacle Actuators. Adv. Funct. Mater. 2020, 30, 2005244. [Google Scholar] [CrossRef]
- Martinez, R.V.; Branch, J.L.; Fish, C.R.; Jin, L.; Shepherd, R.F.; Nunes, R.M.; Suo, Z.; Whitesides, G. Robotic Tentacles with Three-Dimensional Mobility Based on Flexible Elastomers. Adv. Mater. 2013, 25, 205–212. [Google Scholar] [CrossRef]
- Chortos, A.; Mao, J.; Mueller, J.; Hajiesmaili, E.; Lewis, J.A.; Clarke, D.R. Printing Reconfigurable Bundles of Dielectric Elastomer Fibers. Adv. Funct. Mater. 2021, 31, 2010643. [Google Scholar] [CrossRef]
- Shaukat, U.; Rossegger, E.; Schlögl, S. A Review of Multi-Material 3D Printing of Functional Materials via Vat Photopolymerization. Polymers 2022, 14, 2449. [Google Scholar] [CrossRef]
- Knowlton, S.; Yu, C.H.; Ersoy, F.; Emadi, S.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic chips with patterned, cell-laden hydrogel constructs. Biofabrication 2016, 8, 025019. [Google Scholar] [CrossRef]
- Rafiee, M.; Farahani, R.D.; Therriault, D. Multi-Material 3D and 4D Printing: A Survey. Adv. Sci. 2020, 7, 1902307. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.A.; Catterlin, J.; Scherer, A.; Kartalov, E.P. Simulations of 3D-printable biomimetic artificial muscles based on microfluidic microcapacitors for exoskeletal actuation and stealthy underwater propulsion. Sens. Actuators A Phys. 2021, 325, 112700. [Google Scholar] [CrossRef]
- Coltelli, M.A.; Kartalov, E.P. Scalable microfluidic double-helix weave architecture for wiring of microscopic arrays in 3D-printable biomimetic artificial muscles. Sens. Actuators A. Phys. 2022, 340, 113543. [Google Scholar] [CrossRef]
- Bottauscio, O.; Chiampi, M.; Manzin, A. Different Finite-Element Approaches for Electromechanical Dynamics. IEEE Trans. Magn. 2004, 40, 541–544. [Google Scholar] [CrossRef]
- Ren, Z.; Razek, A. Force calculation by Maxwell stress tensor in 3D hybrid finite element-boundary integral formulation. IEEE Trans. Magn. 1990, 26, 2774–2776. [Google Scholar] [CrossRef]
- Kartalov, E.P.; Anderson, W.F.; Scherer, A. The analytical approach to polydimethylsilixane microfluidic technology and its biological applications. J. Nanosci. Nanotechnol. 2006, 6, 2265–2277. [Google Scholar] [CrossRef]
- Kartalov, E.P.; Zhong, J.F.; Scherer, A.; Quake, S.R.; Taylor, C.R.; Anderson, W.F. High-throughput multi-antigen microfluidic fluorescence immunoassays. BioTechniques 2006, 40, 85–90. [Google Scholar] [CrossRef]
- Kartalov, E.P.; Maltezos, G.; Anderson, W.F.; Taylor, C.R.; Scherer, A. Electrical microfluidic pressure gauge for elastomer microelectromechanical systems. J. Appl. Phys. 2007, 102, 084909. [Google Scholar] [CrossRef]
- Kartalov, E.P.; Scherer, A.; Quake, S.R.; Taylor, C.R.; Anderson, W.F. Experimentally validated quantitative linear model for the device physics of elastomeric microfluidic valves. J. Appl. Phys. 2007, 101, 064505. [Google Scholar] [CrossRef]
- Lin, D.H.; Taylor, C.R.; Anderson, W.F.; Scherer, A.; Kartalov, E.P. Internally calibrated quantification of VEGF in human plasma by fluorescence immunoassays in disposable elastomeric microfluidic devices. J. Chromatogr. B 2010, 878, 258–263. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Taylor, C.R.; Scherer, A.; Kartalov, E.P. Elastomeric microfluidic diode and rectifier work with Newtonian fluids. J. Appl. Phys. 2009, 106, 114311. [Google Scholar] [CrossRef] [PubMed]
- Raub, C.B.; Lee, C.; Kartalov, E. Sequestration of bacteria from whole blood by optimized microfluidic cross-flow filtration for Rapid Antimicrobial Susceptibility Testing. Sens. Actuators B 2015, 210, 120–123. [Google Scholar] [CrossRef]
- Maltezos, G.; Lee, J.; Rajagopal, A.; Scholten, K.; Kartalov, E.; Scherer, A. Microfluidic blood filtration device. Biomed. Microdevices 2011, 13, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Kerrebrock, P.A.; Anderson, J.M.; Parry, J.R. Application requirements of artificial muscles for swimming robots. In Smart Structures and Materials 2001: Electroactive Polymer Actuators and Devices; SPIE: Bellingham, WA, USA, 2001; Volume 4329, pp. 364–374. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coltelli, M.A.; Keeven, J.M.; Leckie, J.M.; Catterlin, J.K.; Sadagic, A.; Kartalov, E.P. Output Force Density Saturation in COMSOL Simulations of Biomimetic Artificial Muscles. Appl. Sci. 2023, 13, 9286. https://doi.org/10.3390/app13169286
Coltelli MA, Keeven JM, Leckie JM, Catterlin JK, Sadagic A, Kartalov EP. Output Force Density Saturation in COMSOL Simulations of Biomimetic Artificial Muscles. Applied Sciences. 2023; 13(16):9286. https://doi.org/10.3390/app13169286
Chicago/Turabian StyleColtelli, Michelangelo A., Joshua M. Keeven, Jacob M. Leckie, Jeffrey K. Catterlin, Amela Sadagic, and Emil P. Kartalov. 2023. "Output Force Density Saturation in COMSOL Simulations of Biomimetic Artificial Muscles" Applied Sciences 13, no. 16: 9286. https://doi.org/10.3390/app13169286




