Extraction of Dietary Fibers from Plant-Based Industry Waste: A Comprehensive Review
Abstract
:Featured Application
Abstract
1. Introduction
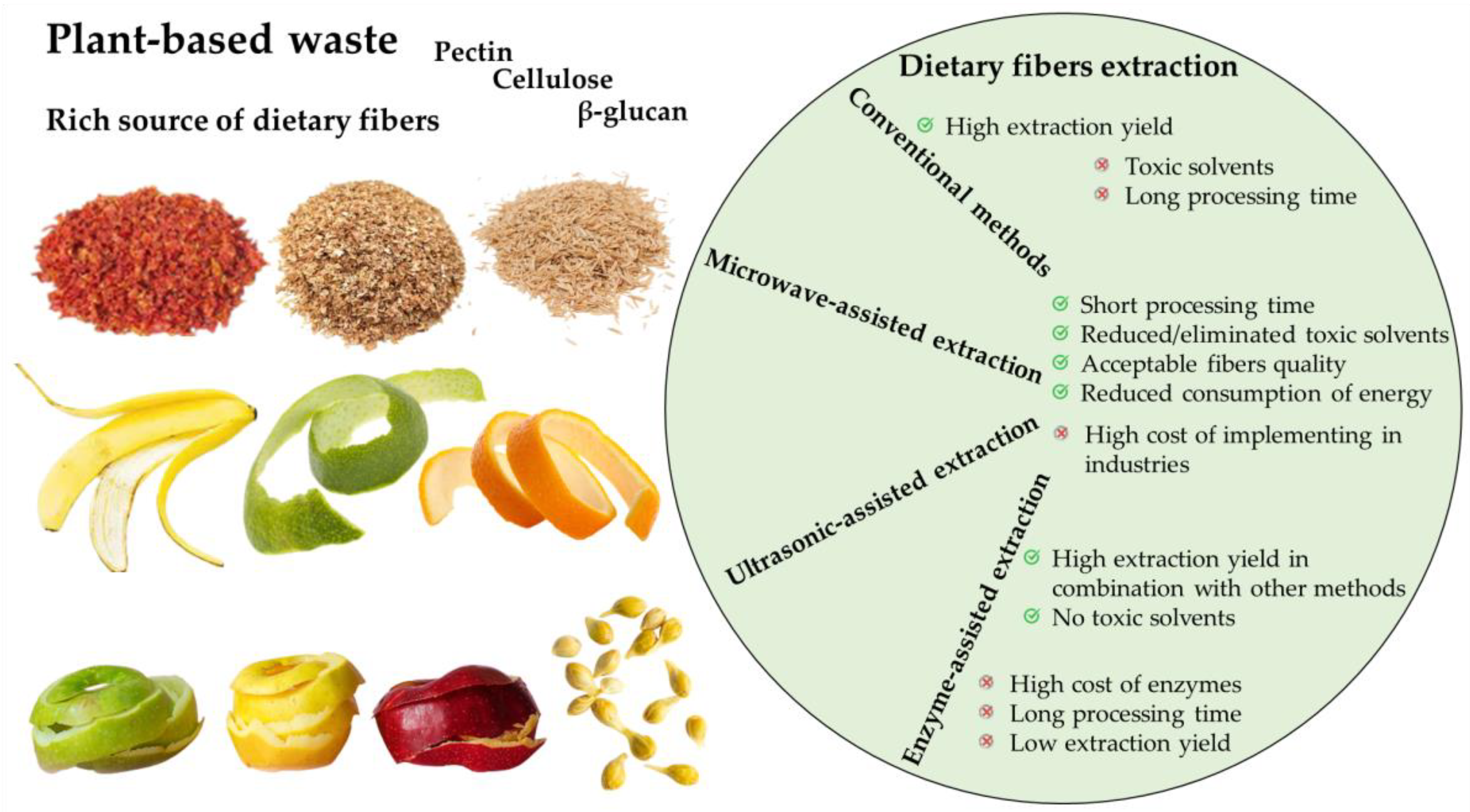
2. Dietary Fibers
2.1. Soluble Dietary Fibers
2.1.1. Pectin
2.1.2. β-Glucan
2.1.3. Gums and Mucilage
2.2. Insoluble Dietary Fibers
2.2.1. Cellulose
2.2.2. Hemicellulose
2.2.3. Lignin
3. Extraction Methods
3.1. Subcritical Water Extraction
3.2. Ultrasound Assisted-Extraction
3.3. Microwave-Assisted Extraction
3.4. Enzyme-Assisted Extraction
3.5. Ohmic Heating Extraction
3.6. Pulsed Electric Field Assisted-Extraction
4. Dietary Fibers Extraction
4.1. Fiber Mixtures Extraction
4.2. Pectin Extraction
4.3. β-Glucan Extraction
4.4. Cellulose Extraction
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gómez, V.; González-Barrio, R.; Periago, M.J. Interaction between Dietary Fibre and Bioactive Compounds in Plant By-Products: Impact on Bioaccessibility and Bioavailability. Antioxidants 2023, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019, 294, 339–346. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists. The definition of dietery fibar. Cereal Foods World 2001, 46, 112–116. [Google Scholar]
- Maphosa, Y.; Jideani, V.A. Dietary fiber extraction for human nutrition—A review. Food Rev. Int. 2015, 32, 98–115. [Google Scholar] [CrossRef]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary Fiber from Underutilized Plant Resources—A Positive Approach for Valorization of Fruit and Vegetable Wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Roussis, I.; Bilalis, D.; Priniotakis, G. Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization. Processes 2023, 11, 1580. [Google Scholar] [CrossRef]
- Quiros-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Gonzalez-Cordova, A.F.; Gonz´alez-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063. [Google Scholar] [CrossRef]
- Cui, J.; Lian, Y.; Zhao, C.; Du, H.; Han, Y.; Gao, W.; Xiao, H.; Zheng, J. Dietary Fibers from Fruits and Vegetables and Their Health Benefits via Modulation of Gut Microbiota. CRFSFS 2019, 18, 1514–1532. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of dietary fiber on human health. Food Sci. Hum. Wellness 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Advances in the Functional Characterization and Extraction Processes of Dietary Fiber. Food Eng. Rev. 2016, 8, 251–271. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wu, L.-X.; Cai, W.-D.; Xiao, G.-S.; Duan, Y.; Zhang, H. Subcritical water extraction-based methods affect the physicochemical and functional properties of soluble dietary fibers from wheat bran. Food Chem. 2019, 298, 124987. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Sousa, R.C.S.; Dias, M.M.S.; Coimbra, J.S.R. Extraction of Pectin from Passion Fruit Peel. Food Eng. Rev. 2020, 12, 460–472. [Google Scholar] [CrossRef]
- Ganguly, P.; Sengupta, S.; Das, P.; Bhowal, A. Valorization of food waste: Extraction of cellulose, lignin and their application in energy use and water treatment. Fuel 2020, 280, 118581. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; You, M.; Liu, X. Comparison of physicochemical properties of β-glucans extracted from hull-less barley bran by different methods. Int. J. Biol. Macromol. 2021, 182, 1192–1199. [Google Scholar] [CrossRef]
- Robledo, R.; Castro Vázquez, L.I. Pectin-Extraction, Purification, Characterization and Applications. In Pectins-Extraction, Purification, Characterization and Applications; Masuelli, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Tromp, R.H.; de Kruif, C.G.; Van Eijk, M.; Rolin, C. On the mechanism of stabilisation of acidified milk drinks by pectin. Food Hydrocoll. 2004, 18, 565–572. [Google Scholar] [CrossRef]
- Yoo, H.-U.; Ko, M.-J.; Chung, M.-S. Hydrolysis of beta-glucan in oat flour during subcritical-water extraction. Food Chem. 2020, 308, 125670. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Ren, Y.; Li, Y.; Fan, M.; Qian, H.; Wang, L.; Wu, G.; Zhang, H.; Qi, X.; Xu, M.; et al. Physiological functionalities and mechanisms of β-glucans. Trends Food Sci. Technol. 2019, 88, 57–66. [Google Scholar] [CrossRef]
- Geller, A.; Shrestha, R.; Yan, J. Yeast-Derived β-Glucan in Cancer: Novel Uses of a Traditional Therapeutic. Int. J. Mol. Sci. 2019, 20, 3618. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, P.; Wang, R.; Zhou, M.; Pang, N.; Cui, X.; Ge, X.; Liu, X.; Huang, X.; Yu, Y. Three Different Types of β-Glucans Enhance Cognition: The Role of the Gut-Brain Axis. Front. Nutr. 2022, 9, 848930. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, G.; Sowrirajan, S.; Joseph, B. Extraction and Isolation of β-Glucan from Grain Sources—A Review. J. Food Sci. 2017, 82, 1535–1545. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef]
- Mu, R.; Hong, X.; Ni, Y.; Li, Y.; Pang, J.; Wang, Q.; Xiao, J.; Zheng, Y. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2018, 271, 462–472. [Google Scholar] [CrossRef]
- Tanaka, M.; Takamizu, A.; Hoshino, M.; Sasaki, M.; Goto, M. Extraction of dietary fiber from Citrus junos peel with subcritical water. Food Bioprod. Process. 2012, 90, 180–186. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Applying ultrasound-assisted processing to obtain cellulose fibres from rice straw to be used as reinforcing agents. Innov. Food Sci. Emerg. Technol. 2022, 76, 102932. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Wang, H.; Ding, J.; Ren, N. Recent advances in microwave-assisted extraction of trace organic pollutants from food and environmental samples. TrAC Trends Anal. Chem. 2016, 75, 197–208. [Google Scholar] [CrossRef]
- Gerschenson, L.N.; Rojas, A.M.; Fissore, E.N. Conventional and Emerging Extraction Technologies. In Dietary Fiber: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2019; pp. 199–245. [Google Scholar] [CrossRef]
- Sharma, N.; Pooja; Yadav, S.K. Conventional and Emerging Novel Techniques for the Extraction of Pectin and Applications of Pectin. Austin J. Biotechnol. Bioeng. 2022, 9, 1115. [Google Scholar]
- Li, J.; Niu, D.; Zhang, Y.; Zeng, X.-A. Physicochemical properties, antioxidant and antiproliferative activities of polysaccharides from Morinda citrifolia L. (Noni) based on different extraction methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar] [CrossRef]
- Fan, R.; Wang, L.; Fan, J.; Sun, W.; Dong, H. The Pulsed Electric Field Assisted-Extraction Enhanced the Yield and the Physicochemical Properties of Soluble Dietary Fiber from Orange Peel. Front. Nutr. 2022, 9, 925642. [Google Scholar] [CrossRef]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave assisted extraction with three modifications on structural and functional properties of soluble dietary fibers from grapefruit peel. Food Hydrocoll. 2019, 101, 105549. [Google Scholar] [CrossRef]
- Cheikh Rouhou, M.; Abdelmoumen, S.; Thomas, S.; Attia, H.; Ghorbel, D. Use of green chemistry methods in the extraction of dietary fibers from cactus rackets (Opuntia ficus indica): Structural and microstructural studies. Int. J. Biol. Macromol. 2018, 116, 901–910. [Google Scholar] [CrossRef]
- Capitani, M.I.; Sportono, V.; Nolasco, S.M.; Tomás, M.C. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. LWT-Food Sci. Technol. 2012, 45, 94–102. [Google Scholar] [CrossRef]
- Campos, B.E.; Dias Ruivo, T.; da Silva Scapim, M.R.; Madrona, G.S.; Bergamasco, R.D.C. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Liu, J.-P.; Huang, X.; Du, L.-P.; Shi, F.-L.; Dong, R.; Huang, X.-T.; Zheng, K.; Yang, L.; Cheong, K.-L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT Food Sci. Technol. 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Pectin extraction from common fig skin by different methods: The physicochemical, rheological, functional, and structural evaluations. Int. J. Biol. Macromol. 2019, 136, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kumar Kalakandan, S. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2019, 61, 104812. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzeto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.E.; Hosseini, S.S. Optimization of aqueous pectin extraction from Citrus medica peel. Carbohydr. Polym. 2017, 178, 27–33. [Google Scholar] [CrossRef]
- Sabater, C.; Sabater, V.; Olano, A.; Montilla, A.; Corzo, N. Ultrasound-assisted extraction of pectin from artichoke by-products. An artificial neural network approach to pectin characterisation. Food Hydrocoll. 2019, 98, 105238. [Google Scholar] [CrossRef]
- Chaharbaghi, E.; Khodaiyan, F.; Hosseini, S.S. Optimization of pectin extraction from pistachio green hull as a new source. Carbohydr. Polym. 2017, 173, 107–113. [Google Scholar] [CrossRef]
- Do Nascimento Oliveira, A.; de Almeida Paula, D.; Basílio de Oliveira, E.; Henriques Saraiva, S.; Stringheta, P.C.; Mota Ramos, A. Optimization of pectin extraction from Ubá mango peel through surface response methodology. Int. J. Biol. Macromol. 2018, 113, 395–402. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, S.; Shi, J.; Langrish, T.A.G. Comparisons between different techniques for water-based extraction of pectin from orange peels. Desalination 2008, 218, 229–237. [Google Scholar] [CrossRef]
- Petkowicz, C.L.O.; Vriesmann, L.C.; Williams, P.A. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Priya, B.; Al-Dhabi, N.A.; Ponmurugan, K.; Ganesh Moothy, I.; Sivarajasekar, N. Ultrasound Assisted Citric Acid mediated Pectin Extraction from Industrial Waste of Musa Balbisiana. Ultrason. Sonochem. 2016, 35, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Freitas de Oliveira, C.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Ferreira Marczak, L.D. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Ultrasound-assisted extraction of pectin from sisal waste. Carbohydr. Polym. 2015, 115, 732–738. [Google Scholar] [CrossRef]
- Dranca, F.; Vargas, M.; Oroian, M. Physicochemical properties of pectin from Malus domestica “Fălticeni” apple pomace as affected by non-conventional extraction techniques. Food Hydrocoll. 2019, 100, 105383. [Google Scholar] [CrossRef]
- Yuliarti, O.; Goh, K.K.T.; Matia-Merino, L.; Mawson, J.; Brennan, C. Extraction and characterisation of pomace pectin from gold kiwifruit (Actinidia chinensis). Food Chem. 2015, 187, 290–296. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef]
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2020, 58, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-H.; Jung, H.-T.; Lee, B.-H.; Kim, H.-S.; Rhee, J.-K.; Yoo, S.-H. Green process development for apple-peel pectin production by organic acid extraction. Carbohydr. Polym. 2018, 204, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Raji, Z.; Khodaiyan, F.; Rezaei, K.; Kiani, H.; Hosseini, S.S. Extraction optimization and physicochemical properties of pectin from melon peel. Int. J. Biol. Macromol. 2017, 98, 709–716. [Google Scholar] [CrossRef]
- Swamy, G.J.; Muthukumarappan, K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017, 220, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Udonne, J.D.; Ajani, O.O.; Akinyemi, O.P. A comparative study of extraction of pectin from wet and dried peels using water based and microwave methods. Int. J. Eng. Res. 2016, 7, 416–432. [Google Scholar]
- Bee Lin, C.; Yek Cze, C. Drying Kinetics and Optimisation of Pectin Extraction from Banana Peels via Response Surface Methodology. MATEC Web Conf. 2018, 152, 01002. [Google Scholar] [CrossRef]
- Goudar, G.; Sharma, P.; Janghu, S.; Longvah, T. Effect of processing on barley β-glucan content, its molecular weight and extractability. Int. J. Biol. Macromol. 2020, 162, 1204–1216. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Dexter, J.E. Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products. Food Res. Int. 2008, 41, 850–868. [Google Scholar] [CrossRef]
- Vasanthan, T.; Temelli, F. Grain fractionation technologies for cereal β-glucan concentration. Food Res. Int. 2008, 41, 876–881. [Google Scholar] [CrossRef]
- Sibakov, J.; Abecassis, J.; Barron, C.; Poutanen, K. Electrostatic separation combined with ultra-fine grinding to produce β-glucan enriched ingredients from oat bran. Innov. Food Sci. Emerg. Technol. 2014, 26, 445–455. [Google Scholar] [CrossRef]
- Ghotra, B.S.; Vasanthan, T.; Temelli, F. Structural characterization of barley β-glucan extracted using a novel fractionation technique. Food Res. Int. 2008, 41, 957–963. [Google Scholar] [CrossRef]
- Mejía, S.M.V.; de Francisco, A.; Bohrer, B. A comprehensive review on cereal β-glucan: Extraction, characterization, causes of degradation, and food application. Crit. Rev. Food Sci. Nutr. 2020, 60, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Climova, A.; Ibrahim, M.N.G.; Salamahina, A.; Savin, A.M.; Dukhinova, M.S.; Barakova, N.V.; Krivoshapkina, E.F. Application of extracted β-glucan from oat for β-carotene encapsulation. J. Food Sci. Technol. 2021, 58, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Shoda, T.; Machmudah, S.; Wahyudiono Kanda, H.; Goto, M. Enhancing pressurized water extraction of β-glucan from barley grain by adding CO2 under hydrothermal conditions. Chem. Eng. Process. Process Intensif. 2015, 97, 45–54. [Google Scholar] [CrossRef]
- Kodama, S.; Shoda, T.; Machmudah, S.; Wahyudiono Kanda, H.; Goto, M. Extraction of β–glucan by hydrothermal liquidization of barley grain in a semi-batch reactor. Sep. Sci. Technol. 2015, 51, 278–289. [Google Scholar] [CrossRef]
- Ren, Y.; Xie, H.; Liu, L.; Jia, D.; Yao, K.; Chi, Y. Processing and Prebiotics Characteristics of β-Glucan Extract from Highland Barley. Appl. Sci. 2018, 8, 1481. [Google Scholar] [CrossRef]
- Karimi, R.; Azizi, M.H.; Xu, Q. Effect of different enzymatic extractions on molecular weight distribution, rheological and microstructural properties of barley bran β-glucan. Int. J. Biol. Macromol. 2019, 126, 298–309. [Google Scholar] [CrossRef]
- Harasym, J.; Żyła, E.; Dziendzikowska, K.; Gromadzka-Ostrowska, J. Proteinaceous Residue Removal from Oat β-Glucan Extracts Obtained by Alkaline Water Extraction. Molecules 2019, 24, 1729. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Liu, H.M.; Hou, J.; Yao, Y.G.; Ma, Y.X.; Wang, X.D. Cellulose fibers extracted from sesame hull using subcritical water as a pretreatment. Arab. J. Chem. 2021, 14, 103178. [Google Scholar] [CrossRef]
- Song, X.; Jiang, Y.; Rong, X.; Wei, W.; Wang, S.; Nie, S. Surface characterization and chemical analysis of bamboo substrates pretreated by alkali hydrogen peroxide. Bioresour. Technol. 2016, 216, 1098–1101. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Yan, D.; Zhang, C.; Nie, S. Enzyme assisted mechanical production of cellulose nanofibrils: Thermal stability. Cellulose 2018, 25, 5049–5061. [Google Scholar] [CrossRef]
- Borrega, M.; Nieminen, K.; Sixta, H. Effects of hot water extraction in a batch reactor on the delignification of birch wood. Bioresources 2011, 6, 1890–1903. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum Alkaline Treatment Parameters for the Extraction of Cellulose and Production of Cellulose Nanocrystals from Apple Pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Cheng, T.-Y.; Yang, W.-G.; Ma, P.-T.; He, H.-Z.; Yin, X.-C.; Yu, X.-X. Characteristics and environmentally friendly extraction of cellulose nanofibrils from sugarcane bagasse. Ind. Crops Prod. 2018, 111, 285–291. [Google Scholar] [CrossRef]
- Moradi, E.; Fathi, M. Production of cellulose nanocrystals from tomato pomace as a food waste and their application for stabilizing of Pickering emulsions. Bioact. Carbohydr. Diet Fibre 2023, 30, 100378. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Bicu, I.; Mustata, F. Cellulose extraction from orange peel using sulfite digestion reagents. Bioresour. Technol. 2011, 102, 10013–10019. [Google Scholar] [CrossRef]
| Plant Resource | Reference | |
|---|---|---|
| Musa balbisiana waste | [56] | |
| Method | Ultrasound-assisted with citric acid-mediated extraction. | |
| Conditions | Optimized conditions were as follows: 323 W—ultrasound power; 3.2—pH; 28 min—extraction time; and 1:15 g/mL—solid: liquid ratio. | |
| Observations | The experimental pectin yield was 8.99% while the predicted yield was 9.02%. | |
| Passion fruit peel | [57] | |
| Method | Ultrasound-assisted extraction. | |
| Conditions | The conditions were as follows: 644 W/cm2—power intensity; 85 °C temp; 1:30—dried peel: solvent ratio; and 10 min—sonication. | |
| Observations | The achieved pectin yield under these conditions was 12.67% and the esterification degree and galacturonic acid content were 60.36% and 66.65%, respectively. The authors made a comparison of this extraction method with the conventional method under similar conditions and the obtained pectin yield was higher with the ultrasound-assisted extraction. | |
| Lime peel | [58] | |
| Method | Microwave-assisted and conventional extraction. | |
| Conditions | Hydrochloric and citric acids—solvents; 1:20 or 1:40—peel: solvent ratio; 95 °C—temp for conventional method; 700 W—microwave oven power. | |
| Observations | Pectin yield was higher with the conventional method with hydrochloric acid. Microwave-assisted extraction with citric acid resulted in pectin without loss in quality (higher equivalent weight and degree of esterification) and this method also resulted in a reduced energy consumption due to the shortened extraction time. | |
| Sisal waste | [59] | |
| Method | Ultrasound-assisted extraction. | |
| Conditions | Optimized conditions were: 61 W—ultrasonic power; 50 °C temp; 26 min—time of sonification; and 1:28 g/mL—solid: liquid ratio. | |
| Observations | The experimental pectin yield was 29.32%, and the authors highlighted the advantages of this method due to reduced energy consumption, lower temperatures, and a shorter extraction time. | |
| Malus domestica ‘Fălticeni’ apple pomace | [60] | |
| Method |
| |
| Conditions |
| |
| Observations | The highest extraction yield was obtained with microwave-assisted extraction while the lowest was with enzyme-assisted extraction (with Celluclast 1.5 L). Pectin obtained via microwave-assisted extraction had a high galacturonic acid content, acceptable color parameters, increased equivalent weight, high molecular weight, and degree of esterification. Pectin obtained through ultrasound-assisted extraction also had a high galacturonic acid content, degree of esterification, and molecular weight. Pectin obtained with enzyme-assisted extraction had a lower degree of esterification and was classified as low-methoxylated pectin. | |
| Gold kiwifruit (Actinidia chinensis) | [61] | |
| Method | Conventional citric acid extraction, water extraction, and enzyme-assisted extraction. | |
| Conditions | Acid extraction: pH 2.2; 1:3 w/v—solid: liquid ratio; 50 °C temp; and 60 min duration. Water extraction: pH 3.7; 1:3 w/v—solid: liquid ratio; 25 °C temp; and 30 min duration. Enzymatic extraction: Celluclast 1.5 L; pH 3.7; 1:3 w/v—solid: liquid ratio; 25 °C temp; and 30 min duration. | |
| Observations | Pectin extracted with water exhibited the properties closest to its native form. The highest extraction yield was obtained with enzyme-assisted extraction. | |
| Sour orange peel | [62] | |
| Method | Microwave-assisted extraction. | |
| Conditions | Optimized conditions: pH 1.5; 700 W—microwave power; and 3 min—irradiation time. | |
| Observations | Pectin yield was 29.1% and degree of esterification ranged from 1.7% to 37.5%, which indicated low-methoxylated pectin. Emulsifying activity and galacturonic acid content was 40.7% and 71%, respectively. | |
| Lemon, mandarin and kiwi peels | [63] | |
| Method | Microwave-assisted extraction and ultrasound-assisted extraction. | |
| Conditions | Hydrochloric acid and nitric acid—solvents. For microwave-assisted extraction: 360–600 W—microwave power; and 1, 2 and 3 min—irradiation time. For ultrasound-assisted extraction: 60 °C and 75 °C temp; and 15, 30 and 45 min—sonification. | |
| Observations | The highest yield was obtained for kiwi peel using hydrochloric acid (17.97% for microwave-assisted extraction at 360 W for 3 min and 17.30% for ultrasound-assisted extraction at 75 °C for 45 min, respectively). Generally, for all materials, microwave-assisted extraction resulted in higher extraction yields. | |
| Apple peel | [64] | |
| Method | Organic acid extraction. | |
| Conditions | Citric, malic, and tartaric acids—solvents; 85 °C temp. | |
| Observations | Citric acid extraction resulted in pectin with the highest molecular weight and apparent viscosity. Pectin obtained with all tested organic acids was highly methoxylated. | |
| Melon peel | [65] | |
| Method | Conventional citric acid extraction. | |
| Conditions | Optimized conditions: pH 1; 95 °C temp; 10 v/w ratio; and 200 min—duration. | |
| Observations | The extraction yield was 29.48% and the galacturonic acid content was 48%, respectively. The emulsifying activity was 35% and at concentrations of 1% w/v, the obtained pectin behaved as a weak gel. | |
| Banana peel | [66] | |
| Method | Microwave-assisted extraction (continuous and intermittent). | |
| Conditions | Optimized conditions for continuous extraction: pH 3; 900 W—microwave power; and 100 s duration. Optimized conditions for intermittent extraction: pH 3; 900 W—microwave power; and 0.5—pulse ratio. | |
| Observations | The highest extraction yield for continuous extraction was 2.18%, while for the intermittent extraction it was 2.58%, respectively. | |
| Lemon wet and dried peels | [67] | |
| Method |
| |
| Conditions |
| |
| Observations | The maximum pectin yield with water-based extraction was obtained at 95 °C, pH of 1, for 90 min for both wet and dried lemon peels, at 46% and 16%, respectively. For microwave extraction, the maximum yield was obtained using EDTA at an extraction time of 15 min. | |
| Banana peel | [68] | |
| Method | Ultrasound-assisted extraction. | |
| Conditions | The conditions were as follows: 0.1 M of citric acid with pH 1.5, solid: liquid ratio 1:33.3 g/mL, temperature of 75 °C, and sonicated for 23 min. | |
| Observations | Using the optimal extraction conditions, the achieved pectin yield was 6.08%. The obtained pectin was high-methoxyl pectin with improved gelling time. | |
| Plant Resource | Reference | |
|---|---|---|
| Highland barley | [78] | |
| Method | Alkaline-acid-alcohol extraction method. | |
| Conditions | Barley bran was mixed with water at pH 8 adjusted with NaOH and incubated for 2.5 h at 80 °C. The mixture was centrifuged two times (after the first one, the pH of the supernatant was adjusted to 4.5 with HCl, and after the second, the supernatant was reduced at 100 mL and mixed with ethanol) and after 10 h of incubation at 4 °C the precipitate was freeze dried. | |
| Observations | β-glucan extract showed great thermal and pH stability and its solubility was influenced by temperature. | |
| Hull-less barley bran | [18] | |
| Method |
| |
| Conditions |
| |
| Observations | The microwave-ultrasound-assisted extraction resulted in the highest extraction yield (2.16%) in the shortest amount of time. β-glucan obtained with this method had the highest apparent viscosity, stronger foam stability and emulsifying properties than β-glucan obtained with ultrasound-assisted extraction, which had the stronger foaming capability. | |
| Barley bran | [79] | |
| Method | Enzyme-assisted extraction. | |
| Conditions | α-amylase—pH 6.5, 96 °C temp; glucoamylase—pH 4.5, 50 °C temp; protease—pH 7.5, 60 °C temp; pullulanase—4.5, 50 °C; two types of xylanase—pH 4.75, 50 °C temp. | |
| Observations | Results showed that the three-step purification using α-amylase, protease, and xylanase for 4 h increased the β-glucan content, and removed starch, protein, and pentosans. Also, the highest β-glucan purity (around 89%), as well as the lowest molecular weight, (2 × 104 g/mol) were achieved with this method. | |
| Oat bran | [80] | |
| Method | Alkaline extraction. | |
| Conditions | Endogenous enzyme inactivation and fat removal: water/ethanol (50:50 w/w)—solvent; and 80 °C temp. Extraction: pH 8.5 (NaOH/water, 1:40 w/w). Deproteinization: at pH 4.5 and centrifugation. Enzymatic treatment: pancreatin, thermostable α-amylase, and amyloglucosidase. | |
| Observations | The obtained β-glucan was successfully purified, and it can be concluded that a complex process, which includes different enzymes, is required for the removal of residuals from β-glucan. | |
| Plant Resource | Reference | |
|---|---|---|
| Tomato pomace | [88] | |
| Method | Alkaline and bleaching treatment for cellulose production and extraction of cellulose nanocrystals with acid hydrolysis. | |
| Conditions | Acid hydrolysis-optimized conditions: 45 °C temp; 30 min duration. | |
| Observations | The obtained crystallinity was 97% and the particle average diameter was 104 nm. The results showed that tomato pomace, as food waste, could be used for the extraction of cellulose nanocrystals, which is an environmentally friendly material. | |
| Peanut shell | [17] | |
| Method | Alkaline and bleaching treatment for cellulose production. | |
| Conditions | For alkaline treatment: 0.5 M sodium hydroxide; 90 °C temp. Washing with nitric acid in ethanol and finally bleaching with sodium hypochlorite (10%). | |
| Observations | A total of 0.39 g/g of cellulose (dry wt%) was extracted from the tested material. | |
| Mengkuang leaves (Pandanus tectorius) | [89] | |
| Method | Alkaline and bleaching treatment for cellulose production and extraction of nanocrystals with acid hydrolysis. | |
| Conditions | For alkaline treatment: 4% sodium hydroxide; 125 °C temp; and 2 h duration. Bleaching treatment: 1.7 w/v% NaClO2; pH 4.5; 125 °C; and 4 h duration. Acid hydrolysis: 60 wt% H2SO4; 45 °C temp; and 45 min duration. | |
| Observations | The raw material contained 37.3% cellulose. After alkaline treatment and bleaching treatment, cellulose content was 57.5% and 81.6%, respectively. | |
| Rice husk | [15] | |
| Method | Alkaline and bleaching treatment for cellulose production and extraction of nanocrystals with acid hydrolysis. | |
| Conditions | For alkaline treatment: 4 wt% sodium hydroxide; 2 h duration. Bleaching treatment: buffer solution of aqueous chlorite (1.7 wt%), acetic acid and distilled water; 4 h duration; and 100–130 °C temp. Acid hydrolysis: 10 M sulfuric acid 50 °C temp; 40 min duration. | |
| Observations | The raw material contained 35% cellulose. After alkaline treatment and bleaching treatment, cellulose content was 57% and 96%, respectively. | |
| Orange peel | [90] | |
| Method | Extraction with sodium sulfite and sodium metabisulfite. | |
| Conditions | The applied conditions were as follows: 98 °C temp; 7.5:1—liquid: solid ratio. Bleaching treatment was performed under an alkaline medium, slightly acid medium, and hydrogen peroxide at 25 °C temp for 240 min, 120 min, and 60 min, respectively. | |
| Observations | The optimum yields were 45.2% and 40.4% for sodium metabisulfite and sodium sulfite, respectively. The results showed good purity, whiteness, water retention, molecular weights, and low crystallinities. | |
| Lemon (Citrus limon) seeds | [29] | |
| Method | Water extraction of cellulose and preparation of cellulose nanocrystals through sulfuric acid hydrolysis, ammonium persulfate oxidation, and TEMPO oxidation. | |
| Conditions | Extraction of cellulose from lemon seed powder with water at 80 °C for 2 h. Lignin was removed with 5% w/v NaClO2, pH 3.8–4, at 75 °C for 5 h. Hemicellulose and residual lignin were removed with 10% w/v NaOH at 30 °C for 12 h. Sulfuric acid (64% w/w) hydrolysis was conducted for 1.5 h at 45 °C. Ammonium persulfate (1 M) oxidation was performed at 70 °C for 14 h. TEMPO oxidation was conducted with the TEMPO/NaBr/NaClO system in water (pH 10) for 4 h. | |
| Observations | The lemon seed cellulose yield was 14.6% (w/w). The yield of cellulose nanocrystals obtained with sulfuric acid hydrolysis, ammonium persulfate oxidation, and TEMPO oxidation was 27.61%, 13.02%, and 52.01%, respectively. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buljeta, I.; Šubarić, D.; Babić, J.; Pichler, A.; Šimunović, J.; Kopjar, M. Extraction of Dietary Fibers from Plant-Based Industry Waste: A Comprehensive Review. Appl. Sci. 2023, 13, 9309. https://doi.org/10.3390/app13169309
Buljeta I, Šubarić D, Babić J, Pichler A, Šimunović J, Kopjar M. Extraction of Dietary Fibers from Plant-Based Industry Waste: A Comprehensive Review. Applied Sciences. 2023; 13(16):9309. https://doi.org/10.3390/app13169309
Chicago/Turabian StyleBuljeta, Ivana, Drago Šubarić, Jurislav Babić, Anita Pichler, Josip Šimunović, and Mirela Kopjar. 2023. "Extraction of Dietary Fibers from Plant-Based Industry Waste: A Comprehensive Review" Applied Sciences 13, no. 16: 9309. https://doi.org/10.3390/app13169309
APA StyleBuljeta, I., Šubarić, D., Babić, J., Pichler, A., Šimunović, J., & Kopjar, M. (2023). Extraction of Dietary Fibers from Plant-Based Industry Waste: A Comprehensive Review. Applied Sciences, 13(16), 9309. https://doi.org/10.3390/app13169309








