Cytotoxic Effect of L-Methioninase from Brevibacterium linens BL2 in Combination with Etoposide against Glioblastoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Bacterial Strain and Growth Conditions
2.2.2. Partial Purification of L-Methioninase Enzyme
2.2.3. Enzyme Activity Assay
2.2.4. Protein Content Analysis
2.2.5. SDS-PAGE Analysis
2.2.6. Cell Culture Conditions
2.2.7. Cell Viability Assay
2.2.8. Investigation of the Effect of L-Methioninase on Cancer Cells
2.2.9. Migration Assay
2.2.10. Colony Formation Assay
2.2.11. Giemsa Staining
2.2.12. DAPI and F-Actin Staining
2.2.13. RNA Isolation and cDNA Synthesis
2.2.14. RT-qPCR Analysis
2.2.15. Statistical Analysis
3. Results and Discussion
3.1. Enzyme Activity and Protein Content of Enzyme Solution
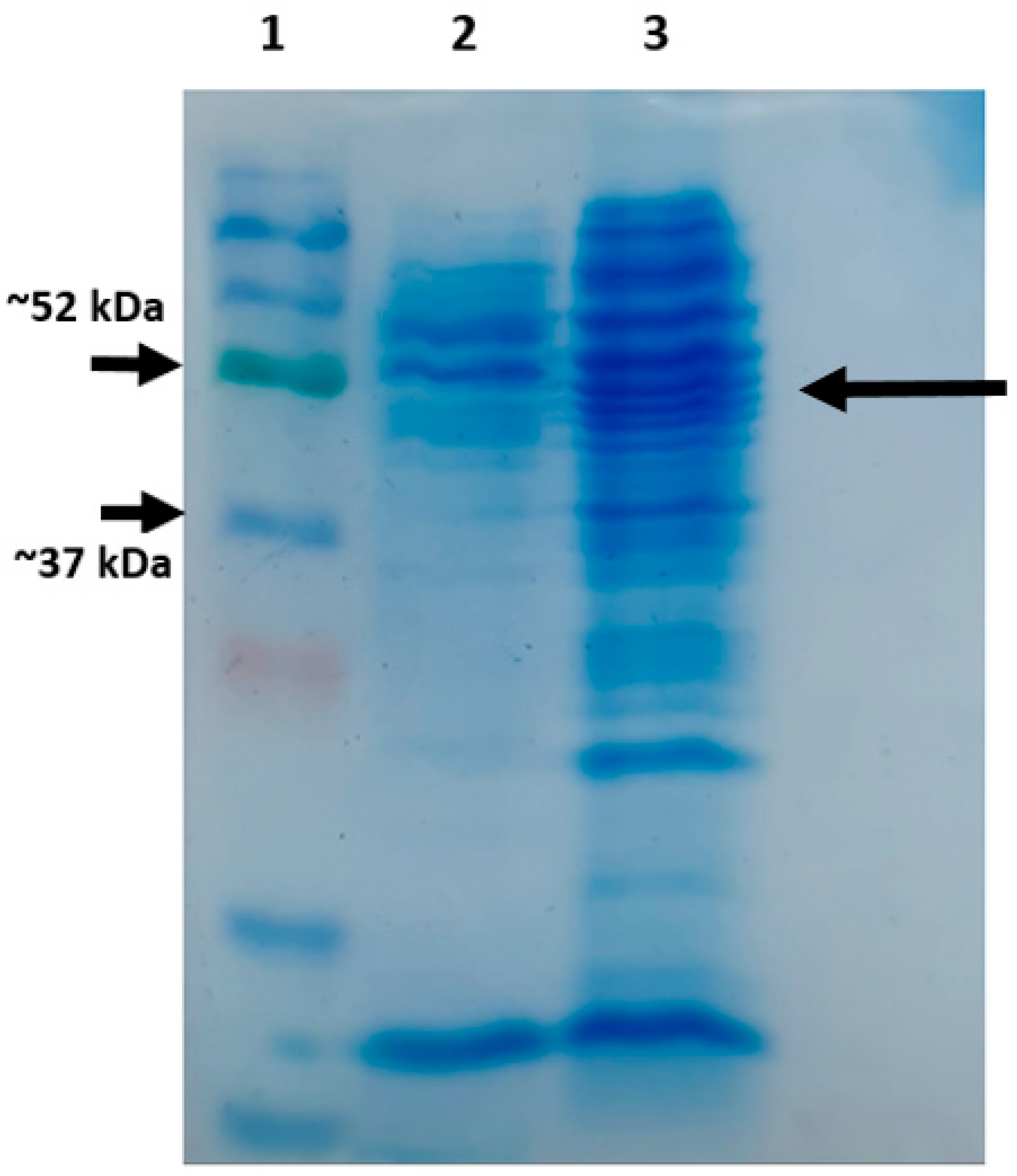
3.2. SDS-PAGE Analysis
3.3. IC50 Graph of L-Methioninase on Glioblastoma Cell Lines
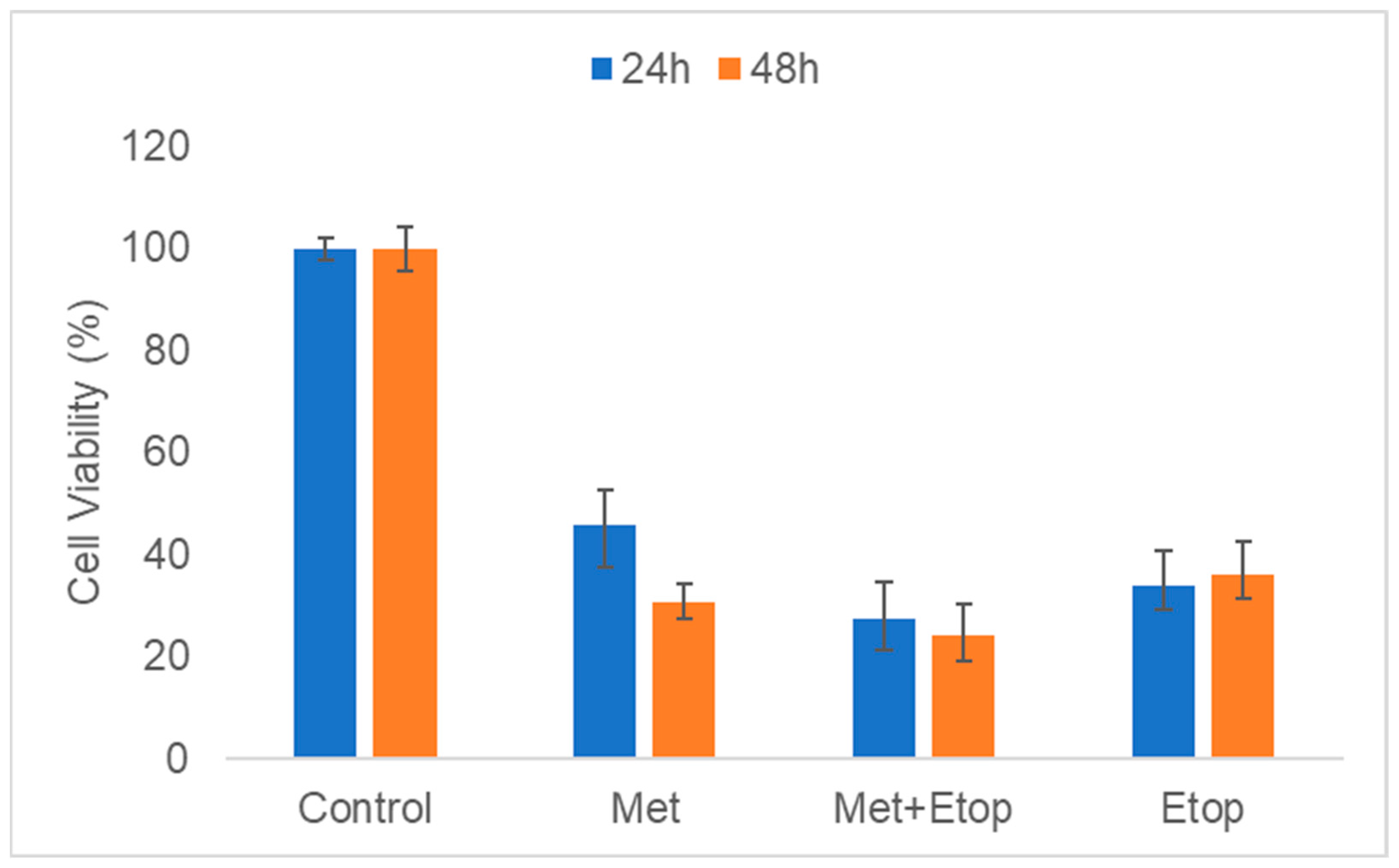
3.4. The Concurrent Effect of L-Methioninase and Etoposide on Cell Viability
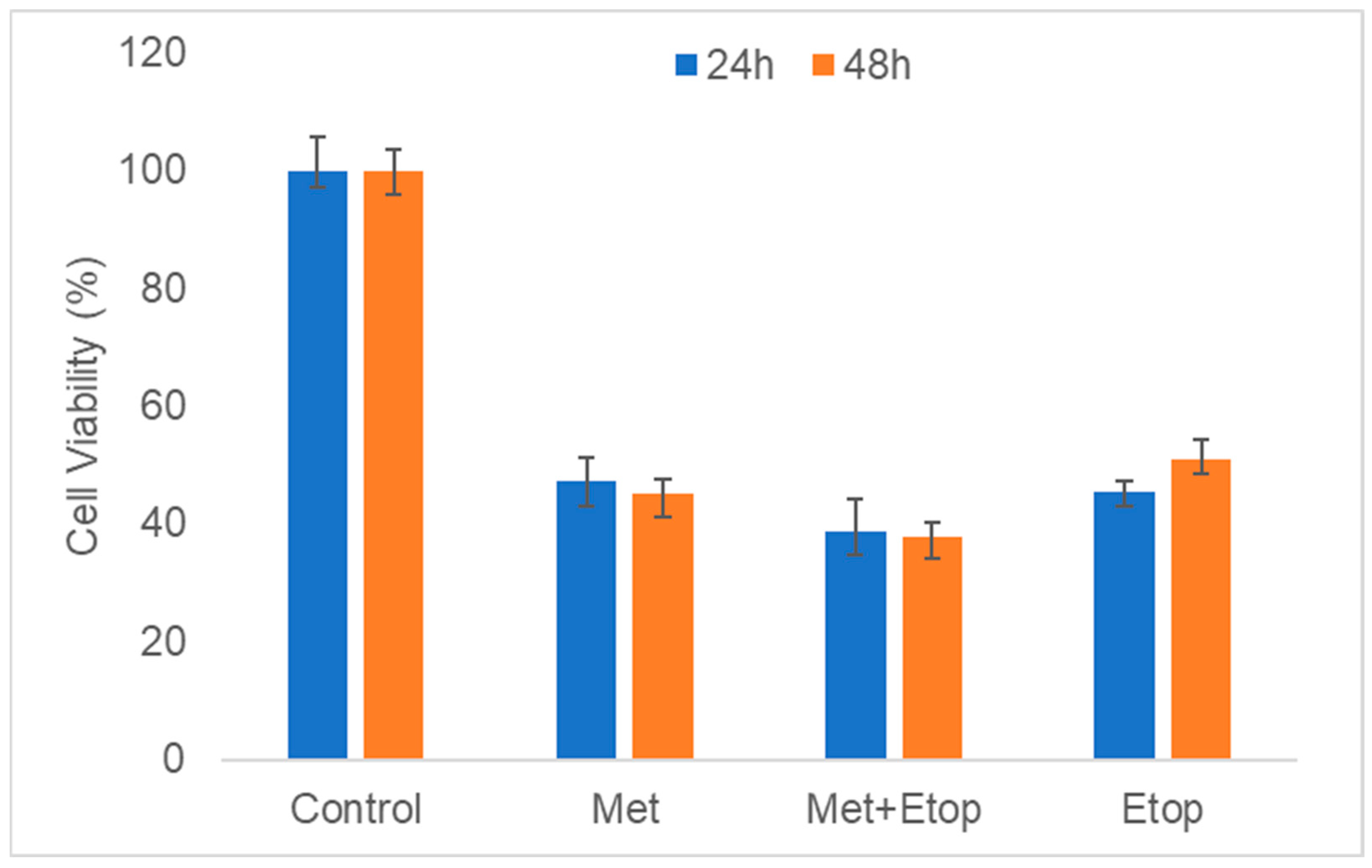
3.5. Evaluation of L-Methioninase with Etoposide on Mouse Embryonic Fibroblast (MEF) and Human Keratinocyte (HaCaT) Cells
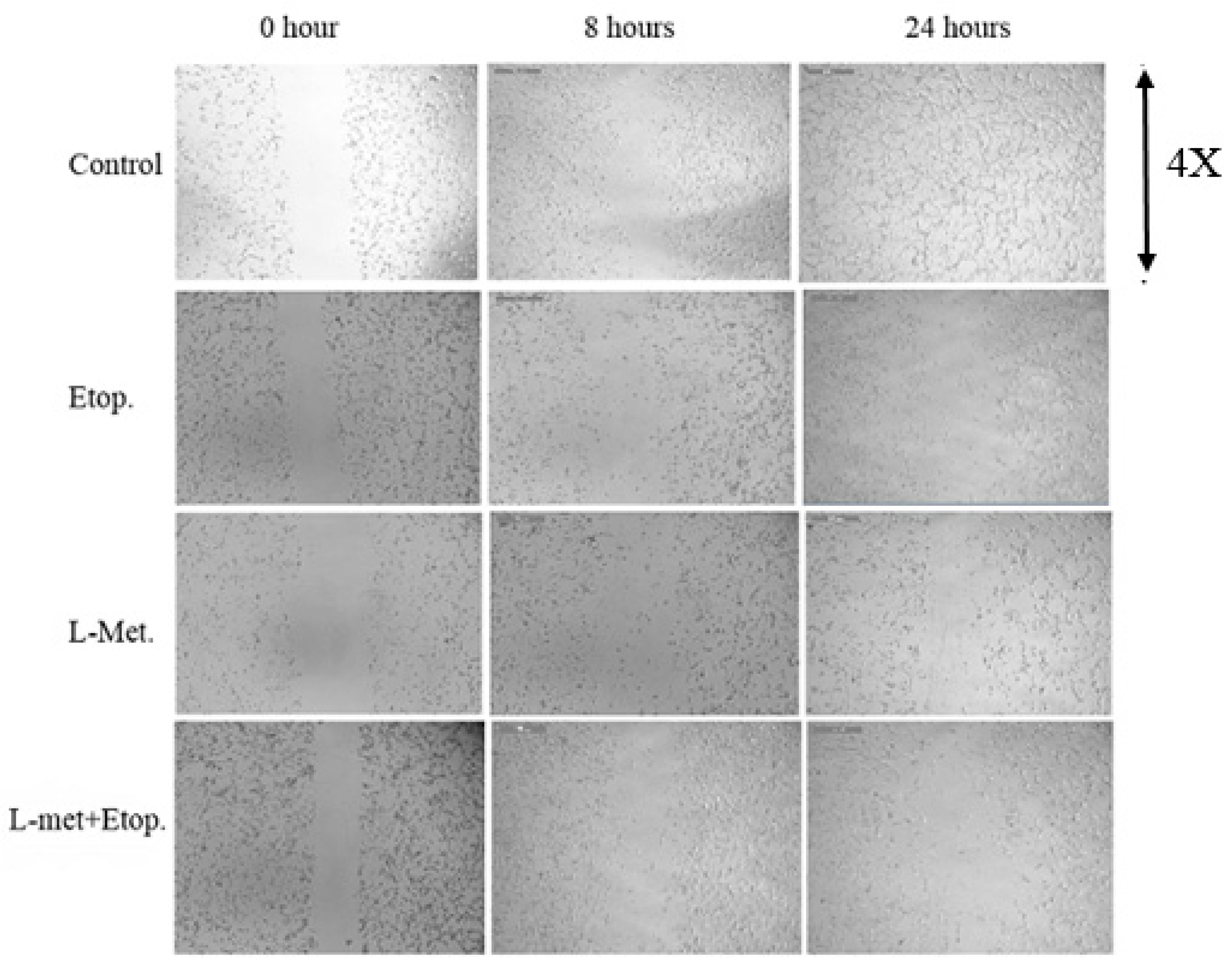
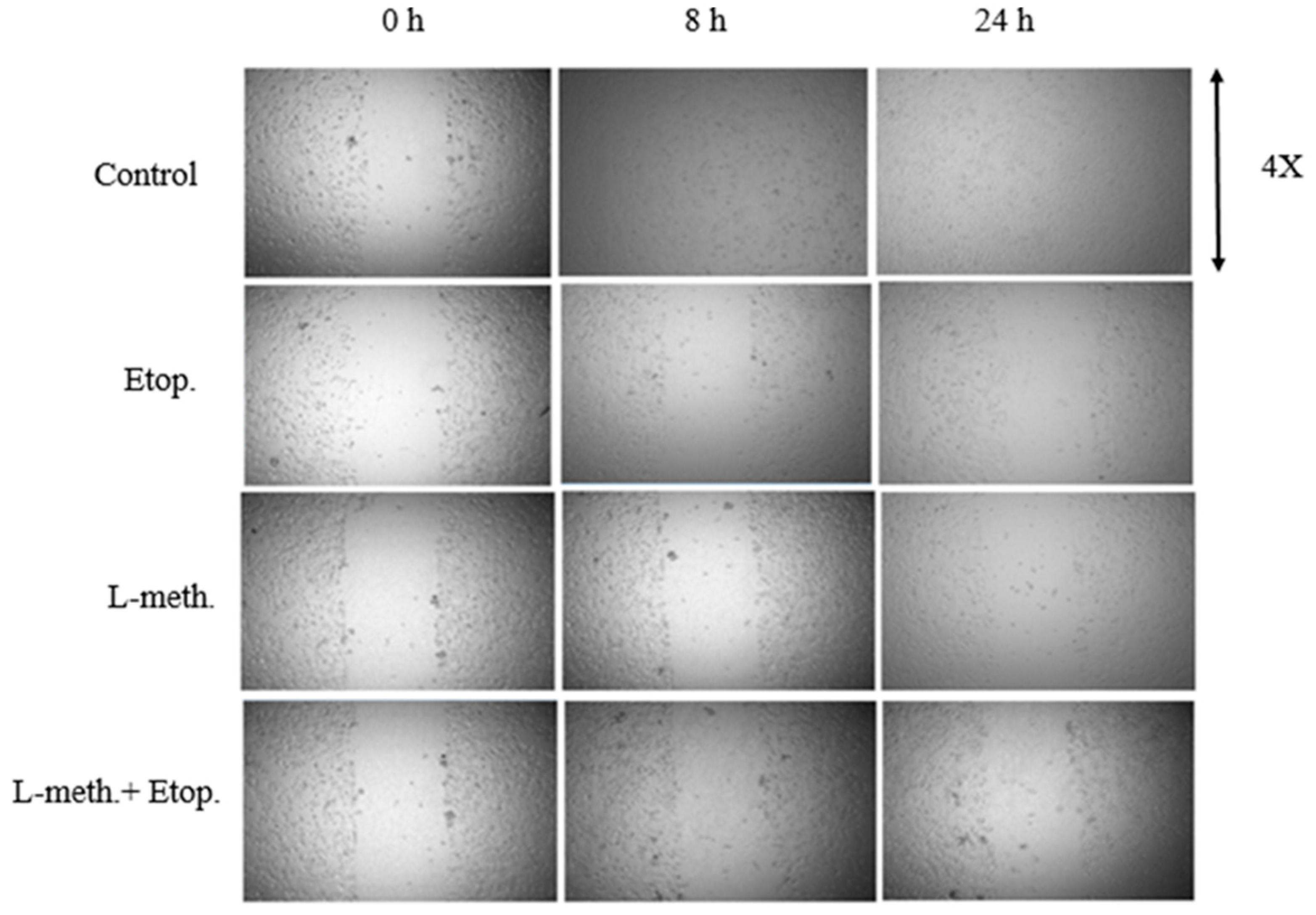
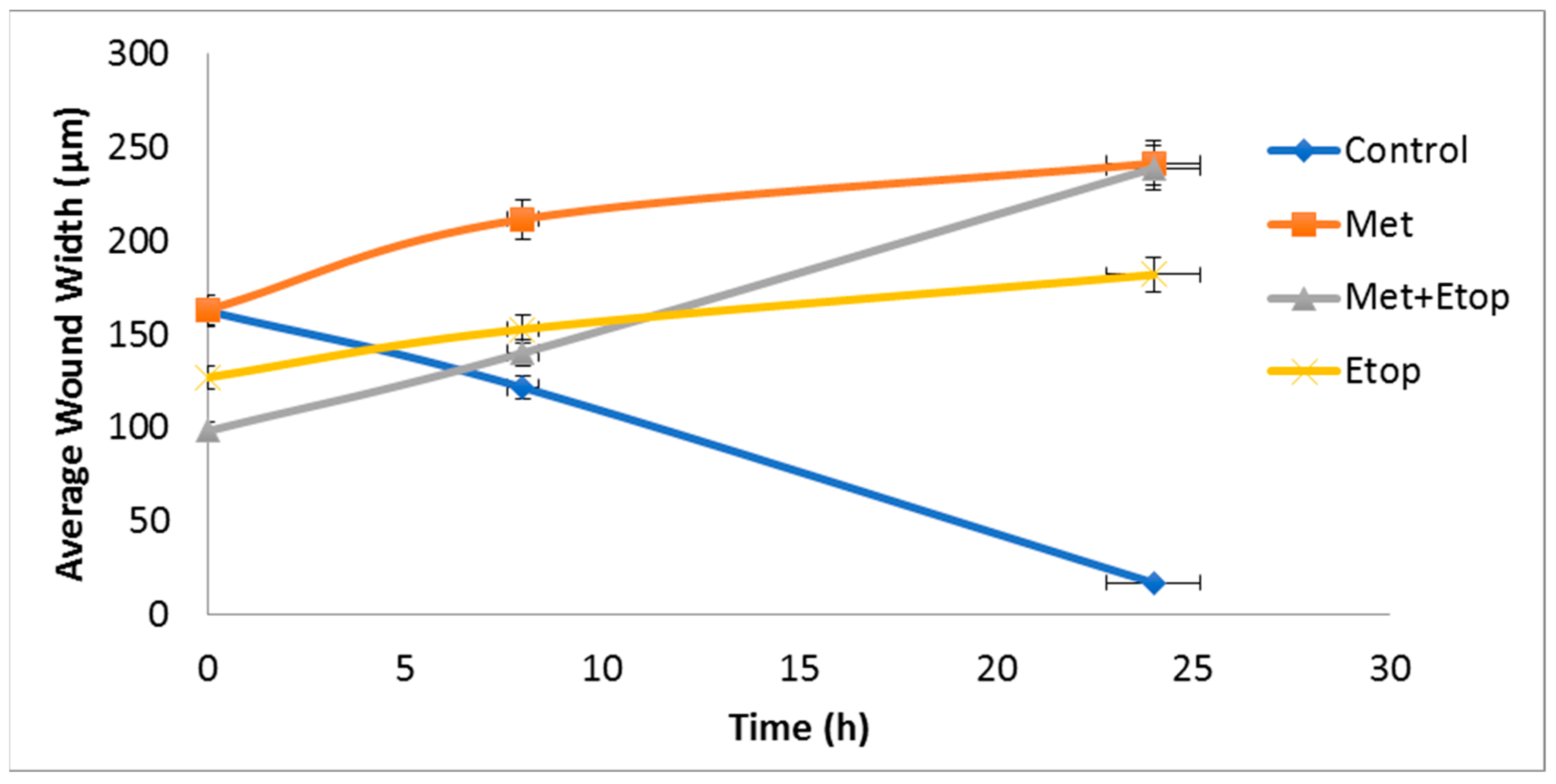
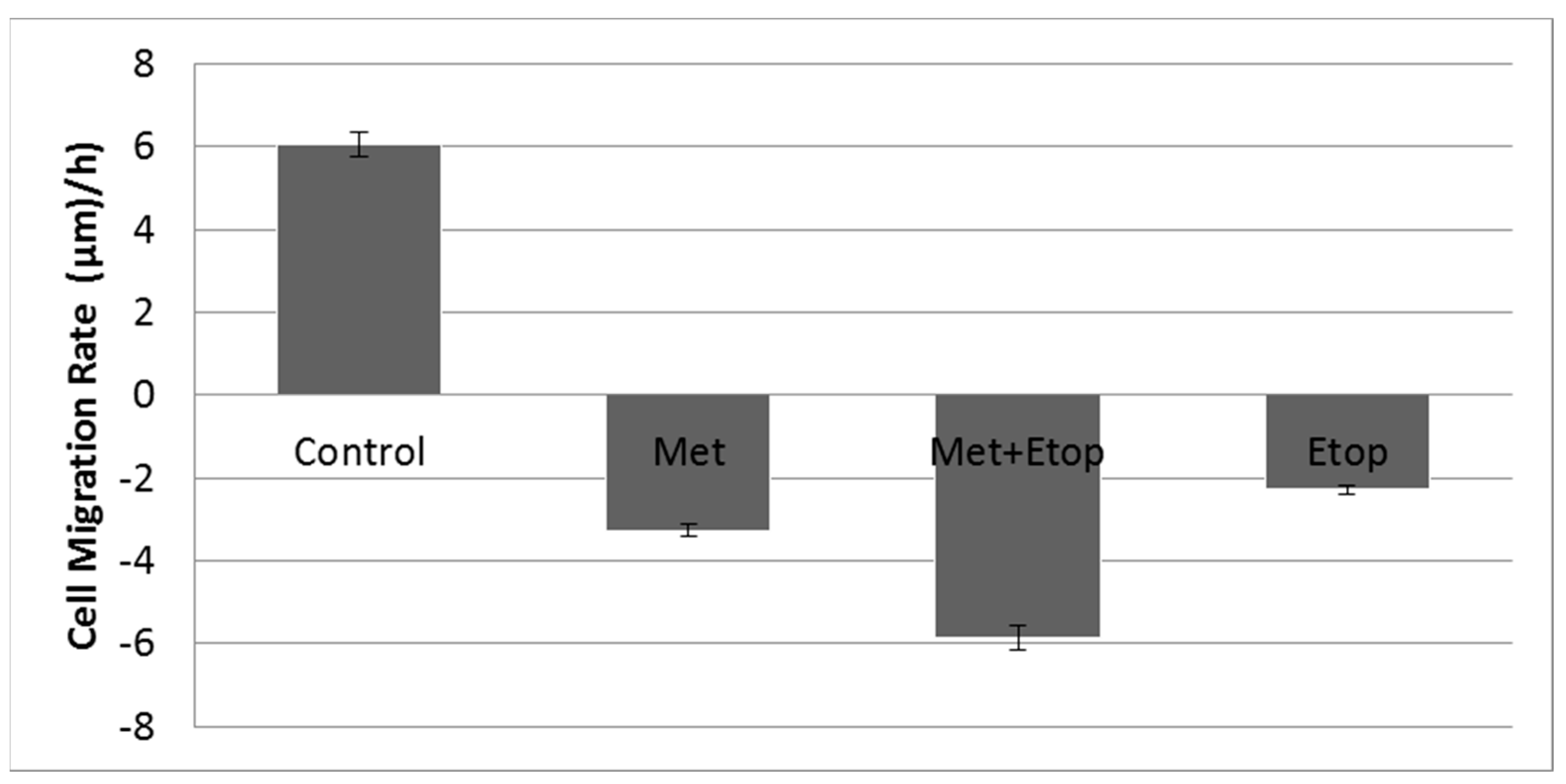
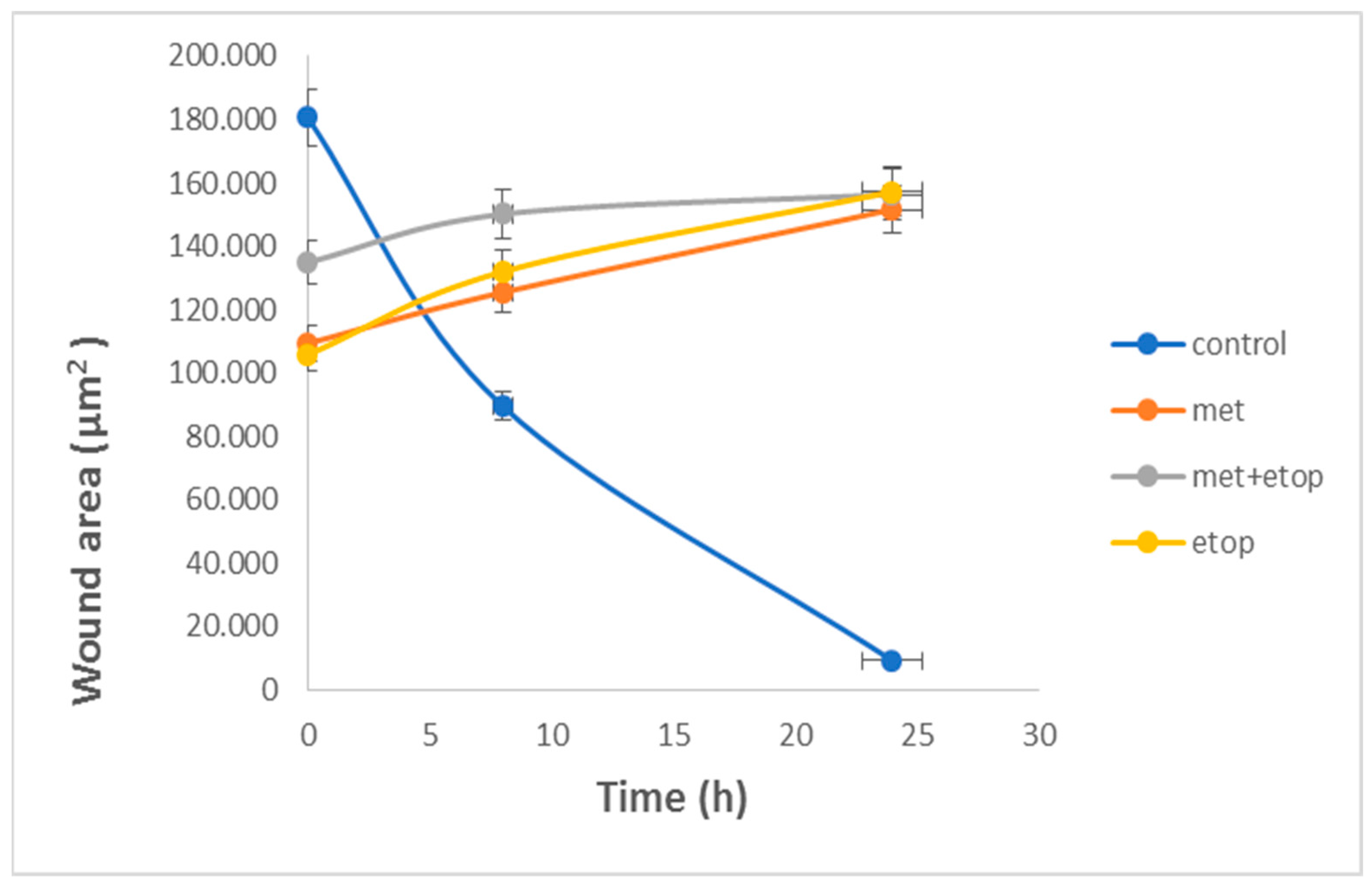
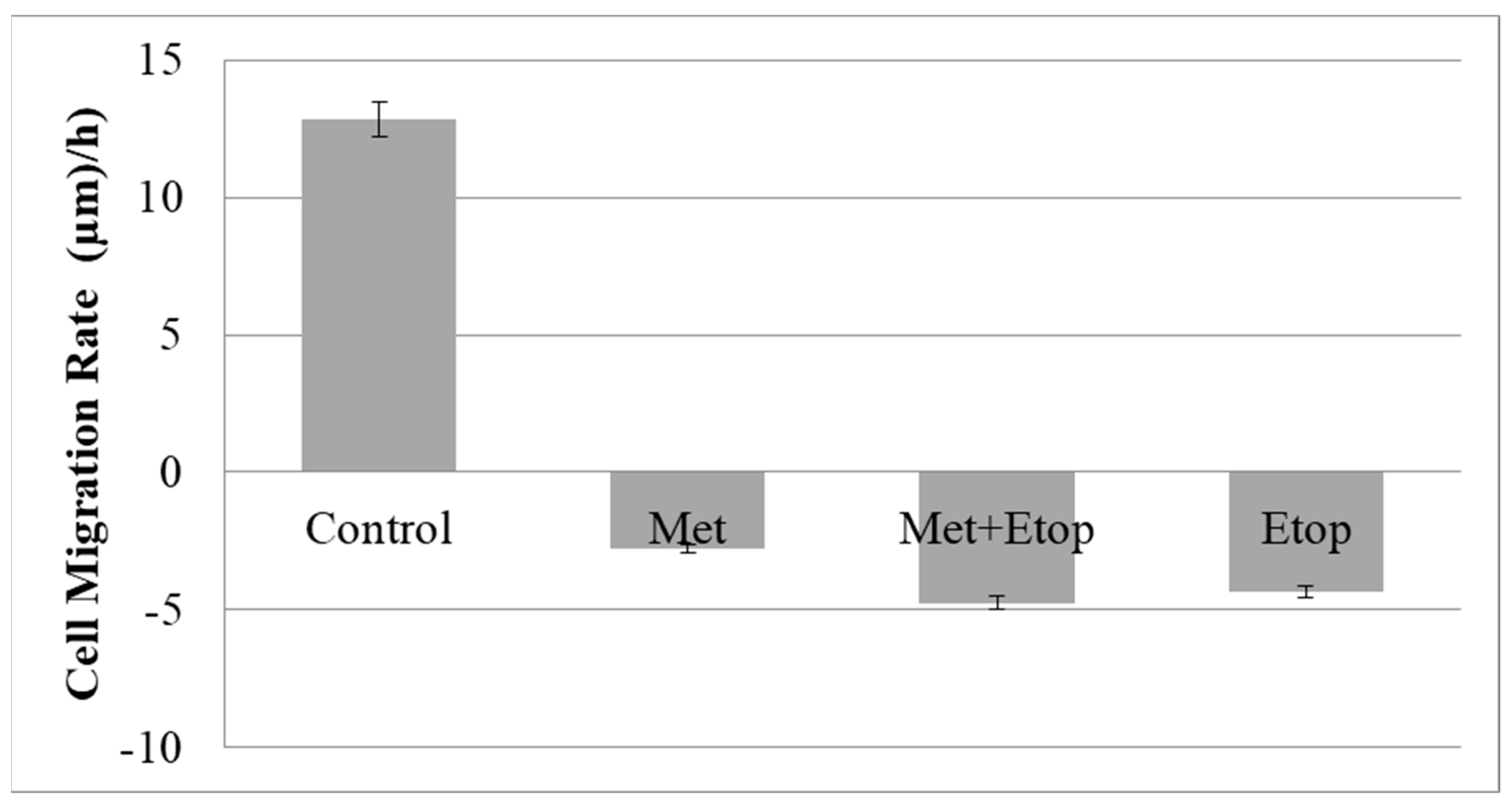
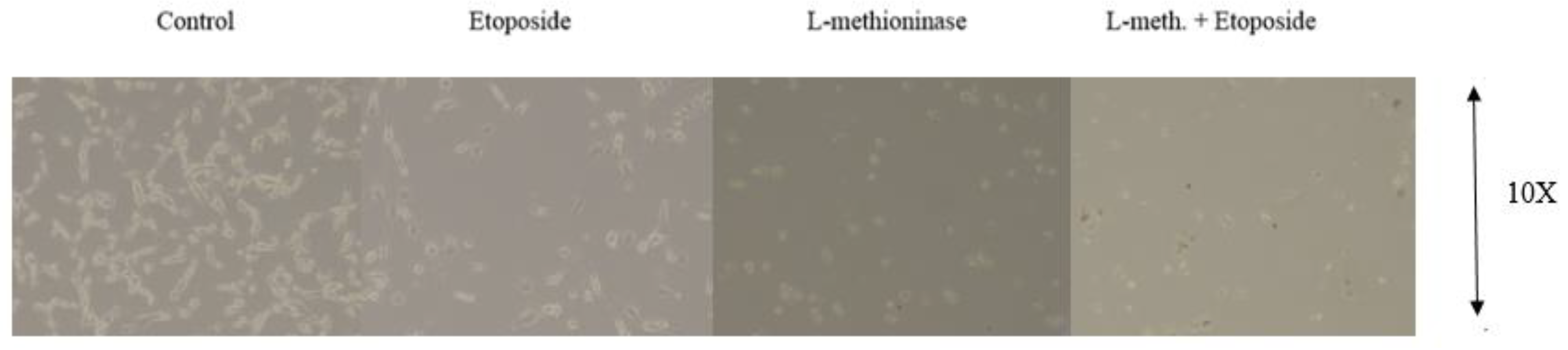
3.6. Evaluation of the Effect of L-Methioninase on Migration Characteristics of Glioblastoma Cells
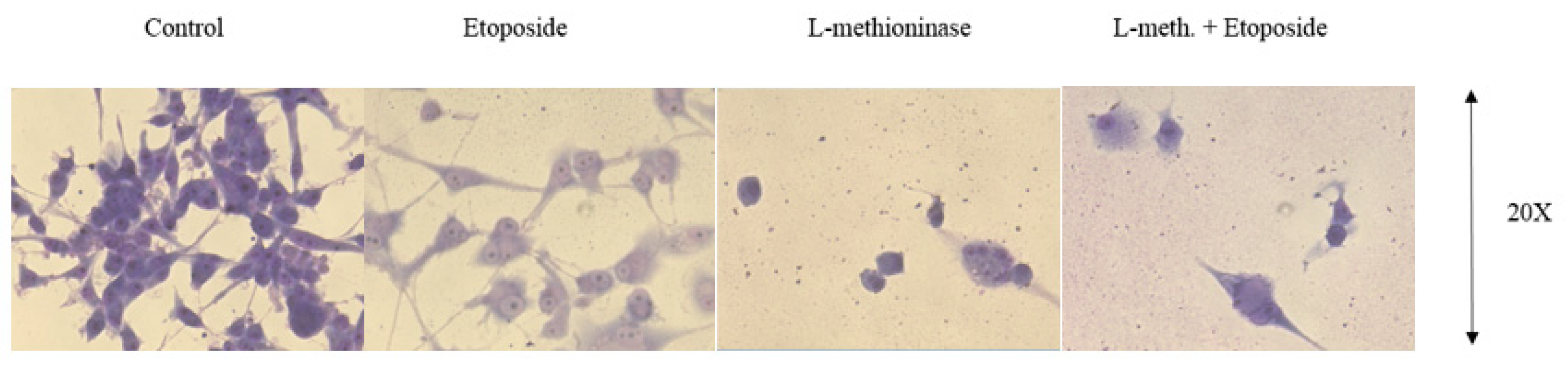
3.7. Giemsa Staining Images of Glioblastoma Cells after L-Methioninase and Etoposide Administration
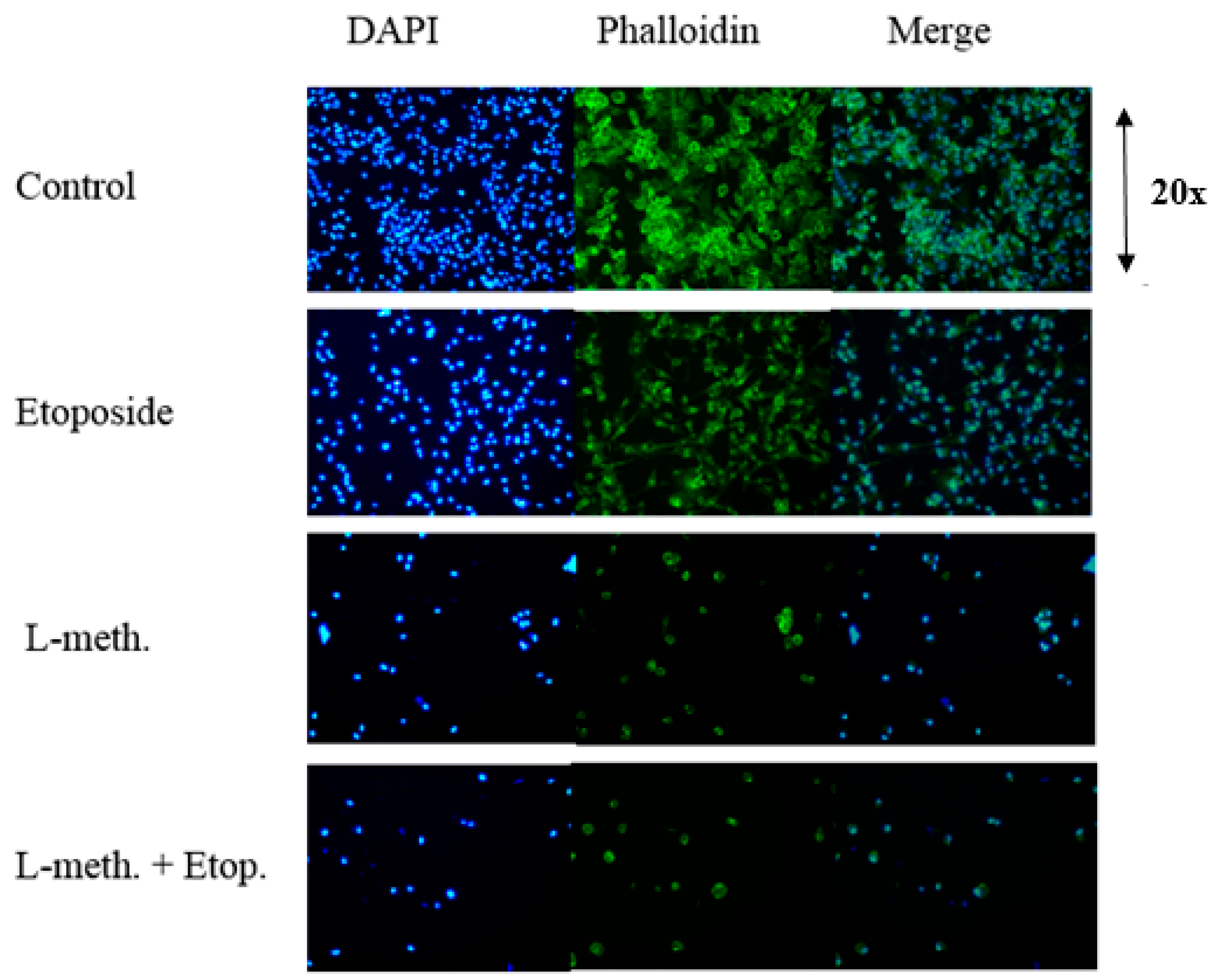
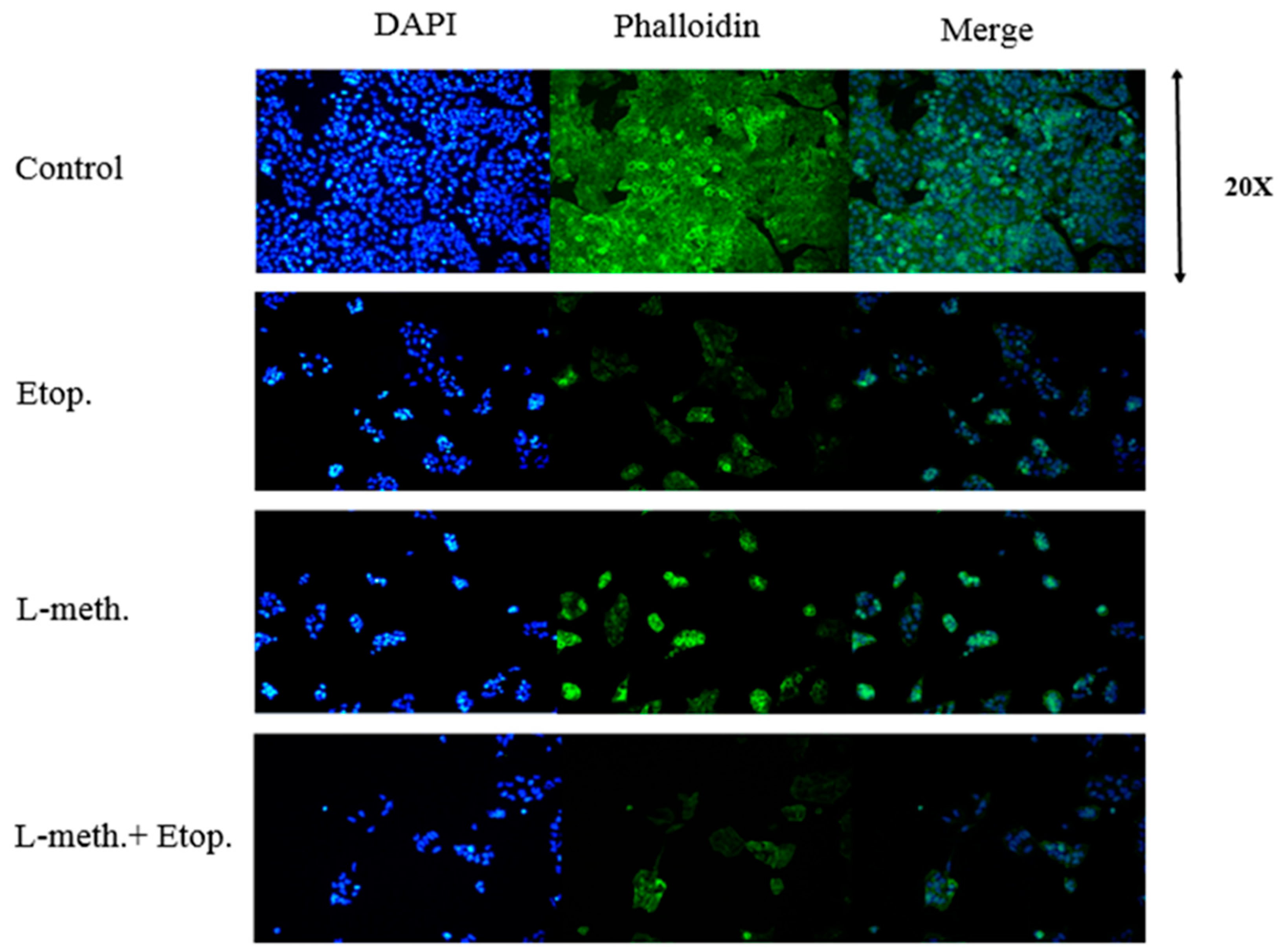
3.8. DAPI and F-Actin Staining Images after L-Methioninase and Etoposide Administration
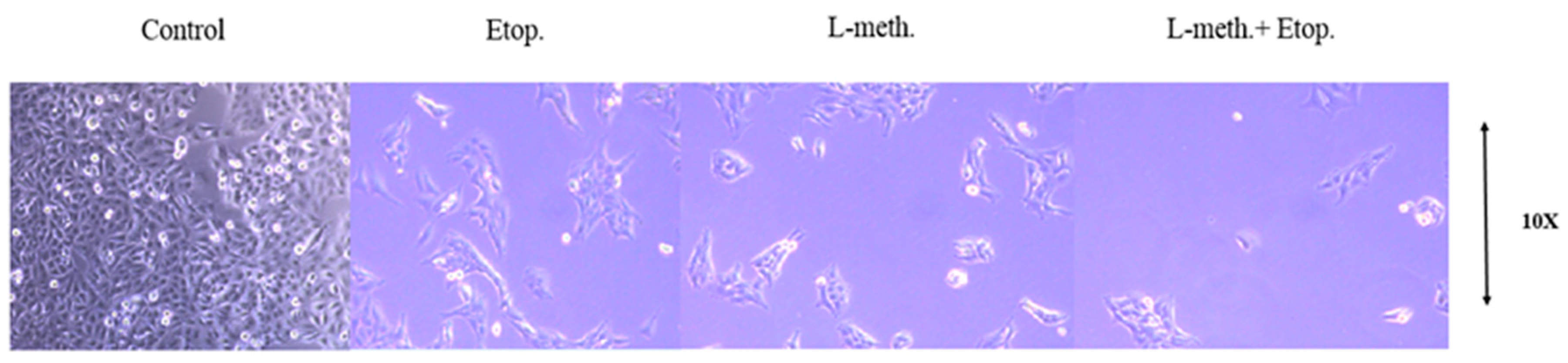
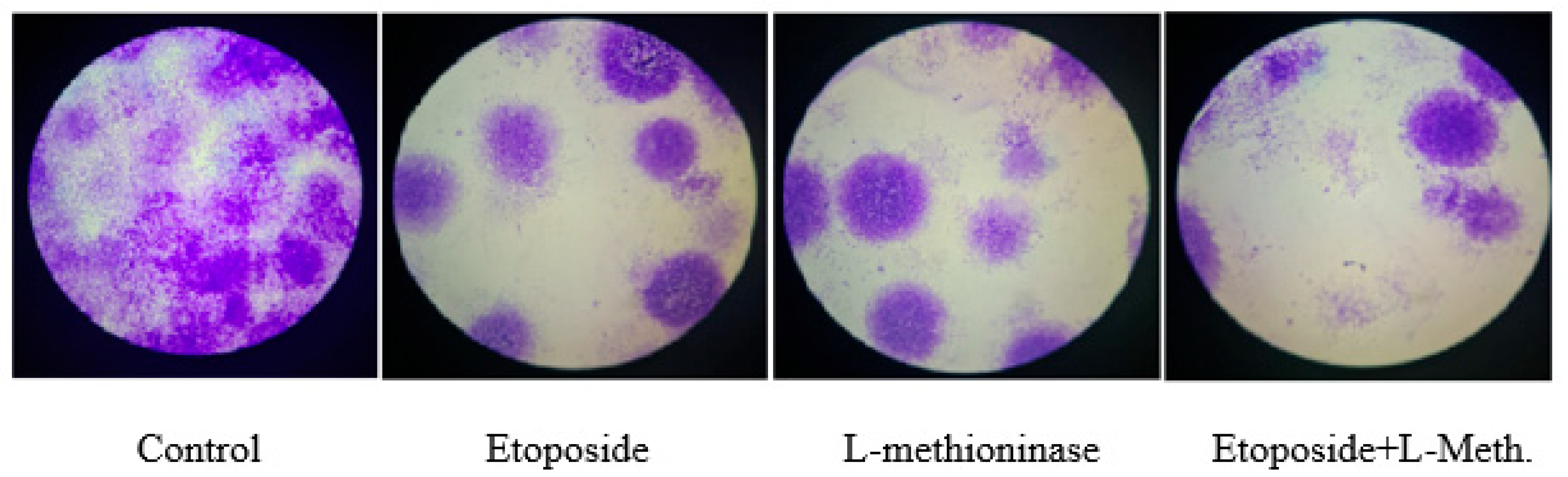
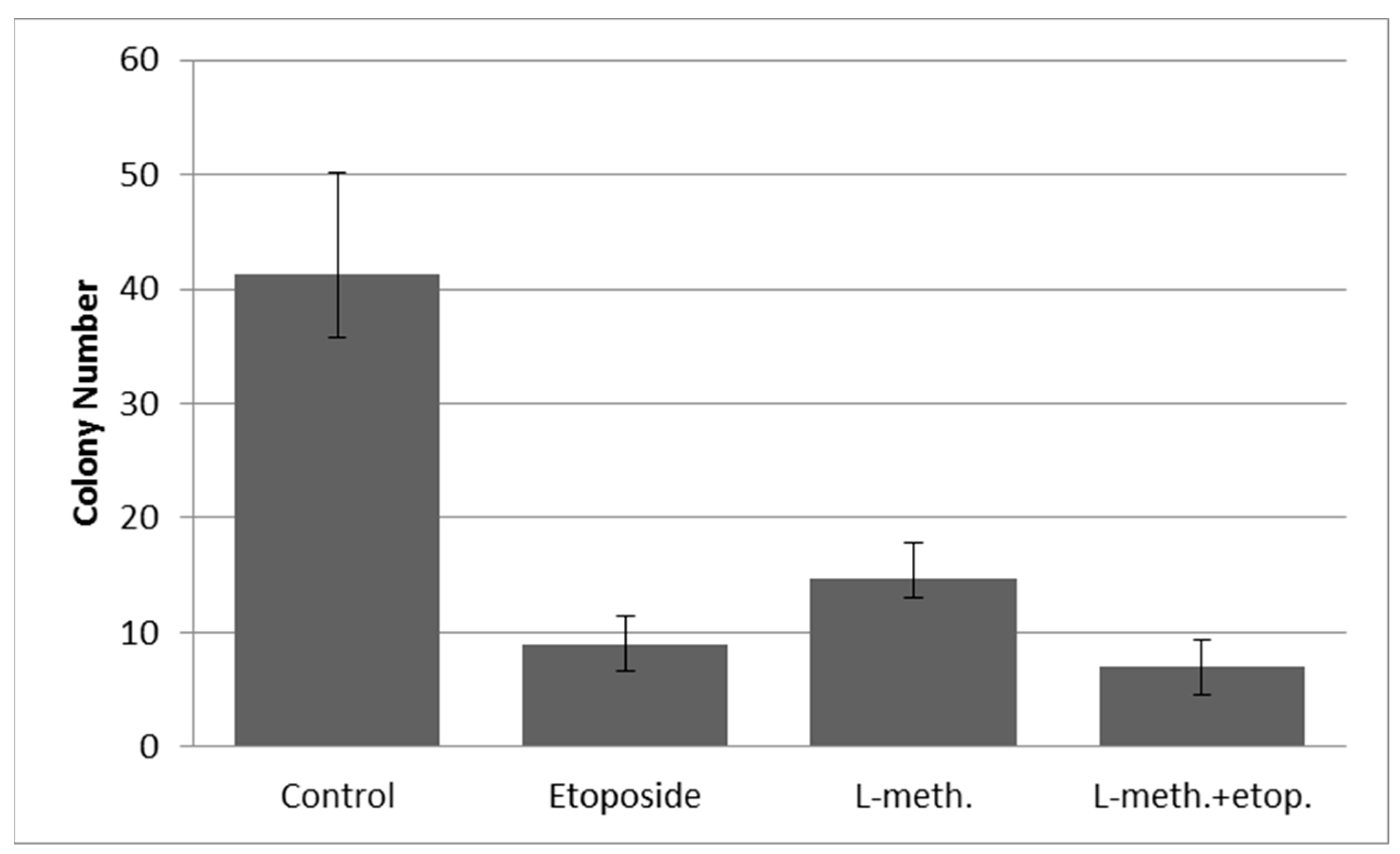
3.9. Clonogenic Assay Images and Colony Numbers after L-Methioninase and Etoposide Administration
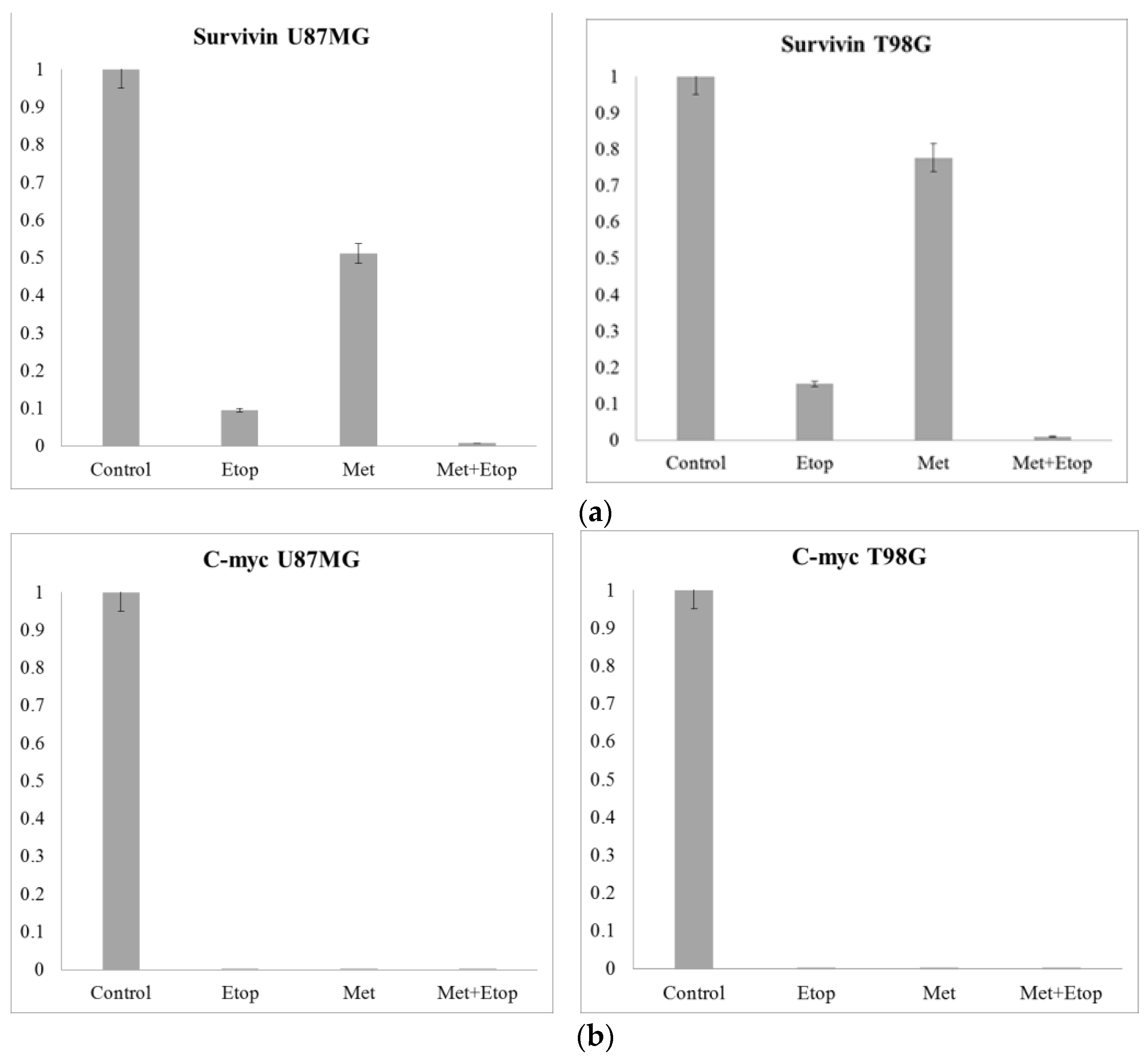
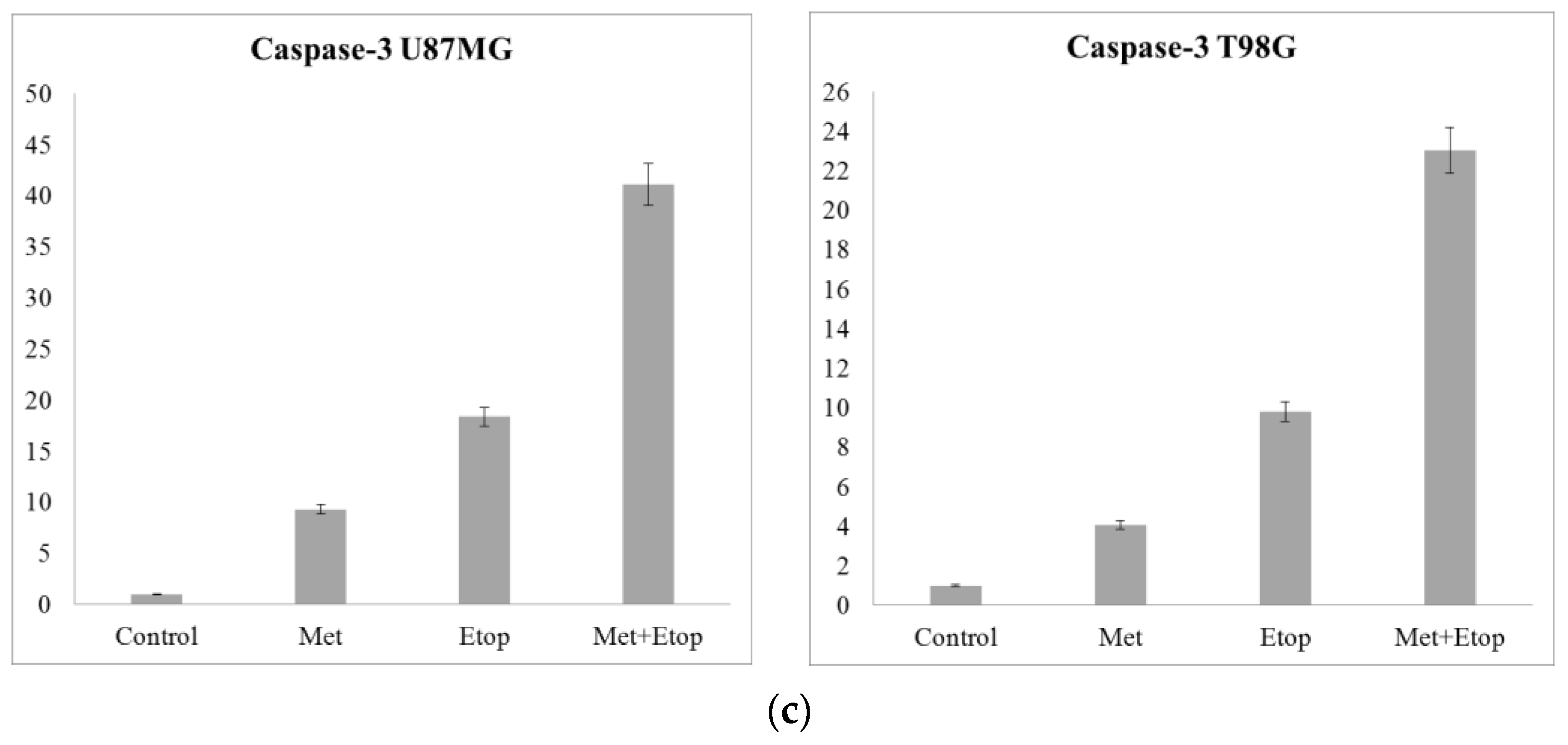
3.10. RT-qPCR Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, R.M. Altered methionine metabolism, DNA methylation and oncogene expression in carcinogenesis: A review and synthesis. Biochim. Biophys. Acta Rev. Cancer 1984, 738, 49–87. [Google Scholar] [CrossRef] [PubMed]
- Mecham, J.O.; Rowitch, D.; Wallace, C.D.; Stern, P.H.; Hoffman, R.M. The metabolic defect of methionine dependence occurs frequently in human tumor cell lines. Biochem. Biophys. Res. Commun. 1983, 117, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Development of recombinant methioninase to target the general cancer-specific metabolic defect of methionine dependence: A 40-year odyssey. Expert. Opin. Biol. Ther. 2015, 15, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Miyake, K.; Han, Q.; Li, S.; Tan, Y.; Igarashi, K.; Kiyuna, T.; Miyake, M.; Higuchi, T.; Oshiro, H.; et al. Oral recombinant methioninase (o-rMETase) is superior to injectable rMETase and overcomes acquired gemcitabine resistance in pancreatic cancer. Cancer Lett. 2018, 432, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Epner, D.E.; Morrow, S.; Wilcox, M.; Houghton, J.L. Nutrient intake and nutritional indexes in adults with metastatic cancer on a phase I clinical trial of dietary methionine restriction. Nutr. Cancer 2002, 42, 158–166. [Google Scholar] [CrossRef]
- Lu, S.; Epner, D.E. Molecular mechanisms of cell cycle block by methionine restriction in human prostate cancer cells. Nutr. Cancer 2000, 38, 123–130. [Google Scholar] [CrossRef]
- Yoshioka, T.; Wada, T.; Uchida, N.; Maki, H.; Yoshida, H.; Ide, N.; Kasai, H.; Hojo, K.; Shono, K.; Maekawa, R.; et al. Anticancer efficacy in vivo and in vitro, synergy with 5-fluorouracil, and safety of recombinant methioninase. Cancer Res. 1998, 58, 2583–2587. [Google Scholar]
- Suganya, K.; Govindan, K.; Prabha, P.; Murugan, M. An extensive review on L-methioninase and its potential applications. Biocatal. Agric. Biotechnol. 2017, 12, 104–115. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, X.; Xu, M.; An, Z.; Tan, X.; Han, Q.; Miljkovic, D.A.; Yang, M.; Hoffman, R.M. Polyethylene glycol conjugation of recombinant methioninase for cancer therapy. Protein Expr. Purif. 1998, 12, 45–52. [Google Scholar] [CrossRef]
- Tanaka, H.; Esaki, N.; Soda, K. Properties of L-methionine gamma-lyase from Pseudomonas ovalis. Biochemistry 1977, 16, 100–106. [Google Scholar] [CrossRef]
- Xin, L.; Yang, W.F.; Zhang, H.T.; Li, Y.F.; Liu, C. The mechanism study of lentiviral vector carrying methioninase enhances the sensitivity of drug-resistant gastric cancer cells to Cisplatin. Br. J. Cancer 2018, 118, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Kreis, W.; Hession, C. Biological effects of enzymatic deprivation of L-methionine in cell culture and an experimental tumor. Cancer Res. 1973, 33, 1866–1869. [Google Scholar] [PubMed]
- Dias, B.; Weimer, B. Purification and Characterization ofl-Methionine γ-Lyase from Brevibacterium linens BL2. Appl. Environ. Microbiol. 1998, 64, 3327–3331. [Google Scholar] [CrossRef] [PubMed]
- Boyaval, P.; Desmazeaud, M.J. Le point des connaissances sur Brevibacterium linens. Le Lait 1983, 63, 187–216. [Google Scholar] [CrossRef]
- Ferchichi, M.; Hemme, D.; Nardi, M. Induction of methanethiol production by Brevibacterium linens CNRZ 918. Microbiology 1986, 132, 3075–3082. [Google Scholar] [CrossRef]
- Hemme, D. Microbial catabolism of amino acids during cheese ripening. Sci. Aliment. 1982, 2, 113–123. [Google Scholar]
- Sharpe, M.E.; Law, B.A.; Phillips, B.A.; Pitcher, D.G. Methanethiol production by coryneform bacteria: Strains from dairy and human skin sources and Brevibacterium linens. Microbiology 1977, 101, 345–349. [Google Scholar] [CrossRef]
- Machover, D.; Rossi, L.; Hamelin, J.; Desterke, C.; Goldschmidt, E.; Chadefaux-Vekemans, B.; Bonnarme, P.; Briozzo, P.; Kopečný, D.; Pierigè, F.; et al. Effects in cancer cells of the recombinant l-methionine gamma-lyase from Brevibacterium aurantiacum. Encapsulation in human erythrocytes for sustained l-methionine elimination. J. Pharmacol. Exp. Ther. 2019, 369, 489–502. [Google Scholar] [CrossRef]
- D’Alessio, A.; Proietti, G.; Sica, G.; Scicchitano, B.M. Pathological and molecular features of glioblastoma and its peritumoral tissue. Cancers 2019, 11, 469. [Google Scholar] [CrossRef]
- Stefano, A.; Leal, A.; Richiusa, S.; Trang, P.; Comelli, A.; Benfante, V.; Cosentino, S.; Sabini, M.G.; Tuttolomondo, A.; Roberto Altieri, R.; et al. Robustness of pet radiomics features: Impact of co-registration with MRI. Appl. Sci. 2021, 11, 10170. [Google Scholar] [CrossRef]
- Stefano, A.; Vitabile, S.; Russo, G.; Ippolito, M.; Sardina, D.; Sabini, M.G.; Gallivanone, F.; Castiglioni, I.; Gilardi, M.C. A graph-based method for PET image segmentation in radiotherapy planning: A pilot study. In Proceedings of the Image Analysis and Processing–ICIAP 2013: 17th International Conference, Naples, Italy, 9–13 September 2013; Springer: Berlin/Heidelberg, Germany; pp. 711–720. [Google Scholar]
- Montecucco, A.; Zanetta, F.; Biamonti, G. Molecular mechanisms of etoposide. EXCLI J. 2015, 14, 95. [Google Scholar]
- Onbasli, K.; Erkısa, M.; Demirci, G.; Muti, A.; Ulukaya, E.; Sennaroglu, A.; Acar, H.Y. The improved killing of both androgen-dependent and independent prostate cancer cells by etoposide loaded SPIONs coupled with NIR irradiation. Biomater. Sci. 2022, 10, 3951–3962. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Woźniak, K. Natural polyphenols as modulators of etoposide anti-cancer activity. Int. J. Mol. Sci. 2021, 22, 6602. [Google Scholar] [CrossRef]
- Laakso, S.; Nurmikko, V. A spectrophotometric assay for demethiolating activity. Anal. Biochem. 1976, 72, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ulagesan, S.; Kim, H.J. Antibacterial and antifungal activities of proteins extracted from seven different snails. Appl. Sci. 2018, 8, 1362. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Al-Khedhairy, A.A.; Wahab, R. Size-dependent cytotoxic and molecular study of the use of gold nanoparticles against liver cancer cells. Appl. Sci. 2022, 12, 901. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Ozdemir, M.D.; Gokturk, D. The effect of Rosmarinus officinalis and chemotherapeutic etoposide on glioblastoma (U87 MG) cell culture. Turk. Neurosurg. 2018, 28, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Sevim, H.; Parkinson, J.F.; McDonald, K.L. Etoposide-mediated glioblastoma cell death: Dependent or independent on the expression of its target, topoisomerase II alpha? J. Cancer Res. Clin. Oncol. 2011, 137, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Azmi, A.S.; Aboukameel, A.; Kauffman, M.; Shacham, S.; Abou-Samra, A.B.; Mohammad, R.M. Nuclear retention of Fbw7 by specific inhibitors of nuclear export leads to Notch1 degradation in pancreatic cancer. Oncotarget 2014, 5, 3444. [Google Scholar] [CrossRef]
- Mobahat, M.; Narendran, A.; Riabowol, K. Survivin as a preferential target for cancer therapy. Int. J. Mol. Sci. 2014, 15, 2494–2516. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Yang, P.; Wang, K.; Liu, Y.; Liu, X.; Shan, X.; Huang, R.; Zhang, K.; Wang, J. Survivin is a prognostic indicator in glioblastoma and may be a target of microRNA-218. Oncol. Lett. 2019, 18, 359–367. [Google Scholar] [CrossRef]
- Conde, M.; Michen, S.; Wiedemuth, R.; Klink, B.; Schröck, E.; Schackert, G.; Temme, A. Chromosomal instability induced by increased BIRC5/Survivin levels affects tumorigenicity of glioma cells. BMC Cancer 2017, 17, 889. [Google Scholar] [CrossRef]
- Sheng, L.; Wan, B.; Feng, P.; Sun, J.; Rigo, F.; Bennett, C.F.; Akerman, M.; Krainer, A.R.; Hua, Y. Downregulation of Survivin contributes to cell-cycle arrest during postnatal cardiac development in a severe spinal muscular atrophy mouse model. Hum. Mol. Genet. 2018, 27, 486–498. [Google Scholar] [CrossRef]
- Wang, L.; Huang, W.; Zhan, J. Grape seed proanthocyanidins induce autophagy and modulate survivin in HepG2 cells and inhibit xenograft tumor growth in vivo. Nutrients 2019, 11, 2983. [Google Scholar] [CrossRef]
- Chiou, S.K.; Jones, M.K.; Tarnawski, A.S. Survivin–An anti-apoptosis protein: Its biological roles and implications for cancer and beyond. Signature 2003, 9, 47. [Google Scholar]
- Shin, S.; Sung, B.J.; Cho, Y.S.; Kim, H.J.; Ha, N.C.; Hwang, J.I.; Chung, C.-W.; Jung, Y.K.; Oh, B.-H. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 2001, 40, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yuan, B.; Shimada, R.; Hayashi, H.; Si, N.; Zhao, H.Y.; Bian, B.; Takagi, N. Cytocidal effects of arenobufagin and hellebrigenin, two active bufadienolide compounds, against human glioblastoma cell line U-87. Int. J. Oncol. 2018, 53, 2488–2502. [Google Scholar] [CrossRef] [PubMed]
- Khaw, A.K.; Hande, M.P.; Kalthur, G.; Hande, M.P. Curcumin inhibits telomerase and induces telomere shortening and apoptosis in brain tumour cells. J. Cell. Biochem. 2013, 114, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, M.D.; Göktürk, D. The concurrent effect of acyclovir and rosemary on glioblastoma cell culture. Cell. Mol. Biol. 2019, 65, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Otevřelová, P.; Brodská, B. Chemotherapy-Induced Survivin Regulation in Acute Myeloid Leukemia Cells. Appl Sci 2021, 11, 460. [Google Scholar] [CrossRef]
- Cadoret, A.; Desbois-Mouthon, C.; Wendum, D.; Leneuve, P.; Perret, C.; Tronche, F.; Housset, C.; Holzenberger, M. c-myc-induced hepatocarcinogenesis in the absence of IGF-I receptor. Int. J. Cancer 2005, 114, 668–672. [Google Scholar] [CrossRef]
- Jamerson, M.H.; Johnson, M.D.; Dickson, R.B. Of mice and Myc: c-Myc and mammary tumorigenesis. J. Mammary Gland Biol. Neoplasia 2004, 9, 27–37. [Google Scholar] [CrossRef]
- Liu, S.Q.; Yu, J.P.; Yu, H.G.; Lv, P.; Chen, H.L. Activation of Akt and ERK signalling pathways induced by etoposide confer chemoresistance in gastric cancer cells. Dig. Liver Dis. 2006, 38, 310–318. [Google Scholar] [CrossRef]
- McEwan, M.V.; Eccles, M.R.; Horsfield, J.A. Cohesin is required for activation of MYC by estradiol. PLoS ONE 2012, 7, e49160. [Google Scholar] [CrossRef]
- Rehman, N.; Khan, S.; Manzoor, S.; Abubakar, M.; Sami, R.; Alharthy, S.A.; Baty, R.S.; Jafri, I.; Alsubhi, N.H.; Qari, S.H.; et al. Estrogen Induces c-myc Transcription by Binding to Upstream ERE Element in Promoter. Appl. Sci. 2022, 12, 6853. [Google Scholar] [CrossRef]
- Thompson, E.B. The many roles of c-Myc in apoptosis. Annu. Rev. Physiol. 1998, 60, 575–600. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Olopade, O.I. MYC and breast cancer. Genes Cancer 2010, 1, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Azrak, R.G.; Frank, C.L.; Ling, X.; Slocum, H.K.; Li, F.; Foster, B.A.; Rustum, Y.M. The mechanism of methylselenocysteine and docetaxel synergistic activity in prostate cancer cells. Mol. Cancer Ther. 2006, 5, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell. Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Özdaş, S. Knockdown of SET Domain, Bifurcated 1 suppresses head and neck cancer cell viability and wound-healing ability in vitro. Turk. J. Biol. 2019, 43, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kavya, D.; Nadumane, V.K. A combination of semi-purified L-methioninase with tamoxifen citrate to ameliorate breast cancer in athymic nude mice. Mol. Biol. Rep. 2023, 50, 2925–2932. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İpek, S.L.; Özdemir, M.D.; Göktürk, D. Cytotoxic Effect of L-Methioninase from Brevibacterium linens BL2 in Combination with Etoposide against Glioblastoma Cells. Appl. Sci. 2023, 13, 9382. https://doi.org/10.3390/app13169382
İpek SL, Özdemir MD, Göktürk D. Cytotoxic Effect of L-Methioninase from Brevibacterium linens BL2 in Combination with Etoposide against Glioblastoma Cells. Applied Sciences. 2023; 13(16):9382. https://doi.org/10.3390/app13169382
Chicago/Turabian Styleİpek, Semih Latif, Meryem Damla Özdemir, and Dilek Göktürk. 2023. "Cytotoxic Effect of L-Methioninase from Brevibacterium linens BL2 in Combination with Etoposide against Glioblastoma Cells" Applied Sciences 13, no. 16: 9382. https://doi.org/10.3390/app13169382
APA Styleİpek, S. L., Özdemir, M. D., & Göktürk, D. (2023). Cytotoxic Effect of L-Methioninase from Brevibacterium linens BL2 in Combination with Etoposide against Glioblastoma Cells. Applied Sciences, 13(16), 9382. https://doi.org/10.3390/app13169382






