Nutritional Quality and Overall Acceptability Optimization of Ultra-Fast Air-Superchilled Golden Rainbow Trout (Oncorhynchus mykiss, Stevanovski) Using the Response Surface Methodology
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
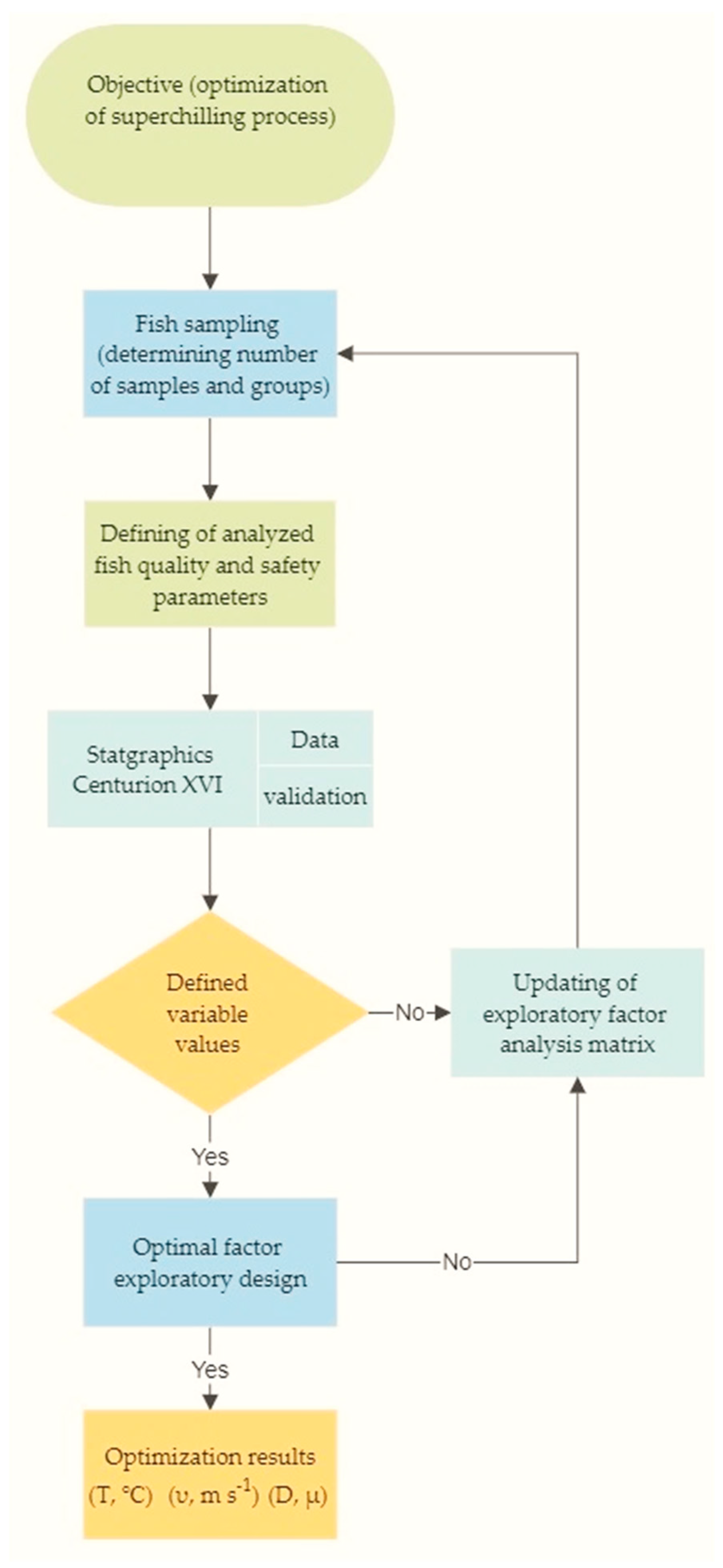
2.1. Flow Chart of the Optimization Process of Golden Rainbow Trout Air-Jet Superchilling Regime
2.2. Auxiliary Materials
2.3. Exploratory Factor Analyses Design
2.4. Sensory Analyses
2.5. Determination of Volatile Base Nitrogen (TVB-N)
2.6. Determination of Total Viable Count (TVC) of the Aerobic Mesophilic Microorganisms
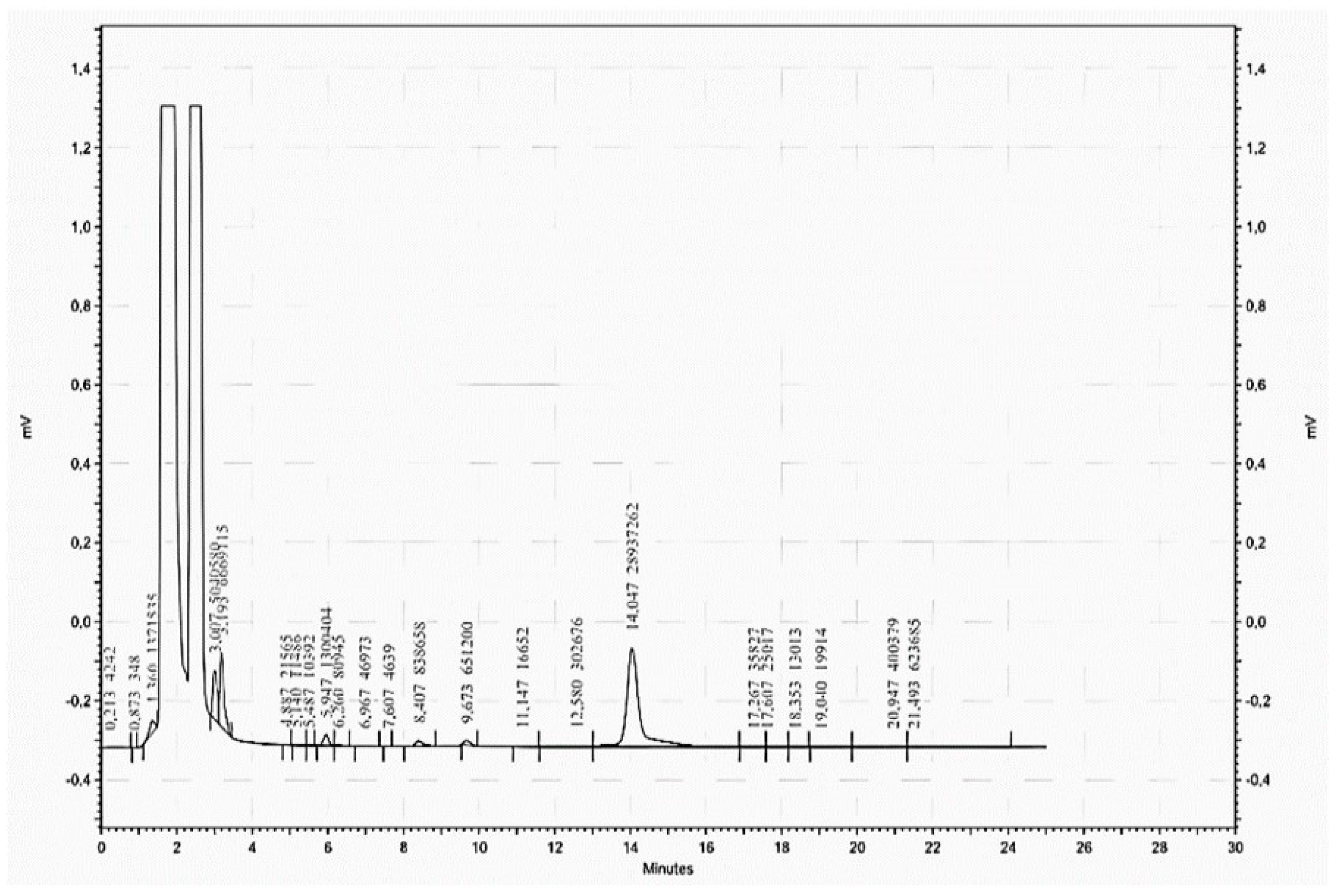
2.7. Determination of Biogenic Amines
2.8. Statistical Analyses
3. Results
3.1. Optimization of the Ultra-Fast Air-Superchilling Process of Golden Rainbow Trout Referring to Quality and Safety Parameters
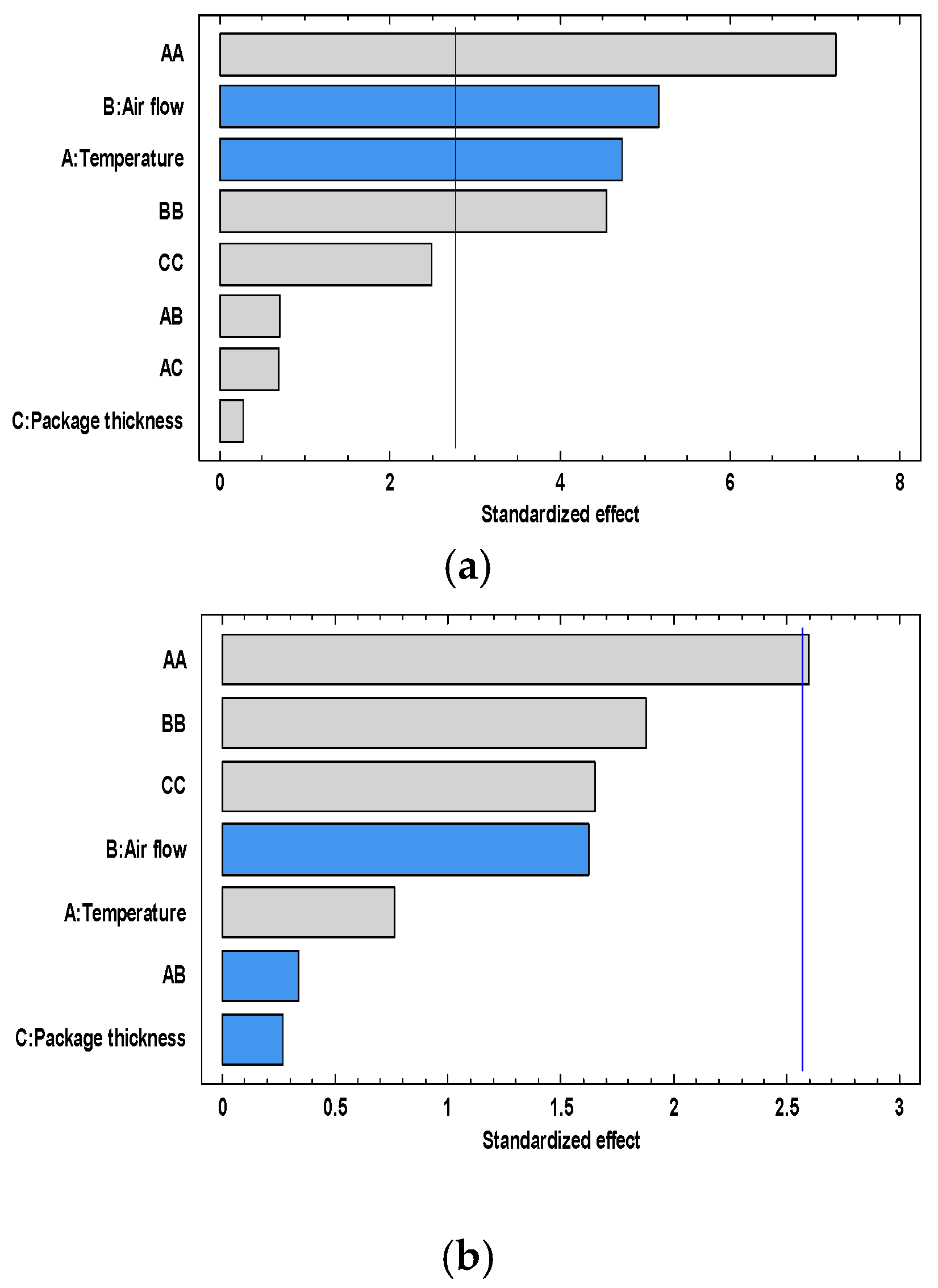
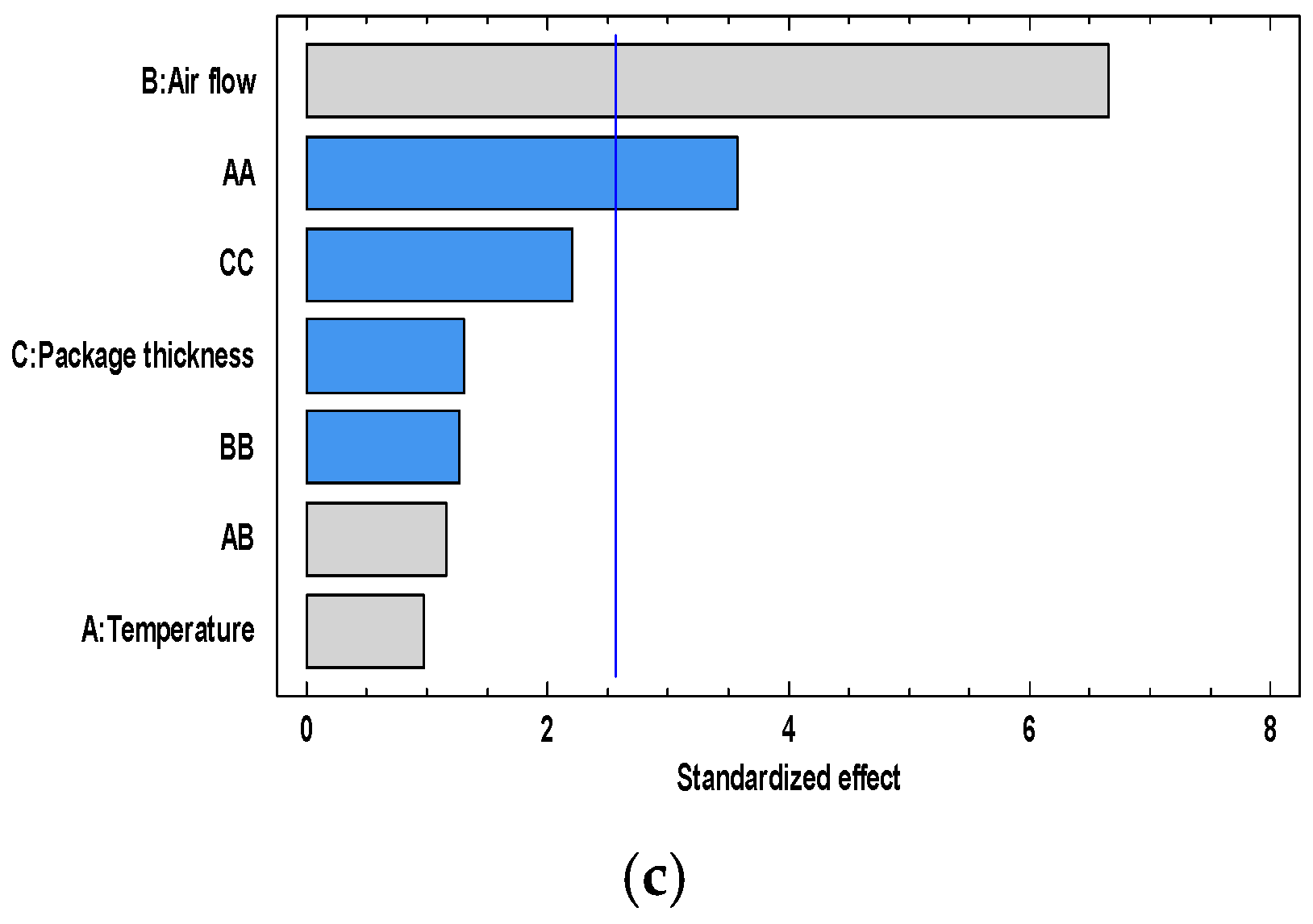
3.2. Influence of the Superchilling Process Variables on the Condition of Quality and Safety Parameters of Golden Rainbow Trout during Storage
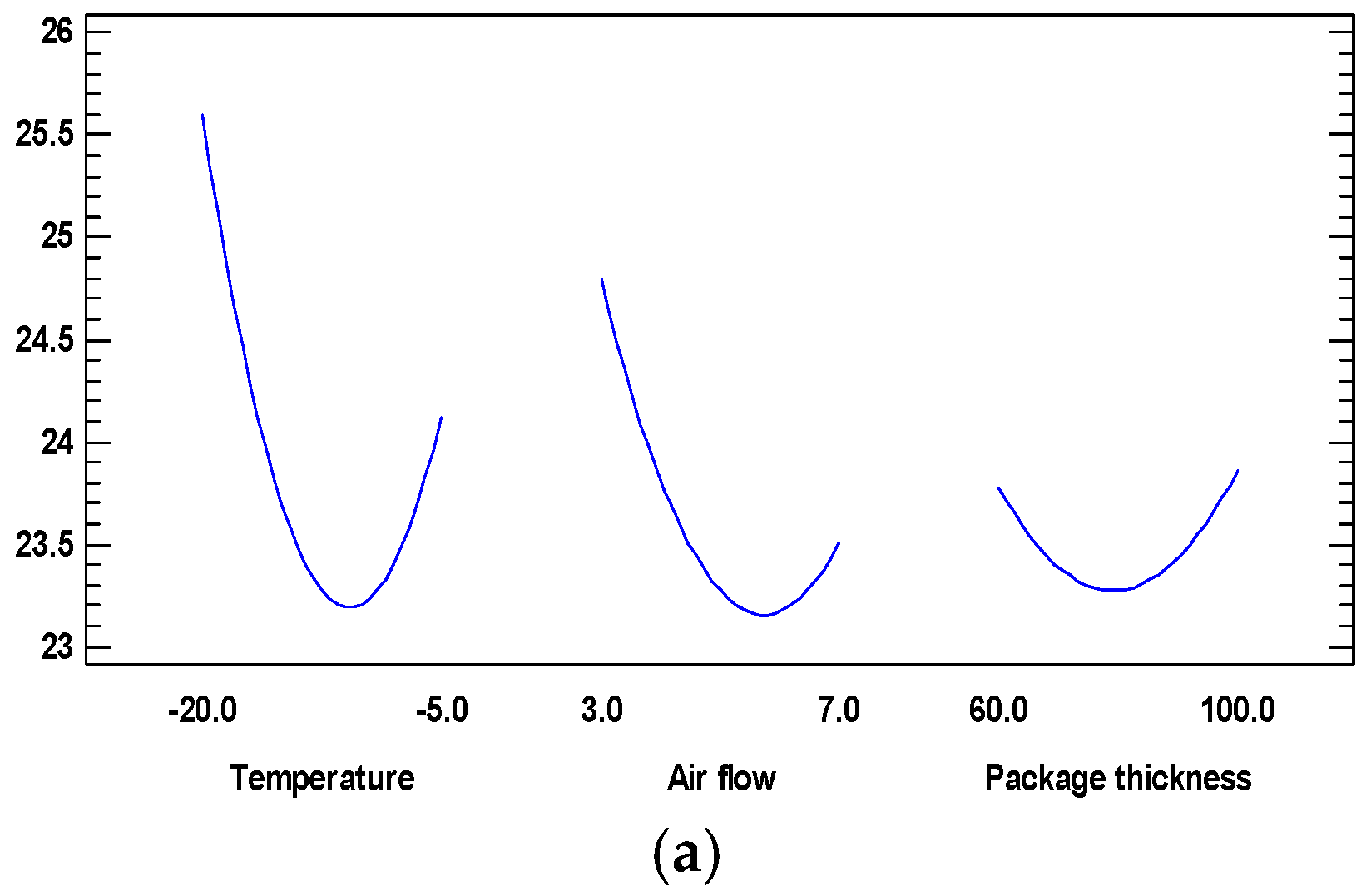
3.3. Mathematical Optimization of the Ultra-Fast Air-Superchilling Process of Golden Rainbow Trout
3.4. Presence of Biogenic Amines in Golden Rainbow Trout (Oncorhynchus mykiss, Stevanovski) Superchilled at Optimal Ultra-Fast Air Regime
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hattori, R.S.; Yoshinaga, T.T.; Butzge, A.J.; Hattori-Ihara, S.; Tsukamoto, R.Y.; Takahashi, N.S.; Tabata, Y.A. Generation of a white-albino phenotype from cobalt blue and yellow-albino rainbow trout (Oncorhynchus mykiss): Inheritance pattern and chromatophores analysis. PLoS ONE 2020, 15, e0214034. [Google Scholar] [CrossRef] [PubMed]
- Massimo, O.; Marco, G.; Elena, S.; Michela, B.; Mirco, C.; Bianchi, S.D.T.; Guido, M.; Gian, E.M.; Jacob, G.S. Effect of temperature on transfer of Midichloria-like organism and development of red mark syndrome in rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 560, 738577. [Google Scholar] [CrossRef]
- Palti, Y.; Gao, G.; Miller, M.R.; Vallejo, R.L.; Wheeler, P.A.; Quillet, E.; Yao, J.; Thorgaard, G.H.; Salem, M.; Rexroad, C.E., III. A resource of single-nucleotide polymorphisms for rainbow trout generated by restriction-site associated DNA sequencing of doubled haploids. Mol. Ecol. Resour. 2014, 14, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Kitanovski, V.D.; Kitanovski, D.P.; Dragoev, S.G.; Balev, D.K.; Vlahova-Vangelova, D.B. Fatty acid composition of the Californian rainbow trout and its hybrid golden rainbow trout. In Proceedings of the 61st International Scientific Conference on Food Technology, Plovdiv, Bulgaria, 24–25 October 2014; University of Food Technologies: Plovdiv, Bulgaria, 2014; Volume 61, pp. 133–136. Available online: https://uft-plovdiv.bg/site_files/file/scienwork/scienworks_2014/docs/part_1/1_Texнoлoгия_кoнтpoл_и_кaчecтвo/27_Kitanovski_Dragoev.pdf (accessed on 17 August 2023).
- Kitanovski, V.D.; Vlahova-Vangelova, D.B.; Dragoev, S.G.; Kitanovski, D.P. Alternations in quality parameters of rainbow trout (Oncorhynchus mykiss) compared to albino golden rainbow trout stored at 0 to 4 °C. In Proceedings of the 63rd International Scientific Conference on Food Technology, Plovdiv, Bulgaria, 27–28 October 2016; Volume 63, pp. 76–82. Available online: https://uft-plovdiv.bg/site_files/file/scienwork/scienworks_2016/docs/original%20SWUFT201601134%20(final).pdf (accessed on 17 August 2023).
- Kiossev, D.; Dragoev, S.G. Technology of Fish and Fish Products, 2nd ed.; Publishing House Food Industry: Sofia, Bulgaria, 2013; pp. 76–90. ISBN 978-954-90533-6-4. (In Bulgarian) [Google Scholar]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Kolsaker, K. Super chilling of food: A review. J. Food Eng. 2011, 107, 141–146. [Google Scholar] [CrossRef]
- Kler, R.; Gangurde, R.; Elmirzaev, S.; Hossain, S.; Vo, N.; Nguyen, T.; Kumar, N. Optimization of Meat and Poultry Farm Inventory Stock Using Data Analytics for Green Supply Chain Network, Discrete Dyn. Nat. Soc. 2022, 1, 1–8. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- Saleh, N.; Elham, A.; Heba, W.; Abdel-Mohsen, H.H. Chapter nine-Sustainable Fish and Seafood Production and Processing. In Sustainable Fish Production and Processing; Galanakis, C., Ed.; Academic Press: Athens, Greece, 2022; pp. 259–291. ISBN 9780128242964. [Google Scholar] [CrossRef]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of food waste, fish waste and food processing waste for China’s aquaculture industry: Needs and challenge. Sci. Total Environ. 2018, 613, 635–643. [Google Scholar] [CrossRef]
- Beheshti Foroutani, M.; Parrish, C.C.; Wells, J.; Taylor, R.G.; Rise, M.L.; Shahidi, F. Minimizing marine ingredients in diets of farmed Atlantic salmon (Salmo salar): Effects on growth performance and muscle lipid and fatty acid composition. PLoS ONE 2018, 13, e0198538. [Google Scholar] [CrossRef] [PubMed]
- Edvins, T.; Raimonda, S.; Ivars, V.; Dagnija, B. Analysis of fish refrigeration electricity consumption. Energy Procedia. 2018, 147, 649–653. [Google Scholar] [CrossRef]
- Beaufort, A.; Cardinal, M.; Le-Bail, A.; Midelet-Bourdin, G. The effects of superchilled storage at −2 °C on the microbiological and organoleptic properties of cold-smoked salmon before retail display. Int. J. Refrig. 2009, 32, 1850–1857. [Google Scholar] [CrossRef]
- Dalgaard, P.; Madsen, H.L.; Samieian, N.; Emborg, J. Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone)-effect of modified atmosphere packaging and previous frozen storage. J. Appl. Microbiol. 2006, 101, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.N.D.; Arason, S.; Thórarinsdóttir, K.A. Effects of dry ice and super chilling on quality and shelf life of Arctic charr (Salvelinus alpinus) fillets. Int. J. Food Eng. 2007, 3, 1–27. [Google Scholar] [CrossRef]
- Hansen, A.Å.; Mørkøre, T.; Rudi, K.; Langsrud, Ø.; Eie, T. The combined effect of superchilling and modified atmosphere packaging using CO2 emitter on quality during chilled storage of pre-rigor salmon fillets (Salmo salar). J. Sci. Food Agric. 2009, 89, 1625–1633. [Google Scholar] [CrossRef]
- Banerjee, R.; Maheswarappa, N.B. Superchilling of muscle foods: Potential alternative for chilling and freezing. Crit. Rev. Food Sci. Nutr. 2019, 59, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Jornet, L.; Rustad, T.; Barat, J.M.; Fito, P.; Escriche, I. Effect of superchilled storage on the freshness and salting behaviour of Atlantic salmon (Salmo salar) fillets. Food Chem. 2007, 103, 1268–1281. [Google Scholar] [CrossRef]
- Garimella, S.V.; Schroeder, V.P. Local heat transfer distributions in confined multiple air jet impingement. J. Electron. Packag. 2000, 123, 165–172. [Google Scholar] [CrossRef]
- Sarkar, A.; Nitin, N.; Karwe, M.V.; Singh, R.P. Fluid flow and heat transfer in ultra-fast air in food processing. J. Food Sci. 2004, 69, 113–122. [Google Scholar] [CrossRef]
- Kitanovski, V.D.; Vlahova-Vangelova, D.B.; Dragoev, S.G.; Nikolov, H.N.; Balev, D.K. Extension the shelf-life of fresh golden rainbow trout via ultra-fast air or cryogenic carbon dioxide superchilling. J. Aquac. Res. Dev. 2017, 8, 481. [Google Scholar] [CrossRef]
- Erikson, U.; Standal, I.B.; Aursand, I.G.; Veliyulin, E.; Aursand, M. Use of NMR in fish processing optimization: A review of recent progress. Magn. Reson. Chem. 2012, 50, 471–480. [Google Scholar] [CrossRef]
- Fabrigar, L.R.; Wegener, D.T. Exploratory Factor Analysis (Understanding Statistics); Oxford University Press Inc.: New York, NY, USA, 2011; pp. 39–83. ISBN 9780199734177. [Google Scholar]
- Gaskin, C.J.; Happell, B. On exploratory factor analysis: A review of recent evidence, an assessment of current practice, and recommendations for future use. Int. J. Nur. Stud. 2014, 51, 511–521. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, C. Factor screening and response surface exploration. Stat. Sin. 2001, 11, 553–580. Available online: http://www.jstor.org/stable/24306827 (accessed on 17 August 2023).
- Archer, M. Sensory Assessment Scoresheets for Fish and Shellfish 2010; Torry & QIM: Edinburgh, UK, 2010; Available online: http://www.seafish.org/media/publications/sensory_assessment_scoresheets_14_5_10.pdf (accessed on 17 August 2023).
- Goulas, A.E.; Kontominas, M.G. Effect of salting and smoking method on the keeping quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Food Chem. 2005, 93, 511–520. [Google Scholar] [CrossRef]
- Gelabert, J.; Gou, P.; Guerrero, L.; Arnau, J. Effect of sodium chloride replacement on some characteristics of fermented sausages. Meat Sci. 2003, 65, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.R.; Rahimi, R.; Mirghaed, A.T. Determination of histamine content in muscle tissue of rainbow trout (Oncorhynchus mykiss) during ice storage. Comp. Clin. Pathol. 2014, 23, 775–778. [Google Scholar] [CrossRef]
- Ocaño-Higuera, V.M.; Marquez-Rios, E.; Canizales-Davila, M.; Castillo-Yanes, F.J.; Pacheco-Aguilar, R.; Lugo-Sanchez, M.E.; Garsia-Orizco, K.D.; Graciano-Verdugo, A.Z. Postmortem chances in cazon fish muscle stored on ice. Food Chem. 2009, 116, 933–938. [Google Scholar] [CrossRef]
- Mi, H.; Qian, C.; Zhao, Y.; Liu, C.; Mao, L. Comparison of super chilling and freezing on microstructure, muscle quality and protein denaturation of grass carp (Ctenopharyngodon idellus). J. Food Process. Preserv. 2013, 37, 546–554. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Moosavi-Nasab, M.; Nassiri, S.M.; Azimifar, Z. Determination of total viable count in rainbow-trout fish fillets based on hyperspectral imaging system and different variable selection and extraction of reference data methods. Food Anal. Methods 2018, 11, 3481–3494. [Google Scholar] [CrossRef]
- Olafsdóttir, G.; Lauzon, H.L.; Martinsdóttir, E.; Oehlenschláuger, J.; Kristbergsson, K. Evaluation of shelf life of superchilled cod (Gadus morhua) fillets and the influence of temperature fluctuations during storage on microbial and chemical quality indicators. J. Food Sci. 2006, 71, 97–109. [Google Scholar] [CrossRef]
- Shafel, T.; Lee, S.H.; Jun, S. Food preservation technology at subzero temperatures: A Review. J. Biosys. Eng. 2015, 40, 261270. [Google Scholar] [CrossRef]
- Erikson, U. Superchilling of rested Atlantic salmon: Different chilling strategies and effects on fish and fillet quality. Food Chem. 2011, 127, 1427–1437. [Google Scholar] [CrossRef]
- Cyprian, O.; Lauzon, H.L.; Johannsson, R.; Sveinsdottir, K.; Arason, S.; Martinsdottir, E. Shelf life of air and modified atmosphere-packaged fresh tilapia (Oreochromis niloticus) fillets stored under chilled and superchilled conditions. Food Sci. Nutr. 2013, 1, 130–140. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/fsn3.18 (accessed on 17 August 2023). [CrossRef] [PubMed]
- Cheftel, J.C.; Lévy, J.; Dumay, E. Pressure-assisted freezing and thawing: Principles and potential applications. Food Rev. Int. 2006, 16, 453–483. [Google Scholar] [CrossRef]
- Waterman, J.J.; Taylor, D.H. Superchilling 2001. Ministry of Technology, Torry Research Station, Torry Advisory Note No. 32, Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/wairdocs/tan/x5910e/x5910e00.htm (accessed on 17 August 2023).
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Nordtvedt, T.S.; Bardal, T. Ice crystal development in pre-rigor Atlantic salmon fillets during super chilling process and following storage. Food Control 2013, 31, 491–498. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, Y.; Liu, X.; Luo, Y.; Gao, L. Quality assessment of rainbow trout (Oncorhynchus mykiss) fillets during super chilling and chilled storage. J. Food Sci. Technol. 2015, 52, 5204–5211. [Google Scholar] [CrossRef]
- Duun, A.S.; Rustad, T. Quality changes during superchilled storage of cod (Gadus morhua) fillets. Food Chem. 2007, 105, 10671075. [Google Scholar] [CrossRef]
- Bahuaud, D.; Mørkøre, T.; Langsrud, Ø.; Sinnes, K.; Veiseth, E.; Ofstad, R.; Thomassen, M.S. Effects of −1.5 °C super-chilling on quality of Atlantic salmon (Salmo salar) pre-rigor fillets: Cathepsin activity, muscle histology, texture and liquid leakage. Food Chem. 2008, 111, 329–339. [Google Scholar] [CrossRef]
- Emborg, J.; Dalgaard, P. Modelling the effect of temperature, carbon dioxide, water activity and pH on growth and histamine formation by Morganella psychrotolerans. Int. J. Food Microbiol. 2008, 128, 226–233. [Google Scholar] [CrossRef]



| X1 | X2 | X3 | (T, °C) | (υ, m s−1) | (D, μm) | |
|---|---|---|---|---|---|---|
| 1 | − | − | − | −20.0 | 3.0 | 60.0 |
| 2 | + | − | − | −5.0 | 3.0 | 60.0 |
| 3 | − | + | − | −20.0 | 7.0 | 60.0 |
| 4 | + | + | − | −5.0 | 7.0 | 60.0 |
| 5 | − | − | + | −20.0 | 3.0 | 100.0 |
| 6 | + | − | + | −5.0 | 3.0 | 100.0 |
| 7 | − | + | + | −20.0 | 7.0 | 100.0 |
| 8 | + | + | + | −5.0 | 7.0 | 100.0 |
| 9 | −a | 0 | 0 | −25.0 | 5.0 | 80.0 |
| 10 | +a | 0 | 0 | 0 | 5.0 | 80.0 |
| 11 | 0 | −a | 0 | −12.5 | 1.6 | 80.0 |
| 12 | 0 | +a | 0 | −12.5 | 8.4 | 80.0 |
| 13 | 0 | 0 | −a | −12.5 | 5.0 | 46.4 |
| 14 | 0 | 0 | +a | −12.5 | 5.0 | 113.6 |
| 15 | 0 | 0 | 0 | −12.5 | 5.0 | 80.0 |
| 16 | 0 | 0 | 0 | −12.5 | 5.0 | 80.0 |
| 17 | 0 | 0 | 0 | −12.5 | 5.0 | 80.0 |
| Independent Variables | Upper Factor Levels | Lower Factor Levels | Centre of the EFA Design | Minimal Upper Factor Level | Maximal Lower Factor Level | Intervals of Variations |
|---|---|---|---|---|---|---|
| Temperature X1 (T, °C) | −5.0 | −20.0 | −12.5 | −25.0 | 0.0 | ± 7.5 |
| Airflow velocity X2 (υ, m s−1) | 7.0 | 3.0 | 5.0 | 1.6 | 8.4 | ± 2.0 |
| Package thickness X3 (D, μm) | 100.0 | 60.0 | 80.0 | 46.4 | 113.6 | ± 20.0 |
| No | T °C | υ m s−1 | D μm | TVB-N mg N2 100 g−1 | TVC log cfu g−1 | Sensory Scores |
|---|---|---|---|---|---|---|
| 1 | −20.0 | 3.0 | 60.0 | 27.88 ± 1.12 a | 4.76 e | 7.25 ± 0.18 g |
| 2 | −5.0 | 3.0 | 60.0 | 25.83 ± 0.98 b | 4.92 e | 7.15 ± 0.13 g |
| 3 | −20.0 | 7.0 | 60.0 | 26.73 ± 1.22 b | 4.95 e | 8.00 ± 0.15 g,h |
| 4 | −5.0 | 7.0 | 60.0 | 24.76 ± 0.87 c | 5.01 e | 8.45 ± 0.19 i |
| 5 | −20.0 | 3.0 | 100.0 | 27.80 ± 1.14 c,a | 4.77 e | 7.00 ± 0.12 g |
| 6 | −5.0 | 3.0 | 100.0 | 25.82 ± 1.25 c,b | 4.94 e | 7.25 ± 0.11 g |
| 7 | −20.0 | 7.0 | 100.0 | 25.91 ± 0.98 c,b | 4.98 e | 7.75 ± 0.14 g,h |
| 8 | −5.0 | 7.0 | 100.0 | 25.17 ± 1.07 c,b | 4.57 e | 8.25 ± 0.17 g,h |
| 9 | −25.0 | 5.0 | 80.0 | 23.23 ± 0.82 d | 4.13 f | 8.50 ± 0.19 i |
| 10 | 0 | 5.0 | 80.0 | 29.88 ± 1.12 c | 5.72 e | 7.00 ± 0.18 g |
| 11 | −12.5 | 1.6 | 80.0 | 28.83 ± 0.98 c | 5.11 e | 7.25 ± 0.13 g |
| 12 | −12.5 | 8.4 | 80.0 | 27.73 ± 1.22 b,c | 4.81 e | 7.75± 0.15 g,h |
| 13 | −12.5 | 5.0 | 46.4 | 29.76 ± 0.87 c | 5.22 e | 8.00 ± 0.19 g |
| 14 | −12.5 | 5.0 | 113.6 | 26.82 ± 1.14 b | 4.93 e | 7.50 ± 0.12 g |
| 15 | −12.5 | 5.0 | 80.0 | 25.19 ± 1.25 c | 5.01 e | 7.75 ± 0.11 g,h |
| 16 | −12.5 | 5.0 | 80.0 | 25.28 ± 1.23 c | 4.89 e | 7.75 ± 0.11 g,h |
| 17 | −12.5 | 5.0 | 80.0 | 25.32 ± 1.21 c | 4.97 e | 7.75 ± 0.11 g,h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitanovski, V.D.; Dragoev, S.G.; Nikolov, H.N.; Vlahova-Vangelova, D.B.; Balev, D.K. Nutritional Quality and Overall Acceptability Optimization of Ultra-Fast Air-Superchilled Golden Rainbow Trout (Oncorhynchus mykiss, Stevanovski) Using the Response Surface Methodology. Appl. Sci. 2023, 13, 9504. https://doi.org/10.3390/app13179504
Kitanovski VD, Dragoev SG, Nikolov HN, Vlahova-Vangelova DB, Balev DK. Nutritional Quality and Overall Acceptability Optimization of Ultra-Fast Air-Superchilled Golden Rainbow Trout (Oncorhynchus mykiss, Stevanovski) Using the Response Surface Methodology. Applied Sciences. 2023; 13(17):9504. https://doi.org/10.3390/app13179504
Chicago/Turabian StyleKitanovski, Vladimir D., Stefan G. Dragoev, Hristo N. Nikolov, Desislava B. Vlahova-Vangelova, and Dessislav K. Balev. 2023. "Nutritional Quality and Overall Acceptability Optimization of Ultra-Fast Air-Superchilled Golden Rainbow Trout (Oncorhynchus mykiss, Stevanovski) Using the Response Surface Methodology" Applied Sciences 13, no. 17: 9504. https://doi.org/10.3390/app13179504
APA StyleKitanovski, V. D., Dragoev, S. G., Nikolov, H. N., Vlahova-Vangelova, D. B., & Balev, D. K. (2023). Nutritional Quality and Overall Acceptability Optimization of Ultra-Fast Air-Superchilled Golden Rainbow Trout (Oncorhynchus mykiss, Stevanovski) Using the Response Surface Methodology. Applied Sciences, 13(17), 9504. https://doi.org/10.3390/app13179504







