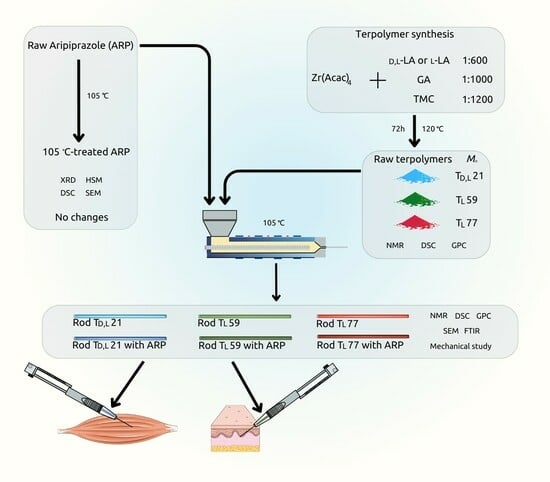
Hot Melt Extrusion as a Formulation Method of Terpolymer Rods with Aripiprazole: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study of ARP
2.1.1. X-ray Diffraction Study
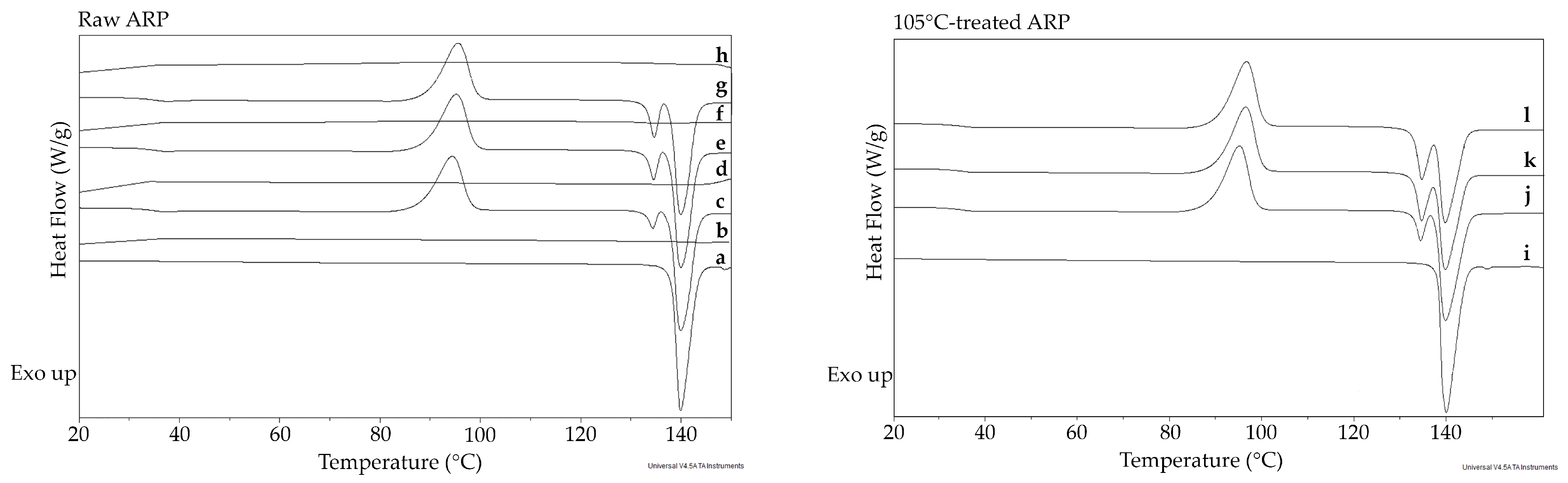
2.1.2. Thermal Study
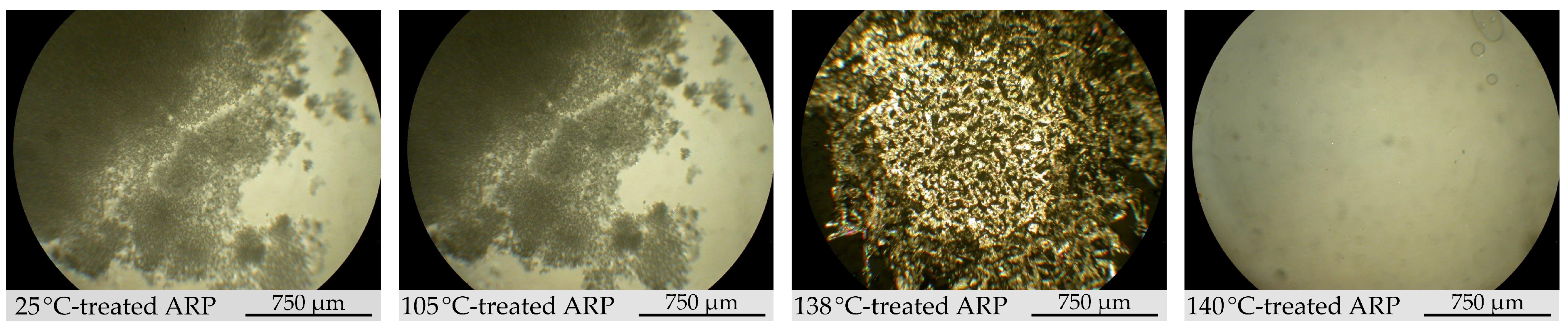
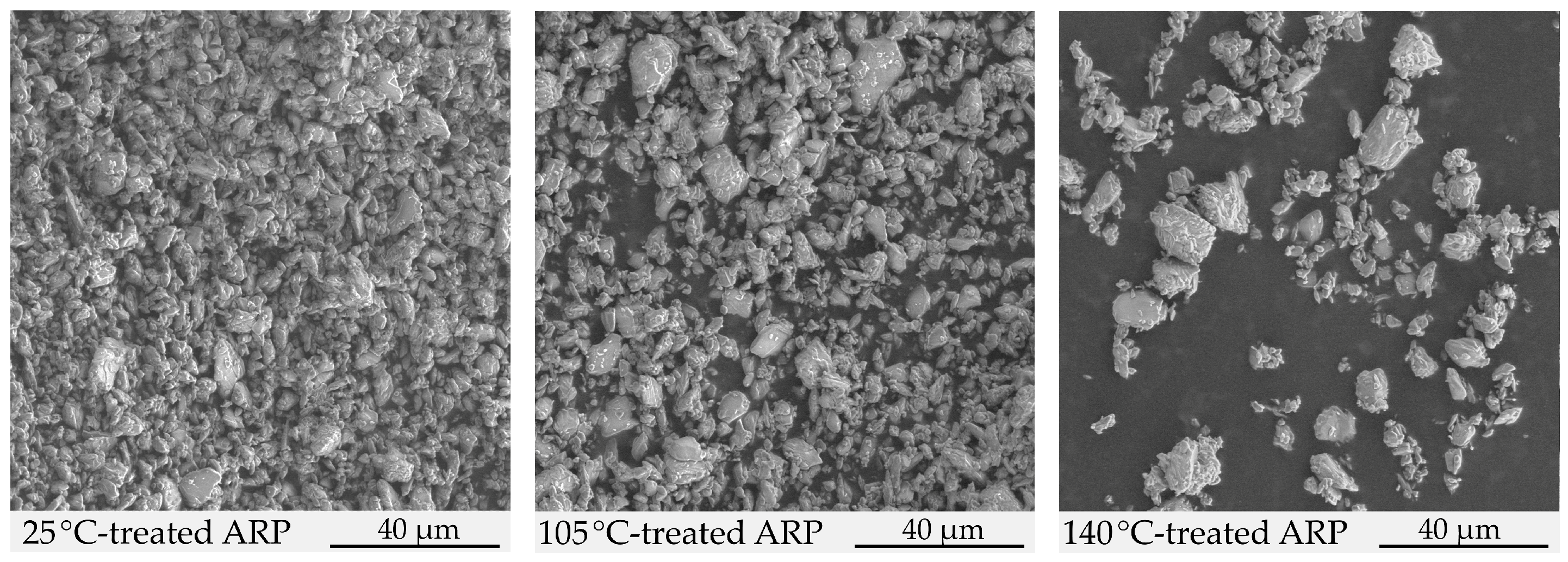
2.1.3. Morphology Study
2.2. Terpolymer Synthesis
2.3. Hot Melt Extrusion
2.4. Study of Raw Terpolymers and Rods
2.4.1. Composition Study
2.4.2. Thermal Study
2.4.3. Molecular Weight Study
2.4.4. Terpolymers–ARP Interaction Study
2.4.5. Morphology Study
2.4.6. Mechanical Study
2.4.7. Rod Hydrolytic Degradation Study
3. Results and Discussion
3.1. Structural Characterization of ARP
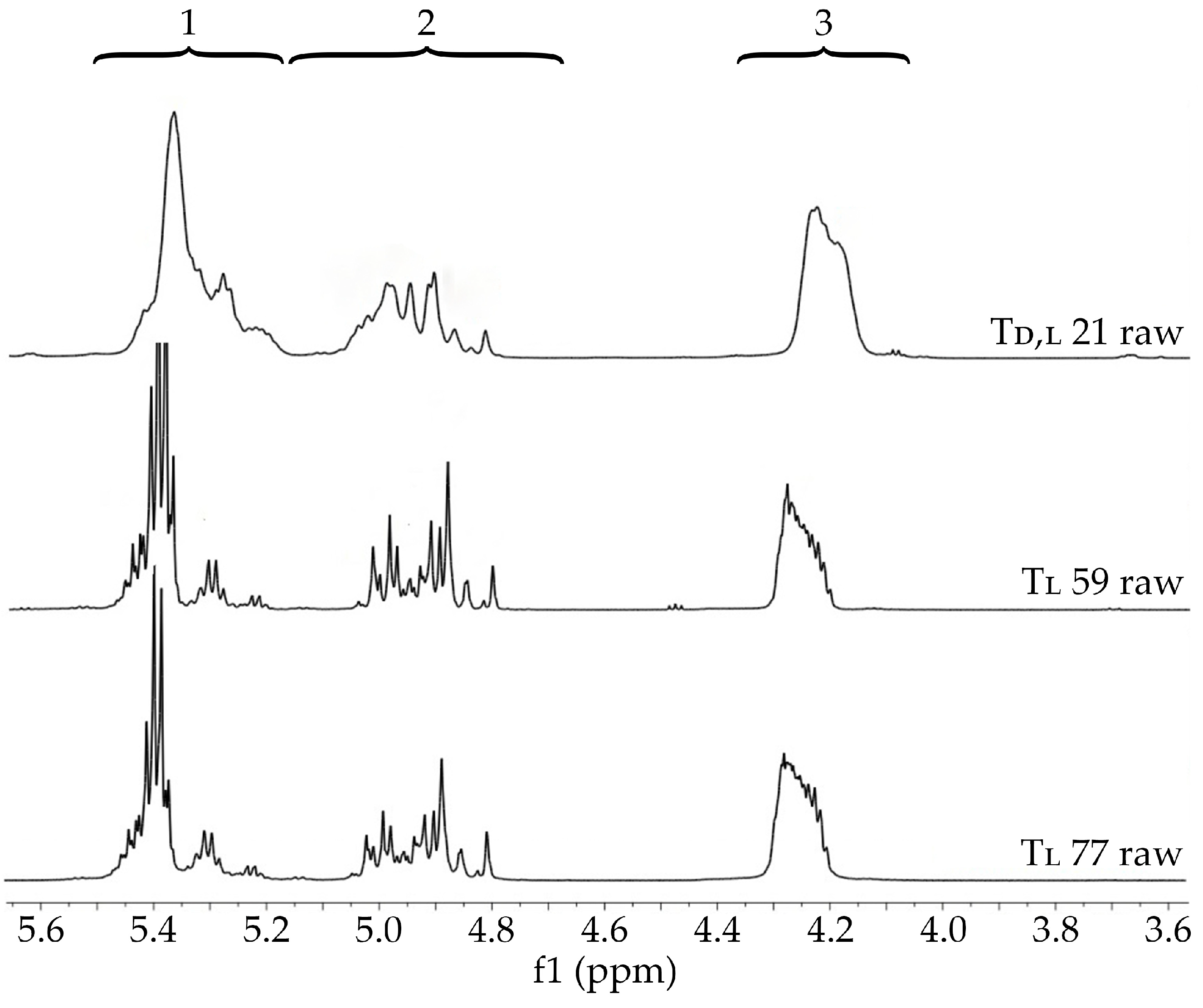
3.2. Characterization of Raw Terpolymers
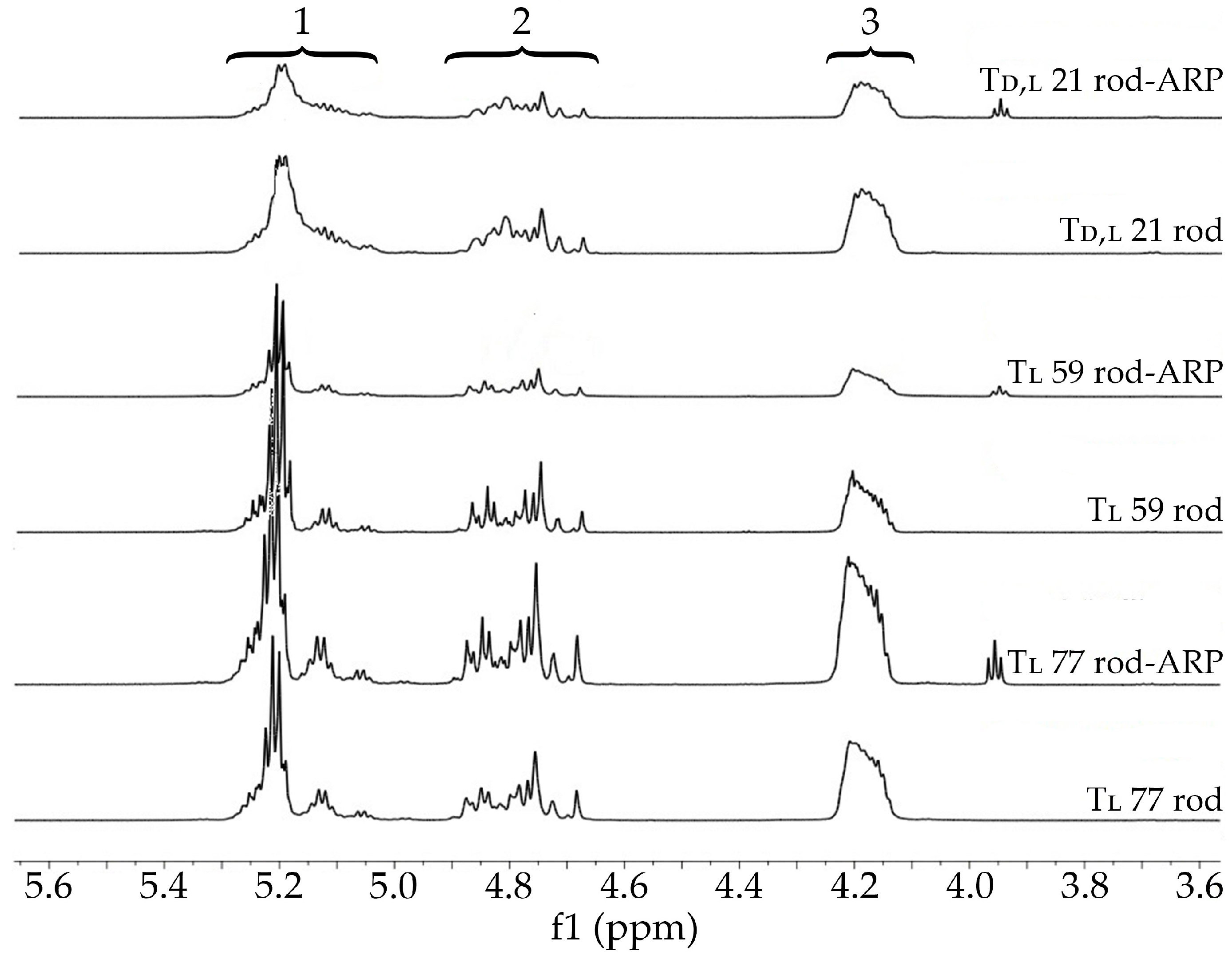
3.3. Characterization of the Rods and Rod with ARP
3.3.1. Terpolymer Composition
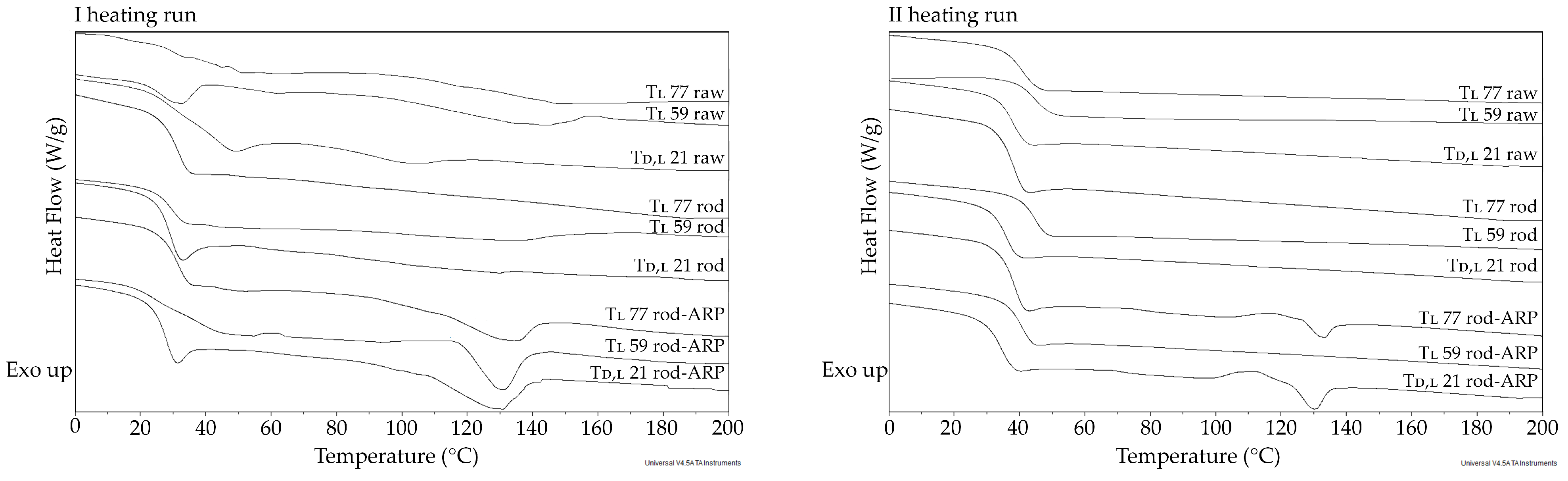
3.3.2. Thermal Study
3.3.3. Mn Study
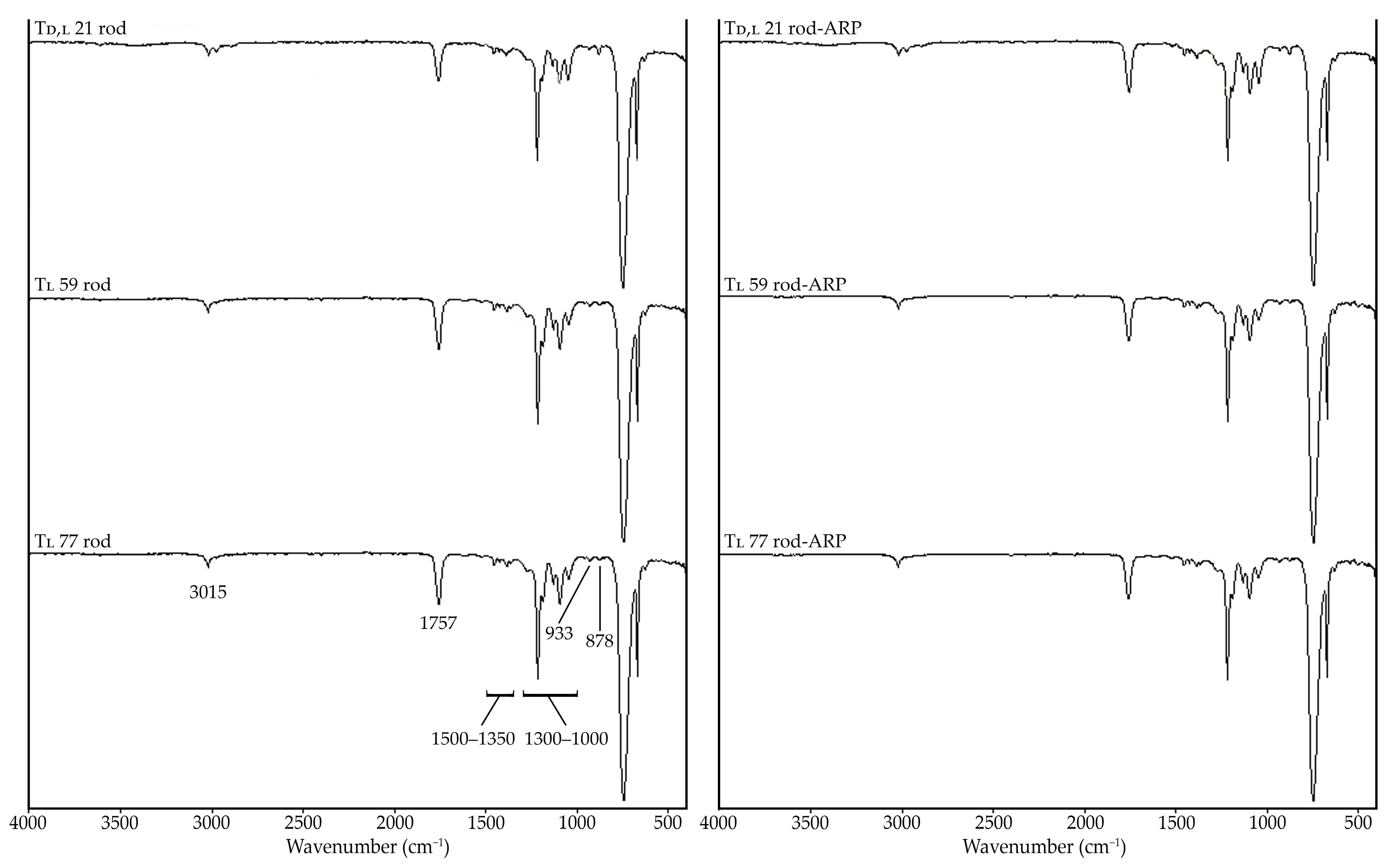
3.3.4. Terpolymer-ARP Interaction Study
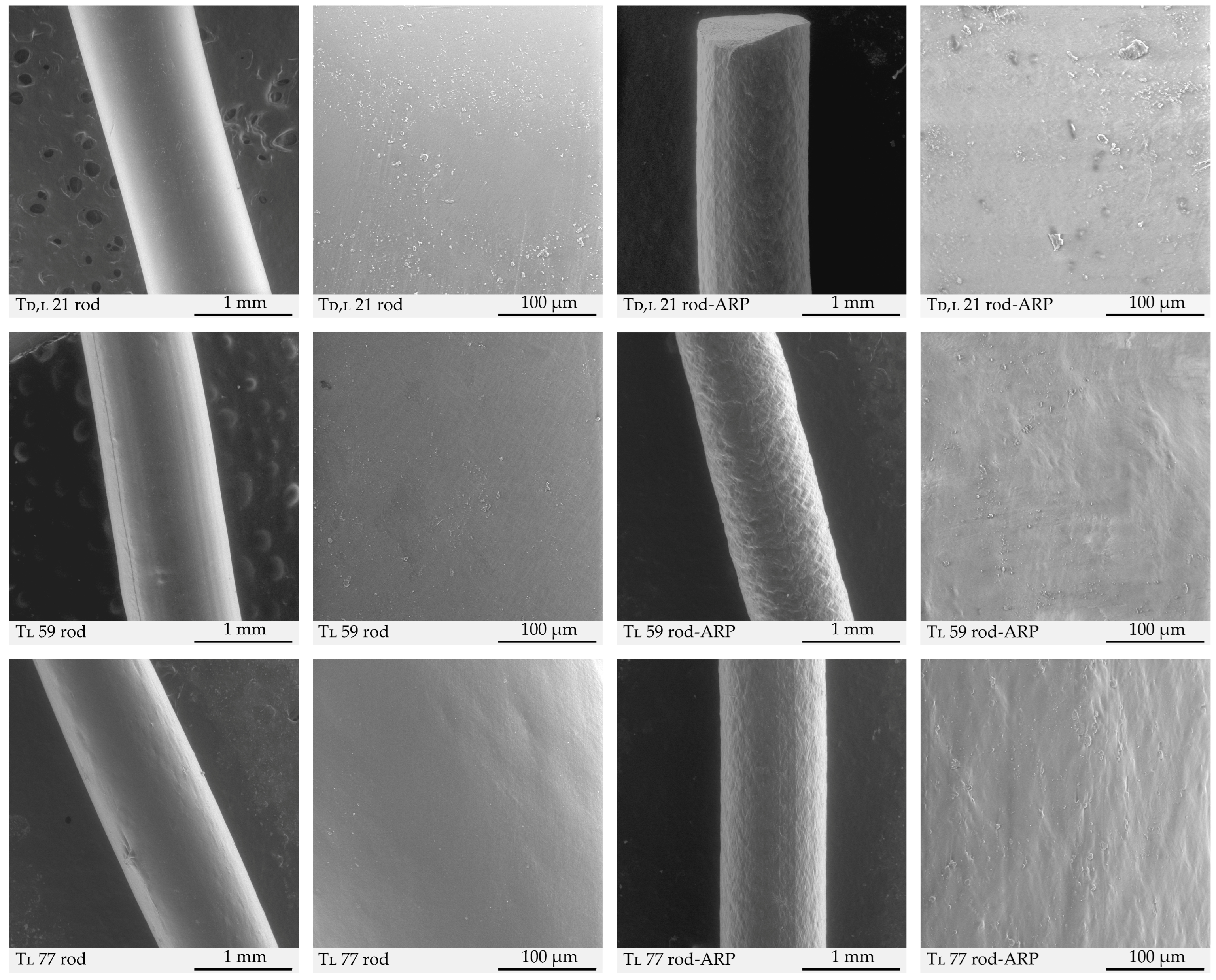
3.3.5. Morphology Study
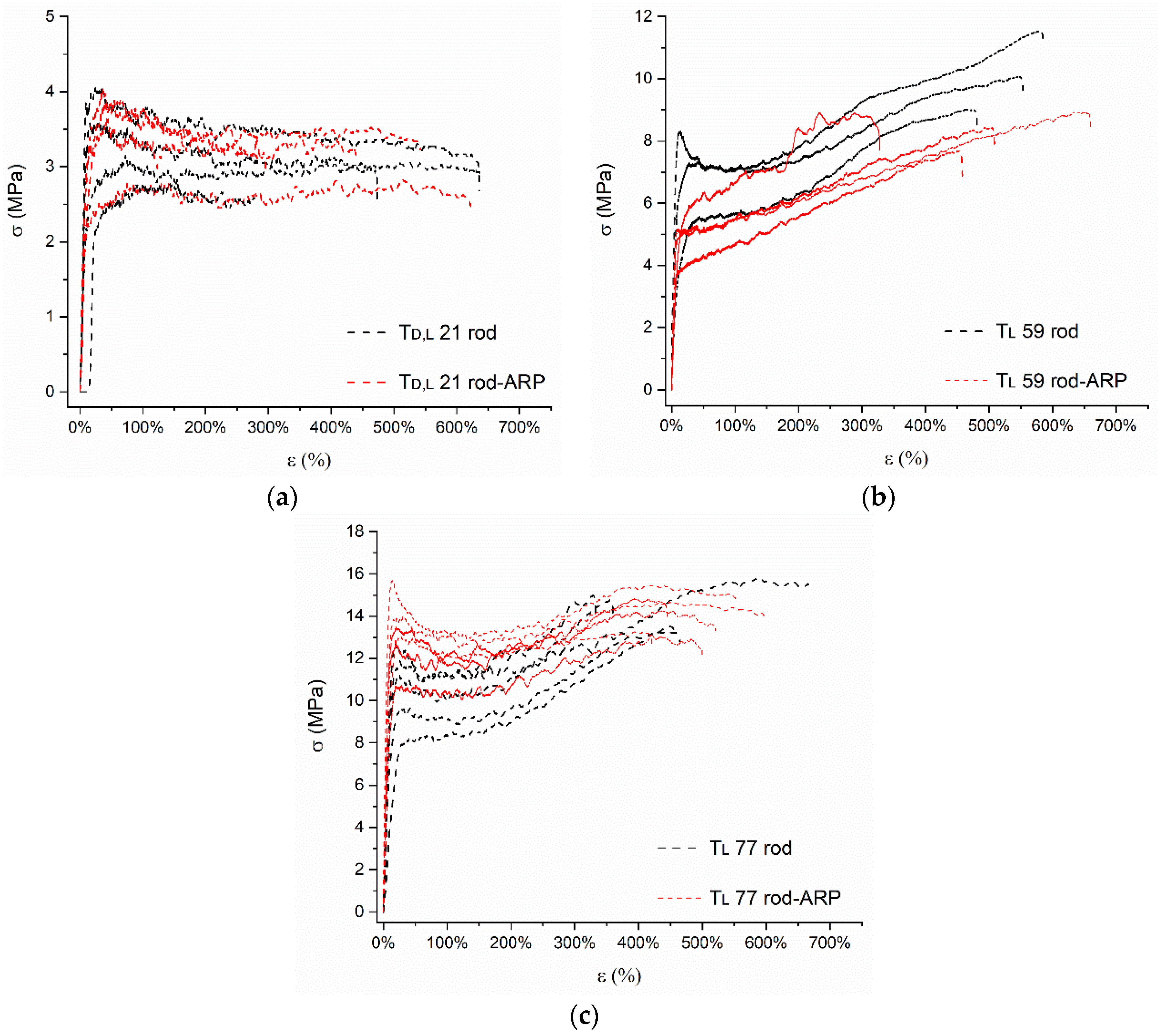
3.3.6. Mechanical Study
3.3.7. Rod Hydrolytic Degradation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casey, A.B.; Canal, C.E. Classics in chemical neuroscience: Aripiprazole. ACS Chem. Neurosci. 2017, 8, 1135–1146. [Google Scholar] [CrossRef]
- Biagi, E.; Capuzzi, E.; Colmegna, F.; Mascarini, A.; Brambilla, G.; Ornaghi, A.; Santambrogio, J.; Clerici, M. Long-acting injectable antipsychotics in schizophrenia: Literature review and practical perspective, with a focus on aripiprazole once-monthly. Adv. Ther. 2017, 34, 1036–1048. [Google Scholar]
- Frampton, J.E. Aripiprazole lauroxil: A review in schizophrenia. Drugs 2017, 77, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M.; Perry, C.M. Aripiprazole (ABILIFY MAINTENA®): A review of its use as maintenance treatment for adult patients with schizophrenia. Drugs 2014, 74, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Hard, M.L.; Mills, R.J.; Sadler, B.M.; Turncliff, R.Z.; Citrome, L. Aripiprazole lauroxil: Pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J. Clin. Psychopharmacol. 2017, 37, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Talegaonkar, S.; Mittal, A.; Parmar, S.; Aqil, M. Aripiprazole loaded polymeric biodegradable microspheres: Formulation and in vitro characterization. J. Dispers. Sci. Technol. 2009, 30, 1198–1202. [Google Scholar] [CrossRef]
- Babu, A.C.; Babu, P.K.; Sudhakar, K.; Subha, M.C.; Rao, K.C. Aripiprazole loaded PLGA nanoparticles for controlled release studies: Effect of co-polymer ratio. Int. J. Drug Deliv. 2014, 6, 151–155. [Google Scholar]
- Hiraoka, S.; Uchida, S.; Namiki, N. Preparation and characterization of high-content aripiprazole-loaded core-shell structure microsphere for long-release injectable formulation. Chem. Pharm. Bull. 2014, 62, 654–660. [Google Scholar] [CrossRef]
- Sawant, K.; Pandey, A.; Patel, S. Aripiprazole loaded poly(caprolactone) nanoparticles: Optimization and in vivo pharmacokinetics. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 230–243. [Google Scholar] [CrossRef]
- Braun, D.E.; Gelbrich, T.; Kahlenberg, V.; Tessadri, R.; Wieser, J.; Griesser, U.J. Stability of solvates and packing systematics of nine crystal forms of the antipsychotic drug aripiprazole. Cryst. Growth Des. 2009, 9, 1054–1065. [Google Scholar] [CrossRef]
- Kamali, H.; Atamanesh, M.; Kaffash, E.; Mohammadpour, F.; Khodaverdi, E.; Hadizadeh, F. Elimination of residual solvent from PLGA microspheres containing risperidone using supercritical carbon dioxide. J. Drug Deliv. Sci. Technol. 2020, 57, 101702. [Google Scholar] [CrossRef]
- Turek, A.; Borecka, A.; Janeczek, H.; Sobota, M.; Kasperczyk, J. Formulation of delivery systems with risperidone based on biodegradable terpolymers. Int. J. Pharm. 2018, 548, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Turek, A.; Rech, J.; Borecka, A.; Wilińska, J.; Kobielarz, M.; Janeczek, H.; Kasperczyk, J. The role of the mechanical, structural, and thermal properties of poly(L-lactide-co-glycolide-co-trimethylene carbonate) in the development of rods with aripiprazole. Polymers 2021, 13, 3556. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.; Frijlink, H.W.; Hinrichs, W.L. Polymeric formulations for drug release prepared by hot melt extrusion: Application and characterization. Drug. Discov. Today 2015, 20, 812–823. [Google Scholar]
- Łaszcz, M.; Witkowska, A. Studies of phase transitions in the aripiprazole solid dosage form. J. Pharm. Biomed. Anal. 2016, 117, 298–303. [Google Scholar] [CrossRef]
- Braun, D.E.; Gelbrich, T.; Kahlenberg, V.; Tessadri, R.; Wieser, J.; Griesser, U.J. Conformational polymorphism in aripiprazole: Preparation, stability and structure of five modifications. J. Pharm. Sci. 2009, 98, 2010–2026. [Google Scholar] [CrossRef]
- Gębarowska, K.; Kasperczyk, J.; Dobrzyński, P.; Scandola, M.; Zini, E.; Li, S. NMR analysis of the chain microstructure of biodegradable terpolymers with shape memory properties. Eur. Polym. J. 2011, 47, 1315–1327. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Eklund, M. Influence of molecular structure on the degradation mechanism of degradable polymers: In vitro degradation of poly(trimethylene carbonate), poly(trimethylene carbonate-co-caprolactone), and poly(adipic anhydride). J. Appl. Polym. Sci. 1995, 57, 87–103. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Poot, A.A.; Grijpma, D.W.; Feijen, J. In vitro degradation of trimethylene carbonate based (co)polymers. Macromol. Biosci. 2002, 2, 411–419. [Google Scholar] [CrossRef]
- Turek, A.; Kasperczyk, J.; Jelonek, K.; Borecka, A.; Janeczek, H.; Libera, M.; Gruchlik, A.; Dobrzyński, P. Thermal properties and morphology changes in degradation process of poly(L-lactide-co-glycolide) matrices with risperidone. Acta Bioeng. Biomech. 2015, 17, 11–20. [Google Scholar]
- Turek, A.; Olakowska, E.; Borecka, A.; Janeczek, H.; Sobota, M.; Jaworska, J.; Kaczmarczyk, B.; Jarząbek, B.; Gruchlik, A.; Libera, M.; et al. Shape-memory terpolymer rods with 17-β-estradiol for the treatment of neurodegenerative diseases: An in vitro and in vivo study. Pharm. Res. 2016, 33, 2967–2978. [Google Scholar]
- Zhang, Y.; Liang, R.; Xu, J.; Shen, L.; Gao, J.; Wang, X.; Wang, N.; Shou, D.; Hu, Y. Efficient induction of antimicrobial activity with vancomycin nanoparticle-loaded poly(trimethylene carbonate) localized drug delivery system. Int. J. Nanomed. 2017, 12, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Turek, A.; Stoklosa, K.; Borecka, A.; Paul-Samojedny, M.; Kaczmarczyk, B.; Marcinkowski, A.; Kasperczyk, J. Designing biodegradable wafers based on poly(L-lactide-co-glycolide) and poly(glycolide-co-ε-caprolactone) for the prolonged and local release of idarubicin for the therapy of glioblastoma multiforme. Pharm. Res. 2020, 37, 90. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska, B.; Dobrzynski, P.; Bero, M. Interaction of cells with L-lactide/glycolide copolymers synthesized with the use of tin or zirconium compounds. J. Biomed. Mater. Res. A 2005, 74, 591–597. [Google Scholar] [CrossRef]
- Liu, G.; McEnnis, K. Glass transition temperature of PLGA particles and the influence on drug delivery applications. Polymers 2022, 14, 993. [Google Scholar] [CrossRef]
- Olakowska, E.; Właszczuk, A.; Turek, A.; Borecka, A.; Liśkiewicz, A.; Wawro, D.; Kasperczyk, J.; Jędrzejowska-Szypułka, H.J. Effects of 17-β-estradiol released from shape-memory terpolymer rods on sciatic nerve regeneration after injury and repair with chitosan nerve conduit in female rats. J. Appl. Biomed. 2022, 20, 87–97. [Google Scholar]
- Mainardes, R.M.; Gremião, M.P.; Evangelista, R.C. Thermoanalytical study of praziquantel-loaded PLGA nanoparticles. Rev. Bras. Cienc. Farm. 2006, 42, 523–530. [Google Scholar] [CrossRef]
- Ellä, V.; Nikkola, L.; Kellomäki, M. Process-induced monomer on a medical-grade polymer and its effect on short-term hydrolytic degradation. J. Appl. Polym. Sci. 2011, 119, 2996–3003. [Google Scholar] [CrossRef]
- Snejdrova, E.; Drastik, M.; Dittrich, M.; Kastner, P.; Nguyenova, J. Mucoadhesive plasticized system of branched poly(lactic-co-glycolic acid) with aciclovir. Drug. Dev. Ind. Pharm. 2016, 42, 1653–1659. [Google Scholar] [CrossRef]
- Prudic, A.; Lesniak, A.K.; Ji, Y.; Sadowski, G. Thermodynamic phase behaviour of indomethacin/PLGA formulations. Eur. J. Pharm. Biopharm. 2015, 93, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Cicero, J.A.; Dorgan, J.R. Physical properties and fiber morphology of poly(lactic acid) obtained from continuous two-step melt spinning. J. Polym. Environ. 2001, 9, 1–10. [Google Scholar] [CrossRef]
- Gogolewski, S.; Jovanovic, M.; Perren, S.M.; Dillon, J.G.; Hughes, M.K. The effect of melt-processing on the degradation of selected polyhydroxyacids: Polylactides, polyhydroxybutyrate, and polyhydroxybutyrate-co-valerates. Polym. Degrad. Stab. 1993, 40, 313–322. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar]
- Nuutinen, J.P.; Clerc, C.; Virta, T.; Törmälä, P. Effect of gamma, ethylene oxide, electron beam, and plasma sterilization on the behaviour of SR-PLLA fibres in vitro. J. Biomater. Sci. Polym. Ed. 2002, 13, 1325–1336. [Google Scholar] [CrossRef]
- Rothen-Weinhold, A.; Besseghir, K.; Gurny, R. Analysis of the influence of polymer characteristics and core loading on the in vivo release of a somatostatin analogue. Eur. J. Pharm. Biopharm. 1997, 5, 303–313. [Google Scholar]
- Rothen-Weinhold, A.; Besseghir, K.; Vuaridel, E.; Sublet, E.; Oudry, N.; Kubel, F.; Gurny, R. Injection-molding versus extrusion as manufacturing technique for the preparation of biodegradable implants. Eur. J. Pharm. Biopharm. 1999, 48, 113–121. [Google Scholar]
- Simpson, R.L.; Nazhat, S.N.; Blaker, J.J.; Bismarck, A.; Hill, R.; Boccaccini, A.R.; Hansen, U.N.; Amis, A.A. A comparative study of the effects of different bioactive fillers in PLGA matrix composites and their suitability as bone substitute materials: A thermo-mechanical and in vitro investigation. J. Mech. Behav. Biomed. Mater. 2015, 50, 277–289. [Google Scholar]
- Yen, H.J.; Tseng, C.S.; Hsu, S.H.; Tsai, C.L. Evaluation of chondrocyte growth in the highly porous scaffolds made by fused deposition manufacturing (FDM) filled with type II collagen. Biomed. Microdevices 2009, 11, 615–624. [Google Scholar]
- Yuan, X.; Mak, A.F.; Kwok, K.W.; Yung, B.K.; Yao, K. Characterization of poly(L-lactic acid) fibers produced by melt spinning. J. Appl. Polym. Sci. 2001, 81, 251–260. [Google Scholar] [CrossRef]
- Nakashima, A.; Izumi, T.; Ohya, K.; Kondo, K.; Niwa, T. Design of highly dispersible PLGA microparticles in aqueous fluid for the development of long-acting release injectables. Chem. Pharm. Bull. 2017, 65, 157–165. [Google Scholar] [CrossRef][Green Version]
- Gao, L.; Li, Q.; Zhang, J.; Huang, Y.; Deng, L.; Li, C.; Tai, G.; Ruan, B. Local penetration of doxorubicin via intrahepatic implantation of PLGA based doxorubicin-loaded implants. Drug Deliv. 2019, 26, 1049–1057. [Google Scholar] [CrossRef]
- He, P.; Xu, S.; Guo, Z.; Yuan, P.; Liu, Y.; Chen, Y.; Zhang, T.; Que, Y.; Hu, Y. Pharmacodynamics and pharmacokinetics of PLGA-based doxorubicin-loaded implants for tumor therapy. Drug Deliv. 2022, 29, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.; Mallery, S.R.; Schwendeman, S.P. Formulation and characterization of injectable poly(DL-lactide-co-glycolide) implants loaded with N-acetylcysteine, a MMP inhibitor. Pharm. Res. 2008, 25, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Steele, T.W.; Widjaja, E.; Boey, F.Y.; Venkatraman, S.S.; Loo, J.S. The influence of additives in modulating drug delivery and degradation of PLGA thin films. NPG Asia Mater. 2013, 5, e54. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Jing, D.; Ding, J. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. A 2006, 76, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Borecka, A.; Rech, J.; Janeczek, H.; Wilińska, J.; Kasperczyk, J.; Kobielarz, M.; Grieb, P.; Turek, A. Development of the latanoprost solid delivery system based on poly(l-lactide-co-glycolide-co-trimethylene carbonate) with shape memory for glaucoma treatment. Appl. Sci. 2023, 13, 7562. [Google Scholar] [CrossRef]


| Sample | Lattice Parameters [Å] | Cell Volume (Å 3) | Crystal System | |||||
|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | A (°) | β (°) | γ (°) | |||
| Raw ARP | 10.161 | 12.143 | 18.714 | 82.197 | 82.570 | 82.863 | 2,255.213 | Triclinic |
| 105 °C-treated ARP | 10.163 | 12.142 | 18.714 | 82.189 | 82.570 | 81.863 | 2,255.219 | Triclinic |
| Heating Run | Tm1 (°C) | ΔH1 (J/g) | Tm2 (°C) | ΔH2 (J/g) | Tcr (°C) | ΔHcr (J/g) | |
|---|---|---|---|---|---|---|---|
| Raw ARP | First | ND | ND | 140.0 | 95.7 | ND | ND |
| Second | 134.4 | 7.2 | 140.0 | 90.1 | 94.4 | 91.8 | |
| Third | 134.7 | 7.6 | 139.9 | 90.5 | 95.6 | 90.2 | |
| Fourth | 134.8 | 7.5 | 139.9 | 90.7 | 96.6 | 90.2 | |
| 105 °C-treated ARP | First | ND | ND | 140.1 | 95.7 | ND | ND |
| Second | 134.6 | 7.6 | 139.9 | 91.8 | 95.2 | 91.8 | |
| Third | 139.6 | 5.4 | 139.6 | 91.1 | 95.6 | 90.9 | |
| Fourth | 134.7 | 6.5 | 139.9 | 90.5 | 96.6 | 90.2 |
| Sample | FLL (mol%) | FGG (mol%) | FTMC (mol%) | Tm (°C) | ΔH (J/g) | Tg (°C) | Mn (kDa) | D | |
|---|---|---|---|---|---|---|---|---|---|
| Td,l 21 | Raw | 65.4 | 14.7 | 19.9 | ND | ND | 36.3 | 21.4 | 1.970 |
| Rod | 63.9 | 15.7 | 20.4 | ND | ND | 35.1 | 20.4 | 2.030 | |
| rod-ARP | 63.5 | 15.9 | 20.6 | 128.7 | 8.4 | 33.7 | 18.7 | 1.998 | |
| Tl 59 | Raw | 64.9 | 16.2 | 18.9 | ND | ND | 46.7 | 59.4 | 1.950 |
| Rod | 64.9 | 16.2 | 18.9 | ND | ND | 44.1 | 57.7 | 1.604 | |
| rod-ARP | 65.4 | 16.3 | 18.3 | 133.4 | 5.2 | 41.8 | 55.2 | 2.012 | |
| Tl 77 | Raw | 57.1 | 18.3 | 24.6 | ND | ND | 39.9 | 76.8 | 2.001 |
| Rod | 56.2 | 18.3 | 25.5 | ND | ND | 37.3 | 46.8 | 2.301 | |
| rod-ARP | 56.2 | 18.3 | 25.5 | 133.6 | 3.4 | 37.4 | 43.9 | 1.928 | |
| Sample | Area (µm2) | Heterogeneity (%) | Circularity | Solidity | |
|---|---|---|---|---|---|
| Td,l 21 | rod | 35,394.7 | 12.9 | 0.043 | 0.83 |
| rod-ARP | 35,492.7 | 19.9 | 0.042 | 0.85 | |
| Tl 59 | rod | 35,871.9 | 16.5 | 0.039 | 0.81 |
| rod-ARP | 35,179.7 | 36.5 | 0.039 | 0.83 | |
| Tl 77 | rod | 35,611.1 | 15.5 | 0.039 | 0.85 |
| rod-ARP | 35,719.9 | 23.6 | 0.029 | 0.87 | |
| Sample | σy (MPa) | εy (%) | σm (MPa) | εm (%) | σb (MPa) | εb (%) | E (MPa) | |
|---|---|---|---|---|---|---|---|---|
| Td,l 21 | rod | 4.21 ± 1.14 | 20.3 ± 10.9 | 4.32 ± 1.10 | 108.0 ± 138.5 | 3.20 ± 0.78 | 488.8 ± 118.5 | 62.03 ± 28.33 |
| rod-ARP | 3.62 ± 0.58 | 28.0 ± 9.8 | 3.77 ± 0.50 | 101.0 ± 152.0 | 2.62 ± 0.29 | 402.0 ± 157.7 | 18.90 ± 7.96 | |
| Tl 59 | rod | 7.04 ± 1.39 | 28.3 ± 16.0 | 10.20 ± 1.26 | 533.2 ± 57.1 | 9.64 ± 1.36 | 539.7 ± 53.3 | 194.92 ± 100.21 |
| rod-ARP | 5.15 ± 1.01 | 23.0 ± 19.1 | 8.48 ± 0.58 | 460.3 ± 173.7 | 7.58 ± 0.74 | 488.5 ± 137.2 | 128.67 ± 33.52 | |
| Tl 77 | rod | 10.67 ± 1.71 | 28.4 ± 7.6 | 14.44 ± 1.07 | 436.4 ± 100.2 | 13.28 ± 1.36 | 456.4 ± 131.3 | 85.01 ± 22.09 |
| rod-ARP | 13.24 ± 1.61 | 18.7 ± 4.1 | 14.30 ± 0.97 | 350.3 ± 167.1 | 13.12 ± 1.02 | 507.0 ± 67.1 | 221.94 ± 88.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilińska, J.; Turek, A.; Rech, J.; Janeczek, H.; Pastusiak, M.; Kordyka, A.; Borecka, A.; Kobielarz, M.; Kasperczyk, J. Hot Melt Extrusion as a Formulation Method of Terpolymer Rods with Aripiprazole: A Preliminary Study. Appl. Sci. 2023, 13, 9521. https://doi.org/10.3390/app13179521
Wilińska J, Turek A, Rech J, Janeczek H, Pastusiak M, Kordyka A, Borecka A, Kobielarz M, Kasperczyk J. Hot Melt Extrusion as a Formulation Method of Terpolymer Rods with Aripiprazole: A Preliminary Study. Applied Sciences. 2023; 13(17):9521. https://doi.org/10.3390/app13179521
Chicago/Turabian StyleWilińska, Justyna, Artur Turek, Jakub Rech, Henryk Janeczek, Małgorzata Pastusiak, Aleksandra Kordyka, Aleksandra Borecka, Magdalena Kobielarz, and Janusz Kasperczyk. 2023. "Hot Melt Extrusion as a Formulation Method of Terpolymer Rods with Aripiprazole: A Preliminary Study" Applied Sciences 13, no. 17: 9521. https://doi.org/10.3390/app13179521
APA StyleWilińska, J., Turek, A., Rech, J., Janeczek, H., Pastusiak, M., Kordyka, A., Borecka, A., Kobielarz, M., & Kasperczyk, J. (2023). Hot Melt Extrusion as a Formulation Method of Terpolymer Rods with Aripiprazole: A Preliminary Study. Applied Sciences, 13(17), 9521. https://doi.org/10.3390/app13179521