Highlights
What are the main findings?
- Nano-manganese dioxide modified biochar was prepared.
- It is proved that nano-manganese dioxide modified biochar can effectively reduce the effective arsenic content in soil.
What is the implication of the main finding?
- When biochar, potassium permanganate and manganese sulfate monohydrate are mixed in a mass ratio of 1:0.18:0.29, manganese can be loaded onto the surface of biochar more efficiently.
- It can promote the prevention and control of high arsenic groundwater. Among them, the preparation of passivation materials and the experimental design of potting can also provide certain reference value for the passivation and restoration of arsenic-contaminated soil.
Abstract
[Objective] The irrigation area in northern Henan is an important grain producing area in China. Native high arsenic groundwater exists in the area and has long been used for agricultural irrigation. Increased soil arsenic (As) content under long-term irrigation threatens the quality and safety of crop products. Soil passivation is the use of adding passivators to the soil to fix pollutants to achieve the purpose of limiting their migration. Therefore, the preparation of an efficient and clean passivator and its arsenic fixation effect in soil are important research areas to reduce the risk of high arsenic groundwater. [Method] Firstly, nano-manganese dioxide (MnO2)-modified biochar was prepared via the pyrolysis of sawdust biochar, potassium permanganate and manganese sulfate monohydrate at a mass ratio of 1:0.18:0.29. Secondly, the adsorption characteristics were explored using adsorption kinetics and adsorption isothermal experiments. Scanning electron microscopy (SEM), X-ray powder diffraction (XRD) feature mapping and other characterization methods were used to study its physical properties and adsorption mechanism. Finally, a potting experiment was designed to explore the changes in arsenic content in soil when the passivator dosages were 0%, 1% and 5%. [Results] (1) The nano-MnO2 modified biochar could reach the adsorption dynamic equilibrium after 180 min, and its maximum adsorption capacity was 58.12 μg/g. (2) When the dosing ratio was 1%, the fixed efficiency of soil effective As content in potted crops of unplanted crops and planted crops was 4.18–5.51% and 1.99–3.83%. When the dosing ratio was 5%, it was 7.48–8.75% and 5.58–9.58%. [Conclusions] The results show that when the addition ratio is 0–5%, the passivation effect of soil effective As is positively correlated with the passivator dosage.
1. Introduction
Arsenic is a harmful metalloid, and long-term exposure to high arsenic can easily cause chronic poisoning or skin cancer [1]. In arid and semi-arid areas such as Inner Mongolia, Shanxi and Henan, there native high arsenic groundwater and soil arsenic pollution [2]. According to the 2014 National Soil Pollution Survey Communiqué, arsenic is one of the main soil pollutants, with an excess rate of 2.7% [3]. Arsenic mostly exists in soil in the form of oxygen-containing anions such as arsenate (AsO43−) and arsenite (AsO33−), showing different environmental behaviors. Arsenic generally remains and accumulates in soil in the form of arsenides such as iron (Fe-As), aluminum (Al-As), calcium (Ca-As), water-soluble (AsO43− and AsO33−), exchangeable and residue (O-As) [4]. Among them, water-soluble arsenide and exchangeable arsenide can be absorbed by crops, so they are generally regarded as soil effective arsenic indicators. While residue As is the most stable in soil and is not easily migrated and transformed to be absorbed by crops, it is generally considered to convert the effective arsenic in the soil into residual arsenic when remediating soil arsenic pollution, so as to reduce the risk of arsenic exposure in crops [5]. Therefore, it is of great significance to explore a passivation material that can greatly reduce the effective arsenic content in soil for soil arsenic pollution remediation.
Compared with electric remediation, heat treatment and chemical leaching technology, soil passivation remediation in solidification/stabilization technology has the advantages of low economic cost, simple operation, significant passivation effect, easy recognition by farmers and suitability for large-scale promotion, so it is the most widely used [6]. The principle of soil passivation remediation is to reduce the effective arsenic content in the soil by adding a passivator to the soil, which is manifested by the water-soluble and exchangeable arsenic in the soil being fixed by the passivator into a residual state. There are many types of passivators, which can be divided into organic materials such as biochar, livestock and poultry manure, and inorganic materials such as clay minerals and iron–manganese compounds [7,8,9]. Amin [10] used rice husks and sawdust agricultural wastes as raw materials to prepare biochars, and further modified with KMnO4 solution and applied them to arsenic-contaminated soil. Through column leaching experiments, they found that this type of modified biochar can reduce the mobility of As in soil, and also observed that the passivation effect was positively correlated with the addition ratio. Chen [11] used two soil amendments, oyster shell waste and biochar, to carry out passivation tests in highly arsenic-contaminated soil, and both oyster shell waste and biochar effectively reduced the leaching capacity of arsenic in acidic soil, and reduced the exchangeable arsenic content from 105.8 mg/kg to 54.0 mg/kg. Qin Songyan [12] applied ordinary peat charcoal and iron-modified carbon with a soil mass fraction of 20% to potted plants, and found that the content of water-soluble arsenic in soil decreased by 2.6–19.6% and 3.9–19.0%, respectively, indicating that iron modification had little effect on the passivation ability of biochar. MnO2 has strong oxidation and affinity for As, and it is easy to oxidize As(III) to As(V) with lower toxicity, and biochar then adsorbs As(V) [13]. Yu [14] used permanganate and biochar as raw materials to prepare manganese-oxide-modified biochar composites via pyrolysis and studied their adsorption characteristics in red soil, and found that manganese oxides improved the hydrogen/carbon ratio (H/C), oxygen/carbon ratio (O/C), surface hydrophilicity and surface adsorption capacity of biochar, indicating that manganese oxides play a vital role in arsenic removal. At present, the preparation and modification technology of biochar is relatively mature, but due to the influence of the modification method, the arsenic fixing effect of metal on the soil after biochar modification needs to be further explored.
In view of this, this paper uses aspen sawdust as a raw material to prepare biochar, and uses metal manganese for modification to explore the arsenic fixing effect of manganese-modified biochar in soil, and the experimental results provide a reference for the selection of passivation agents in the process of arsenic-contaminated soil passivation and remediation.
2. Materials and Methods
2.1. Test Material
2.1.1. Preparation Process of Nano-MnO2-Modified Biochar Passivator
First, the aspen sawdust was screened with a 60–80 mesh standard sieve, washed with water and dried in an environment of 80 °C for 24 h. A certain quantity of sawdust was weighed into the crucible, the crucible was placed in a muffle furnace with a heating temperature of 500 °C, and sawdust biochar was obtained after slow pyrolysis for 1 h in an oxygen-free environment. Then, sawdust biochar, potassium permanganate and manganese sulfate monohydrate were added to the beaker at a mass ratio of 1:0.18:0.29 to form a suspension. Under the condition of a water bath temperature of 80 °C, a magnetic stirrer with a speed of 150 rpm was used to stir the suspension liquid for 2 h to fully mix, and it was stirred evenly, cooled to room temperature, and then filtered to obtain the biochar matrix. Finally, the biochar matrix was put into a blast drying oven with a temperature of 80 °C and dried for 12–18 h, and the required passivator could be obtained after drying.
2.1.2. Test Soil and Crops
The test soil was collected from the surface layer of agricultural planting land in Xinxiang City, Henan Province, and the sampling depth was about 5–20 cm. The collected soil samples were brought back to the laboratory to initially remove large particles of stones and crop residues in the soil samples, placed in a ventilated place to dry naturally, mashed and ground, and finally passed through a 20-mesh sieve and mixed well for later use. The soil tested in this experiment was a weakly alkaline semi-leaching soil (cinnamon soil), with a pH of 7.69, cation exchange capacity (CEC) of 15.01 cmol·kg−1, conductivity (EC) and organic matter content (OM) of 725.33 μS·cm−1 and 2.28 g·kg−1 and total nitrogen (TN) and total phosphorus (TP) of 0.85 mg·kg−1 and 0.80 mg·kg−1, respectively [15]. Referring to the determination of total mercury, total arsenic and total lead in soil quality, the atomic fluorescence method (GB/T22105.2-2008), part 2: determination method of total arsenic in soil [16], could find that the arsenic content of the test soil was 16.4 mg·kg−1 (Table 1).

Table 1.
Physical and chemical properties of the experimental soil.
The test crops were Brassica rapa var. parachinensis cv. Shanghaiqing and Sijiucaixin. Shanghaiqing Brassica rapa was purchased from Xinxiang Jinwangjie Seed Industry Co., Ltd. (Xinxiang, China), Sijiucaixin Brassica rapa was purchased from Shenyang Boster Agricultural Technology Co., Ltd. (Shenyang, China), and both crops were seasonal varieties and could grow normally in the test soil.
2.1.3. Test Water
According to the spatial characteristics of arsenic distribution in groundwater in the study area in 2020, high arsenic groundwater was mainly distributed in Yanjin County and Fengqiu County of Xinxiang City. The water used for this test was taken from a well in Dongxinzhuang Village, Zhaocheng Town, Yanjin County, Xinxiang City, and the arsenic content of the well reached 160 μg/L (Table 2). This far exceeds China’s agricultural irrigation water standard of 50 μg/L [17].

Table 2.
Analysis of various components of high arsenic groundwater.
2.2. Experimental Design
Clean pots of the same specification were first selected (d × h = 30 cm × 25 cm), and equal quantities of experimental sample soil were added to each pot (5 kg), and then the blank control group CK and the experimental group SY series were set up (Table 3), two replicates were set up in each group and the sequential arrangement method was adopted. The dosage of the passivating agent was 0% (0 g), 1% (50 g) and 5% (250 g) of the soil quantity, respectively, and was fully mixed with the soil, placed in a dry and ventilated place, and the weighing method used to regularly replenish water during the period to maintain the soil moisture content at 50–70%. All pots were sealed with plastic wrap and had 3–5 evenly spaced small holes, so that the soil in the pot was not lost via evaporation under the premise of ensuring ventilation. After 3 days’ stabilization, crops were planted in pots, the seeds soaked in 1% hydrogen peroxide for 24 h, and 15 seeds per pot evenly spread after sterilization treatment in the above pots.

Table 3.
Design of potted experiments.
After the crop had been sown, we put the potted plant in the cold light source greenhouse, adjusted the temperature to 20–28 °C according to the crop growth conditions and maintained ventilation during the period. After the seedlings grew, we selected 10 seedlings with consistent growth in each pot to retain, and removed the excess seedlings. The soil moisture content was maintained at 60% until the plants’ growth was complete. Due to the small number of experimental crops planted in pots, and considering the impact of fertilizer addition on the passivation effect, no fertilizer was added.
2.3. Sampling and Sample Determination
After the crop had matured, each pot was sampled by triangulation at a depth of 5 cm. That is, the same amount of soil was taken at 3 positions in each pot and mixed into a soil sample, representing this sampling point. The soil sample was packed into a ziplock bag and sealed, with the corresponding sampling number and sampling date written, respectively. This time, 9 experimental types and corresponding parallel experiments were designed. Therefore, each pot had three sampling positions to test 1 sample after mixing, and 18 samples needed to be tested in 18 pots. The soil effective arsenic content was detected by referring to Part 2 of ‘Determination of Total Mercury, Total Arsenic and Total Lead in Soil Atomic Fluorescence Method’ (GB/T22105.2-2008): ‘Determination method of total arsenic in soil’ [16]. The testing unit was the Mineral Resources Supervision and Testing Center of the Ministry of Natural Resources, the instrument and equipment used was an AFS-8510 atomic fluorescence photometer and the soil detection limit was 0.01 mg/kg.
Specific test process for soil effective state As: First, 5.00 g of soil samples that had been sieved through 20 mesh screens were weighed into 150 mL plastic bottles. Then, we added a concentration of 0.1 mol/L HCl extract to the plastic bottle, mixed it with a ratio of soil to liquid volume of 1:10, and tightened the cap. The bottle was placed on a speed shaker with a speed of 250 r/min and shaken for 120 min, and after the end of the shaking, it was immediately filtered with a filter membrane with a pore size of 0.45 μm and the filtrate was taken on an atomic fluorescence photometer to determine the effective arsenic concentration in soil.
2.4. Adsorption Model
The results of adsorption kinetic experiments were simulated with pseudo-first-order and pseudo-second-order adsorption kinetic models [18]. The adsorption phenomenon that occurs on the solid surface under constant temperature conditions is often used by the Langmuir and Freundlich equations to characterize the relationship between the adsorption capacity on the surface and the equilibrium concentration of solute in the medium.
Langmuir equation: 1/q = 1/qm k1 c + 1/qm,
In the formula, c is the equilibrium mass concentration (mg/L); q is the equilibrium adsorption capacity of the adsorbent to As (mg/g); qm is the maximum adsorption capacity (mg/g); and k1 is the adsorption constant, which reflects the affinity of the adsorbent for As.
Freundlich equation: lgq = 1/n lgc + lgk,
In the formula, c is the equilibrium mass concentration (mg/L); q is the equilibrium adsorption capacity of the adsorbent to As (mg/g); k is Freudrich’s constant; n is constant, usually n > 1, with the increase in temperature; and the adsorption index 1/n tends to 1, and it is generally believed that 1/n is between 0.1 and 0.5, and it is easily adsorbed, and 1/n > 2 substances are difficult to adsorb.
2.5. Characterization Methods
Scanning electron microscopy (SEM, S4800 and SU8000, Hitachi High-Tech Corporation, Tokyo, Japan) was used to observe the surface structure morphology before and after biochar modification. The content and distribution of elements such as C, O and Mn on the surface of biochar were analyzed with an energy dispersive X-ray spectrometer (EDS, HORIBA EX-350, Kyoto, Japan); X-ray powder diffraction (XRD, Bruker D8advance, Karlsruhe, Germany) was used to characterize the structure of biochar crystals before and after modification.
2.6. Data Processing and Computing
The experimental data were collated using Excel 2019, plotted with Origin 2021 and CorelDraw 2019 software, and the Pearson correlation coefficient in SPSS 26 was analyzed for correlation, with ** indicating that the correlation was significant at the p < 0.01 level (double-tailed). The arsenic fixing efficiency of the passivator can be expressed as:
In the formula, X1 is the effective As content of soil remediated by adding passivator; X2 is the soil effective As content in the control group.
3. Results
3.1. Study on the Adsorption Characteristics of Biochar
3.1.1. Experimental Exploration of Adsorption Kinetics
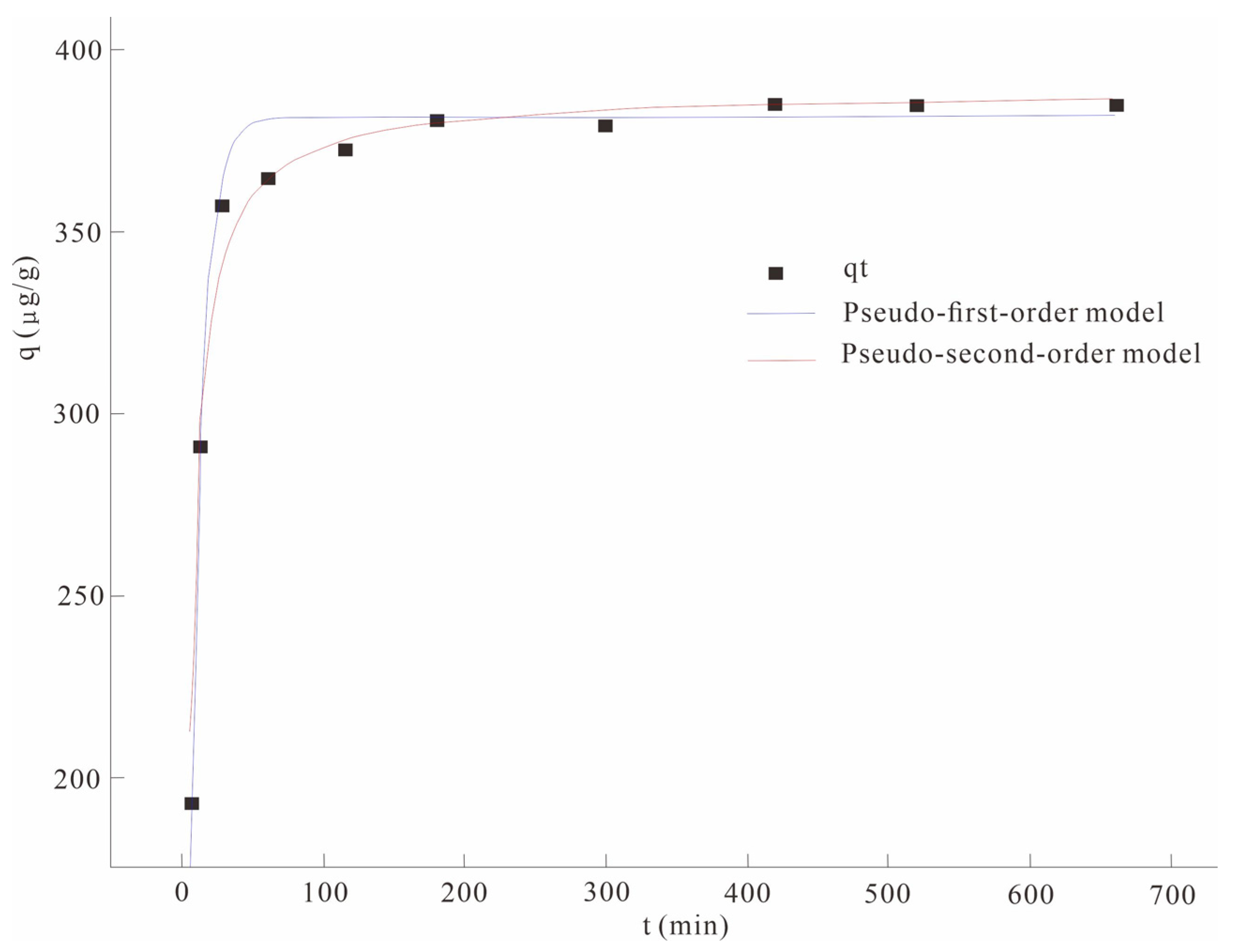
The solution with the initial concentration of As(V) was configured in the laboratory in advance, and the nano-MnO2-modified biochar was added to the solution at a solid/liquid ratio of 3 g/L for adsorption kinetic evaluation, and the adsorption curve of the adsorption material to As(V) in solution was obtained by fitting the pseudo-first-order model and pseudo-second-order kinetic model (Figure 1).
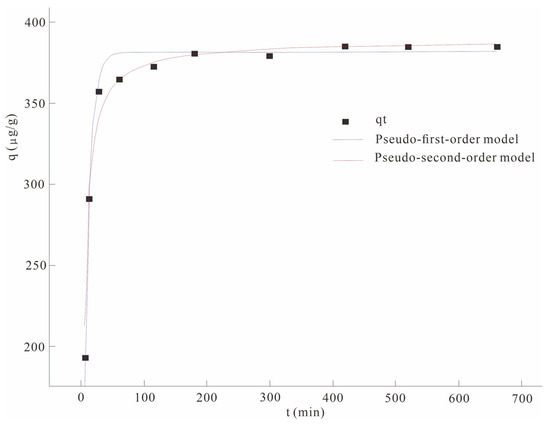
Figure 1.
Comparison of pseudo-first-order and pseudo-second-order adsorption kinetic models.
The adsorption capacity of Nano MnO2-modified biochar for As(V) increased rapidly 50 min before the adsorption started, and reached adsorption equilibrium after 180 min. The analysis found that in the initial stage of adsorption, there were a large number of active sites (hydroxyl bonds) on the surface of the nano-MnO2-modified biochar, which could react with As(V) to form a chelate with stability and affinity and be adsorbed on the surface of biochar. In addition, manganese oxide can combine with As to form ligands or co-precipitate, enhancing its fixation ability to As [19]. However, with the progress of the adsorption process, the active site of the biochar surface was gradually occupied, the adsorption rate was gradually reduced, and the adsorption process gradually tended to a dynamic equilibrium. Therefore, after 180 min of adsorption, the adsorption capacity of As(V) hardly changes; that is, the adsorption process reaches dynamic equilibrium.
The R2 values of the pseudo-first-order model and the pseudo-second-order model were 0.81 and 0.97, respectively (Table 4). The pseudo-second-order fitting correlation coefficient R2 was higher than that of the pseudo-first-order fitting coefficient, indicating that the pseudo-second-order kinetics better describe the adsorption behavior of As(V) on the material.

Table 4.
Fitting parameters for pseudo-first-order and pseudo-second-order models.
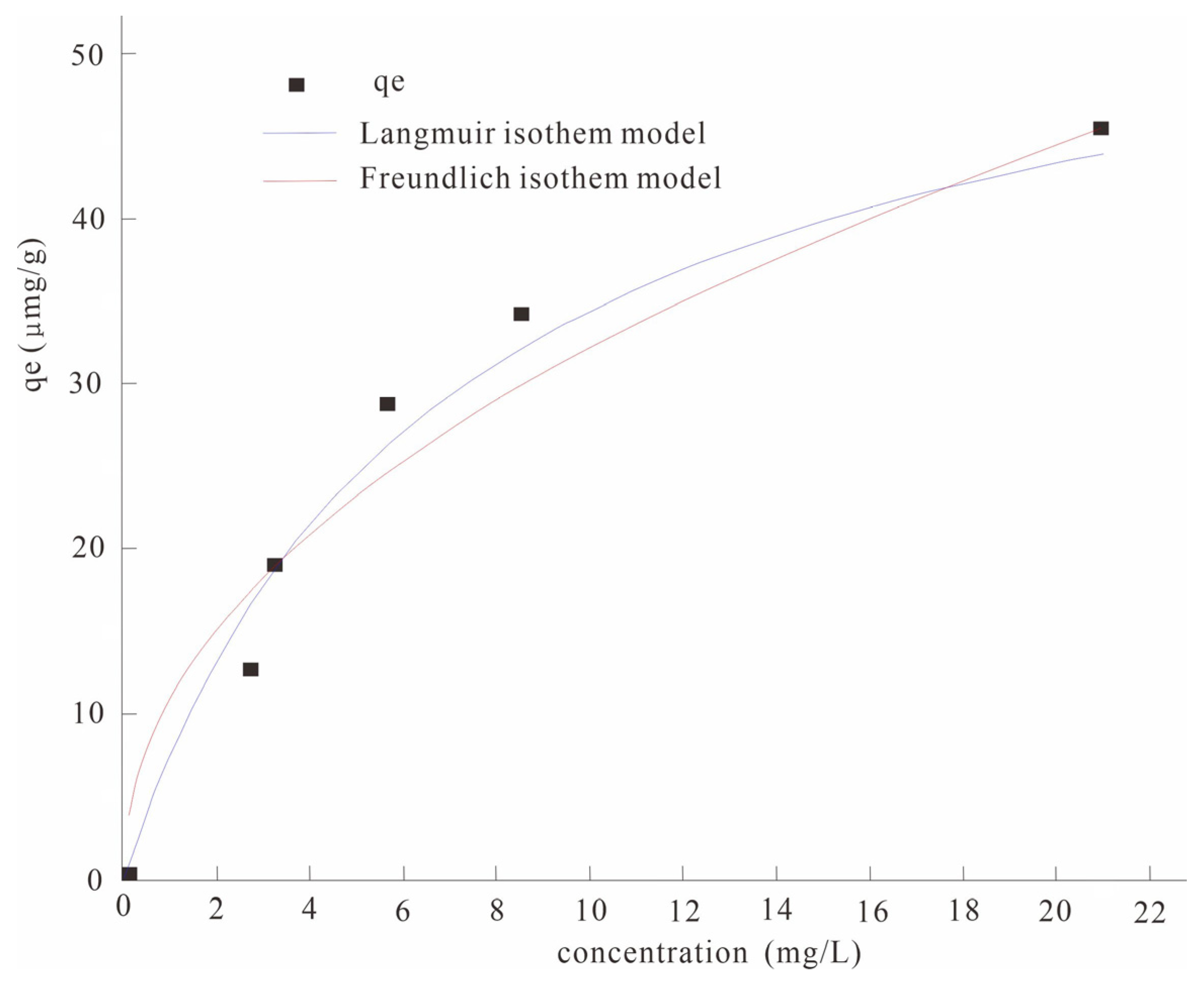
3.1.2. Experimental Exploration of Adsorption Isotherms
The adsorption isotherm experimental data were fitted with the Langmuir and Freundlich isothermal adsorption models, respectively (Figure 2). The results show that both the Langmuir equation and the Freundlich equation can describe the isothermal characteristics of arsenic adsorption by modified manganese dioxide modified biochar well, and their fitting correlation coefficient R2 values reached more than 0.90 (Table 5). It was indicated that the adsorption of As(V) in water by the nano-MnO2-modified biochar belonged to monolayer adsorption. The maximum adsorption capacity of the nano-MnO2-modified biochar was 58.12 μg/g through the Langmuir isothermal model.
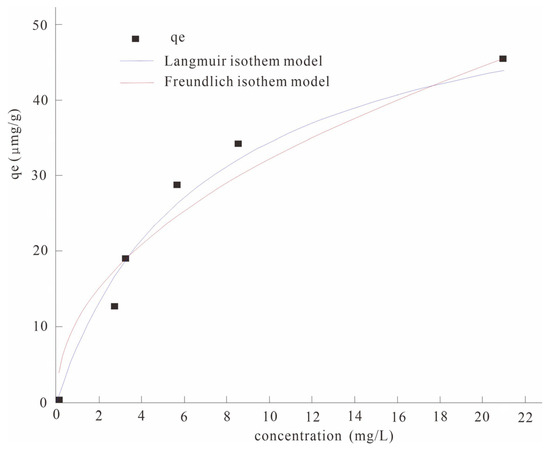
Figure 2.
Comparison of adsorption isotherms of the Langmuir and Freundlich models.

Table 5.
Correlation fitting parameters for Langmuir and Freundlich models.
3.2. Effect of Passivator on Soil Effective Arsenic Content under High Arsenic Water Irrigation
3.2.1. Analysis of Changes in Soil Effective Arsenic Content under Different Addition Ratios
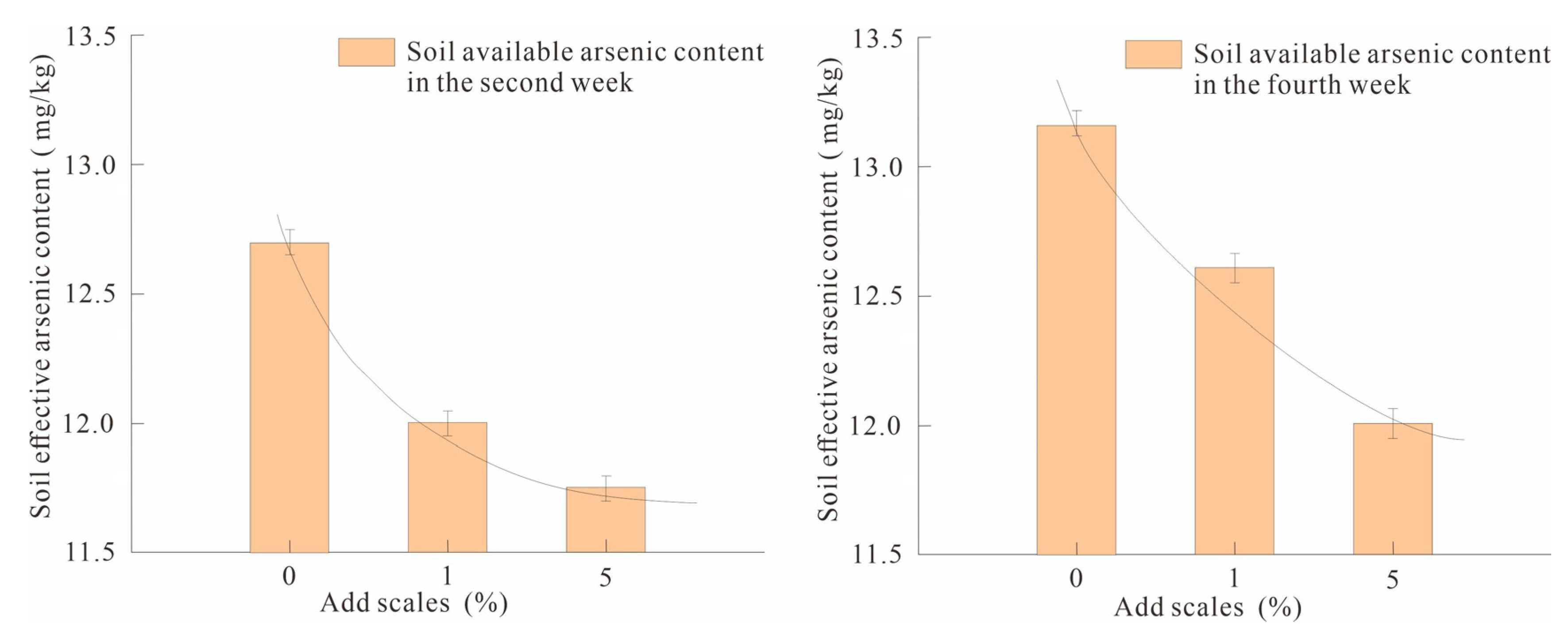
When the passivator was added at 0%, 1% and 5%, respectively, the soil was sampled and tested for effective arsenic content in the second and fourth weeks. The results showed that the soil effective arsenic contents in the second week and the fourth week of CK0-1 were 12.70 and 13.15 mg/kg, respectively. SY1-1 values were 12.00 and 12.60 mg/kg, respectively; SY5-1 was 11.75 and 12.00 mg/kg, respectively (Figure 3). According to the arsenic efficiency calculation formula (Equation (3)) of nano-MnO2-modified biochar, the fixed efficiency range when adding 1% was 4.18–5.51%; when adding 5%, it was 7.48–8.75%. This shows that in the range of 0–5%, the dosage is positively correlated with the fixed efficiency.
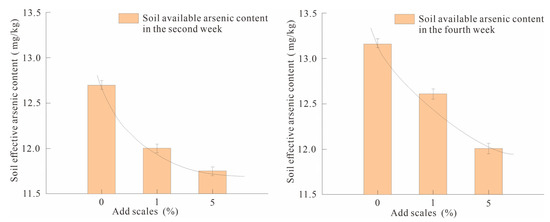
Figure 3.
Comparison of soil available arsenic content in the second week and the fourth week under different addition ratios.
The correlations between addition ratio, soil effective arsenic content and arsenic fixation efficiency in the second and fourth weeks of Pearson correlation analysis showed that the correlation was significant at p < 0.01, and the correlation coefficients were −0.83 and −0.95, respectively. When the addition ratio was 0–5%, the passivator dosing ratio was significantly negatively correlated with the effective arsenic content in the soil, indicating that the effective arsenic content in the soil decreased significantly with the increase in the addition ratio. In this experiment, when the passivator added 5%, the solid arsenic effect was better, up to 8.75%.
3.2.2. Study on the Arsenic Fixation Effect of the Passivator under Planting Crop Conditions
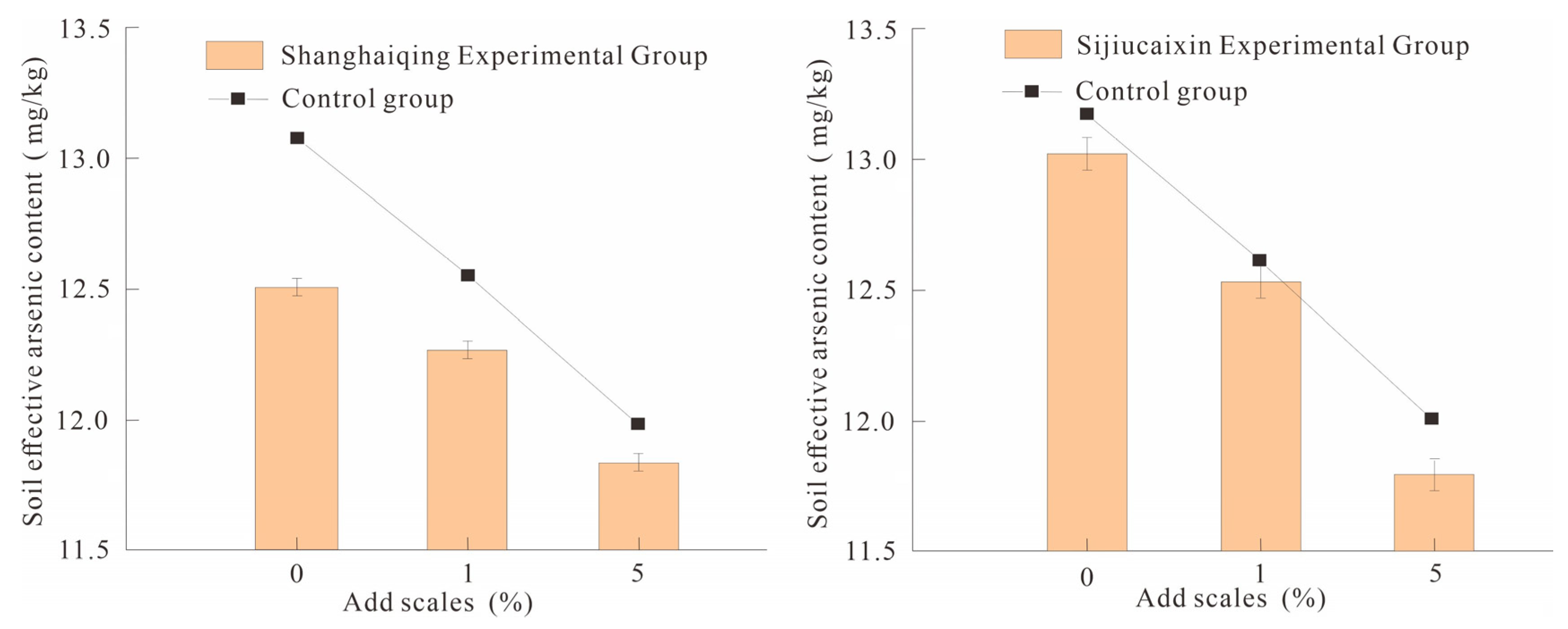
In the potting experiment, the original seeds of Shanghaiqing Brassica rapa and Sijiucaixin Brassica rapa were planted and the rhizosphere soil after crop maturity was taken after four weeks and the effective arsenic content was measured. The results showed that the soil available arsenic contents in CK0-2 and CK0-3 pots were 12.55 and 13.05 mg/kg, respectively. SY1-2 and SY1-3 had 12.30 and 12.55 mg/kg, respectively. SY5-2 and SY5-3 had 11.85 and 11.80 mg/kg, respectively (Figure 4).
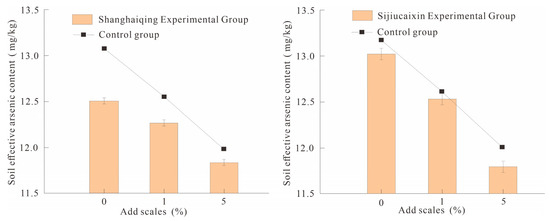
Figure 4.
Comparison of soil available arsenic contents under different planting crop conditions.
The fixed efficiency values of SY1-2 and SY5-2 can be calculated as 1.99% and 5.58%, respectively. The fixed efficiency values of SY1-3 and SY5-3 were 3.83% and 9.58%, respectively. From the perspective of fixed effect, when the addition ratio was 1%, the fixed efficiency only accounted for 1/2 of the unplanted crop, which was 1.99–3.83%; when the addition ratio was 5%, the Shanghaiqing experimental group decreased by 1.90% compared with the unplanted crop, and the Sijiucaixin experimental group increased slightly, by 0.83%. It can be seen that under the premise of planting crops, the passivation effect of the passivator in the soil will be reduced to a certain extent, indicating that the arsenic fixing ability of the passivator in the soil is inhibited.
4. Discussion
4.1. The Arsenic Fixation Mechanism of Modified Biochar
In this study, the modified biochar had a significant effect on soil As fixation, which was mainly attributed to the characteristics of nano-MnO2. MnO2 is a relatively common and highly adsorptive substance in soil [20]. Therefore, it is used to modify biochar and add it to the soil, and it is not easy to destroy the original properties. However, the physicochemical properties of biochar modified with MnO2 and the fixed adsorption mechanism of soil As need to be further explored.
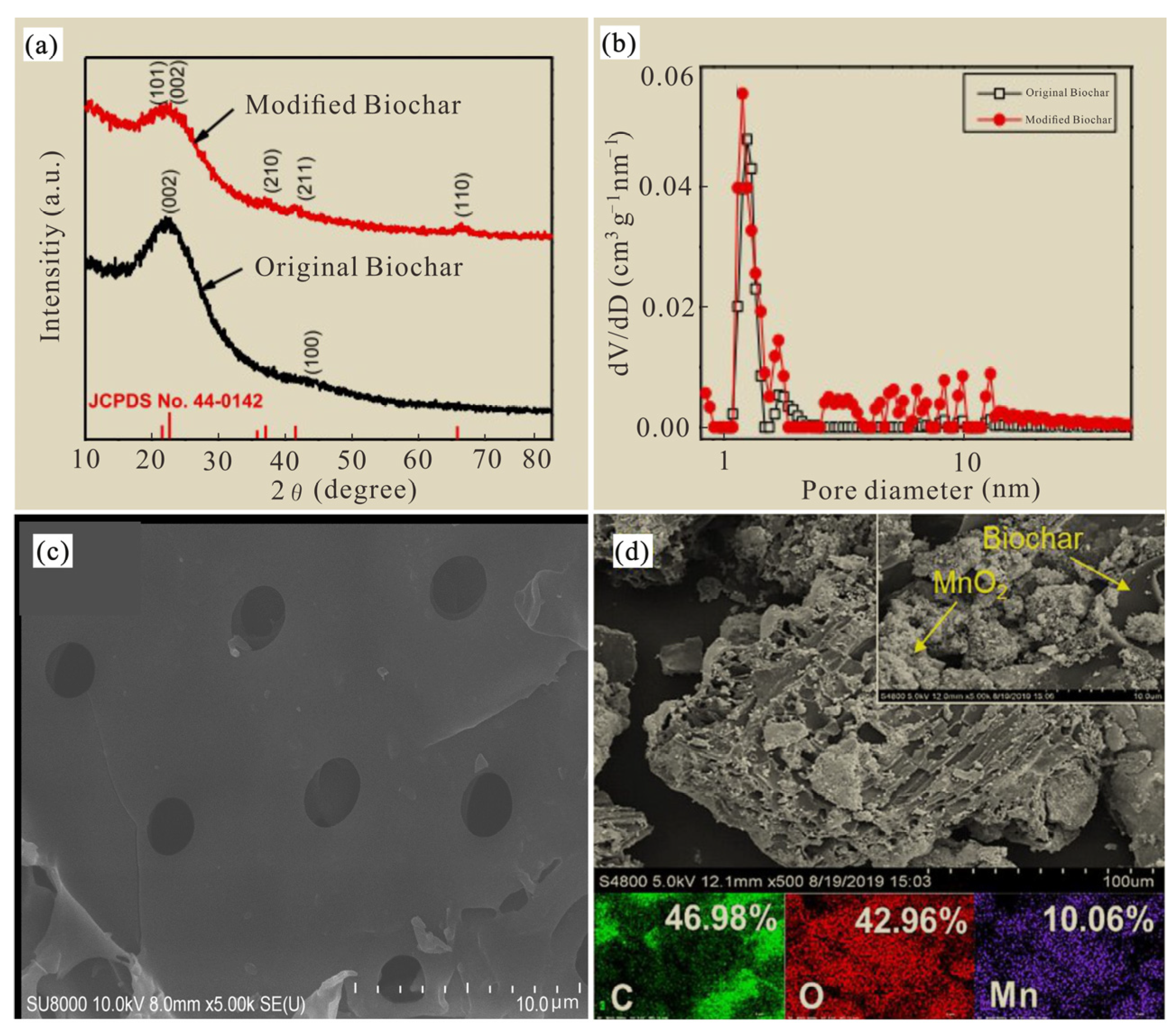
The XRD characteristic map (Figure 5a) shows that MnO2-modified biochar (MBC) had a strong diffraction peak of MnO2 characteristics compared to the original biochar, which is consistent with previous results [21,22]. It was shown that the metal manganese in potassium permanganate and manganese sulfate monohydrate were successfully loaded into the original biochar. The particle size of the laboratory-synthesized MnO2 modified biochar was mainly distributed in the 1–10 nm range (Figure 5b), which was easier to mix and make uniform and promote the fixation of soil arsenic.
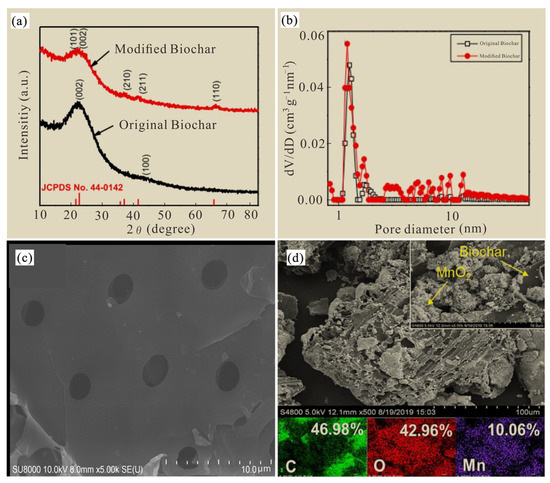
Figure 5.
XRD characteristic map images (a), particle size distribution images (b) and scanning-electron microscopy images (c,d) of biochar and MnO2 modified biochar.
The surface structure and morphology of unmodified and MnO2-modified biochar were observed with an SEM diagram, and the original biochar was irregular in shape, rough in surface and accompanied by a certain pore structure (Figure 5c). The surface of the modified biochar was composed of more small particles (Figure 5d), which indicates that the manganese element was successfully loaded onto the surface of the biochar, which increased the surface roughness of the modified biochar and thus provided a large number of adsorption sites, which was conducive to improving its adsorption capacity for As [23]. In this study, the C, O and Mn elements of MnO2-modified biochar accounted for 46.98%, 42.96% and 10.06%, respectively. The results show that after the aspen sawdust is carbonized by slow high-temperature pyrolysis, the biomass will release a higher heat to flush out the pores, the pore structure will increase significantly and the pore density will gradually increase with the increase in pyrolysis temperature [24]. The complex pore structure can improve the surface roughness and porosity of biochar, and then increase the specific surface area, while the number of surface oxygen-containing functional groups (Mn-O) can also be increased after manganese oxide loading to biochar [25,26], and Mn-O functional groups can produce tetranuclear ions rich in hydroxyl ligands [27], so the modification of biochar can usually promote its adsorption and fixation of arsenic. In addition, As can also co-precipitate with manganese oxide, or combine with manganese oxide by forming ligands, enhancing its fixation ability regarding soil As [28].
4.2. Influencing Factors of Effective Arsenic Content Change in Soil Systems
The variation in effective arsenic content in soil is related to many factors, and it is necessary to control not only the proportion of passivating agent added during the experiment, but also the effects of soil pH, dissolved organic matter (DOM) content and crop root exudates [29]. Studies have shown that the mobility and solubility of As in soil are closely related to pH value. When the soil pH value increases, it is more conducive to the release of As, which in turn increases the content of effective As in the system, which negatively affects the growth and development of crops [30]. Compared to the acidic soils of southern China, most farmland in northern China is alkaline. Therefore, it is recommended to use a pH-neutral passivator when improving the soil of alkaline farmland [7,8].
In soil, DOM easily forms complexes with As to change the morphology of As, improving its mobility and bioavailability in soil [31]. Ru Shuhua’s [32] research results show that the application of manganese-modified biochar to soil can significantly reduce the DOM content of soil [10], which is more conducive to the fixation of As and helps modified biochar to passivate the effective arsenic in soil. In this study, with the increase in the addition ratio of nano-MnO2-modified biochar, it is obvious that the passivation effect of SY5 series experiments is better than that of SY1 series experiments. It can be seen that the application of modified biochar may cause changes in soil pH and DOM, which directly affects the mobility of soil As and thus changes the effective arsenic content in the system.
In addition, the fixing effect of modified biochar on soil As is also related to the characteristics of the soil itself. If there are inorganic ions such as PO43− and SO42− or organic ions such as degradants derived from plant residues in the soil, they will compete with As for adsorption points to varying degrees, thereby inhibiting the fixed adsorption of arsenic by the passivator [33]. In this study, the passivation effect of SY1-2, SY1-3 and SY5-2 in the experimental group was reduced, which may be due to the presence of degradants of some plant residues in the relevant experimental pots, and the upper adsorption point on the surface of the biochar was reduced, which reduced the arsenic fixing effect of the nano-MnO2-modified biochar.
In this study, the successful loading of manganese oxide to the surface of biochar played a key role in the passivation restoration experiment, which effectively improved the fixation efficiency of nano-MnO2-modified biochar on soil effective state As, and provided useful information for the application of nano-MnO2-modified biochar in soil. However, it is necessary to further explore the micromolecular mechanism of the binding of soil effective As with modified biochar and study the growth and development of crops before and after biochar addition to evaluate their stability and practicality.
5. Conclusions
The addition of modified biochar to the soil can reduce the content of available arsenic and limit the migration of arsenic in the soil. Therefore, the preparation of an efficient and clean passivator was the main goal of this study.
Potassium permanganate and manganese sulfate monohydrate were used to modify sawdust biochar, and the characteristics of the passivator were characterized using SEM and XRD. The results clearly showed that after biochar loading MnO2, the surface roughness and pore structure increased, and the maximum adsorption capacity of arsenic from the original biochar was increased to 58.12 μg/g. At the same time, the particle size was in nanometers, which can effectively improve the degree of mixing between the passivator and the soil and play a role in promoting the fixation of the effective state As in the soil.
The application effect of this material was explored through pot experiments, and the results showed that the passivator could reduce the effective As content in soil after adding the passivator. When the addition ratio was 1%, the fixed efficiencies of soil effective state As content in unplanted crops and planted crops were 4.18–5.51% and 1.99–3.83%, respectively. When the addition ratio was 5%, the values were 7.48–8.75% and 5.58–9.58%, respectively. This shows that the passivation effect has a significant increase with the increase in the addition ratio.
Author Contributions
Conceptualization, Y.R. and Y.L.; methodology, Y.R., Y.L., S.X. and X.Z.; validation, Y.L. and S.X.; writing—original draft preparation, Y.L. and S.X.; writing—review and editing, Y.L., S.X. and J.Q.; visualization, S.X., X.Z. and J.Q.; supervision, Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by the National Key R&D Program of China (2022YFC3703701), the National Natural Science Foundation of China (No. 41972262) and Hebei Natural Science Foundation for Excellent Young Scholars (D2020504032).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We sincerely thank the Chinese Academy of Geological Sciences for the support of the hydrogeological environment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, G. Research progress on endemic arsenic poisoning in China. J. Environ. Health 2009, 26, 1035–1036. [Google Scholar]
- Guo, H.; Guo, Q.; Jia, Y.; Liu, Z.; Jiang, Y. Chemical characteristics and geochemical processes of high arsenic groundwater in different regions of China. J. Earth Sci. Environ. 2013, 35, 83–96. [Google Scholar]
- Report on the National General Survey of Soil Contamination; Ministry of Environmental Protection: Singapore, 2014. Available online: http://www.zhb.goVcn/gkml/hbb/qt/201404/t20140417_270670.htm (accessed on 20 May 2023).
- Liu, G.; Chen, M.; Li, W.; Gong, W. A critical review on the speciation and development of sequential extraction procedures for arsenic in soils. J. Agro-Environ. Sci. 2018, 37, 2629–2638. [Google Scholar]
- Hu, L. Study on Species Transformation and Phyto-Availability of Arsenic in Soil. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2008. [Google Scholar]
- Ren, Y.; Cao, W.; Xiao, S.; Li, X.; Pan, D.; Wang, S. Research progress on distribution, harm and control technology of heavy metals in soil. Geol. China 2023, 10, 231. [Google Scholar] [CrossRef]
- Li, Y.; Shang, J.; Huang, Y.; Wang, N. Research progress on passivation materials for cadmium-arsenic co-contamination in soil. Acta Pedol. Sin. 2021, 58, 837–850. [Google Scholar]
- Yang, J.; Bonheur, G.; Jiang, N.; Zu, Y. The immobilization remediation of cadmium and arsenic combined contaminated soils: A review. Environ. Poll. Control 2021, 43, 1189–1195+1200. [Google Scholar]
- Collivignarelli, M.; Sorlini, S.; Milanese, C.; Iiiankoon, W.; Caccamo, F.; Calatroni, S. Rice Industry By-Products as Adsorbent Materials for Removing Fluoride and Arsenic from Drinking Water-A Review. Appl. Sci. 2022, 12, 3166. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, M.; Farooqi, A.; Hussain, Q.; Ahmad, M.; AI-Wabel, M.; Saleem, H. Arsenic release in contaminated soil amended with unmodified and modified biochars derived from sawdust and rice husk. J. Soils Sediments 2020, 20, 3358–3367. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Lv, Z.; Xie, R.; Huang, L.; Jiang, J. Impacts of biochar and oyster shells waste on the immobilization of arsenic in highly contaminated soils. J. Environ. Manag. 2018, 217, 646–653. [Google Scholar] [CrossRef]
- Qin, S.; Xia, D.; Zhao, L. Stable remediafion of arsenic-contaminated soil by sludge-based biochar. J. Ecolo. Rural Environ. 2021, 37, 1481–1486. [Google Scholar]
- Oscarson, D.W.; Huang, P.M.; Liaw, W.K. The Oxidation of Arsenite by Aquatic Sediments. J. Environ. Qual. 1980, 9, 700–703. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, L.; Huang, Y.; Song, Z.; Qiu, W. Effects of a manganese oxide-modified biochar composite on adsorption of arsenic in red soil. J. Environ. Manag. 2015, 163, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Gao, F.; Wang, Y.; Ma, H.; Zhang, Q.; Hu, C.; Cui, B.; Liu, C. Changes in soil physicochemical properties and urease activity as affected by reclaimed water irrigation and nitrogen fertilization. J. Irrig. Drain 2022, 4l, 95–100. [Google Scholar]
- GB/T 22105.2-2008; Soil Quality-Analysis of Total Mercury, Arsenic and Lead Contents in Soils-Atomic Fluorescence Spectrometry-Part 2: Analysis of Total Arsenic Contents in Soils. Standards Press of China: Beijing, China, 2008.
- GB/T 14848-2017; Standard for Groundwater Quality. Standards Press of China: Beijing, China, 2017.
- Sun, R.; Wang, J.; Peng, Y.; Wang, H.; Chen, Q. Mitigation of arsenic accumulation in arugula (Eruca sativa Mill.) using Fe/Al/Zn impregnated biochar composites. Environ. Sci. Pollut. R. 2021, 28, 4136–4146. [Google Scholar] [CrossRef]
- Liu, M.; Sun, F.; Lv, Y.; Xu, Y.; Li, M.; Wang, Y.; Yin, X.; Jiang, H. Remediation of arsenic-contaminated soil by nano-zirconia modified biochar. Environ. Sci. Pollut. R. 2021, 28, 68792–68803. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Bian, Z.; Tian, X.; Zhang, J. Study on absorption of arsenic by immobilized nano-manganese oxide. Technol. Water Treat. 2013, 1, 60–64. [Google Scholar]
- Liang, X.; Gao, Z.; Fu, Q.; Liu, Y.; Zhu, J.; Hu, H. Passivation of arsenic-cadmium combined pollution in alkaline soil by manganese-modified rice straw biochar. J. Nat. Sci. Hunan Normal Univ. 2022, 45, 93–100. [Google Scholar]
- Yu, Z.; Huang, Y.; Lian, F.; Xie, L.; Liu, S.; Song, Z. Adsorption of arsenic(Ⅲ) on biochar-manganese oxide composites. J. Agro-Environ. Sci. 2015, 34, 155–161. [Google Scholar]
- Gao, X.; Peng, Y.; Guo, L.; Wang, Q.; Guan, C.Y.; Yang, F.; Chen, Q. Arsenic adsorption on layered double hydroxides biochars and their amended red and calcareous soils. J. Environ. Manag. 2020, 271, 111045. [Google Scholar] [CrossRef]
- Lu, X.; Wu, J.; Guo, Y. Advances in remediation of heavy metal contaminated soil by biochar. Appl. Chem. Ind. 2019, 48, 1172–1177. [Google Scholar]
- Wu, P.; Cui, P.; Zhang, Y.; Alves, M.E.; Liu, C.; Zhou, D.; Wang, Y. Unraveling the molecular mechanisms of Cd sorption onto MnOx—Loaded biochar produced from the Mn-hyperaccumulator Phytolacca americana. J. Hazard. Mater. 2022, 423, 127157. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, G.; Wu, Q.; Wang, D. Facile fabrication of nanostructured cerium-manganese binary oxide for enhanced arsenite removal from water. Chem. Eng. J. 2018, 334, 1518–1526. [Google Scholar] [CrossRef]
- Liang, T.; Li, L.; Zhu, C.; Ye, J. Cerium-manganese modified biochar immobilizes arsenic in farmland soils. Environ. Sci. 2019, 40, 5114–5123. [Google Scholar]
- Ouvrard, S.; Donato, P.; Simonnot, M.O.; Begin, S.; Ghanbaja, J.; Alnot, M.; Duval, Y.B.; Lhote, F.; Barres, O.; Sardin, M. Natural manganese oxide: Combined analytical approach for solid characterization and arsenic retention. Geochim. Cosmochim. Acta 2005, 69, 2715–2724. [Google Scholar] [CrossRef]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J.C. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.; Lim, J.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.; Ok, Y. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.; Tang, J.; Cotner, J.; Xu, Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ru, S.; Zhao, O.; Hou, L.; Xiao, G.; Wang, C.; Sun, S.; Zhang, G.; Wang, L.; Liu, L. Effects of eight kinds of passivators on properties and cadmium availability in different cadmium-contaminated soil. Ecol. Environ. Sci. 2021, 30, 2085–2092. [Google Scholar]
- Chen, X. The Research on Mixture Leaching Remediation Technology of Arsenic Contaminated Soil. Master’s Thesis, Hunan University, Changsha, China, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).