Electroencephalographic Characterization by Covariance Analysis in Men with Parkinson’s Disease Reveals Sex- and Age-Related Differences
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electroencephalographic Records
2.2. Volunteers Overview
2.3. Covariance Wavelet Analysis and Wavelet Transform
2.4. Data Preprocessing
2.5. Continuous Wavelet Transform and Cross-Wavelet Transform
2.6. Global Wavelet Profiles
2.7. Statistical Analysis
3. Results
3.1. Global Wavelet Profiles of Control and Parkinson’s Disease Men under 60 Years Old (<60)
3.2. Global Wavelet Profiles of Control and Parkinson’s Disease Men over 60 Years Old (>60)
3.3. Comparison of the Global Wavelet Profiles from Control and Parkinson’s Disease Men under 60 Years Old (<60) and over 60 Years Old (>60)
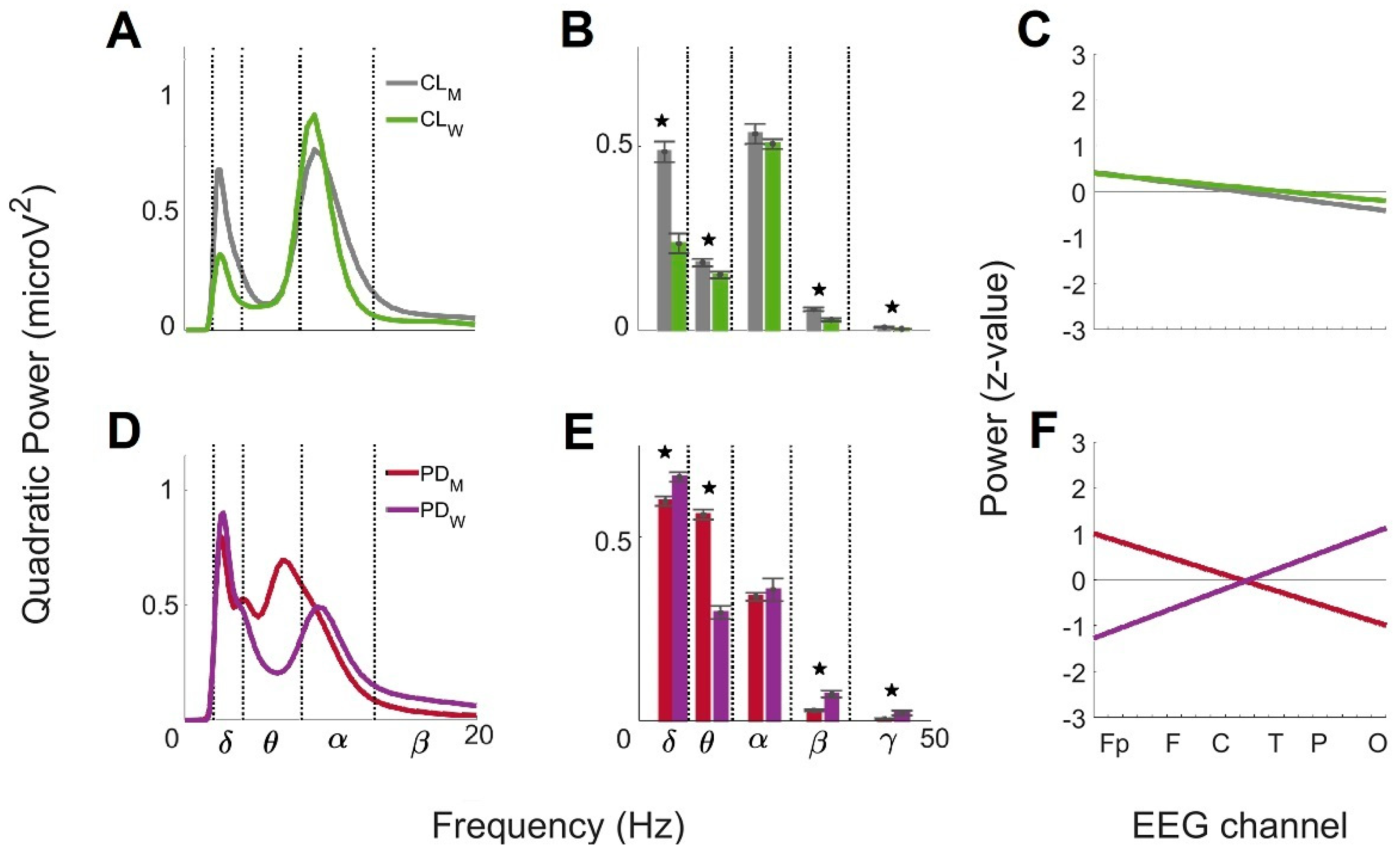
3.4. Sex Differences of the Global Wavelet Profiles from Control and with Parkinson’s Disease under 60 Years Old (<60)
3.5. Sex Differences of the Global Wavelet Profiles from Control and Parkinson’s Disease Group over 60 Years Old (>60)
4. Discussion
4.1. Differences between Men with PD and CL Groups at Age < 60
4.2. Differences between Men with PD and CL Groups at Age > 60
4.3. Sex-Related Differences between Control and PD Groups < 60
4.4. Sex-Related Differences between Controls and PD Groups > 60
4.5. Comparison of Different Intelligent Algorithms in EEG Analysis
4.5.1. Comparison between ICA and the Inverse WT in EEG Analysis
4.5.2. Comparison between ML/DL and Wavelet Algorithms in EEG Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| PD | Parkinson’s Disease |
| EEG | Electroencephalographic records |
| Fp | Frontopolar |
| F | Frontal |
| T | Temporal |
| C | Central |
| P | Parietal |
| O | Occipital |
| CWT | Continuous Wavelet Transform |
| XWT | Cross Wavelet Transform |
| PWS | Power Wavelet Spectrum |
| GWS | Global Wavelet Spectrum |
Appendix A. Covariance Wavelet Analysis
References
- Maserejian, N.; Vinikoor-Imler, L.; Dilley, A. Estimation of the 2020 Global Population of Parkinson’s Disease (PD). In Proceedings of the MDS Virtual Congress 2020, Virtual, 12–16 September 2020; Volume 35. Available online: https://www.mdsabstracts.org/abstract/estimation-of-the-2020-global-population-of-parkinsons-disease-pd/ (accessed on 14 March 2023).
- Phillipson, O.T. Management of the aging risk factor for Parkinson’s disease. Neurobiol. Aging 2014, 35, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ageing and Health. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. (accessed on 23 January 2023).
- Orozco, J.L.; Valderrama-Chaparro, J.A.; Pinilla-Monsalve, G.D.; Molina-Echeverry, M.I.; Pérez Castaño, A.M.; Ariza-Araújo, Y.; Prada, S.I.; Takeuchi, Y. Parkinson’s disease prevalence, age distribution and staging in Colombia. Neurol. Int. 2020, 12, 8401. [Google Scholar] [CrossRef]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the difference? J. Park. Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.; Okun, M.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Reekes, T.H.; Higginson, C.I.; Ledbetter, C.R.; Sathivadivel, N.; Zweig, R.M.; Disbrow, E.A. Sex specific cognitive differences in Parkinson disease. NPJ Park. Dis. 2020, 6, 7. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Sastre, M.; Del Tredici, K. Development of α- Synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007, 114, 231–241. [Google Scholar] [CrossRef]
- Askanas, V.; Engel, W.K.; Alvarez, R.B.; McFerrin, J.; Broccolini, A. Novel immunolocalization of α- Synuclein in human muscle of inclusion-body myositis, regenerating and necrotic muscle fibers, and at neuromuscular junctions. J. Neuropathol. Exp. Neurol. 2000, 59, 592–598. [Google Scholar] [CrossRef]
- Ramirez-Soto, R.; Ortega-Aguilar, A. Skeletal Muscle is a Source of α-Synuclein with a Sarcolemmal Non-Lipid Raft Distribution. Cell Physiol. Biochem. 2022, 56, 382–400. [Google Scholar]
- Tian, C.; Liu, G.; Gao, L.; Soltys, D.; Pan, C.; Stewart, T.; Shi, M.; Xie, Z.; Liu, N.; Feng, T.; et al. Erythrocytic α- Synuclein as a potential biomarker for Parkinson’s disease. Transl. Neurodegener. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Peelaerts, W.; Bousset, L.; Van der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van den Haute, C.; Melki, R.; Baekelandt, V. Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Teravskis, P.J.; Covelo, A.; Miller, E.C.; Singh, B.; Martell-Martínez, H.A.; Benneyworth, M.A.; Gallardo, C.; Oxnard, B.R.; Araque, A.; Lee, M.K.; et al. A53T mutant alpha-synuclein induces Tau-dependent postsynaptic impairment independently of neurodegenerative changes. J. Neurosci. 2018, 38, 9754–9767. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.A.; Lees, A.J.; Schrag, A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov. Disord. 2010, 25, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.J.; Pavese, N. Imaging biomarkers in Parkinson’s disease. Prog. Neurobiol. 2011, 95, 614–628. [Google Scholar] [CrossRef] [PubMed]
- NICE. National Institute for Health and Care Excellence Guideline. In Parkinson’s Disease in Adults; NICE: Manchester, UK, 2020. [Google Scholar]
- Secretaría de Salud. Diagnóstico y Tratamiento de la Enfermedad de Parkinson Inicial y Avanzada en el Tercer Nivel de Atención; Secretaría de Salud: Mexico City, Mexico, 2010. [Google Scholar]
- DeLong, M.R.; Wichmann, T. Circuits, and circuit disorders of the basal ganglia. Arch. Neurol. 2007, 64, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Cozac, V.V.; Gschwandtner, U.; Hatz, F.; Hardmeier, M.; Rüegg, S.; Fuhr, P. Quantitative EEG and cognitive decline in Parkinson’s disease. Park. Dis. 2016, 2016, 9060649. [Google Scholar] [CrossRef] [PubMed]
- Shirahige, L.; Berenguer-Rocha, M.; Mendonça, S.; Rocha, S.; Rodrigues, M.C.; Monte-Silva, K. Quantitative electroencephalography characteristics for Parkinson’s disease: A systematic review. J. Park. Dis. 2020, 10, 455–470. [Google Scholar] [CrossRef]
- González-González, G.; Velasco-Herrera, V.M.; Ortega-Aguilar, A. Use of Covariance Analysis in Electroencephalogram Reveals Abnormalities in Parkinson’s Disease. Appl. Sci. 2021, 11, 9633. [Google Scholar] [CrossRef]
- Iwaki, H.; Nishikawa, N.; Nagai, M.; Tsujii, T.; Yabe, H.; Kubo, M.; Ieiri, I.; Nomoto, M. Pharmacokinetics of levodopa/benserazide versus levodopa/carbidopa in healthy subjects and patients with Parkinson’s disease. Neurol. Clin. Neurosci. 2015, 3, 68–73. [Google Scholar] [CrossRef]
- Stathis, P.; Konitsiotis, S.; Antonini, A. Dopamine agonists early monotherapy for the delay of development of levodopa-induced dyskinesias. Expert. Rev. Neurother. 2015, 15, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Fitzer-Attas, C.J.; Hauser, R.; Jankovic, J.; Lang, A.; Langston, J.W.; Melamed, E.; Poewe, W.; Stocchi, F.; Tolosa, E.; et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease (the ADAGIO study): Prespecified and post-hoc analyses of the need for additional therapies, changes in UPDRS scores, and non-motor outcomes. Lancet Neurol. 2011, 10, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Torrence, C.; Compo, G.P. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Postnikov, E.B.; Lebedeva, E.A.; Lavrova, A.I. Computational implementation of the inverse continuous wavelet transform without a requirement of the admissibility condition. Appl. Math. Comput. 2016, 282, 128–136. [Google Scholar] [CrossRef]
- Velasco, V.M.; Soon, W.; Velasco, G.; Traversi, R.; Horiuchi, K. Generalization of the cross-wavelet function. New Astron. 2017, 56, 86–93. [Google Scholar] [CrossRef]
- Han, C.X.; Wang, J.; Yi, G.S.; Che, Y.Q. Investigation of EEG abnormalities in the early stage of Parkinson’s disease. Cogn. Neurodyn. 2013, 7, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Zanto, T.P.; Gazzaley, A. Aging of the frontal lobe. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 163, pp. 369–389. [Google Scholar]
- Rotte, M. Age-related differences in the areas of Broca and Wernicke using functional magnetic resonance imaging. Age Ageing 2005, 34, 609–613. [Google Scholar] [CrossRef]
- Lopes da Silva, F.H. Neural mechanisms underlying brain waves: From neural mechanisms to networks. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 81–93. [Google Scholar] [CrossRef]
- Bollimunta, A.; Chen, Y.; Schroeder, C.; Ding, M. Mechanisms of cortical alpha oscillations in awake-behaving macaques. J. Neurosci. 2008, 28, 9976–9988. [Google Scholar] [CrossRef]
- Buzsaki, G.; Bickford, R.; Ponomareff, G.; Thal, L.; Mandel, R.; Gage, F. Nucleus basalis and Thalamic Control of Neocortical Activity in the Freely Moving Rat. J. Neurosci. 1988, 8, 4007–4026. [Google Scholar] [CrossRef]
- Liu, A.K.L.; Chang, R.C.C.; Pearce, R.K.B.; Gentleman, S.M. Nucleus basalis of Meynert revisited: Anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015, 129, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, P.; Marciani, M.G.; Maschio, M.; Bassetti, M.A.; Spanedda, F.; Pierantozzi, M.; Semprini, R.; Bernardi, G. Quantitative EEG changes in non-demented Pakinson’s disease patients before and during L-dopa therapy. Eur. J. Neurol. 1996, 3, 354–362. [Google Scholar] [CrossRef]
- Weinberger, M.; Hutchison, W.D.; Lozano, A.M.; Hodaie, M.; Dostrovsky, J.O. Increased gamma oscillatory activity in the subthalamic nucleus during tremor in Parkinson’s disease patients. J. Neurophysiol. 2009, 101, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Alberico, S.L.; Cassell, M.D.; Narayanan, N.S. The Vulnerable Ventral Tegmental Area in Parkinson’s Disease. Basal Ganglia 2015, 5, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jacobs, J. Traveling Theta Waves in the Human Hippocampus. J. Neurosci. 2015, 35, 12477–12487. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, H.S.; Zare-Shahabadi, A.; Rahmani, F.; Rezaei, N. Neurotransmission systems in Parkinson’s disease. Rev. Neurosci. 2017, 28, 509–536. [Google Scholar] [CrossRef] [PubMed]
- Klass, D.W.; Brenner, R.P. Electroencephalography of the Elderly. J. Clin. Neurophysiol. 1995, 12, 116–131. [Google Scholar] [CrossRef]
- Scally, B.; Burke, M.R.; Bunce, D.; Delvenne, J.F. Resting state EEG power and connectivity are associated with alpha peak frequency slowing in healthy aging. Neurobiol. Aging. 2018, 71, 149–155. [Google Scholar] [CrossRef]
- Hughes, S.W.; Crunelli, V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist 2005, 11, 357–372. [Google Scholar] [CrossRef]
- Hughes, E.J.; Bond, J.; Svrckova, P.; Makropoulos, A.; Ball, G.; Sharp, D.J.; Edwards, A.D.; Hajnal, J.V.; Counsell, S.J. Regional changes in thalamic shape and Vol. with increasing age. NeuroImage 2012, 63, 1134–1142. [Google Scholar] [CrossRef]
- Melgari, J.M.; Curcio, G.; Mastrolilli, F.; Salomone, G.; Trotta, L.; Tombini, M.; di Biase, L.; Scrascia, F.; Fini, R.; Fabrizio, E.; et al. Alpha and beta EEG power reflects L-dopa acute administration in parkinsonian patients. Front. Aging Neurosci. 2014, 6, 302. [Google Scholar] [CrossRef] [PubMed]
- Kan, D.P.X.; Croarkin, P.E.; Phang, C.K.; Lee, P.F. EEG differences between eyes-closed and eyes-open conditions at the resting stage for euthymic participants. Neurophysiology 2017, 49, 432–440. [Google Scholar] [CrossRef]
- Ishii, R.; Canuet, L.; Aoki, Y.; Hata, M.; Iwase, M.; Ikeda, S.; Nishida, K.; Ikeda, M. Healthy and pathological brain aging: From the perspective of oscillations, functional connectivity, and signal complexity. Neuropsychobiology 2017, 75, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Murty, D.V.P.S.; Manikandan, K.; Kumar, W.S.; Ramesh, R.G.; Purokayastha, S.; Javali, M.; Rao, N.P. Gamma oscillations weaken with age in healthy elderly in human EEG. NeuroImage 2020, 215, 116826. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, S.; Nolfe, G. The EEG in the normal elderly: A contribution to the interpretation of aging and dementia. Electroencephalogr. Clin. Neurophysiol. 1986, 63, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Polunina, A.G.; Leftrova, N.P. Gender differences in resting state electroencephalography characteristics. Curr. Trends Neurol. 2012, 6, 51–60. [Google Scholar]
- Brenner, R.P.; Ulrich, R.F.; Reynolds, C.F. EEG spectral findings in healthy, elderly men and women- sex differences. Electroencephalogr. Clin. Neurophysiol. 1995, 94, 1–5. [Google Scholar] [CrossRef]
- Sang, F.; Chen, Y.; Chen, K.; Dang, M.; Gao, S.; Zhang, Z. Sex Differences in Cortical Morphometry and White Matter Microstructure During Brain Aging and Their Relationships to Cognition. Cereb. Cortex 2021, 31, 5253–5262. [Google Scholar] [CrossRef]
- Simmonite, M.; Carp, J.; Foerster, B.R.; Ossher, L.; Petrou, M.; Weissman, D.H.; Polk, T.A. Age-related declines in occipital GABA are associated with reduced fluid processing ability. Acad. Radiol. 2019, 26, 1053–1061. [Google Scholar] [CrossRef]
- Hyvarinen, A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 1999, 10, 626–634. [Google Scholar] [CrossRef]
- Morabito, F.C. Independent component analysis and feature extraction for NDT data. Mater. Eval. 2000, 58, 85–92. [Google Scholar]
- Islam, M.S.; Hussain, I.; Rahman, M.M.; Park, S.J.; Hossain, M.A. Explainable Artificial Intelligence Model for Stroke Prediction Using EEG Signal. Sensors 2022, 22, 9859. [Google Scholar] [CrossRef]
- Surya, S.; Yamini, B.; Rajendran, T.; Narayanan, K. A Comprehensive Method for Identification of Stroke using Deep Learning. Turk. J. Comput. Math. Educ. 2021, 12, 647–652. [Google Scholar]
- Miller, A.; Blott, B.; Hames, T. Review of neural network applications in medical imaging and signal processing. Med. Biol. Eng. Comput. 1992, 30, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Subasi, A. Automatic recognition of alertness level from EEG by using neural network and wavelet coefficients. Expert. Syst. Appl. 2005, 28, 701–711. [Google Scholar] [CrossRef]
- Djemili, R.; Bourouba, H.; Korba, M.A. Application of empirical mode decomposition and artificial neural network for the classification of normal and epileptic EEG signals. Biocybern. Biomed. Eng. 2016, 36, 285–291. [Google Scholar] [CrossRef]
- Subasi, A. Automatic detection of epileptic seizure using dynamic fuzzy neural networks. Expert. Syst. Appl. 2006, 31, 320–328. [Google Scholar] [CrossRef]
- Subasi, A. EEG signal classification using wavelet feature extraction and a mixture of expert model. Expert. Syst. Appl. 2007, 32, 1084–1093. [Google Scholar] [CrossRef]
- Adeli, H.; Zhou, Z.; Dadmehr, N. Analysis of EEG records in an epileptic patient using wavelet transform. J. Neurosci. Methods 2003, 123, 69–87. [Google Scholar] [CrossRef]
- Akay, M. Wavelet applications in medicine. IEEE Spectr. 1997, 34, 50–56. [Google Scholar] [CrossRef]
- Subasi, A. Epileptic seizure detection using dynamic wavelet network. Expert. Syst. Appl. 2005, 29, 343–355. [Google Scholar] [CrossRef]
- Alturki, F.A.; AlSharabi, K.; Abdurraqeeb, A.M.; Aljalal, M. EEG Signal Analysis for Diagnosing Neurological Disorders Using Discrete Wavelet Transform and Intelligent Techniques. Sensors 2020, 20, 2505. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Hurley, N.; Silvestre, G. Single-trial EEG classification for brain-computer interface using wavelet decomposition. In Proceedings of the European Signal Processing Conference, Antalya, Turkey, 4–8 September 2005; pp. 1–4. [Google Scholar]
- Khan, Y.U.; Rafiuddin, N.; Farooq, O. Automated seizure detection in scalp EEG using multiple wavelet scales. In Proceedings of the 2012 IEEE International Conference on Signal Processing, Computing and Control, Solan, India, 15–17 March 2012; pp. 1–5. [Google Scholar]
- Sheng, Y. Wavelet Transform. In The Transforms and Applications Handbook; Poularikas, A., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 747–828. [Google Scholar]
- Jevrejeva, S.; Moore, J.; Grinsted, A. Influence of the Arctic Oscillation and El Niño-Southern Oscillation (ENSO) on ice conditions in the Baltic Sea: The wavelet approach. J. Geophys. Res. 2003, 108, 4677. [Google Scholar] [CrossRef]
- Grinsted, A.; Moore, J.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Hramov, A.; Koronovskii, A.; Makarov, V.; Pavlov, A.; Sitnikova, E. Wavelets in Neuroscience; Springer-Verlag: Berlin, Heidelberg, 2015; p. 331. [Google Scholar]
- Velasco Herrera, V.M.; Perez-Peraza, J.; Soon, W.; Márquez-Adame, J.C. The quasi-biennial oscillation of 1.7 years in ground level enhancement events. New Astron. 2017, 60, 7–13. [Google Scholar] [CrossRef]
- Einstein, A. Method for the determination of the statistical values of observations concerning quantities subject to irregular fluctuatƒions. Arch. Des Sci. Nat. 1914, 37, 254–256. [Google Scholar]

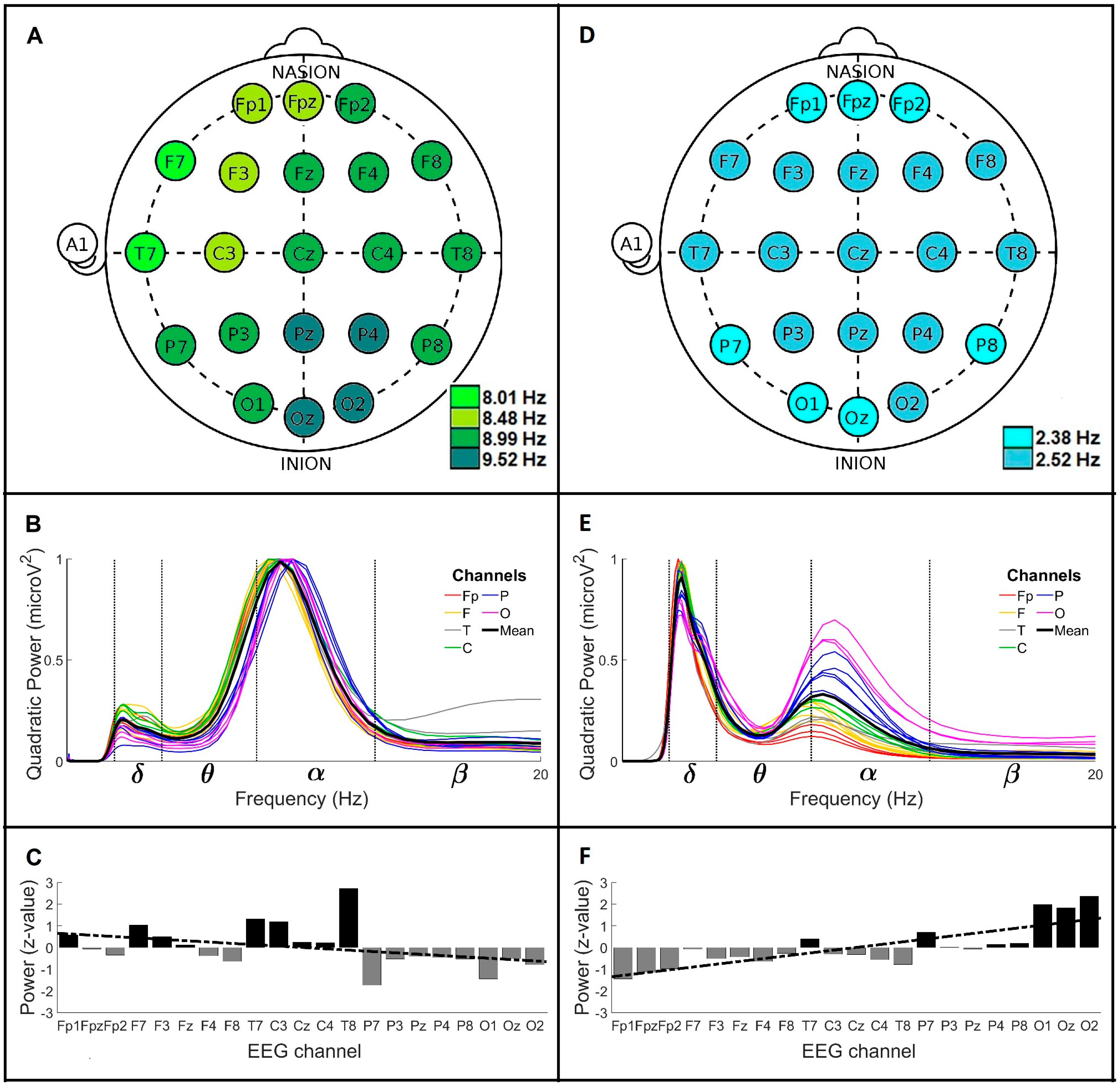
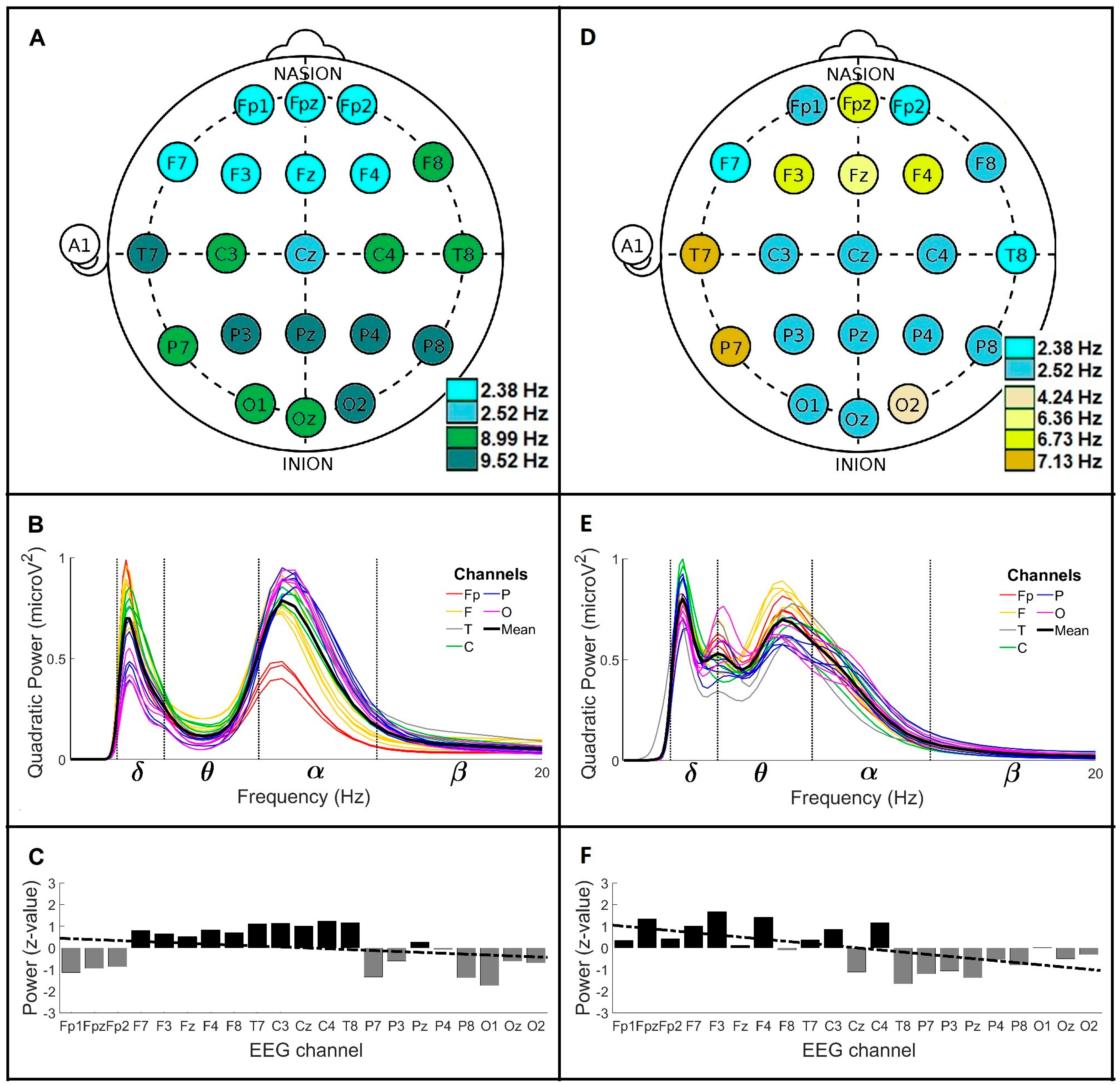
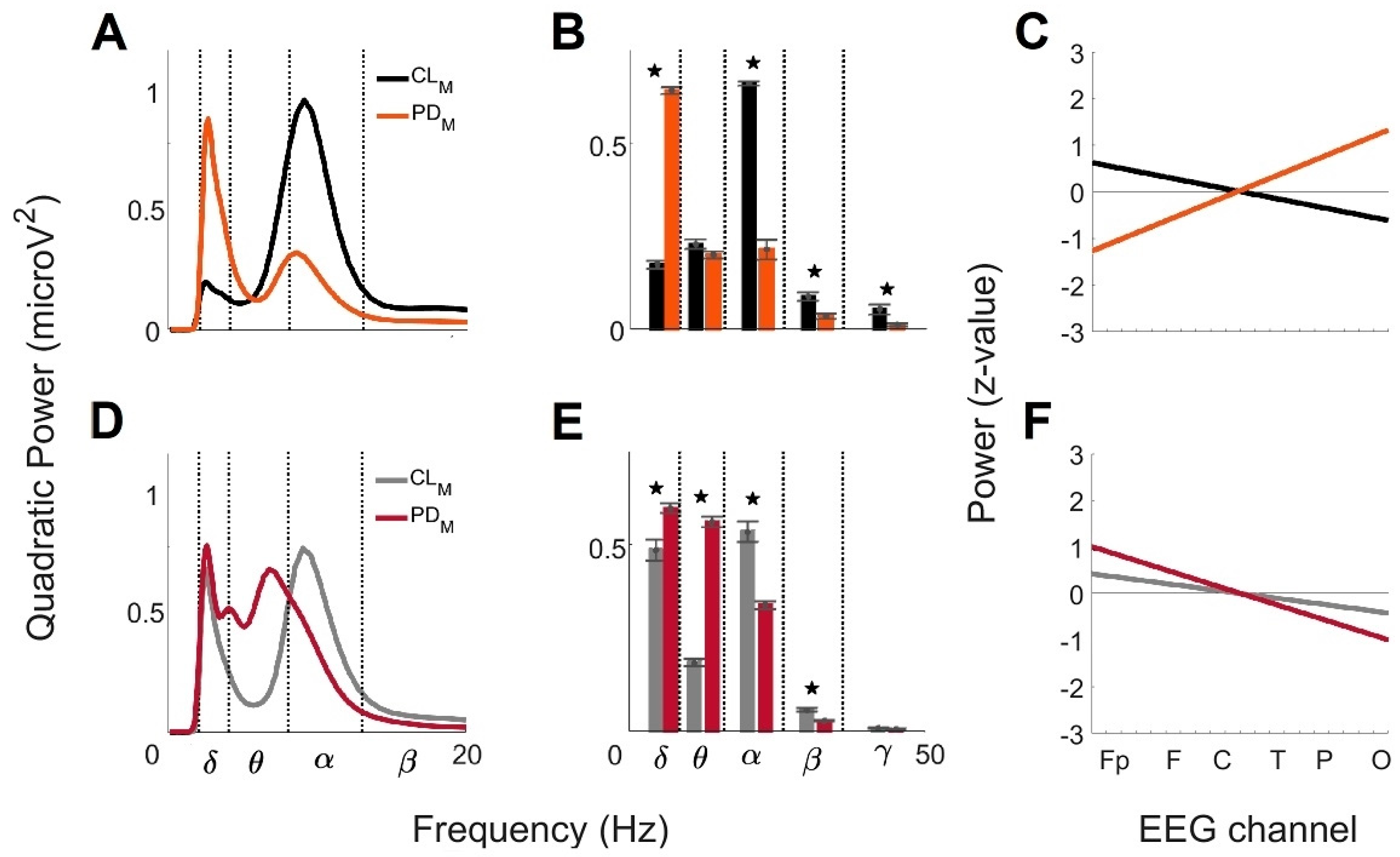
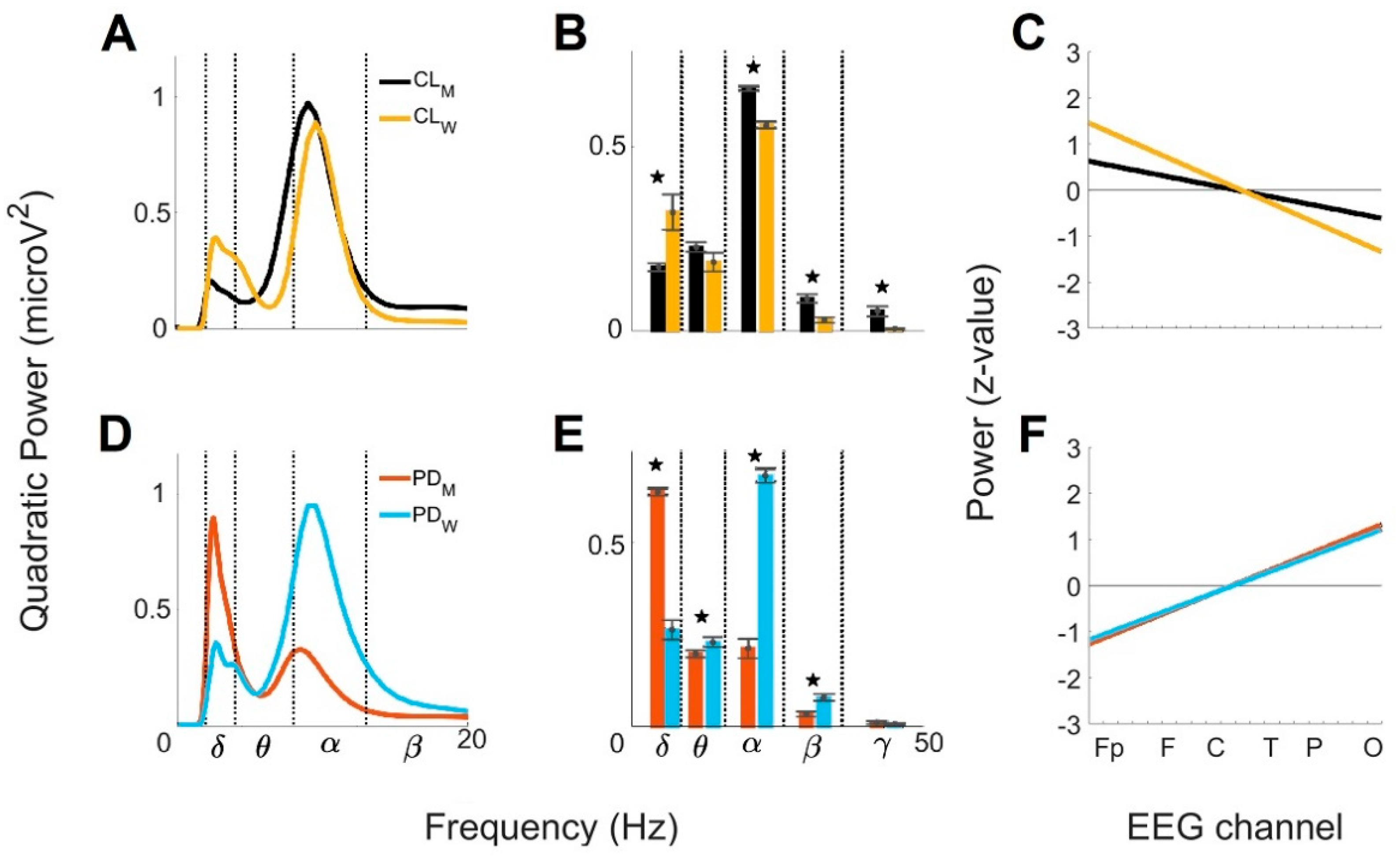
| Group (Men) | MF a (Hz) | GWC b | PA c | LR d (Slope/y-Intercept) | BP e (μV2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Frequencies (Mean ± SD; Hz) | Power (μV2) | + | − | ≈0 | δ | θ | α | β | γ | |||
| (Brain Regions) | ||||||||||||
| CL < 60 | 8.01 8.48 8.99 9.52 | 8.99 ± 1.86 2.38 ± 0.50 | 0.98 0.20 | F T C | P O | Fp | −0.06/0.6 | 0.17 | 0.22 | 0.65 | 0.08 | 0.05 |
| CL > 60 | 2.38 2.52 8.99 9.52 | 8.99 ± 2.34 2.52 ± 0.98 | 0.78 0.69 | F T C | Fp P O | - | −0.04/0.4 | 0.48 | 0.18 | 0.53 | 0.05 | 7.9 × 10−3 |
| PD < 60 | 2.38 2.52 8.48 ± 2.22 2.52 ± 1.05 0.33 0.90 | 8.48 ± 2.22 2.52 ± 1.05 | 0.33 0.90 | P O | Fp F T C | - | 0.13/−1.4 | 0.64 | 0.19 | 0.21 | 0.03 | 0.01 |
| PD > 60 | 2.38 2.52 4.24 6.36 6.73 7.13 | 4.00 ± 0.70 6.73 ± 3.27 2.52 ± 0.47 | 0.53 0.69 0.79 | Fp F C | T P O | - | −0.10/1.1 | 0.59 | 0.56 | 0.33 | 0.02 | 5.2 × 10−3 |
| Group (Women) | MF a (Hz) | GWC b | PA c | LR d (Slope/y-Intercept) | BP e (μV2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Frequencies (mean ± SD; Hz) | Power (μV2) | + | − | ≈0 | δ | θ | α | β | γ | |||
| (Brain Regions) | ||||||||||||
| CL < 60 | 8.99 9.52 10.09 | 9.52 ± 1.81 2.67 ± 0.55 | 0.89 0.39 | Fp F T | P O | C | −0.14/1.6 | 0.32 | 0.18 | 0.56 | 0.02 | 4.4 × 10−3 |
| CL > 60 | 8.48 8.99 | 8.99 ± 1.36 2.52 ± 0.46 | 0.93 0.33 | Fp T O | F C P | - | −0.03/0.4 | 0.23 | 0.15 | 0.50 | 0.02 | 2.9 × 10−3 |
| PD < 60 | 8.99 9.52 10.09 | 9.52 ± 1.96 2.67 ± 0.49 | 0.95 0.35 | T P O | Fp F C | - | 0.12/−1.3 | 0.26 | 0.23 | 0.68 | 0.07 | 8.5 × 10−3 |
| PD > 60 | 2.38 2.52 2.67 8.99 | 8.99 ± 2.41 2.67 ± 0.61 | 0.49 0.90 | T P O | Fp | F C | 0.12/−1.4 | 0.66 | 0.29 | 0.35 | 0.07 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, G.; Velasco Herrera, V.M.; Ortega-Aguilar, A. Electroencephalographic Characterization by Covariance Analysis in Men with Parkinson’s Disease Reveals Sex- and Age-Related Differences. Appl. Sci. 2023, 13, 9618. https://doi.org/10.3390/app13179618
González-González G, Velasco Herrera VM, Ortega-Aguilar A. Electroencephalographic Characterization by Covariance Analysis in Men with Parkinson’s Disease Reveals Sex- and Age-Related Differences. Applied Sciences. 2023; 13(17):9618. https://doi.org/10.3390/app13179618
Chicago/Turabian StyleGonzález-González, Gabriela, Víctor Manuel Velasco Herrera, and Alicia Ortega-Aguilar. 2023. "Electroencephalographic Characterization by Covariance Analysis in Men with Parkinson’s Disease Reveals Sex- and Age-Related Differences" Applied Sciences 13, no. 17: 9618. https://doi.org/10.3390/app13179618






