Coumarins of Ferulopsis hystrix: LC–MS Profiling and Gastroprotective and Antioxidant Activities of Skimmin and Peucenidin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Material
2.2. Extract Preparation
2.3. LC–MS Analysis and Quantification
2.4. Animal Study Design
2.5. Macroscopic Study of the Gastric Mucosa
2.6. Biochemical Study
2.6.1. Determination of Malondialdehyde Content
2.6.2. Catalase Activity Determination
2.6.3. Determination of Reduced Glutathione Content
2.7. Statistical Analysis
3. Results
3.1. LC–MS Profiling of the F. hystrix Root Extract
3.2. Gastroprotective and Antioxidant Activities of Skimmin and Peucenidin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butov, M.A.; Zhestkova, T.V.; Esakova, E.M.; Efanova, L.V. Assessment of ten-year dynamics of cases of hospitalizations of patients with peptic ulcer disease, chronic gastritis and chronic duodenitis. Ter. Arkh. 2022, 94, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Hamid, K.A.; Altaf, D.M.; Ahmad, M.M. Gastric ulcer: An overview. Int. J. Curr. Res. Physiol. Pharmacol. 2023, 7, 1–7. Available online: https://ijcrpp.com/index.php/ijcrpp/article/view/63 (accessed on 15 June 2023).
- Majumdar, D.; Bebb, J. Helicobacter pylori infection and peptic ulcers. Medicine 2019, 47, 292–300. [Google Scholar] [CrossRef]
- Narayanan, M.; Reddy, K.M.; Marsicano, E. Peptic ulcer disease and Helicobacter pylori infection. Mo. Med. 2018, 115, 219–224. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6140150 (accessed on 15 June 2023). [PubMed]
- Siddique, O.; Ovalle, A.; Siddique, A.S.; Moss, S.F. Helicobacter pylori infection: An update for the internist in the age of increasing global antibiotic resistance. Am. J. Med. 2018, 131, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Xiaomin, Z.; Songze, D.; Ruobing, H. The related study on the pathogenesis of gastrointestinal diseases in gastrointestinal flora and the risk of gastric ulcer carcinogenesis. J. Biomater. Tissue. Eng. 2021, 11, 1418–1428. [Google Scholar] [CrossRef]
- Naumov, A.V.; Tkacheva, O.N.; Khovasova, N.O. Safety of nonsteroidal anti-inflammatory drugs in patients with cardiovascular risk. Ter. Arkh. 2019, 91, 108–113. [Google Scholar] [CrossRef]
- Sanaei, M.-J.; Shirzad, H.; Soltani, A.; Abdollahpour-Alitappeh, M.; Shafigh, M.-H.; Rahimian, G.; Mirzaei, Y.; Bagheri, N. Up-regulated CCL18, CCL28 and CXCL13 expression is associated with the risk of gastritis and peptic ulcer disease in Helicobacter pylori infection. Am. J. Med. Sci. 2021, 361, 43–54. [Google Scholar] [CrossRef]
- Levitan, B.N.; Skvortsov, V.V.; Samokhvalova, P.D. Modern approaches in treatment of helicobacteriosis in patients with peptic ulcer. Med. Alph. 2021, 40, 7–13. [Google Scholar] [CrossRef]
- Brătucu, M.N.; Prunoiu, V.-M.; Strâmbu, V.; Brătucu, E.; Răvaş, M.-M.; Simion, L.; Petre, R. Unusual complicated gastric ulcers. Medicina 2021, 57, 1345. [Google Scholar] [CrossRef]
- Tarasconi, A.; Coccolini, F.; Biffl, W.L.; Tomasoni, M.; Ansaloni, L.; Picetti, E.; Molfino, S.; Shelat, V.; Cimbanassi, S.; Weber, D.G.; et al. Perforated and bleeding peptic ulcer: WSES guidelines. World J. Emerg. Surg. 2020, 15, 3. [Google Scholar] [CrossRef]
- Vu, T.B.; Tran, T.N.Q.; Tran, T.Q.A.; Vu, D.L.; Hoang, V.T. Antibiotic resistance of Helicobacter pylori in patients with peptic ulcer. Medicina 2023, 59, 6. [Google Scholar] [CrossRef] [PubMed]
- Ercan, G.; Tartar, R.I.; Solmaz, A.; Gulcicek, O.B.; Karagulle, O.O.; Meric, S.; Cayoren, H.; Kusaslan, R.; Kemik, A.; Kayali, D.G.; et al. Potent therapeutic effects of ruscogenin on gastric ulcer established by acetic acid. Asian J. Surg. 2020, 43, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Lee, J.H.; Song, J.; Kim, H. Gastroprotective effects of Inulae flos on HCl/ethanol-induced gastric ulcers in rats. Molecules 2020, 25, 5623. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, D.H.; Kim, S.-Y.; Kang, K.S.; Lee, S.; Seo, H.J.; Park, S.J.; Jung, K. Potential and beneficial effects of Cinnamomum cassia on gastritis and safety: Literature review and analysis of standard extract. Appl. Biol. Chem. 2021, 64, 95. [Google Scholar] [CrossRef]
- Li, C.F.; Zhang, F.R.; Zhu, N.; Cui, J.Z.; Tang, S.H.; Li, Z.Y. Network pharmacology study of Yi medicine Jinweitai Capsules in treating gastrointestinal diseases. China J. Chin. Mater. Med. 2021, 46, 865–876. [Google Scholar] [CrossRef]
- Park, J.U.; Cho, J.S.; Kim, J.S.; Kim, H.K.; Jo, Y.H.; Rahman, M.A.A.; Lee, Y.I. Synergistic effect of Rubus crataegifolius and Ulmus macrocarpa against Helicobacter pylori clinical isolates and gastritis. Front. Pharmacol. 2020, 11, 4. [Google Scholar] [CrossRef]
- Anbari, K.; Hasanvand, A.; Andevari, A.N.; Moghadasi, M.; Abbaszadeh, S. Concise overview: A review on natural antioxidants and important herbal plants on gastrointestinal system. Res. J. Pharm. Technol. 2019, 12, 841–847. [Google Scholar] [CrossRef]
- Matole, V.; Thorat, Y.; Ghurghure, S.; Ingle, S.; Birajdar, A.; Nangare, G.; Safwan, M.; Madur, S.; Patil, S.; Bagalkote, Z.; et al. A brief review on herbal medicines. Res. J. Pharmacogn. Phytochem. 2021, 13, 101–102. [Google Scholar] [CrossRef]
- Park, J.U.; Kang, J.H.; Rahman, M.A.A.; Hussain, A.; Cho, J.S.; Lee, Y.I. Gastroprotective effects of plants extracts on gastric mucosal injury in experimental sprague-dawley rats. Biomed. Res. Int. 2019, 2019, 8759708. [Google Scholar] [CrossRef]
- Blinova, K.F.; Kuvaev, V.B. Medicinal plants of Tibetan medicine in Transbaikalia. Quest. Pharmacog. 1965, 3, 163–178. [Google Scholar]
- Shul’ts, E.E.; Ganbaatar, Z.; Petrova, T.N.; Shakirov, M.M.; Bagryanskaya, I.Y.; Taraskin, V.V.; Radnaeva, L.D.; Otgonsuren, D.; Pokrovskii, A.G.; Tolstikov, G.A. Plant coumarins. IX. Phenolic compounds of Ferulopsis hystrix growing in Mongolia. Cytotoxic activity of 8,9-dihydrofurocoumarins. Chem. Nat. Compd. 2012, 48, 211–217. [Google Scholar] [CrossRef]
- Aseeva, T.A.; Dashiev, D.B.; Dashiev, A.D.; Nikolaev, S.M.; Surkova, N.A.; Chirikova, G.V.; Yurina, T.A. Tibetan Medicine of the Buryats, 1st ed.; Publishing House of the Siberian Branch of the Russian Academy of Sciences: Novosibirsk, Russia, 2008; pp. 164–166. [Google Scholar]
- Batorova, S.M.; Yakovlev, G.P.; Aseeva, T.A. Reference Book of Medicinal Plants of Traditional Tibetan Medicine, 1st ed.; Nauka: Novosibirsk, Russia, 2013. [Google Scholar]
- Markova, L.P.; Belenovskaya, L.M.; Nadezhina, T.P.; Sinitsky, V.S.; Ligaa, W.; Sokolov, P.D.; Bakina, L.A. Wild Useful Plants of the Mongolian People’s Republic Flora, 1st ed.; Nauka Publishing House: Leningrad, Russia, 1985; pp. 204–208. [Google Scholar]
- Serenot, S.K. Tuvan Folk Medicine: Medicinal Plants, Herbs, Lichens, Mushrooms with a Parallel Description of Their Use in Chinese, Mongolian and Tibetan Medicine, 1st ed.; Tuvan Book Publisher: Kyzvl, Russia, 2009; pp. 56–58. [Google Scholar]
- Salchak, S.M.; Razuvaeva, Y.G.; Arakchaa, K.D.; Toropova, A.A.; Olennikov, D.N. Pharmacological efficacy Ferulopsis hystrix (Bunge) Pimenov dry extract in experimental of indomethacin-induced gastropathy. Probl. Biol. Med. Pharm. Chem. 2019, 22, 47–52. [Google Scholar] [CrossRef]
- Salchak, S.M.; Razuvaeva, Y.G.; Toropova, A.A.; Arakchaa, K.D.; Olennikov, D.N.; Nikolaev, I.G. Gastroprotective effect of Ferulopsis hystrix (Bunge) Pimenov in ethanol-induced gastropathy. Bull. Sib. Med. 2020, 19, 151–157. [Google Scholar] [CrossRef]
- Salchak, S.M.; Razuvaeva, Y.G.; Toropova, A.A.; Arakchaa, K.-K.D. Morphofunctional estimation of the gastroprotective activity of the Ferulopsis hystrix dry extract in neurogenic ulcer. Exp. Clin. Gastroenterol. 2020, 175, 71–75. [Google Scholar] [CrossRef]
- Salchak, S.M.; Razuvaeva, Y.G.; Toropova, A.A.; Nikolaeva, I.G.; Olennikov, D.N. The gastroprotective effect of Ferulopsis hystrix (Bunge) Pimenov dry extract. Exp. Clin. Gastroenterol. 2020, 83, 12–16. [Google Scholar] [CrossRef]
- Salchak, S.M.; Razuvaeva, Y.G.; Toropova, A.A.; Arakchaa, K.D. Anti-exudative activity of Ferulopsis hystrix (Bunge) Pimenov. Sci. Innovat. 2018, 1, 205–208. [Google Scholar]
- Salchak, S.M.; Razuvaeva, Y.G.; Arakchaa, K.D.; Toropova, A.A.; Sambueva, Z.G.; Nikolaeva, I.G. Pharmacological effects of Ferulopsis hystrix dry extract. Iss. Qual. Assur. Med. 2019, 23, 63–68. [Google Scholar]
- Salchak, S.M.; Toropova, A.A.; Arakchaa, K.D.; Razuvaeva, Y.G.; Nikolaeva, I.G. Stress-protective and antioxidant effect of the dry extract Ferulopsis hystrix. J. Tradit. Mong. Med. 2017, 8, 117–119. [Google Scholar]
- Salchak, S.M.; Toropova, A.A.; Razuvaeva, Y.G.; Arakchaa, K.D. Antioxidant activity of dry extract of Ferulopsis hystrix (Bunge) Pimenov in experimental reflux gastritis. Russ. J. Biopharm. 2021, 13, 34–38. [Google Scholar] [CrossRef]
- Plant Resources of Russia: Wild Flowering Plants, Their Composition and Biological Activity; Belenovskaya, L.M.; Lesiovskaya, E.E. (Eds.) KMK Association of Scientific Publications: St. Petersburg–Moscow, Russia, 2010; Volume 3, p. 223. [Google Scholar]
- Bhattarai, N.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer potential of coumarin and its derivatives. Mini Rev. Med. Chem. 2021, 21, 2996–3029. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Qin, H.-L.; Zhang, Z.-W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef] [PubMed]
- Radha, G.V.; Sadhana, B.; Trideva, S.K.; Ganapaty, S. Bioactive umbelliferone and its derivatives: An update. J. Pharmacogn. Phytochem. 2019, 8, 59–66. [Google Scholar]
- Rostom, B.; Karaky, R.; Kassab, I.; Sylla-Iyarreta Veitía, M. Coumarins derivatives and inflammation: Review of their effects on the inflammatory signaling pathways. Eur. J. Pharmacol. 2022, 922, 174867. [Google Scholar] [CrossRef]
- Banikazemi, Z.; Mirazimi, S.M.; Dashti, F.; Mazandaranian, M.R.; Akbari, M.; Morshedi, K.; Aslanbeigi, F.; Rashidian, A.; Chamanara, M.; Hamblin, M.R.; et al. Coumarins and gastrointestinal cancer: A new therapeutic option? Front. Oncol. 2021, 11, 752784. [Google Scholar] [CrossRef] [PubMed]
- Majouli, K.; Hamdi, A.; Abdelhamid, A.; Bouraoui, A.; Kenani, A. Anti-inflammatory activity and gastroprotective effect of Hertia cheirifolia L. roots extract. J. Ethnopharmacol. 2018, 217, 7–10. [Google Scholar] [CrossRef]
- Parfenov, E.A.; Trapkov, V.A.; Shabanov, P.D. Redox regulation as a reliable platform for the search and development of new types of drugs. Search for gastroprotectors among substituted coumarins. Rev. Clin. Pharmacol. Drug Ther. 2014, 12, 22–42. [Google Scholar] [CrossRef]
- Di Stasi, L.C. Coumarin derivatives in inflammatory bowel disease. Molecules 2021, 26, 422. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. New compounds from flowers of Phlojodicarpus sibiricus. Chem. Nat. Compd. 2020, 56, 628–632. [Google Scholar] [CrossRef]
- Olennikov, D.N. New coumarins from roots and fruit of Peucedanum morisonii. Chem. Nat. Compd. 2022, 58, 816–821. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Fedorov, I.A.; Kashchenko, N.I.; Chirikova, N.K.; Vennos, C. Khellactone derivatives and other phenolics of Phlojodicarpus sibiricus (Apiaceae): HPLC-DAD-ESI-QQQ-MS/MS and HPLC-UV profile, and antiobesity potential of dihydrosamidin. Molecules 2019, 24, 2286. [Google Scholar] [CrossRef] [PubMed]
- Pauls, F.; Wick, A.N.; Mackey, E.M. An assay method for anti-ulcer substances. Gastroenterology 1947, 8, 774–782. [Google Scholar] [PubMed]
- Gavrilov, V.B.; Gavrilova, A.R.; Mazhul, L.M. Analysis of methods for determining the products of lipid peroxidation in blood serum according to the test with thiobarbituric acid. Quest. Med. Chem. 1987, 33, 118–122. [Google Scholar]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Kamyshnikov, V.S.; Alekhnovich, L.I.; Vasiliou-Svetlitskaya, S.G.; Volotovskaya, O.A.; Dalnova, T.S.; Khodyukova, A.B.; Zubovskaya, E.T.; Kuzmenko, A.T.; Kokhnovich, N.N.; Stepanova, Y.I.; et al. Clinical Laboratory Diagnostics (Methods and Interpretation of Laboratory Studies), 2nd ed.; MED-Press-Inform: Moscow, Russia, 2017; pp. 378–390. [Google Scholar]
- Shaik, I.H.; Mehvar, R. Rapid determination of reduced and oxidized glutathione levels using a new thiol-masking reagent and the enzymatic recycling method: Application to the rat liver and bile samples. Anal. Bioanal. Chem. 2006, 385, 105–113. [Google Scholar] [CrossRef]
- Olennikov, D.N. Coumarins of lovage roots (Levisticum officinale W.D.J.Koch): LC-MS profile, quantification, and stability during postharvest storage. Metabolites 2023, 13, 3. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. Hogweed seed oil: Physico–chemical characterization, LC-MS profile, and neuroprotective activity of Heracleum dissectum nanosuspension. Life 2023, 13, 1112. [Google Scholar] [CrossRef]
- Razuvaeva, Y.G.; Markova, K.V.; Toropova, A.A.; Kashchenko, N.I.; Olennikov, D.N. Chemical constituents, neuroprotective and antioxidant potential of Klasea centauroides leaves. Appl. Sci. 2023, 13, 860. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Metabolites of Geum aleppicum and Sibbaldianthe bifurca: Diversity and α-glucosidase inhibitory potential. Metabolites 2023, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N. Separation, characterization and mammal pancreatic lipase inhibitory potential of cucumber flower flavonoids. Separations 2023, 10, 255. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacolog. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Shahnoza, H. Literature review of modern treatment of rheumatoid arthritis. Web of Scientist. Int. Sci. Res. J. 2022, 3, 1073–1085. [Google Scholar] [CrossRef]
- Varrassi, G.; Pergolizzi, J.V.; Dowling, P.; Paladini, A. Ibuprofen safety at the golden anniversary: Are all NSAIDs the same? A narrative review. Adv. Ther. 2020, 37, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I.; Scarpignato, C.; Holmgren, E.; Olszewski, M.; Rainsford, K.D.; Lanas, A. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology 2018, 154, 500–514. [Google Scholar] [CrossRef]
- García-Rayado, G.; Navarro, M.; Lanas, A. NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs. Expert. Rev. Clin. Pharmacol. 2018, 11, 1031–1043. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Warner, T.D. COX isoforms in the cardiovascular system: Understanding the activities of non-steroidal anti-inflammatory drugs. Nat. Rev. Drug. Discov. 2006, 5, 75–86. [Google Scholar] [CrossRef]
- Sinha, M.; Gautam, L.; Shukla, P.K.; Kaur, P.; Sharma, S.; Singh, T.P. Current perspectives in NSAID-induced gastropathy. Mediat. Inflamm. 2013, 2013, 258209. [Google Scholar] [CrossRef]
- Tomita, T.; Sadakata, H.; Tamura, M.; Matsui, H. Indomethacin-induced generation of reactive oxygen species leads to epithelial cell injury before the formation of intestinal lesions in mice. J. Physiol. Pharmacol. 2014, 65, 435–440. Available online: https://www.jpp.krakow.pl/journal/archive/06_14/pdf/435_06_14_article.pdf (accessed on 15 June 2023).
- Fang, Y.F.; Xu, W.L.; Wang, L.; Lian, Q.W.; Qiu, L.F.; Zhou, H.; Chen, S.J. Effect of hydrotalcite on indometacin-induced gastric injury in rats. Biomed. Res. Int. 2019, 2019, 4605748. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Jeong, C.S. Gastroprotective activities of sennoside A and sennoside B via the Up-regulation of prostaglandin E2 and the inhibition of H+/K+-ATPase. Biomol. Ther. 2015, 23, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Tlaskalová-Hogenová, H.; Stěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčíř, T.; Kverka, M.; Zákostelská, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Triste, N.E.; Gonzalez-Garcia, M.P.; Jimenez-Andrade, J.M.; Castaneda-Hernandez, G.; Chavez-Pina, A.E. Pharmacological evidence for the participation of NO-cGMP-KATP pathway in the gastric protective effect of curcumin against indomethacin-induced gastric injury in the rat. Eur. J. Pharmacol. 2014, 730, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Dey, S.; Pal, C.; Goyal, M.; Alam, A.; Iqbal, M.S.; Sarkar, S.; Azhar Siddiqui, A.; Banerjee, C.; et al. Nonsteroidal anti-inflammatory drug induces proinflammatory damage in gastric mucosa through NF-κB activation and neutrophil infiltration: Anti-inflammatory role of heme oxygenase-1 against nonsteroidal anti-inflammatory drug. Free Radic. Biol. Med. 2013, 65, 456–467. [Google Scholar] [CrossRef]
- Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, S.H.; Kim, C.J.; Han, J.H.; Kwak, M.S.; Yoon, J.Y.; Jeon, J.W. Evaluating the mucoprotective effect of polydeoxyribonucleotide against indomethacin-induced gastropathy via the MAPK/NF-kappaB signaling pathway in rats. Eur. J. Pharmacol. 2020, 874, 172952. [Google Scholar] [CrossRef]
- McEvoy, L.; Carr, D.F.; Pirmohamed, M. Pharmacogenomics of NSAID-induced upper gastrointestinal toxicity. Front. Pharmacol. 2021, 12, 684162. [Google Scholar] [CrossRef]
- Scarpignato, C.; Hunt, R.H. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: Clinical picture, pathogenesis, and prevention. Gastroenterol. Clin. N. Am. 2010, 39, 433. [Google Scholar] [CrossRef]
- Chakraborty, S.; Yadav, S.K.; Saha, B.; Tyagi, M.; Singh Rathee, J.; Chattopadhyay, S. A bis-resorcinol resveratrol congener prevents indomethacin-induced gastric ulceration by inhibiting TNF-α as well as NF-κB and JNK pathways. Free Radic. Res. 2019, 53, 596–610. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Nabil, M.; Abdo, W.; Abdelfattah, M.A.O.; El-Shazly, A.M.; El Kharrassi, Y.; Sobeh, M. Syzygium samarangense leaf extract mitigates indomethacin-induced gastropathy via the NF-κB signaling pathway in rats. Biomed. Pharmacother. 2021, 139, 111675. [Google Scholar] [CrossRef]
- Narum, S.; Westergren, T.; Klemp, M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open 2014, 4, e004587. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, F.; Roth, P. Safety, tolerability, and use of steroids. In Central Nervous System Metastases; Ahluwalia, M., Metellus, P., Soffietti, R., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Bissell, B.D.; Gilbert, B. Glucocorticoids. In Pharmacology in Clinical Neurosciences; Prabhakar, H., Mahajan, C., Kapoor, I., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Boutemine, I.-M.; Amri, M.; Amir, Z.-C.; Fitting, C.; Mecherara-Idjeri, S.; Layaida, K.; Sennoun, N.; Berkane, S.; Cavaillon, J.-M. Touil-Boukoffa, C. Gastro-protective, therapeutic and anti-inflammatory activities of Pistacia lentiscus L. fatty oil against ethanol-induced gastric ulcers in rats. J. Ethnopharmacol. 2018, 224, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Dou, Y.; Wu, X.; Li, H.; Wu, J.; Huang, Q.; Luo, D.; Yi, T.; Liu, Y.; Su, Z.; et al. Prophylactic efficacy of patchoulene epoxide against ethanol-induced gastric ulcer in rats: Influence on oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2018, 283, 30–37. [Google Scholar] [CrossRef]
- Yerznkyan, G.G.; Shakeyev, K.T.; Tatina, E.S. Biochemical manifestations of oxidative stress in peptic ulcer. Succes. Modern Sci. Educ. 2016, 6, 78–81. [Google Scholar]
- Cruz, L.F.; de Figueiredo, G.F.; Pedro, L.P.; Amorin, Y.M.; Andrade, J.T.; Passos, T.F.; Rodrigues, F.F.; Souza, I.L.A.; Gonçalves, T.P.R.; dos Santos Lima, L.A.R.; et al. Umbelliferone (7-hydroxycoumarin): A non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed. Pharmacother. 2020, 129, 110432. [Google Scholar] [CrossRef]
- Manon, B.; Sharma, P. Design, synthesis and evaluation of diclofenac-antioxidant mutual prodrugs as safer NSAIDs. Indian J. Chem. 2009, 48, 1279–1287. Available online: https://nopr.niscpr.res.in/handle/123456789/6045 (accessed on 15 June 2023).
- Brumbarova, T.; Bauer, P.; Ivanov, R. Molecular mechanisms governing arabidopsis iron uptake. Trends Plant Sci. 2015, 20, 124–133. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, Ş.; Gülçin, İ. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Faculty Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Tomasz, K.; Rafał, P.; Monika, S. Natural and synthetic coumarins and their pharmacological activity. Eur. J. Clin. Exp. Med. 2017, 15, 169–175. [Google Scholar] [CrossRef]
- Cheriyan, B.V., Sr.; Kadhirvelu, P., Sr.; Nadipelly, J., Jr.; Shanmugasundaram, J.; Sayeli, V., Sr.; Subramanian, V., Sr. Anti-nociceptive effect of 7-methoxy coumarin from Eupatorium triplinerve vahl (Asteraceae). Pharmacogn. Mag. 2017, 13, 81–84. [Google Scholar] [CrossRef]
- Revankar, H.M.; Bukhari, S.N.A.; Kumar, G.B.; Qin, H.-L. Coumarins scaffolds as COX inhibitors. Bioorg. Chem. 2017, 71, 146–159. [Google Scholar] [CrossRef]
- Dawood, D.H.; Batran, R.Z.; Farghaly, T.A.; Khedr, M.A.; Abdulla, M.M. New coumarin derivatives as potent selective cox-2 inhibitors: Synthesis, anti-inflammatory, qsar, and molecular modeling studies. Arch. Pharm. 2015, 348, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Gagliotti Vigil de Mello, S.V.; Frode, T.S. In vitro and in vivo experimental model-based approaches for investigating anti-inflammatory properties of coumarins. Curr. Med. Chem. 2018, 25, 1446–1476. [Google Scholar] [CrossRef]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural product coumarins: Biological and pharmacological perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Nicolotti, O.; Mangiatordi, G.F.; Tognolini, M.; Karalaki, F.; Giorgio, C.; Patsilinakos, A.; Carotti, A.; Hadjipavlou-Litina, D.; Barocelli, E. Studies on the antiplatelet and antithrombotic profile of anti-inflammatory coumarin derivatives. J. Enzyme Inhib. Med. Chem. 2015, 30, 925–933. [Google Scholar] [CrossRef]
- Borisov, Y.Y. State of the protective mucous barrier and stomach secretory activity in patients with duodenal peptic ulcer. Sci. Rev. Med. Sci. 2015, 1, 85. Available online: https://science-medicine.ru/ru/article/view?id=647 (accessed on 15 June 2023).
- Samsonov, A.A.; Golubev, N.N.; Andreev, N.G.; Shcherbakova, N.A. Protective barrier of the gastric mucosa and the possibility of drug cytoprotection. Handb. Polyclin. Doct. 2018, 6, 40–48. [Google Scholar]
- Gyires, K.; Laszlo, S.B.; Lazar, B.; Zadori, Z.S. Similar and distinct mechanisms in the protective processes of upper and lower gastrointestinal tract. Curr. Pharm. Des. 2018, 24, 1936–1946. [Google Scholar] [CrossRef]
- Khoder, G.; Al-Menhali, A.A.; Al-Yassir, F.; Karam, S.M. Potential role of probiotics in the management of gastric ulcer. Exp. Ther. Med. 2016, 12, 3–17. [Google Scholar] [CrossRef]
- Grover, J.; Jachak, S. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015, 5, 38892–38905. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Devi, U.; Kumar, V.R.; Kumar, V.; Anwar, F.; Kaithwas, G. Dual inhibition of arachidonic acid pathway by mulberry leaf extract. Inflammopharmacology 2015, 23, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, A.; Shehzadi, S.A.; Saeed, F.; Khan, I. Developing hybrid molecule therapeutics for diverse enzyme inhibitory action: Active role of coumarin-based structural leads in drug discovery. Bioorg. Med. Chem. 2018, 26, 3731–3762. [Google Scholar] [CrossRef]
- Srivastava, P.; Vyas, V.K.; Variya, B.; Patel, P.; Qureshi, G.; Ghate, M. Synthesis, anti-inflammatory, analgesic, 5-lipoxygenase (5-LOX) inhibition activities, and molecular docking study of 7-substituted coumarin derivatives. Bioorg. Chem. 2016, 67, 130–138. [Google Scholar] [CrossRef]
- Krishna, R.; Pandagale, A.; Ronad, P.; Honnalli, S.; Kumar Das, B.; Gadad, P. Synthesis and pharmacological evaluation of schiff bases of 7-amino-4-methyl coumarins as novel anti-inflammatory agents. Asian J. Pharm. Parmacol. 2019, 5, 693–700. [Google Scholar] [CrossRef]
- Ovsyannikov, V.I. Neurotransmitters and Hormones of the Gastrointestinal Tract, 1st ed.; Publisher B.I.: St. Petersburg, Russia, 2003; pp. 67–98. [Google Scholar]

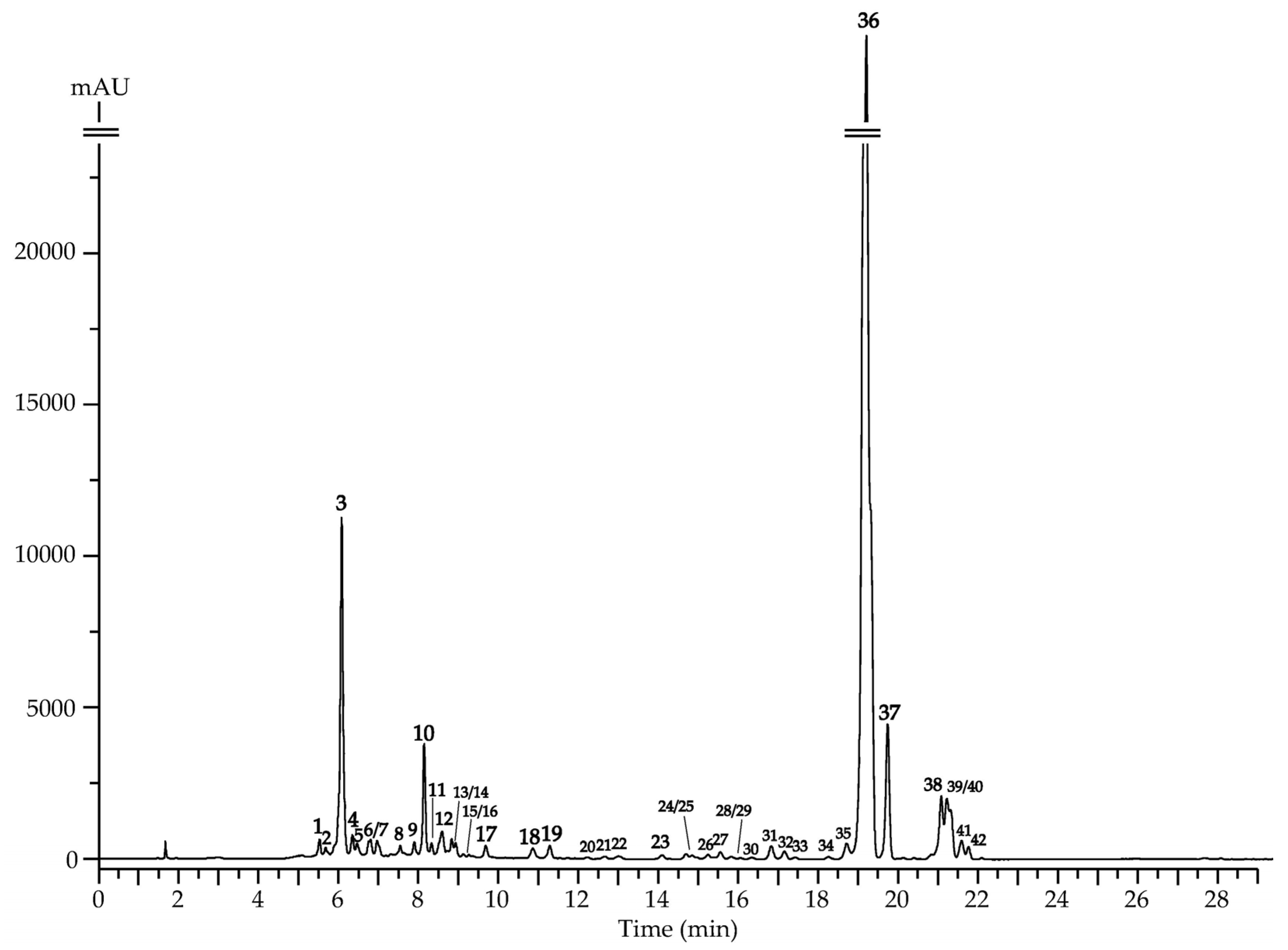
| No. | t, min | ESI-MS, m/z | Compound [Ref.] | IL a | Content, mg/g ± S.D. |
|---|---|---|---|---|---|
| 1 | 5.51 | 495 [M+K]+, 479 [M+Na]+, 463 [M+Li]+, 457 [M+H]+, 325 [(M+H)-Api]+, 163 [(M+H)-Api-Glc]+ | 6′′-Apiosylskimmin (umbelliferone 7-O-(6″-apiosyl)-glucoside) [46] | 1 | 0.85 ± 0.02 |
| 2 | 5.70 | 495 [M+K]+, 479 [M+Na]+, 463 [M+Li]+, 457 [M+H]+, 325 [(M+H)-Pent]+, 163 [(M+H)-Pent-Hex]+ | Umbelliferone O-pentosyl-O-hexoside [46,48] | 2 | 0.26 ± 0.00 |
| 3 | 6.08 | 363 [M+K]+, 347 [M+Na]+, 325 [M+H]+, 163 [(M+H)-Glc]+ | Skimmin (umbelliferone 7-O-glucoside) [48] | 1 | 22.57 ± 0.45 |
| 4 | 6.33 | 595 [M+K]+, 579 [M+Na]+, 563 [M+Li]+, 557 [M+H]+, 425 [(M+H)-Pent]+, 263 [(M+H)-Pent-Hex]+ | Vaginidiol O-pentosyl-O-hexoside [46,47] | 2 | 0.38 ± 0.01 |
| 5 | 6.45 | Vaginidiol O-pentosyl-O-hexoside [46,47] | 0.18 ± 0.00 | ||
| 6 | 6.77 | 537 [M+K]+, 521 [M+Na]+, 505 [M+Li]+, 499 [M+H]+, 457 [(M+H)-Ac]+, 325 [(M+H)-Ac-Pent]+, 163 [(M+H)-Ac-Pent-Hex]+ | Umbelliferone O-acetyl-O-pentosyl-O-hexoside [48] | 2 | 0.41 ± 0.01 |
| 7 | 6.98 | 597 [M+K]+, 581 [M+Na]+, 565 [M+Li]+, 559 [M+H]+, 427 [(M+H)-Api]+, 265 [(M+H)-Api-Glc]+ | Peujaponiside (peucedanol 7-O-(6″-apiosyl)-glucoside) [46,47] | 1 | 0.45 ± 0.01 |
| 8 | 7.51 | 597 [M+K]+, 581 [M+Na]+, 565 [M+Li]+, 559 [M+H]+, 427 [(M+H)-Api]+, 265 [(M+H)-Api-Glc]+ | Peucedanol 2′-O-(6″-apiosyl)-glucoside [46,47] | 1 | 0.18 ± 0.00 |
| 9 | 7.83 | Peucedanol 3′-O-(6″-apiosyl)-glucoside [46,47] | 0.15 ± 0.00 | ||
| 10 | 8.17 | 463 [M+K]+, 447 [M+Na]+, 431 [M+Li]+, 425 [M+H]+, 263 [(M+H)-Glc]+ | Apterin (vaginidiol 1′-O-glucoside) [54] | 1 | 9.14 ± 0.19 |
| 11 | 8.24 | 579 [M+K]+, 563 [M+Na]+, 547 [M+Li]+, 541 [M+H]+, 499 [(M+H)-Ac]+, 457 [(M+H)-2×Ac]+, 325 [(M+H)-2×Ac-Pent]+, 163 [(M+H)-2×Ac-Pent-Hex]+ | Umbelliferone di-O-acetyl-O-pentosyl-O-hexoside [48,54] | 2 | 0.12 ± 0.00 |
| 12 | 8.63 | 639 [M+K]+, 623 [M+Na]+, 607 [M+Li]+, 601 [M+H]+, 559 [(M+H)-Ac]+, 427 [(M+H)-Ac-Pent]+, 265 [(M+H)-Ac-Pent-Hex]+ | Peucedanol O-acetyl-O-pentosyl-O-hexoside [46,47,48] | 2 | 0.41 ± 0.01 |
| 13 | 8.84 | 465 [M+K]+, 449 [M+Na]+, 433 [M+Li]+, 427 [M+H]+, 265 [(M+H)-Glc]+ | Peucedanol 7-O-glucoside [55] | 1 | 0.11 ± 0.00 |
| 14 | 8.90 | 639 [M+K]+, 623 [M+Na]+, 607 [M+Li]+, 601 [M+H]+, 559 [(M+H)-Ac]+, 427 [(M+H)-Ac-Pent]+, 265 [(M+H)-Ac-Pent-Hex]+ | Peucedanol O-acetyl-O-pentosyl-O-hexoside [46,47,48] | 2 | <0.01 |
| 15 | 9.08 | 465 [M+K]+, 449 [M+Na]+, 433 [M+Li]+, 427 [M+H]+, 265 [(M+H)-Glc]+ | Peucedanol 2′-O-glucoside [46,47] | 1 | 0.02 ± 0.00 |
| 16 | 9.11 | Peucedanol 3′-O-glucoside [46,47] | 0.01 ± 0.00 | ||
| 17 | 9.61 | 505 [M+K]+, 489 [M+Na]+, 473 [M+Li]+, 467 [M+H]+, 425 [(M+H)-Ac]+, 263 [(M+H)-Ac-Glc]+ | Vaginidiol O-acetyl-O-hexoside [46,47,48] | 2 | 0.21 ± 0.00 |
| 18 | 10.88 | 301 [M+K]+, 285 [M+Na]+, 269 [M+Li]+, 263 [M+H]+ | Vaginidiol [46,47,48] | 1 | 0.12 ± 0.00 |
| 19 | 11.21 | Vaginidiol isomer [46,47,48] | 2 | 0.14 ± 0.00 | |
| 20 | 12.20 | 303 [M+K]+, 287 [M+Na]+, 271 [M+Li]+, 265 [M+H]+ | Peucedanol [55] | 1 | <0.01 |
| 21 | 12.63 | 315 [M+K]+, 299 [M+Na]+, 283 [M+Li]+, 277 [M+H]+, 263 [(M+H)-CH2]+ | Vaginidiol O-methyl ester [46,47,48,55] | 2 | <0.01 |
| 22 | 13.01 | 383 [M+K]+, 367 [M+Na]+, 351 [M+Li]+, 345 [M+H]+, 245 [(M+H)-C5H8O2]+ | Vaginidiol 9-O-angeloyl/senecioyl ester [46,47,48] | 2 | <0.01 |
| 23 | 14.05 | 329 [M+K]+, 313 [M+Na]+, 297 [M+Li]+, 291 [M+H]+, 277 [(M+H)-CH2]+, 263 [(M+H)-2×CH2]+ | Vaginidiol di-O-methyl ester [46,47,48,55] | 2 | 0.08 ± 0.00 |
| 24 | 14.69 | 329 [M+K]+, 313 [M+Na]+, 297 [M+Li]+, 291 [M+H]+, 263 [(M+H)-C2H4]+ | Vaginidiol O-ethyl ester [46,47,48,55] | 2 | <0.01 |
| 25 | 14.71 | ||||
| 26 | 15.23 | 383 [M+K]+, 367 [M+Na]+, 351 [M+Li]+, 345 [M+H]+, 245 [(M+H)-C5H8O2]+ | Vaginidiol 9-O-angeloyl/senecioyl ester [46,47,48,55] | 2 | <0.01 |
| 27 | 16.52 | 343 [M+K]+, 327 [M+Na]+, 311 [M+Li]+, 305 [M+H]+, 245 [(M+H)-C2H4O2]+ | Vaginidiol 9-O-acetyl ester [55] | 2 | <0.01 |
| 28 | 15.81 | 385 [M+K]+, 369 [M+Na]+, 353 [M+Li]+, 347 [M+H]+, 245 [(M+H)-C5H10O2]+ | Vaginidiol 9-O-isovaleroyl/2-methylbutyroyl ester [46,47,48,56] | 2 | <0.01 |
| 29 | 15.84 | ||||
| 30 | 16.31 | 371 [M+K]+, 355 [M+Na]+, 339 [M+Li]+, 333 [M+H]+, 245 [(M+H)-C4H8O2]+ | Vaginidiol 9-O-isobutyroyl ester [46,47,48,56] | 2 | <0.01 |
| 31 | 16.81 | 385 [M+K]+, 369 [M+Na]+, 353 [M+Li]+, 347 [M+H]+, 287 [(M+H)-C2H4O2]+, 245 [(M+H)-C2H2O]+ | Vaginidiol 9,1′-di-O-acetyl ester [46,47,48] | 2 | 0.72 ± 0.02 |
| 32 | 17.14 | 413 [M+K]+, 397 [M+Na]+, 381 [M+Li]+, 375 [M+H]+, 315 [(M+H)-C2H4O2]+, 245 [(M+H)-C2H4O2-C4H6O]+ | Vaginidiol 9-O-acetyl-1′-O-isobutyroyl ester [46,47,48] | 2 | 0.51 ± 0.01 |
| 33 | 17.42 | 427 [M+K]+, 411 [M+Na]+, 395 [M+Li]+, 389 [M+H]+, 329 [(M+H)-C2H4O2]+, 245 [(M+H)-C2H4O2-C5H8O]+ | Vaginidiol 9-O-acetyl-1′-O-isovaleroyl/2-methylbutyroyl ester [46,47,48] | 2 | <0.01 |
| 34 | 18.26 | ||||
| 35 | 18.72 | 425 [M+K]+, 409 [M+Na]+, 393 [M+Li]+, 387 [M+H]+, 327 [(M+H)-C2H4O2]+, 245 [(M+H)-C2H4O2-C5H6O]+ | Vaginidiol 9-O-acetyl-1′-O-angeloyl ester [46,47,48,57] | 2 | 1.02 ± 0.02 |
| 36 | 19.11 | 425 [M+K]+, 409 [M+Na]+, 393 [M+Li]+, 387 [M+H]+, 327 [(M+H)-C2H4O2]+, 245 [(M+H)-C2H4O2-C5H6O]+ | Peucenidin (vaginidiol 9-O-acetyl-1′-O-senecioyl ester) [46,47,48] | 1 | 170.35 ± 3.42 |
| 37 | 19.77 | 425 [M+K]+, 409 [M+Na]+, 393 [M+Li]+, 387 [M+H]+, 287 [(M+H)-C5H8O2]+, 245 [(M+H)-C5H8O2-C2H2O]+ | Libanotin (vaginidiol 9-O-angeloyl-1′-O-acetyl ester) [46,47,48] | 1 | 8.52 ± 0.17 |
| 38 | 21.02 | 427 [M+K]+, 411 [M+Na]+, 395 [M+Li]+, 389 [M+H]+, 287 [(M+H)-C5H10O2]+, 245 [(M+H)-C5H10O2-C2H2O]+ | Vaginidiol 9-O-isovaleroyl/2-methylbutyroyl-1′-O-acetyl ester [46,47,48] | 2 | 4.25 ± 0.09 |
| 39 | 21.21 | 465 [M+K]+, 449 [M+Na]+, 433 [M+Li]+, 427 [M+H]+, 327 [(M+H)-C5H8O2]+, 245 [(M+H)-C5H8O2-C5H6O]+ | Vaginidiol 9,1′-di-O-angeloyl/senecioyl ester [46,47,48] | 2 | 6.27 ± 0.12 b |
| 40 | 21.34 | ||||
| 41 | 21.64 | 441 [M+K]+, 425 [M+Na]+, 409 [M+Li]+, 403 [M+H]+, 315 [(M+H)-C4H8O2]+, 245 [(M+H)-C4H8O2-C4H6O]+ | Vaginidiol 9,1′-di-O-isobuturoyl ester [46,47,48] | 2 | 1.14 ± 0.02 |
| 42 | 21.73 | 469 [M+K]+, 453 [M+Na]+, 437 [M+Li]+, 431 [M+H]+, 329 [(M+H)-C5H10O2]+, 245 [(M+H)-C5H10O2-C5H8O]+ | Vaginidiol 9,1′-di-O-isovaleroyl/2-methylbutyroyl ester [46,47,48] | 2 | 0.82 ± 0.02 |
| Total umbelliferone content | 24.21 | ||||
| Total peucedanol content | 1.33 | ||||
| Total vaginidiol content | 203.85 | ||||
| Total coumarin content | 229.39 | ||||
| Parameters | Non-Treated Control (H2O), n = 8 | Negative Control (Indomethacin + H2O), n = 8 | Experimental Group I (Indomethacin + Skimmin, 1 mg/kg), n = 8 | Experimental Group II (Indomethacin + Skimmin, 3 mg/kg), n = 8 | Experimental Group III (Indomethacin + Peucenidin, 16 mg/kg), n = 8 | Experimental Group IV (Indomethacin + Peucenidin, 48 mg/kg), n = 8 |
|---|---|---|---|---|---|---|
| Erosions total number, Me (Q1–Q3) | 0 | 13 (10–14.5) p ≤ 0.01 ** | 5.5 (3–6.5) p ≤ 0.01 * | 4 (2.5–7.5) p ≤ 0.01 * | 5 (1.5–9) p ≤ 0.01 * | 6 (4–8) p ≤ 0.01 * |
| Point erosions (1–2 mm) | ||||||
| Number of animals, % | 0 | 100 p ≤ 0.01 ** | 100 | 100 | 100 | 100 |
| Number of erosions, Me (Q1–Q3) | 0 | 6(3.5–9) p ≤ 0.01 ** | 4.5 (2–8) | 2.5 (1.5–5) p ≤ 0.05 * | 4.5 (1.5–7.5) | 4 (1.5–7) |
| Pauls’ index | 0 | 6.3 | 4.9 | 3.3 | 4.5 | 4.3 |
| Large erosions (2–5 mm) | ||||||
| Number of animals, % | 0 | 100 p ≤ 0.01 ** | 25 p ≤ 0.05 * | 75 | 50 p ≤ 0.05 * | 75 |
| Number of erosions, Me (Q1–Q3) | 0 | 4(3–5) p ≤ 0.01 ** | 0 (0–1) p ≤ 0.01 * | 1.5 (0.5–2) p ≤ 0.01 * | 0.5 (0–1.5) p ≤ 0.01 * | 2 (1–2.5) p ≤ 0.01 * |
| Paul’s index | 0 | 4.0 | 0.13 | 0.98 | 0.38 | 1.3 |
| Strip-like erosions (≥5 mm) | ||||||
| Number of animals, % | 0 | 50 p ≤ 0.01 ** | 37.5 | 50 | 0 p ≤ 0.05 * | 0 p ≤ 0.01 * |
| Number of erosions, Me (Q1–Q3) | 0 | 2(0–3) p ≤ 0.01 ** | 0 (0–1) | 0.5 (0–1) | 0 | 0 |
| Paul’s index | 0 | 1.0 | 0.15 | 0.25 | 0 | 0 |
| Parameters | Non-Treated Control (H2O), n = 8 | Negative Control (Ethanol/Prednisolone + H2O), n = 8 | Experimental Group I (Ethanol/Prednisolone + Skimmin, 1 mg/kg), n = 8 | Experimental Group II (Ethanol/Prednisolone + Skimmin, 3 mg/kg), n = 8 | Experimental Group III (Ethanol/Prednisolone + Peucenidin, 16 mg/kg), n = 8 | Experimental Group IV (Ethanol/Prednisolone + Peucenidin, 48 mg/kg), n = 8 |
|---|---|---|---|---|---|---|
| Total number of erosions, Me (Q1–Q3) | 0 | 7(6.5–9) p ≤ 0.01 ** | 3.5 (2.5–4) p ≤ 0.01 * | 5.5 (2.5–9) | 3.5 (2.5–4.5) p ≤ 0.01 * | 3(3–3.5) p ≤ 0.01 * |
| Point erosions (1–2 mm) | ||||||
| Number of animals, % | 0 | 100 p ≤ 0.01 ** | 100 | 100 | 100 | 100 |
| Number of erosions, Me (Q1–Q3) | 0 | 3.5 (2.5–4) p ≤ 0.01 ** | 2.5 (1.5–3.5) | 3.5 (1.5–5) | 3 (2.5–3) | 2 (1–3.5) |
| Paul’s index | 0 | 3.3 | 2.5 | 3.3 | 2.8 | 2.3 |
| Large erosions (2–5 mm) | ||||||
| Number of animals, % | 0 | 100 p ≤ 0.01 ** | 75 | 75 | 50 p ≤ 0.05 * | 50 p ≤ 0.05 * |
| Number of erosions, Me (Q1–Q3) | 0 | 3 (2.5–3) p ≤ 0.01 ** | 1 (0.5–1) p ≤ 0.01 * | 2 (1–2.5) p ≤ 0.05 * | 0.5 (0–1.5) p ≤ 0.01 * | 1(0–2) p ≤ 0.01 * |
| Paul’s index | 0 | 2.8 | 0.6 | 1.3 | 0.4 | 0.5 |
| Strip-like erosions (≥5 mm) | ||||||
| Number of animals, % | 0 | 50 p ≤ 0.05 ** | 0 p ≤ 0.05 * | 25 | 0 * | 0 p ≤ 0.05 * |
| Number of erosions, Me (Q1–Q3) | 0 | 1 (0–2.5) | 0 | 0 (0–1.5) | 0 | 0 |
| Paul’s index | 0 | 0.9 | 0 | 0.2 | 0 | 0 |
| Animal Groups | MDA, µmol/L | Catalase, mkat/L | GSH, µmol/L |
|---|---|---|---|
| Indomethacin gastropathy | |||
| Non-treated control (H2O) | 6.5 ± 0.39 | 17.2 ± 0.94 | 926.2 ± 36.47 |
| Negative control (Indomethacin + H2O) | 23.2 ± 1.08 p ≤ 0.01 ** | 10.4 ± 0.74 p ≤ 0.01 ** | 584.7 ± 38.06 p ≤ 0.01 ** |
| Experimental group I (Indomethacin + skimmin, 1 mg/kg) | 9.5 ± 0.50 p ≤ 0.01 * | 13.8 ± 0.61 p ≤ 0.01 * | 805.7 ± 66.60 p ≤ 0.01 * |
| Experimental group II (Indomethacin + skimmin, 3 mg/kg) | 11.0 ± 0.88 p ≤ 0.01 * | 12.7 ± 0.77 | 812.4 ± 61.85 p ≤ 0.01 * |
| Experimental group III (Indomethacin + peucenidin, 16 mg/kg) | 12.3 ± 1.15 p ≤ 0.01 * | 12.9 ± 0.42 p ≤ 0.05 * | 832.4 ± 66.63 p ≤ 0.01 * |
| Experimental group IV (Indomethacin + peucenidin, 48 mg/kg) | 11.3 ± 0.89 p ≤ 0.01 * | 13.9 ± 0.42 p ≤ 0.01 * | 906.1 ± 53.92 p ≤ 0.01 * |
| Ethanol/steroid gastropathy | |||
| Non-treated control (H2O) | 5.9 ± 0.40 | 18.3 ± 0.71 | 973.1 ± 39.64 |
| Negative control (ethanol/prednisolone + H2O) | 20.2 ± 0.95 p ≤ 0.01 ** | 10.3 ± 0.63 p ≤ 0.01 ** | 671.7 ± 66.60 p ≤ 0.01 ** |
| Experimental group I (ethanol/prednisolone + skimmin, 1 mg/kg) | 12.5 ± 0.68 p ≤ 0.01 * | 13.1 ± 0.62 p ≤ 0.01 * | 932.9 ± 82.46 p ≤ 0.05 * |
| Experimental group II (ethanol/prednisolone + skimmin, 3 mg/kg) | 14.1 ± 0.70 p ≤ 0.01 * | 12.8 ± 0.41 p ≤ 0.01 * | 845.9 ± 56.30 |
| Experimental group III (ethanol/prednisolone + peucenidin, 16 mg/kg) | 14.2 ± 0.63 p ≤ 0.01 * | 14.8 ± 0.51 p ≤ 0.01 * | 926.2 ± 42.57 p ≤ 0.01 * |
| Experimental group IV (ethanol/prednisolone + peucenidin, 48 mg/kg) | 13.1 ± 0.86 p ≤ 0.01 * | 12.9 ± 0.30 p ≤ 0.01 * | 959.7 ± 47.57 p ≤ 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razuvaeva, Y.G.; Toropova, A.A.; Salchak, S.M.; Olennikov, D.N. Coumarins of Ferulopsis hystrix: LC–MS Profiling and Gastroprotective and Antioxidant Activities of Skimmin and Peucenidin. Appl. Sci. 2023, 13, 9653. https://doi.org/10.3390/app13179653
Razuvaeva YG, Toropova AA, Salchak SM, Olennikov DN. Coumarins of Ferulopsis hystrix: LC–MS Profiling and Gastroprotective and Antioxidant Activities of Skimmin and Peucenidin. Applied Sciences. 2023; 13(17):9653. https://doi.org/10.3390/app13179653
Chicago/Turabian StyleRazuvaeva, Yanina G., Anyuta A. Toropova, Saizana M. Salchak, and Daniil N. Olennikov. 2023. "Coumarins of Ferulopsis hystrix: LC–MS Profiling and Gastroprotective and Antioxidant Activities of Skimmin and Peucenidin" Applied Sciences 13, no. 17: 9653. https://doi.org/10.3390/app13179653
APA StyleRazuvaeva, Y. G., Toropova, A. A., Salchak, S. M., & Olennikov, D. N. (2023). Coumarins of Ferulopsis hystrix: LC–MS Profiling and Gastroprotective and Antioxidant Activities of Skimmin and Peucenidin. Applied Sciences, 13(17), 9653. https://doi.org/10.3390/app13179653








