Stents in Congenital Heart Disease: State of the Art and Future Scenarios
Abstract
:1. Introduction
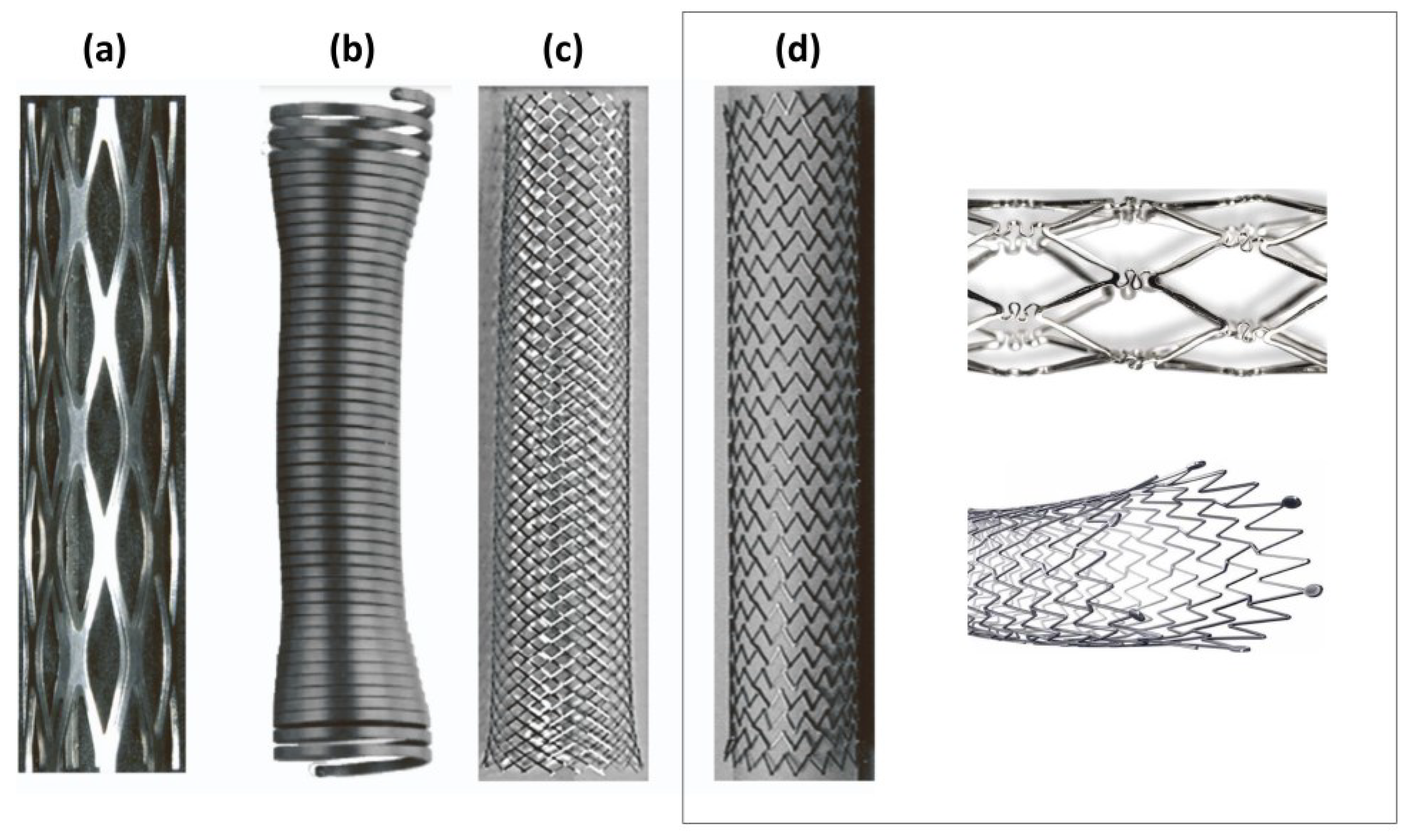
2. Stent Design
2.1. Materials and Deployment Strategy
2.2. Fabrication Method
2.3. Geometry and Biomechanical Properties
3. Congenital Heart Disease
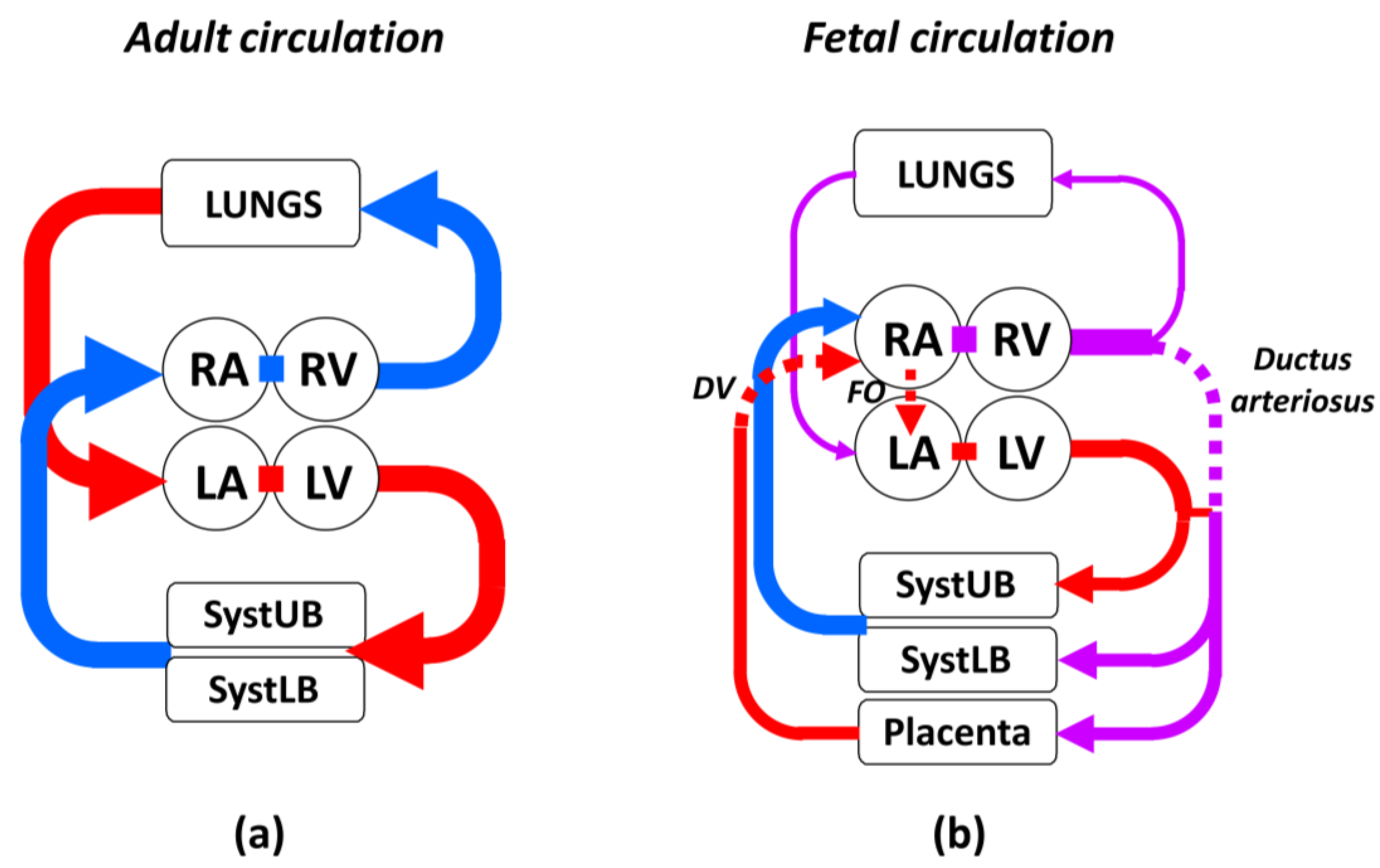
3.1. Characteristic Features of Fetal Circulation
3.2. Defects Amenable to Stenting
3.2.1. Hypoplastic Left Heart Syndrome
3.2.2. Pulmonary Atresia
3.2.3. Aortic Coarctation
3.2.4. Pulmonary Artery Stenosis
4. Stents in the Treatment of Congenital Heart Disease
4.1. Ductal Stenting: HLHS and Pulmonary Atresia
4.2. Great Vessel Stenting: CoA and Pulmonary Artery Stenosis
5. Vision and Future Perspectives
- High conformability to well adapt to duct geometry and shape;
- High radial force to counteract duct constriction keeping duct patency;
- Low profile for implantation at birth through small delivery sheaths;
- Good longitudinal flexibility to allow for delivery and maneuverability in/through torturous vessels during implantation;
- Minimal foreshortening for precise positioning in the duct, avoiding portions of the conduit being left uncovered after stent expansion;
- Minimal recoil to maintain duct patency after expansion.
- Adaptable dimensions to accommodate vessel somatic growth;
- High radial strength to resist external radial forces applied by the stenotic vessel wall;
- Low profile for implantation in small children through small delivery sheaths;
- High longitudinal flexibility to allow for delivery in/through torturous vessels and maneuverability during deployment;
- Minimal foreshortening at maximal expansion for precise positioning in the vessel, to better match the length of the stenotic lesion;
- Excellent durability to withstand long-term working conditions;
- Open-cell configuration to ensure blood flow in side branches.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakamura, K.; Keating, J.K.; Edelman, E.R. Pathology of Endovascular Stents. Interv. Cardiol. Clin. 2016, 5, 391–403. [Google Scholar] [CrossRef]
- Mosayyebi, A.; Manes, C.; Carugo, D.; Somani, B.K. Advances in Ureteral Stent Design and Materials. Curr. Urol. Rep. 2018, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Sagar, J. Role of Colonic Stents in the Management of Colorectal Cancers. World J. Gastrointest. Endosc. 2016, 8, 198–204. [Google Scholar] [CrossRef]
- Vermeulen, B.D.; Siersema, P.D. Esophageal Stenting in Clinical Practice: An Overview. Curr. Treat. Options Gastroenterol. 2018, 16, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Mangiavillano, B.; Pagano, N.; Baron, T.H.; Arena, M.; Iabichino, G.; Consolo, P.; Opocher, E.; Luigiano, C. Biliary and Pancreatic Stenting: Devices and Insertion Techniques in Therapeutic Endoscopic Retrograde Cholangiopancreatography and Endoscopic Ultrasonography. World J. Gastrointest. Endosc. 2016, 8, 143–156. [Google Scholar] [CrossRef]
- Bentham, J.R.; Zava, N.K.; Harrison, W.J.; Shauq, A.; Kalantre, A.; Derrick, G.; Chen, R.H.; Dhillon, R.; Taliotis, D.; Kang, S.L.; et al. Duct Stenting Versus Modified Blalock-Taussig Shunt in Neonates with Duct-Dependent Pulmonary Blood Flow: Associations with Clinical Outcomes in a Multicenter National Study. Circulation 2018, 137, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.L.; Rothman, M.T.; Rees, M.R.; Parsons, J.M.; Blackburn, M.E.; Ruiz, C.E. Stenting of the Arterial Duct: A New Approach to Palliation for Pulmonary Atresia. Heart 1992, 67, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Hascoët, S.; Baruteau, A.; Jalal, Z.; Mauri, L.; Acar, P.; Elbaz, M.; Boudjemline, Y.; Fraisse, A. Stents in Paediatric and Adult Congenital Interventional Cardiac Catheterization. Arch. Cardiovasc. Dis. 2014, 107, 462–475. [Google Scholar] [CrossRef]
- Van Gameren, M.; Witsenburg, M.; Takkenberg, J.J.M.; Boshoff, D.; Mertens, L.; Van Oort, A.M.; De Wolf, D.; Freund, M.; Sreeram, N.; Bökenkamp, R.; et al. Early Complications of Stenting in Patients with Congenital Heart Disease: A Multicentre Study. Eur. Heart J. 2006, 27, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Dotter, C.T.; Judkins, M.P. Percutaneous Transluminal Treatment of Arteriosclerotic Obstruction. Radiology 1965, 84, 631–643. [Google Scholar] [CrossRef]
- Dotter, C.T.; Buschmann, R.W.; McKinney, R.K.; Rösch, J. Transluminal Expandable Nitinol Coil Stent Grafting: Preliminary Report. Radiology 1983, 147, 259–260. [Google Scholar] [CrossRef]
- Tomberli, B.; Mattesini, A.; Baldereschi, G.I.; Di Mario, C. A Brief History of Coronary Artery Stents. Rev. Española Cardiol. 2018, 71, 312–319. [Google Scholar] [CrossRef]
- Raval, A.; Choubey, A.; Engineer, C.; Kothwala, D. Development and Assessment of 316LVM Cardiovascular Stents. Mater. Sci. Eng. A 2004, 386, 331–343. [Google Scholar] [CrossRef]
- Sun, F.; Mantovani, D.; Prima, F. Carbides and Their Role in Advanced Mechanical Properties of L605 Alloy: Implications for Medical Devices. Mater. Sci. Forum 2014, 783–786, 1354–1359. [Google Scholar] [CrossRef]
- O’Brien, B.J.; Stinson, J.S.; Larsen, S.R.; Eppihimer, M.J.; Carroll, W.M. A Platinum-Chromium Steel for Cardiovascular Stents. Biomaterials 2010, 31, 3755–3761. [Google Scholar] [CrossRef] [PubMed]
- Antonini, L.; Poletti, G.; Pennati, G.; Petrini, L. A Review on the Use of Finite Element Simulations for Structural Analyses of Coronary Stenting: What Can We Do Nowadays and What Do We Need to Move Forward? Eur. J. Mech. A Solids 2023, 101, 105071. [Google Scholar] [CrossRef]
- Duerig, T.W.; Wholey, M. A Comparison of Balloon- and Self-Expanding Stents. Minim. Invasive Ther. Allied Technol. 2002, 11, 173–178. [Google Scholar] [CrossRef] [PubMed]
- MacTaggart, J.N.; Phillips, N.Y.; Lomneth, C.S.; Pipinos, I.I.; Bowen, R.; Baxter, B.T.; Johanning, J.; Matthew Longo, G.; Desyatova, A.S.; Moulton, M.J.; et al. Three-Dimensional Bending, Torsion and Axial Compression of the Femoropopliteal Artery during Limb Flexion. J. Biomech. 2014, 47, 2249–2256. [Google Scholar] [CrossRef]
- Smouse, H.B.; Nikanorov, A.; LaFlash, D. Biomechanical Forces in the Femoropopliteal Arterial Segment. Endovasc. Today 2005, 4, 60–66. [Google Scholar]
- Stoeckel, D.; Pelton, A.; Duerig, T. Self-Expanding Nitinol Stents: Material and Design Considerations. Eur. Radiol. 2004, 14, 292–301. [Google Scholar] [CrossRef]
- Stoeckel, D. Nitinol Medical Devices and Implants. Minim. Invasive Ther. Allied Technol. 2000, 9, 81–88. [Google Scholar] [CrossRef]
- Robertson, S.W.; Pelton, A.R.; Ritchie, R.O. Mechanical Fatigue and Fracture of Nitinol. Int. Mater. Rev. 2012, 57, 1–37. [Google Scholar] [CrossRef]
- Korei, N.; Solouk, A.; Nazarpak, M.H.; Nouri, A. A Review on Design Characteristics and Fabrication Methods of Metallic Cardiovascular Stents. Mater. Today Commun. 2022, 31, 103467. [Google Scholar] [CrossRef]
- Stoeckel, D.; Bonsignore, C.; Duda, S.; Stoeckel, D.; Bonsignore, C.; Duda, S. A Survey of Stent Designs. Minim. Invasive Ther. Allied Technol. 2002, 11, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Maleckis, K.; Anttila, E.; Aylward, P.; Poulson, W.; Desyatova, A.; MacTaggart, J.; Kamenskiy, A. Nitinol Stents in the Femoropopliteal Artery: A Mechanical Perspective on Material, Design, and Performance. Ann. Biomed. Eng. 2018, 46, 684–704. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, R.; Tabriz, A.G.; Okereke, M.I.; Douroumis, D. 3D Printing Advances in the Development of Stents. Int. J. Pharm. 2021, 609, 121153. [Google Scholar] [CrossRef]
- Finazzi, V.; Berti, F.; Petrini, L.; Previtali, B.; Demir, A.G. Additive Manufacturing and Post-Processing of Superelastic NiTi Micro Struts as Building Blocks for Cardiovascular Stents. Addit. Manuf. 2023, 70, 103561. [Google Scholar] [CrossRef]
- Zaccaria, A.; Migliavacca, F.; Pennati, G.; Petrini, L. Modeling of Braided Stents: Comparison of Geometry Reconstruction and Contact Strategies. J. Biomech. 2020, 107, 109841. [Google Scholar] [CrossRef]
- Lee, H.J.; Chang, F.C.; Luo, C.B.; Guo, W.Y. Influence of Stenting with Open-Cell Stents vs. Close-Cell Stents on the Outcomes of Patients with Bilateral Carotid Stenosis. J. Chin. Med. Assoc. 2019, 82, 66–71. [Google Scholar] [PubMed]
- Bruneau, B.G. The Developmental Genetics of Congenital Heart Disease. Nature 2008, 451, 943–948. [Google Scholar] [CrossRef]
- Hoffman, J.I.E.; Kaplan, S. The Incidence of Congenital Heart Disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Tennant, P.W.; Pearce, M.S.; Bythell, M.; Rankin, J. 20-Year Survival of Children Born with Congenital Anomalies: A Population-Based Study. Lancet 2010, 375, 649–656. [Google Scholar] [CrossRef]
- Bouma, B.J.; Mulder, B.J.M. Changing Landscape of Congenital Heart Disease. Circ. Res. 2017, 120, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Triedman, J.K.; Newburger, J.W. Trends in Congenital Heart Disease. Circulation 2016, 133, 2716–2733. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.J. The Fetal Circulation. Contin. Educ. Anaesth. Crit. Care Pain 2005, 5, 107–112. [Google Scholar] [CrossRef]
- Gillam-Krakauer, M.; Mahajan, K. Patent Ductus Arteriosus; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rai, V.; Gładki, M.; Dudyńska, M.; Skalski, J. Hypoplastic Left Heart Syndrome [HLHS]: Treatment Options in Present Era. Indian J. Thorac. Cardiovasc. Surg. 2019, 35, 196–202. [Google Scholar] [CrossRef]
- Coats, L.; O’Connor, S.; Wren, C.; O’Sullivan, J. The Single-Ventricle Patient Population: A Current and Future Concern a Population-Based Study in the North of England. Heart 2014, 100, 1348–1353. [Google Scholar] [CrossRef]
- Yabrodi, M.; Mastropietro, C.W. Hypoplastic Left Heart Syndrome: From Comfort Care to Long-Term Survival. Pediatr. Res. 2017, 81, 142–149. [Google Scholar] [CrossRef]
- Wald, R.M.; Mertens, L.L. Hypoplastic Left Heart Syndrome Across the Lifespan: Clinical Considerations for Care of the Fetus, Child, and Adult. Can. J. Cardiol. 2022, 38, 930–945. [Google Scholar] [CrossRef]
- Gobergs, R.; Salputra, E.; Lubaua, I. Hypoplastic Left Heart Syndrome: A Review. Acta Medica Litu. 2016, 23, 86–98. [Google Scholar] [CrossRef]
- Kiserud, T. Physiology of the Fetal Circulation. Semin. Fetal Neonatal Med. 2005, 10, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.L.; Wren, C.; Watterson, K.G.; Hunter, S.; Hamilton, J.R. Stenting of the Arterial Duct Combined with Banding of the Pulmonary Arteries and Atrial Septectomy or Septostomy: A New Approach to Palliation for the Hypoplastic Left Heart Syndrome. Br. Heart J. 1993, 69, 551–555. [Google Scholar] [CrossRef]
- Hsia, T.Y.; Cosentino, D.; Corsini, C.; Pennati, G.; Dubini, G.; Migliavacca, F. Use of Mathematical Modeling to Compare and Predict Hemodynamic Effects between Hybrid and Surgical Norwood Palliations for Hypoplastic Left Heart Syndrome. Circulation 2011, 124, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Chikkabyrappa, S.M.; Loomba, R.S.; Tretter, J.T. Pulmonary Atresia with an Intact Ventricular Septum: Preoperative Physiology, Imaging, and Management. Semin. Cardiothorac. Vasc. Anesth. 2018, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Siblini, G.; Rao, P.S.; Singh, G.K.; Tinker, K.; Balfour, I.C. Transcatheter Management of Neonates with Pulmonary Atresia and Intact Ventricular Septum. Cathet. Cardiovasc. Diagn. 1997, 42, 395–402. [Google Scholar] [CrossRef]
- Alwi, M. Management Algorithm in Pulmonary Atresia with Intact Ventricular Septum. Catheter. Cardiovasc. Interv. 2006, 67, 679–686. [Google Scholar] [CrossRef]
- Nasr, V.G.; DiNardo, J.A. Pulmonary Atresia/Intact Ventricular Septum (PA/IVS). In The Pediatric Cardiac Anesthesia Handbook; John Wiley & Sons, Ltd: Chichester, UK, 2017; pp. 125–129. [Google Scholar]
- Gibbs, J.L.; Uzun, O.; Blackburn, M.E.C.; Wren, C.; Hamilton, J.R.L.; Watterson, K.G. Fate of the Stented Arterial Duct. Circulation 1999, 99, 2621–2625. [Google Scholar] [CrossRef]
- Ovaert, C.; Qureshi, S.A.; Rosenthal, E.; Baker, E.J.; Tynan, M. Growth of the Right Ventricle after Successful Transcatheter Pulmonary Valvotomy in Neonates and Infants with Pulmonary Atresia and Intact Ventricular Septum. J. Thorac. Cardiovasc. Surg. 1998, 115, 1055–1062. [Google Scholar] [CrossRef]
- Alcíbar-Villa, J.; Rubio, A.; Peña, N.; Galdeano, J.M.; Luis, M.; Arriola, J.; Inguanzo, R.; Pérez-Asenjo, J.; Aramendi, J.I.; Barrenechea, J.I. Atresia Pulmonar Con Septo Íntegro. Perforación y Valvuloplastia Pulmonar Mediante Técnica Mecánica Modificada. Seguimiento a Medio Plazo. Rev. Española Cardiol. 2007, 60, 833–840. [Google Scholar] [CrossRef]
- Zampi, J.D.; Hirsch-Romano, J.C.; Goldstein, B.H.; Shaya, J.A.; Armstrong, A.K. Hybrid Approach for Pulmonary Atresia with Intact Ventricular Septum: Early Single Center Results and Comparison to the Standard Surgical Approach. Catheter. Cardiovasc. Interv. 2014, 83, 753–761. [Google Scholar] [CrossRef]
- Sohrabi, B.; Jamshidi, P.; Yaghoubi, A.; Habibzadeh, A.; Hashemi-aghdam, Y.; Moin, A.; Kazemi, B.; Ghaffari, S.; Abdolahzadeh Baghayi, M.R.; Mahmoody, K. Comparison between Covered and Bare Cheatham-Platinum Stents for Endovascular Treatment of Patients with Native Post-Ductal Aortic Coarctation. JACC Cardiovasc. Interv. 2014, 7, 416–423. [Google Scholar] [CrossRef]
- Goldstein, B.H.; Kreutzer, J. Transcatheter Intervention for Congenital Defects Involving the Great Vessels. J. Am. Coll. Cardiol. 2021, 77, 80–96. [Google Scholar] [CrossRef]
- Boe, B.A.; Armstrong, A.K.; Janse, S.A.; Loccoh, E.C.; Stockmaster, K.; Holzer, R.J.; Cheatham, S.L.; Cheatham, J.P.; Berman, D.P. Percutaneous Implantation of Adult Sized Stents for Coarctation of the Aorta in Children ≤ 20 Kg. Circ. Cardiovasc. Interv. 2021, 14, E009399. [Google Scholar] [CrossRef]
- Castaldi, B.; Ciarmoli, E.; Di Candia, A.; Sirico, D.; Tarantini, G.; Scattolin, F.; Padalino, M.; Vida, V.; Di Salvo, G. Safety and Efficacy of Aortic Coarctation Stenting in Children and Adolescents. Int. J. Cardiol. Congenit. Heart Dis. 2022, 8, 100389. [Google Scholar] [CrossRef]
- Butera, G.; Manica, J.L.L.; Marini, D.; Piazza, L.; Chessa, M.; Filho, R.I.R.; Sarmento Leite, R.E.; Carminati, M. From Bare to Covered. Catheter. Cardiovasc. Interv. 2014, 83, 953–963. [Google Scholar] [CrossRef]
- Ringel, R.E.; Gauvreau, K.; Moses, H.; Jenkins, K.J. Coarctation of the Aorta Stent Trial (COAST): Study Design and Rationale. Am. Heart J. 2012, 164, 7–13. [Google Scholar] [CrossRef]
- Bondanza, S.; Calevo, M.G.; Marasini, M. Early and Long-Term Results of Stent Implantation for Aortic Coarctation in Pediatric Patients Compared to Adolescents: A Single Center Experience. Cardiol. Res. Pr. 2016, 2016, 4818307. [Google Scholar] [CrossRef]
- Trivedi, K.R.; Benson, L.N. Interventional Strategies in the Management of Peripheral Pulmonary Artery Stenosis. J. Interv. Cardiol. 2003, 16, 171–188. [Google Scholar] [CrossRef]
- BACHA, E.A.; KREUTZER, J. Comprehensive Management of Branch Pulmonary Artery Stenosis. J. Interv. Cardiol. 2001, 14, 367–376. [Google Scholar] [CrossRef]
- Feltes, T.F.; Bacha, E.; Beekman, R.H.; Cheatham, J.P.; Feinstein, J.A.; Gomes, A.S.; Hijazi, Z.M.; Ing, F.F.; de Moor, M.; Morrow, W.R.; et al. Indications for Cardiac Catheterization and Intervention in Pediatric Cardiac Disease. Circulation 2011, 123, 2607–2652. [Google Scholar] [CrossRef]
- Ruiz, C.E.; Gamra, H.; Zhang, H.P.; Garcia, E.J.; Boucek, M.M. Stenting of the Ductus Arteriosus as a Bridge to Cardiac Transplantation in Infants with the Hypoplastic Left-Heart Syndrome. N. Engl. J. Med. 1993, 328, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Suárez de Lezo, J.; Pan, M.; Romero, M.; Medina, A.; Segura, J.; Lafuente, M.; Pavlovic, D.; Hernández, E.; Melián, F.; Espada, J. Immediate and Follow-up Findings after Stent Treatment for Severe Coarctation of Aorta. Am. J. Cardiol. 1999, 83, 400–406. [Google Scholar] [CrossRef]
- Magee, A.G.; Brzezinska-Rajszys, G.; Qureshi, S.A.; Rosenthal, E.; Zubrzycka, M.; Ksiazyk, J.; Tynan, M. Stent Implantation for Aortic Coarctation and Recoarctation. Heart 1999, 82, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Fogelman, R.; Nykanen, D.; Smallhorn, J.F.; McCrindle, B.W.; Freedom, R.M.; Benson, L.N. Endovascular Stents in the Pulmonary Circulation. Circulation 1995, 92, 881–885. [Google Scholar] [CrossRef]
- Peters, B.; Ewert, P.; Berger, F. The Role of Stents in the Treatment of Congenital Heart Disease: Current Status and Future Perspectives. Ann. Pediatr. Cardiol. 2009, 2, 3. [Google Scholar] [CrossRef]
- Akintürk, H.; Michel-Behnke, I.; Valeske, K.; Mueller, M.; Thul, J.; Bauer, J.; Hagel, K.-J.; Schranz, D. Hybrid Transcatheter–Surgical Palliation. Pediatr. Cardiol. 2007, 28, 79–87. [Google Scholar] [CrossRef]
- Ruiz, C.E. Ductal Stents in the Management of Congenital Heart Defects. Catheter. Cardiovasc. Interv. 2001, 53, 75–80. [Google Scholar] [CrossRef]
- Baba, K.; Chaturvedi, R.; Lee, K.-J.; Caldarone, C.A.; Benson, L.N. Fate of the Ductal Stent after Hybrid Palliation for Hypoplastic Left Heart Syndrome. Ann. Thorac. Surg. 2013, 95, 1660–1664. [Google Scholar] [CrossRef]
- Akintuerk, H.; Michel-Behnke, I.; Valeske, K.; Mueller, M.; Thul, J.; Bauer, J.; Hagel, K.J.; Kreuder, J.; Vogt, P.; Schranz, D. Stenting of the Arterial Duct and Banding of the Pulmonary Arteries: Basis for Combined Norwood Stage I and II Repair in Hypoplastic Left Heart. Circulation 2002, 105, 1099–1103. [Google Scholar] [CrossRef]
- Michel-Behnke, I. Stenting of the Ductus Arteriosus and Banding of the Pulmonary Arteries: Basis for Various Surgical Strategies in Newborns with Multiple Left Heart Obstructive Lesions. Heart 2003, 89, 645–650. [Google Scholar] [CrossRef]
- Hribernik, I.; Thomson, J.; Bentham, J. Deformation and Stenosis of the Sinus-SuperFlex-DS Stent after Ductal Stenting for the Hybrid Stage 1 Procedure. Catheter. Cardiovasc. Interv. 2021, 98, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Bacha, E.A.M.; Hijazi, Z.M. Hybrid Procedures in Pediatric Cardiac Surgery. Pediatr. Card. Surg. Annu. 2005, 8, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Galantowicz, M.; Cheatham, J.P. Lessons Learned from the Development of a New Hybrid Strategy for the Management of Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2005, 26, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Betrián-Blasco, P.; Marti-Aguasca, G.; Giralt-Garcia, G. Sinus Superflex DS © Early Collapse in Systemic Arterial Duct. Cardiol. Young 2020, 30, 436–437. [Google Scholar] [CrossRef]
- Goreczny, S.; Qureshi, S.A.; Rosenthal, E.; Krasemann, T.; Nassar, M.S.; Anderson, D.R.; Morgan, G.J. Comparison of Self-Expandable and Balloon-Expanding Stents for Hybrid Ductal Stenting in Hypoplastic Left Heart Complex. Cardiol. Young 2017, 27, 837–845. [Google Scholar] [CrossRef]
- Goreczny, S.; Qureshi, S.; Rosenthal, E.; Krasemann, T.; Bedair, R.; Salih, C.; Austin, C.; Anderson, D.; Morgan, G.J. Self-Expanding Stent Implantation in Arterial Duct during Hybrid Palliation of Hypoplastic Left Heart Syndrome: Midterm Experience with a Specially Designed Stent. EuroIntervention 2015, 10, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M. Stent Implantation of the Arterial Duct in Newborns with Duct-Dependent Circulation. Eur. Heart J. 1998, 19, 1401–1409. [Google Scholar] [CrossRef]
- Aggarwal, V.; Dhillon, G.S.; Penny, D.J.; Gowda, S.T.; Qureshi, A.M. Drug-Eluting Stents Compared with Bare Metal Stents for Stenting the Ductus Arteriosus in Infants with Ductal-Dependent Pulmonary Blood Flow. Am. J. Cardiol. 2019, 124, 952–959. [Google Scholar] [CrossRef]
- McMullan, D.M.; Permut, L.C.; Jones, T.K.; Johnston, T.A.; Rubio, A.E. Modified Blalock-Taussig Shunt versus Ductal Stenting for Palliation of Cardiac Lesions with Inadequate Pulmonary Blood Flow. J. Thorac. Cardiovasc. Surg. 2014, 147, 397–403. [Google Scholar] [CrossRef]
- Haddad, R.N.; Hanna, N.; Charbel, R.; Daou, L.; Chehab, G.; Saliba, Z. Ductal Stenting to Improve Pulmonary Blood Flow in Pulmonary Atresia with Intact Ventricular Septum and Critical Pulmonary Stenosis after Balloon Valvuloplasty. Cardiol. Young 2019, 29, 492–498. [Google Scholar] [CrossRef]
- Mallula, K.; Vaughn, G.; El-Said, H.; Lamberti, J.J.; Moore, J.W. Comparison of Ductal Stenting versus Surgical Shunts for Palliation of Patients with Pulmonary Atresia and Intact Ventricular Septum. Catheter. Cardiovasc. Interv. 2015, 85, 1196–1202. [Google Scholar] [CrossRef]
- Schranz, D.; Michel-Behnke, I.; Heyer, R.; Vogel, M.; Bauer, J.; Valeske, K.; Akintürk, H.; Jux, C. Stent Implantation of the Arterial Duct in Newborns with a Truly Duct-Dependent Pulmonary Circulation: A Single-Center Experience with Emphasis on Aspects of the Interventional Technique. J. Interv. Cardiol. 2010, 23, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Celebi, A.; Yucel, I.K.; Bulut, M.O.; Kucuk, M.; Balli, S. Stenting of the Ductus Arteriosus in Infants with Functionally Univentricular Heart Disease and Ductal-Dependent Pulmonary Blood Flow: A Single-Center Experience. Catheter. Cardiovasc. Interv. 2017, 89, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.; Palladino, M.T.; Capozzi, G.; Iacono, C.; Russo, M.G.; Calabrò, R. Pulmonary Artery Growth Following Arterial Duct Stenting in Congenital Heart Disease with Duct-Dependent Pulmonary Circulation. Catheter. Cardiovasc. Interv. 2009, 74, 1072–1076. [Google Scholar] [CrossRef]
- Alwi, M.; Choo, K.K.; Latiff, H.A.; Kandavello, G.; Samion, H.; Mulyadi, M.D. Initial Results and Medium-Term Follow-up of Stent Implantation of Patent Ductus Arteriosus in Duct-Dependent Pulmonary Circulation. J. Am. Coll. Cardiol. 2004, 44, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Melvin, T.; Kenny, D.; Gewillig, M.; Fraser, A.G. Orphan Medical Devices and Pediatric Cardiology – What Interventionists in Europe Need to Know, and What Needs to be Done. Pediatr. Cardiol. 2023, 44, 271–279. [Google Scholar] [CrossRef]
- Peiris, V.; Xu, K.; Agler, H.L.; Chen, E.A.; Gopal-Srivastava, R.; Lappin, B.M.; Lewis, D.Y.; Rao, G.R. Children and Adults with Rare Diseases Need Innovative Medical Devices. J. Med. Device 2018, 12, 034701. [Google Scholar] [CrossRef]
- Holzer, R.; Hijazi, Z. The Off-versus on-Label Use of Medical Devices in Interventional Cardiovascular Medicine?: Clarifying the Ambiguity between Regulatory Labeling and Clinical Decision Making, Part III: Structural Heart Disease Interventions. Catheterizatio. Cardiovasc. Interv. 2008, 72, 848–852. [Google Scholar] [CrossRef]
- Matsui, H.; McCarthy, K.; Ho, S. Morphology of the Patent Arterial Duct: Features Relevant to Treatment. Images Paediatr. Cardiol. 2008, 10, 27–38. [Google Scholar]
- Chan, K.C.; Mashburn, C.; Boucek, M.M. Initial Transcatheter Palliation of Hypoplastic Left Heart Syndrome. Catheter. Cardiovasc. Interv. 2006, 68, 719–726. [Google Scholar] [CrossRef]
- Boucek, M.M.; Mashburn, C.; Kunz, E.; Chan, K.-C. Ductal Anatomy. Pediatr. Cardiol. 2005, 26, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Gewillig, M.; Boshoff, D.E.; Dens, J.; Mertens, L.; Benson, L.N. Stenting the Neonatal Arterial Duct in Duct-Dependent Pulmonary Circulation: New Techniques, Better Results. J. Am. Coll. Cardiol. 2004, 43, 107–112. [Google Scholar] [CrossRef]
- Qureshi, A.M.; Goldstein, B.H.; Glatz, A.C.; Agrawal, H.; Aggarwal, V.; Ligon, R.A.; McCracken, C.; McDonnell, A.; Buckey, T.M.; Whiteside, W.; et al. Classification Scheme for Ductal Morphology in Cyanotic Patients with Ductal Dependent Pulmonary Blood Flow and Association with Outcomes of Patent Ductus Arteriosus Stenting. Catheter. Cardiovasc. Interv. 2019, 93, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Hinek, A.; Chaturvedi, R.R.; Almeida, C.L.; Honjo, O.; Koren, G.; Benson, L.N. Rapamycin-Eluting Stents in the Arterial Duct Experimental Observations in the Pig Model. Circulation 2009, 119, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Riede, F.T.; Schneider, P.; Dähnert, I. Transient Sirolimus Serum Levels after Implantation of a Sirolimus Eluting Stent in an Infant. Clin. Res. Cardiol. 2007, 96, 508–510. [Google Scholar] [CrossRef]
- McCrossan, B.A.; McMahon, C.J.; Walsh, K.P. First Reported Use of Drug-Eluting Bioabsorbable Vascular Scaffold in Congenital Heart Disease. Catheter. Cardiovasc. Interv. 2016, 87, 324–328. [Google Scholar] [CrossRef]
- van Kalsbeek, R.J.; Krings, G.J.; Molenschot, M.M.C.; Breur, J.M.P.J. Early and Midterm Outcomes of Bare Metal Stenting in Small Children with Recurrent Aortic Coarctation. EuroIntervention 2021, 16, e1281–e1287. [Google Scholar] [CrossRef]
- Hatoum, I.; Haddad, R.N.; Saliba, Z.; Abdel Massih, T. Endovascular Stent Implantation for Aortic Coarctation: Parameters Affecting Clinical Outcomes. Am. J. Cardiovasc. Dis. 2020, 10, 528–537. [Google Scholar]
- Holzer, R.J.; Gauvreau, K.; McEnaney, K.; Watanabe, H.; Ringel, R. Long-Term Outcomes of the Coarctation of the Aorta Stent Trials. Circ. Cardiovasc. Interv. 2021, 14, E010308. [Google Scholar] [CrossRef]
- Forbes, T.J.; Gowda, S.T. Intravascular Stent Therapy for Coarctation of the Aorta. Methodist Debakey Cardiovasc. J. 2014, 10, 82. [Google Scholar] [CrossRef]
- Sadeghipour, P.; Mohebbi, B.; Firouzi, A.; Khajali, Z.; Saedi, S.; Shafe, O.; Pouraliakbar, H.R.; Alemzadeh-Ansari, M.J.; Shahdi, S.; Samiei, N.; et al. Balloon-Expandable Cheatham-Platinum Stents Versus Self-Expandable Nitinol Stents in Coarctation of Aorta: A Randomized Controlled Trial. JACC Cardiovasc. Interv. 2022, 15, 308–317. [Google Scholar] [CrossRef]
- Ewert, P.; Peters, B.; Nagdyman, N.; Miera, O.; Kühne, T.; Berger, F. Early and Mid-Term Results with the Growth Stent—A Possible Concept for Transcatheter Treatment of Aortic Coarctation from Infancy to Adulthood by Stent Implantation? Catheter. Cardiovasc. Interv. 2008, 71, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zablah, J.E.; Morgan, G.J. Pulmonary Artery Stenting. Interv. Cardiol. Clin. 2019, 8, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Stern, H.J.; Baird, C.W. A Premounted Stent That Can Be Implanted in Infants and Re-Dilated to 20 Mm: Introducing the Edwards Valeo Lifestent. Catheter. Cardiovasc. Interv. 2009, 74, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Ooi, Y.K.; Kim, S.I.H.; Gillespie, S.E.; Kim, D.W.; Vincent, R.N.; Petit, C.J. Premounted Stents for Branch Pulmonary Artery Stenosis in Children: A Short Term Solution. Catheter. Cardiovasc. Interv. 2018, 92, 1315–1322. [Google Scholar] [CrossRef]
- Cheung, Y.F.; Sanatani, S.; Leung, M.P.; Human, D.G.; Chau, A.K.; Culham, J.A. Early and Intermediate-Term Complications of Self-Expanding Stents Limit Its Potential Application in Children with Congenital Heart Disease. J. Am. Coll. Cardiol. 2000, 35, 1007–1015. [Google Scholar] [CrossRef]
- Rajan, P.; Kaur, N.; Barwad, P.; Revaiah, P.; Rohit, M. Coarctation of Aorta Intervention: When Covered Stents Should Have Been First Choice? Ann. Pediatr. Cardiol. 2021, 14, 204. [Google Scholar] [CrossRef]
- Stassen, J.; De Meester, P.; Troost, E.; Roggen, L.; Moons, P.; Gewillig, M.; Van De Bruaene, A.; Budts, W. Covered Stent Placement for Treatment of Coarctation of the Aorta: Immediate and Long-Term Results. Acta Cardiol. 2021, 76, 464–472. [Google Scholar] [CrossRef]
- Cheatham, J.P. Stenting of Coarctation of the Aorta. Catheter. Cardiovasc. Interv. 2001, 54, 112–125. [Google Scholar] [CrossRef]
- Zahn, E.M.; Abbott, E.; Tailor, N.; Sathanandam, S.; Armer, D. Preliminary Testing and Evaluation of the Renata Minima Stent, an Infant Stent Capable of Achieving Adult Dimensions. Catheter. Cardiovasc. Interv. 2021, 98, 117–127. [Google Scholar] [CrossRef]
- Quandt, D.; Knirsch, W.; Michel-Behnke, I.; Kitzmüller, E.; Obradovic, M.; Uhlemann, F.; Kretschmar, O. First-in-Man Pulmonary Artery Stenting in Children Using the Bentley® BeGrowTM Stent System for Newborns and Infants. Int. J. Cardiol. 2019, 276, 107–109. [Google Scholar] [CrossRef]
- Sizarov, A.; Boudjemline, Y. Novel Materials and Devices in the Transcatheter Management of Congenital Heart Diseases—The Future Comes Slowly (Part 1). Arch. Cardiovasc. Dis. 2016, 109, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.R.V.; Welch, T.R.; Nugent, A.W. Biodegradable Stent Use for Congenital Heart Disease. Prog. Pediatr. Cardiol. 2021, 61, 101349. [Google Scholar] [CrossRef]
- Capelli, C.; Sauvage, E.; Giusti, G.; Bosi, G.M.; Ntsinjana, H.; Carminati, M.; Derrick, G.; Marek, J.; Khambadkone, S.; Taylor, A.M.; et al. Patient-Specific Simulations for Planning Treatment in Congenital Heart Disease. Interface Focus 2018, 8, 20170021. [Google Scholar] [CrossRef]
- Biglino, G.; Corsini, C.; Schievano, S.; Dubini, G.; Giardini, A.; Hsia, T.-Y.; Pennati, G.; Taylor, A.M. Computational Models of Aortic Coarctation in Hypoplastic Left Heart Syndrome: Considerations on Validation of a Detailed 3D Model. Int. J. Artif. Organs 2014, 37, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Biglino, G.; Capelli, C.; Bruse, J.; Bosi, G.M.; Taylor, A.M.; Schievano, S. Computational Modelling for Congenital Heart Disease: How Far Are We from Clinical Translation? Heart 2017, 103, 98–103. [Google Scholar] [CrossRef]
- Marsden, A.L.; Feinstein, J.A. Computational Modeling and Engineering in Pediatric and Congenital Heart Disease. Curr. Opin. Pediatr. 2015, 27, 587–596. [Google Scholar] [CrossRef]
- Rigatelli, G.; Chiastra, C.; Pennati, G.; Dubini, G.; Migliavacca, F.; Zuin, M. Applications of Computational Fluid Dynamics to Congenital Heart Diseases: A Practical Review for Cardiovascular Professionals. Expert Rev. Cardiovasc. Ther. 2021, 19, 907–916. [Google Scholar] [CrossRef]
- Levitt, M.R.; McGah, P.M.; Aliseda, A.; Mourad, P.D.; Nerva, J.D.; Vaidya, S.S.; Morton, R.P.; Ghodke, B.V.; Kim, L.J. Cerebral Aneurysms Treated with Flow-Diverting Stents: Computational Models with Intravascular Blood Flow Measurements. Am. J. Neuroradiol. 2014, 35, 143–148. [Google Scholar] [CrossRef]
- Hewlin, R.L.; Kizito, J.P. Development of an Experimental and Digital Cardiovascular Arterial Model for Transient Hemodynamic and Postural Change Studies: “A Preliminary Framework Analysis”. Cardiovasc. Eng. Technol. 2018, 9, 1–31. [Google Scholar] [CrossRef]

| Off-Label Use | Ad Hoc for CHD | ||
|---|---|---|---|
| Balloon-Expandable | Self-Expandable | ||
| HLHS | Palmaz-Schatz (C) [43,49,63] Palmaz-Genesis (C) [68] Herculink (O) [68,69] Nir Royal (C) [69] Mega-Link (O) [69] Libertè (O) [70] Jo stent (C) [68,71,72] Omnilink (O) [68,72] Saxx stent (C) [68,71,72] Cook Formula 414 (O) [73] | Precise (O) [74] Protégé (O) [70,74,75] | Sinus-Repo-DS (C) [68] Sinus-SuperFlex-DS (O) [73,76,77,78]  |
| Pulmonary atresia | Palmaz–Schatz (C) [7,46,49,79] Multi-Link Vision (O) [80,81,82,83] Promus Premier * (O) [80] Driver (O) [83,84,85,86] Tsunami (O) [84] Libertè (O) [84,85] Ephesos (O) [85] Jostent (C) [87] Express (O) [87] Cordis Bx Velocity * (O) [87] AVE S670 (O) [87] VeriFLEX (O) [82,83] Integrity (O) [82,83] Omega (O) [82] Skylor (C) [82] Amazonia * (O) [82] | NA | NA |
| Off-Label Use | Ad Hoc for CHD | ||
|---|---|---|---|
| Balloon-Expandable | Self-Expandable | ||
| CoA | Cook Formula 418 and 535 (O) [99] Palmaz Genesis (C) [100,101,102] Ev3 Maxi (O) [102] Palmaz Genesis XD (C) [55,65] IntraStent Mega LD (O) [55] IntraStent Max LD (O) [55] Palmaz XL (C) [55] Valeo (O) [55] | Sinus-XL (C) [103] | Growth Stent (O) [104] Cheatam Platinum (C) [100,101]  |
| Pulmonary artery stenosis | iCAST Atrium * (C) [105] Palmaz Blue (C) [105] Palmaz Genesis (C) [105] Cook Formula 418 (O) [105] VeriFLEX (O) [105] IntraStent Max (O) [105] IntraStent Mega (O) [105] Edwards Valeo Lifestent (O) [106] | Protégé (O) [105] Zilver Cook (O) [105] Dynalink Biliary (O) [105] | Growth Stent (O) [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brambilla, A.; Pennati, G.; Petrini, L.; Berti, F. Stents in Congenital Heart Disease: State of the Art and Future Scenarios. Appl. Sci. 2023, 13, 9692. https://doi.org/10.3390/app13179692
Brambilla A, Pennati G, Petrini L, Berti F. Stents in Congenital Heart Disease: State of the Art and Future Scenarios. Applied Sciences. 2023; 13(17):9692. https://doi.org/10.3390/app13179692
Chicago/Turabian StyleBrambilla, Alma, Giancarlo Pennati, Lorenza Petrini, and Francesca Berti. 2023. "Stents in Congenital Heart Disease: State of the Art and Future Scenarios" Applied Sciences 13, no. 17: 9692. https://doi.org/10.3390/app13179692
APA StyleBrambilla, A., Pennati, G., Petrini, L., & Berti, F. (2023). Stents in Congenital Heart Disease: State of the Art and Future Scenarios. Applied Sciences, 13(17), 9692. https://doi.org/10.3390/app13179692







