Culturable Diversity and Biological Properties of Bacterial Endophytes Associated with the Medicinal Plants of Vernonia anthelmintica (L.) Willd
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Purification V. anthelmintica Endophytes
2.2. Molecular Identification of the Endophytic Isolates
2.2.1. DNA Isolation
2.2.2. 16S rRNA Gene Amplification
2.2.3. Restriction Fragment Length Polymorphism (RFLP) Analysis
2.2.4. Sequencing and Phylogenetic Analysis
2.3. Incubation and Extraction of Bacterial Culture
2.4. Biological Activities of Endophytic Bacteria
2.4.1. Antimicrobial Activity
2.4.2. Melanin Content Assay and Tyrosinase Activity
2.4.3. Cytotoxic Activity
2.4.4. Anti-Diabetic Activity (PTP-1B Inhibition Assay)
2.4.5. Antioxidant Activity (DPPH Radical-Scavenging Activity)
2.5. Optimization of Culture Condition
2.6. The Effect of Incubation Time on the Synthese Natural Product by Most Active Endophytic Bacteria
2.7. Scanning Electron Microscopy (SEM) Analysis of Endophytic Bacteria
2.8. High-Performace Liquid Chromatography (HPLC) Analysis
2.9. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Endophytic Bacteria
3.2. Antimicrobial Activity
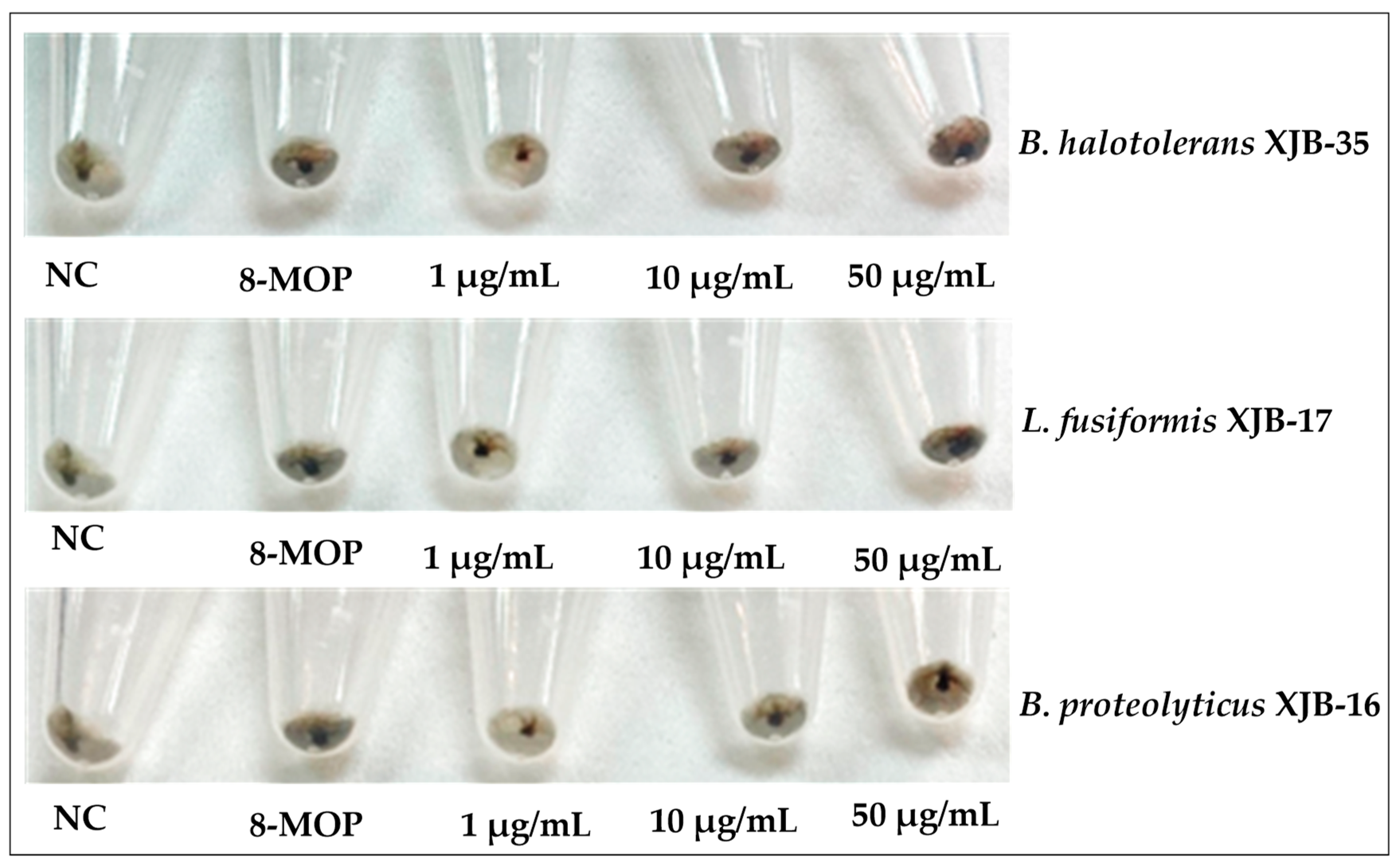
3.3. Melanin Content Assay and Tyrosinase Activity
3.4. Cytotoxic Activity
3.5. Antidiabetic Activity of the Natural Products Synthesis by Endophytic Bacteria
3.6. Antioxidant Activity of the Natural Products’ Synthesis by Endophytic Bacteria
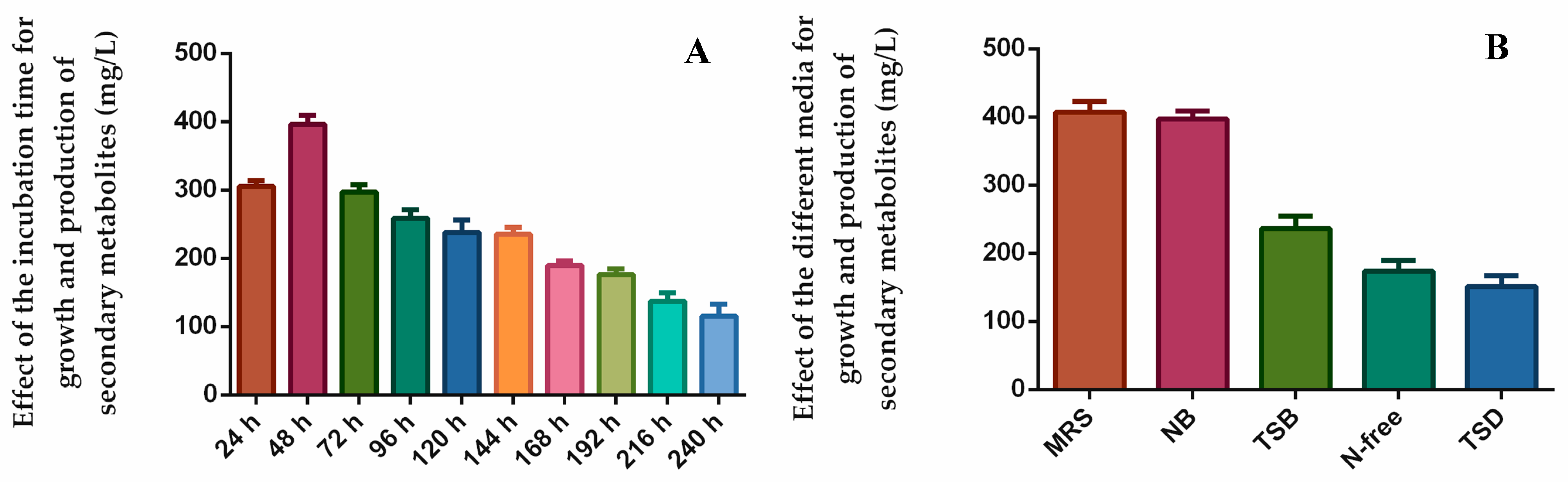
3.7. The Effect of Cultivation Time and Medium Content on the Natural Products Synthesis of Endophytic Bacteria
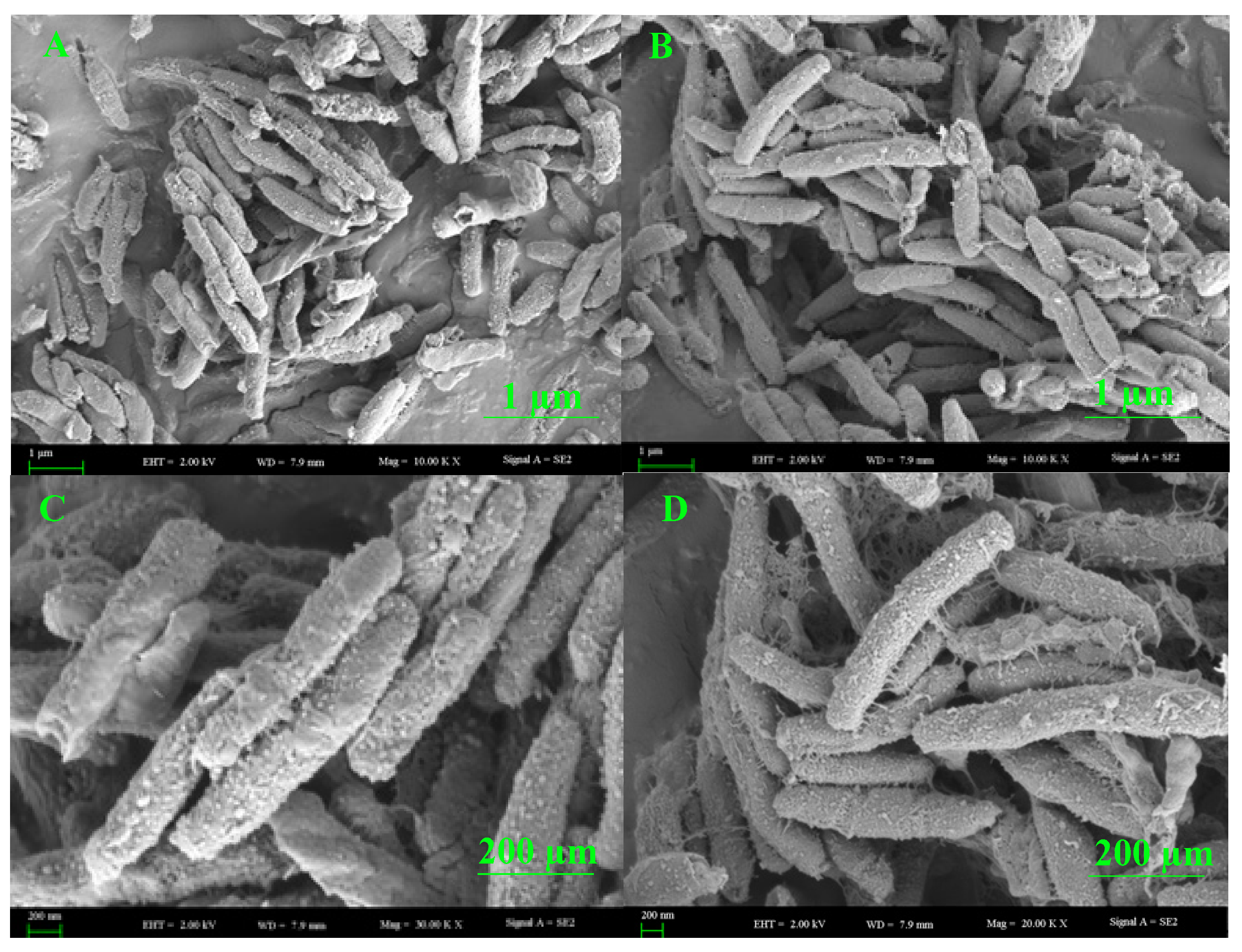
3.8. SEM Analysis of the Endophytic Bacteria B. halotolerans XJB-35
3.9. Secondary Metabolites Produced by the Most Active Endophytic Bacteria B. halotolerans XJB-35 on Different Culture Media HPLC Analysis
3.10. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, A.; Gupta, P.; Rai, N.; Tiwari, R.K.; Kumar, A.; Salvi, P.; Kamble, S.C.; Singh, S.K.; Gautam, V. Assessment of Biological Activities of Fungal Endophytes Derived Bioactive Compounds Isolated from Amoora rohituka. J. Fungi 2022, 8, 285. [Google Scholar] [CrossRef]
- Rustamova, N.; Litao, N.; Bozorov, K.; Sayyed, R.; Aisa, H.A.; Yili, A. Plant-associated endophytic fungi: A source of structurally diverse and bioactive natural products. Plant Cell Biotechnol. Mol. Biol. 2022, 23, 1–19. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Srivastava, A.K.; Tiwari, R.K. Plant growth promoting and antifungal activity in endophytic Bacillus strains from pearl millet (Pennisetum glaucum). Braz. J. Microbiol. 2020, 51, 229–241. [Google Scholar] [CrossRef]
- Rustamova, N.; Bozorov, K.; Efferth, T.; Yili, A. Novel secondary metabolites from endophytic fungi: Synthesis and biological properties. Phytochem. Rev. 2020, 19, 425–448. [Google Scholar] [CrossRef]
- Tyagi, J.; Chaudhary, P.; Mishra, A.; Khatwani, M.; Dey, S.; Varma, A. Role of Endophytes in Abiotic Stress Tolerance: With Special Emphasis on Serendipita indica. Int. J. Environ. Res. 2022, 16, 62. [Google Scholar] [CrossRef]
- Narayanan, Z.; Glick, B.R. Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes. Microorganisms 2022, 10, 2008. [Google Scholar] [CrossRef]
- Liu, S.-S.; Huang, R.; Zhang, S.-P.; Xu, T.-C.; Hu, K.; Wu, S.-H. Antimicrobial secondary metabolites from an endophytic fungus Aspergillus polyporicola. Fitoterapia 2022, 162, 105297. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Segaran, G.; Sathiavelu, M. Chapter 4—Antimicrobial metabolites from endophytic microorganisms and its mode of action. In Biocontrol Mechanisms of Endophytic Microorganisms; Radhakrishnan, E.K., Kumar, A., Aswani, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 75–88. [Google Scholar]
- Siebatcheu, E.C.; Wetadieu, D.; Youassi Youassi, O.; Bedine Boat, M.A.; Bedane, K.G.; Tchameni, N.S.; Sameza, M.L. Secondary metabolites from an endophytic fungus Trichoderma erinaceum with antimicrobial activity towards Pythium ultimum. Nat. Prod. Res. 2022, 37, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Vasundhara, M.; Reddy, M.S.; Kumar, A. Chapter 18—Secondary Metabolites from Endophytic Fungi and Their Biological Activities. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–258. [Google Scholar]
- Gupta, S.; Choudhary, M.; Singh, B.; Singh, R.; Dhar, M.K.; Kaul, S. Diversity and biological activity of fungal endophytes of Zingiber officinale Rosc. with emphasis on Aspergillus terreus as a biocontrol agent of its leaf spot. Biocatal. Agric. Biotechnol. 2022, 39, 102234. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Proksch, P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011, 49, 1–12. [Google Scholar] [CrossRef]
- Tang, Z.; Qin, Y.; Chen, W.; Zhao, Z.; Lin, W.; Xiao, Y.; Chen, H.; Liu, Y.; Chen, H.; Bu, T.; et al. Diversity, Chemical Constituents, and Biological Activities of Endophytic Fungi Isolated from Ligusticum chuanxiong Hort. Front. Microbiol. 2021, 12, 771000. [Google Scholar] [CrossRef]
- Gao, H.; Li, G.; Lou, H.-X. Structural Diversity and Biological Activities of Novel Secondary Metabolites from Endophytes. Molecules 2018, 23, 646. [Google Scholar] [CrossRef]
- Xian, P.-J.; Liu, S.-Z.; Wang, W.-J.; Yang, S.-X.; Feng, Z.; Yang, X.-L. Undescribed specialised metabolites from the endophytic fungus Emericella sp. XL029 and their antimicrobial activities. Phytochemistry 2022, 202, 113303. [Google Scholar] [CrossRef]
- Hagag, A.; Abdelwahab, M.F.; Abd El-kader, A.M.; Fouad, M.A. The endophytic Aspergillus strains: A bountiful source of natural products. J. Appl. Microbiol. 2022, 132, 4150–4169. [Google Scholar] [CrossRef]
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.; Xie, L.; Ran, Y.; Hu, Y. Endophytic Fungi: An Effective Alternative Source of Plant-Derived Bioactive Compounds for Pharmacological Studies. J. Fungi 2022, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Wani, A.K.; Dhanjal, D.S.; Mukherjee, S. Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: Resilience to biotic and abiotic stresses. World J. Microbiol. Biotechnol. 2022, 38, 79. [Google Scholar] [CrossRef]
- Niu, L.; Rustamova, N.; Ning, H.; Paerhati, P.; Lu, C.; Yili, A. Diversity and Biological Activities of Endophytic Fungi from the Flowers of the Medicinal Plant Vernonia anthelmintica. Int. J. Mol. Sci. 2022, 23, 11935. [Google Scholar] [CrossRef]
- Rustamova, N.; Gao, Y.; Zhang, Y.; Yili, A. Biological Activity of Endophytic Fungi from the Roots of the Medicinal Plant Vernonia anthelmintica. Microorganisms 2020, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Rustamova, N.; Bobakulov, K.; Litao, N.; Nuerxiati, R.; Wali, A.; Setzer, W.N.; Yili, A. Secondary Metabolites and their Biological Activities from Endophytic Fungal Strain Aspergillus terreus XJA8 Associated with Vernonia anthelmintica. J. Biol. Act. Prod. Nat. 2022, 12, 421–435. [Google Scholar] [CrossRef]
- Mora-Ruiz, M.D.R.; Font-Verdera, F.; Díaz-Gil, C.; Urdiain, M.; Rodríguez-Valdecantos, G.; González, B.; Orfila, A.; Rosselló-Móra, R. Moderate halophilic bacteria colonizing the phylloplane of halophytes of the subfamily Salicornioideae (Amaranthaceae). Syst. Appl. Microbiol. 2015, 38, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Rustamova, N.; Wubulikasimu, A.; Mukhamedov, N.; Gao, Y.; Egamberdieva, D.; Yili, A. Endophytic Bacteria Associated with Medicinal Plant Vernonia anthelmintica: Diversity and Characterization. Curr. Microbiol. 2020, 77, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. Res. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Jinneman, K.C.; Wetherington, J.H.; Adams, A.M.; Johnson, J.M.; Tenge, B.J.; Dang, N.-L.; Hill, W.E. Differentiation of Cyclospora sp. and Eimeria spp. by Using the Polymerase Chain Reaction Amplification Products and Restriction Fragment Length Polymorphisms. Food and Drug Administration Laboratory Information Bulletin LIB No. 4044. 1996. Available online: http://vm.cfsan.fda.gov/%E2%88%BCmow/kjcs19c.html (accessed on 17 July 2023).
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, S.; Li, Y.; Zheng, M.; Xi, X.; Cao, H.; Cui, X.; Guo, H.; Han, C. Yield enhancement strategies of rare pharmaceutical metabolites from endophytes. Biotechnol. Lett. 2018, 40, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-J.; Li, Y.-S.; Xia, B.; Li, W.-P.; Pang, L.; Tong, Z.-D. Optimization of medium composition and culture conditions for antifungal activity of a tomato endophytic bacterium. Biol. Control. 2015, 82, 69–75. [Google Scholar] [CrossRef]
- Nongkhlaw, F.M.W.; Joshi, S.R. Microscopic study on colonization and antimicrobial property of endophytic bacteria associated with ethnomedicinal plants of Meghalaya. J. Microsc. Ultrastruct. 2017, 5, 132–139. [Google Scholar]
- Pan, Y.; Jin, H.; Yang, S.; Liu, H. Changes of volatile organic compounds and bioactivity of Alternaria brassicae GL07 in different ages. J. Basic Microbiol. 2019, 59, 713–722. [Google Scholar] [CrossRef]
- Seo, W.T.; Lim, W.J.; Kim, E.J.; Yun, H.D.; Lee, Y.H.; Cho, K.M. Endophytic Bacterial Diversity in the Young Radish and Their Antimicrobial Activity against Pathogens. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 493–503. [Google Scholar] [CrossRef]
- Christina, A.; Christapher, V.; Bhore, S.J. Endophytic bacteria as a source of novel antibiotics: An overview. Pharmacogn. Rev. 2013, 7, 11–16. [Google Scholar]
- Wang, P.; Yu, J.-H.; Zhu, K.; Wang, Y.; Cheng, Z.-Q.; Jiang, C.-S.; Dai, J.-G.; Wu, J.; Zhang, H. Phenolic bisabolane sesquiterpenoids from a Thai mangrove endophytic fungus, Aspergillus sp. xy02. Fitoterapia 2018, 127, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, G.; Sun, M.; Yang, X.; Xu, J. A new antimicrobial sesquiterpene isolated from endophytic fungus Cytospora sp. from the Chinese mangrove plant Ceriops tagal. Nat. Prod. Res. 2018, 34, 1404–1408. [Google Scholar] [CrossRef]
- He, J.; Yang, M.-S.; Wang, W.-X.; Li, Z.-H.; Elkhateeb, W.A.M.; Wen, T.-C.; Ai, H.-L.; Feng, T. Anti-phytopathogenic sesquiterpenoid-xanthone adducts from potato endophytic fungus Bipolaris eleusines. RSC Adv. 2019, 9, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-C.; Wang, Y.-L.; Zhang, T.-Y.; Chen, Z.-J.; Yang, T.-M.; Wu, Y.-Y.; Sun, C.-P.; Ma, X.-C.; Zhang, Y.-X. Indole diterpenoids from the endophytic fungus Drechmeria sp. as natural antimicrobial agents. Phytochemistry 2018, 148, 21–28. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Meyskens, F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef]
- Li, H.-R.; Habasi, M.; Xie, L.-Z.; Aisa, H.A. Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef]
- Turak, A.; Maimaiti, Z.; Ma, H.; Aisa, H.A. Pseudo-disesquiterpenoids from seeds of Vernonia anthelmintica and their biological activities. Phytochem. Lett. 2017, 21, 163–168. [Google Scholar] [CrossRef]
- Maimaiti, Z.; Turak, A.; Aisa, H.A. Two new compounds from the seeds of Vernonia anthelmintica. J. Asian Nat. Prod. Res. 2017, 19, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Rustamova, N.; Bobakulov, K.; Begmatov, N.; Turak, A.; Yili, A.; Aisa, H.A. Secondary metabolites produced by endophytic Pantoea ananatis derived from roots of Baccharoides anthelmintica and their effect on melanin synthesis in murine B16 cells. Nat. Prod. Res. 2021, 35, 796–801. [Google Scholar] [CrossRef]
- Ebada, S.S.; El-Neketi, M.; Ebrahim, W.; Mándi, A.; Kurtán, T.; Kalscheuer, R.; Müller, W.E.G.; Proksch, P. Cytotoxic secondary metabolites from the endophytic fungus Aspergillus versicolor KU258497. Phytochem. Lett. 2018, 24, 88–93. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.-Q.; Tang, J.-J.; Zhang, A.-L.; Gao, J.-M. Secondary Metabolites from the Endophytic Botryosphaeria dothidea of Melia azedarach and Their Antifungal, Antibacterial, Antioxidant, and Cytotoxic Activities. J. Agric. Food Chem. 2014, 62, 3584–3590. [Google Scholar] [CrossRef]
- Deng, M.; Tao, L.; Qiao, Y.; Sun, W.; Xie, S.; Shi, Z.; Qi, C.; Zhang, Y. New cytotoxic secondary metabolites against human pancreatic cancer cells from the Hypericum perforatum endophytic fungus Aspergillus terreus. Fitoterapia 2020, 146, 104685. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Huang, C.; Li, J.; Chen, T.; Tang, J.; Liu, W.; Long, Y. Isolation, Structural Characterization and Antidiabetic Activity of New Diketopiperazine Alkaloids from Mangrove Endophytic Fungus Aspergillus sp. Mar. Drugs 2021, 19, 402. [Google Scholar] [CrossRef]
- Ramírez-Espinosa, J.J.; Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Paoli, P.; Camici, G.; Navarrete-Vázquez, G.; Ortiz-Andrade, R.; Estrada-Soto, S. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP–1B: In vitro, in silico, and in vivo approaches. Eur. J. Med. Chem. 2011, 46, 2243–2251. [Google Scholar] [CrossRef]
- Malik, A.; Ardalani, H.; Anam, S.; McNair, L.M.; Kromphardt, K.J.K.; Frandsen, R.J.N.; Franzyk, H.; Staerk, D.; Kongstad, K.T. Antidiabetic xanthones with α-glucosidase inhibitory activities from an endophytic Penicillium canescens. Fitoterapia 2020, 142, 104522. [Google Scholar] [CrossRef]
- Druzian, S.P.; Pinheiro, L.N.; Susin, N.M.B.; Dal Prá, V.; Mazutti, M.A.; Kuhn, R.C.; Terra, L.d.M. Production of metabolites with antioxidant activity by Botryosphaeria dothidea in submerged fermentation. Bioprocess Biosyst. Eng. 2020, 43, 13–20. [Google Scholar] [CrossRef]
- da Silva, A.A.; Polonio, J.C.; Bulla, A.M.; Polli, A.D.; Castro, J.C.; Soares, L.C.; de Oliveira-Junior, V.A.; Vicentini, V.E.P.; de Oliveira, A.J.B.; Gonçalves, J.E.; et al. Antimicrobial and antioxidant activities of secondary metabolites from endophytic fungus Botryosphaeria fabicerciana (MGN23-3) associated to Morus nigra L. Nat. Prod. Res. 2022, 36, 3158–3162. [Google Scholar] [CrossRef]
- Pudjas, N.T.G.; Mubarik, N.R.; Astuti, R.I.; Sudirman, L.I. Antioxidant Activity of Endophytic Bacteria Derived from Hoya multiflora Blume Plant and Their Cellular Activities on Schizosaccharomyces pombe. AYATI J. Biosci. 2022, 29, 214–221. [Google Scholar] [CrossRef]
- Sharma, V.; Singamaneni, V.; Sharma, N.; Kumar, A.; Arora, D.; Kushwaha, M.; Bhushan, S.; Jaglan, S.; Gupta, P. Valproic acid induces three novel cytotoxic secondary metabolites in Diaporthe sp., an endophytic fungus from Datura inoxia Mill. Bioorg. Med. Chem. Lett. 2018, 28, 2217–2221. [Google Scholar] [CrossRef]
- Sun, B.; Jing, R.; Wang, Z.; Tian, L.; Mao, F.; Liu, Y. Diversity and community structure of endophytic Bacillus with antagonistic and antioxidant activity in the fruits of Xisha Wild Noni (Morinda citrifolia L.). Microb. Pathog. 2021, 158, 105065. [Google Scholar] [CrossRef]
- Kumar, V.; Sahai, V.; Bisaria, V.S. High-density spore production of Piriformospora indica, a plant growth-promoting endophyte, by optimization of nutritional and cultural parameters. Bioresour. Technol. 2011, 102, 3169–3175. [Google Scholar] [CrossRef]
- Chandrakar, S.; Gupta, A.K. Actinomycin-Producing Endophytic Streptomyces parvulus Associated with Root of Aloe vera and Optimization of Conditions for Antibiotic Production. Probiotics Antimicrob. Proteins 2019, 11, 1055–1069. [Google Scholar] [CrossRef]
- Deka, D.; Jha, D.K. Optimization of Culture Parameters for Improved Production of Bioactive Metabolite by Endophytic Geosmithia pallida (KU693285) Isolated from Brucea mollis Wall ex. Kurz, An Endangered Medicinal Plant. J. Pure Appl. Microbiol. 2018, 12, 1205–1213. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.-K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Abdelshafy Mohamad, O.A.; Ma, J.-B.; Liu, Y.-H.; Zhang, D.; Hua, S.; Bhute, S.; Hedlund, B.P.; Li, W.-J.; Li, L. Beneficial Endophytic Bacterial Populations Associated with Medicinal Plant Thymus vulgaris Alleviate Salt Stress and Confer Resistance to Fusarium oxysporum. Front. Plant Sci. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Passari, A.K.; Chandra, P.; Leo, V.V.; Kumar, B.; Uthandi, S.; Thankappan, S.; Gupta, V.K.; Singh, B.P. Determination and production of antimicrobial compounds by Aspergillus clavatonanicus strain MJ31, an endophytic fungus from Mirabilis jalapa L. using UPLC-ESI-MS/MS and TD-GC-MS analysis. PLoS ONE 2017, 12, e0186234. [Google Scholar] [CrossRef]
- Alsultan, W.; Vadamalai, G.; Khairulmazmi, A.; Saud, H.M.; Al-Sadi, A.M.; Rashed, O.; Jaaffar, A.K.M.; Nasehi, A. Isolation, identification and characterization of endophytic bacteria antagonistic to Phytophthora palmivora causing black pod of cocoa in Malaysia. Eur. J. Plant Pathol. 2019, 155, 1077–1091. [Google Scholar] [CrossRef]
- Jayakumar, V.; Ramesh Sundar, A.; Viswanathan, R. Biocontrol of Colletotrichum falcatum with volatile metabolites produced by endophytic bacteria and profiling VOCs by headspace SPME coupled with GC–MS. Sugar Tech 2021, 23, 94–107. [Google Scholar] [CrossRef]
- Leylaie, S.; Zafari, D. Antiproliferative and Antimicrobial Activities of Secondary Metabolites and Phylogenetic Study of Endophytic Trichoderma Species from Vinca Plants. Front. Microbiol. 2018, 9, 1484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Antony, A.R.; Kannan, V.R. Exploration of endophytic microorganisms from selected medicinal plants and their control potential to multi drug resistant pathogens. J. Med. Plants Stud. 2015, 3, 49–57. [Google Scholar]



| NB | N-Free | MRS | LB | TSD |
|---|---|---|---|---|
| Peptone 5 g/L | K2HPO4 0.1 g/L | Peptone 10 g/L | Peptone 10 g/L | Tryptone 17 g/L |
| Yeast extract 3 g/L | KH2PO4 0.4 g/L | Yeast extract 0.4 g/L | Yeast extract 5 g/L | Soytone 3 g/L |
| NaCl 5 g/L | MgSO4 0.2 g/L | Beef extract 10 g/L | NaCl 5 g/L | Dextrose 2.5 g/L |
| NaCl 0.1 g/L | Glucose 20 g/L | NaCl 5 g/L | ||
| C2H9NaO5 0.5 g/L | K2HPO4 2.5 g/L | |||
| Tween 80 1 mL | ||||
| K2HPO4 0.2 g/L |
| Isolated Strains Sequences Deposited in GenBank | Closest Match among Bacteria (16S rRNA Genes) (GenBANK) | |||
|---|---|---|---|---|
| Strains | Accession Number | Species | Source | ID% |
| XJB-5 | MW820297 | B. haynesii | stem | 99.26% |
| XJB-16 | MW876136 | B. proteolyticus | stem | 99.79% |
| XJB-35 | MW876143 | B. halotolerans | stem | 99.93% |
| XJB-71 | MW876161 | B. safensis | stem | 100% |
| XJB-7 | MW876130 | P. punonensis | stem | 99.65% |
| XJB-17 | MW876137 | L. fusiformis | stem | 99.86% |
| XJB-66 | MW876158 | S. lutetiensis | stem | 99.79% |
| XJB-12 | MW876133 | L. adecarboxylata | stem | 99.08% |
| XJB-14 | MW876135 | P. alvei | stem | 99.94% |
| XJB-62 | MW876157 | P. agglomerans | stem | 100% |
| Sample | Sample Concentration (μg/mL) | Sample Amount (μL) | C. albicans (mm ZOI) | E. coli (mm ZOI) | S. aureus (mm ZOI) |
|---|---|---|---|---|---|
| Ampicillin | 4.88 | 20 | NT | 12.5 | 10 |
| Amphotericin B | 5 | 20 | 15 | NT | NT |
| B. haynesii XJB-5 | 50 | 20 | 11.5 | 15 | 14 |
| B. proteolyticus XJB-16 | 50 | 20 | 11 | NA | 16 |
| B. halotolerans XJB-35 | 50 | 20 | 10.5 | 17.5 | 18 |
| B. safensis XJB-71 | 50 | 20 | 8.5 | 12.5 | 14 |
| P. punonensis XJB-7 | 50 | 20 | 12 | 10 | 16 |
| L. fusiformis XJB-17 | 50 | 20 | 10 | NA | 16 |
| S. lutetiensis XJB-66 | 50 | 20 | 9.5 | 15 | 17 |
| L. adecarboxylata XJB-12 | 50 | 20 | 10.5 | 8.5 | 18 |
| P. alvei XJB-14 | 50 | 20 | 14 | 13 | 14 |
| P. agglomerans XJB-62 | 50 | 20 | 11 | 11 | 12 |
| Experimental Group | Concentration | Relative Melanin Content (%) |
|---|---|---|
| NC | NC | 100.0 ± 3.465 |
| 8-MOP-50µM | 50 µM | 129.9 ± 4.179 |
| B. haynesii XJB-5 | 50 µg/mL | 197.5 ± 18.65 |
| B. proteolyticus XJB-16 | 50 µg/mL | 168.3 ± 17.5 |
| B. halotolerans XJB-35 | 50 µg/mL | 226.1 ± 16.57 |
| B. safensis XJB-71 | 50 µg/mL | 128.1 ± 4.226 |
| P. punonensis XJB-7 | 50 µg/mL | 123.5 ± 5.616 |
| L. fusiformis XJB-17 | 50 µg/mL | 164.5 ± 15.68 |
| S. lutetiensis XJB-66 | 50 µg/mL | 105.6 ± 7.373 |
| L. adecarboxylata XJB-12 | 50 µg/mL | 152.7 ± 7.989 |
| P. alvei XJB-14 | 50 µg/mL | 108.5 ± 7.363 |
| P. agglomerans XJB-62 | 50 µg/mL | 145.6 ± 7.373 |
| Experimental Group | Concentration | Relative Melanin Content (%) | Relative Tyrosinase Activity (%) |
|---|---|---|---|
| NC | 50 µM | 100.0 ± 5.193 | 100.0 ± 5.193 |
| 8-MOP | 50 µM | 132.0 ± 2.818 | 124.1 ± 3.172 |
| B. halotolerans XJB-35 | 1 µg/mL | 138.6 ± 7.638 | 123.1 ± 3.761 |
| 10 µg/mL | 161.5 ± 3.751 | 130.0 ± 5.653 | |
| 50 µg/mL | 229.9 ± 6.737 | 154.7 ± 3.266 | |
| B. haynesii XJB-5 | 1 µg/mL | 185.6 ± 12.51 | 125.0 ± 4.56 |
| 10 µg/mL | 191.5 ± 76.61 | 131.4 ± 3.94 | |
| 50 µg/mL | 198.2 ± 18.65 | 138.7 ± 2382 | |
| L. fusiformis XJB-17 | 1 µg/mL | 129.3 ± 10.02 | 115.3 ± 7.194 |
| 10 µg/mL | 139.8 ± 1.822 | 118.3 ± 6.790 | |
| 50 µg/mL | 183.8 ± 7.762 | 121.9 ± 2.753 | |
| B. proteolyticus XJB-16 | 1 µg/mL | 118.8 ± 11.50 | 116.9 ± 5.334 |
| 10 µg/mL | 138.1 ± 7.450 | 103.7 ± 6.514 | |
| 50 µg/mL | 179.0 ± 2.288 | 146.6 ± 5.417 |
| Samples | Cell Lines | ||
|---|---|---|---|
| IC50 (μg/mL) | |||
| HT-29 (μg/mL) | MCF-7 (μg/mL) | HeLa (μg/mL) | |
| B. haynesii XJB-5 | 45.10 ± 0.004 | Not active | 65.6 ± 0.15 |
| B. proteolyticus XJB-16 | 32.41 ± 2.20 | Not active | 29.38 ± 1.27 |
| B. halotolerans XJB-35 | 15.07 ± 0.34 | 19.05 ± 0.90 | 11.39 ± 0.23 |
| B. safensis XJB-71 | 23.45 ± 0.15 | 28.72 ± 0.34 | 44.89 ± 0.43 |
| P. punonensis XJB-7 | 78.37 ± 1.82 | 53.98 ± 1.14 | 47.01 ± 2.08 |
| L. fusiformis XJB-17 | 33.098 ± 1.03 | 25.98 ± 1.66 | 27.11 ± 0.34 |
| S. lutetiensis XJB-66 | 36.5 ± 1.10 | 33.9 ± 0.78 | 22.09 ± 0.005 |
| L. adecarboxylata XJB-12 | 34.21 ± 2.20 | 57.6 ± 1.04 | 39.58 ± 1.08 |
| P. alvei XJB-14 | 38.6 ± 1.054 | 29.99 ± 0.89 | 25.29 ± 0.15 |
| P. agglomerans XJB-62 | 36.24 ± 0.85 | 29.55 ± 1.05 | 43.008 ± 0.23 |
| DOX | 0.82 ± 0.041 | 0.17 ± 0.006 | 33.11 ± 0.005 |
| Sample | IC50 (µg/mL) |
|---|---|
| B. haynesii XJB-5 | 13.24 ± 0.53 |
| B. proteolyticus XJB-16 | 19.15 ± 0.23 |
| B. halotolerans XJB-35 | 4.93 ± 0.29 |
| B. safensis XJB-71 | 16.54 ± 0.77 |
| P. punonensis XJB-7 | 6.18 ± 1.45 |
| L. fusiformis XJB-17 | 18.08 ± 0.78 |
| S. lutetiensis XJB-66 | 29.17 ± 0.57 |
| L. adecarboxylata XJB-12 | 8.08 ± 0.81 |
| P. alvei XJB-14 | 7.62 ± 0.46 |
| P. agglomerans XJB-62 | No effect |
| PTP1B inhibitor | 1.59 ± 0.40 |
| Sample | IC50 (μg/mL) |
|---|---|
| B. haynesii XJB-5 | 78.38 ± 1.06 |
| B. proteolyticus XJB-16 | 161.58 ± 2.06 |
| B. halotolerans XJB-35 | 35.453 ± 3.25 |
| B. safensis XJB-71 | 56.41 ± 0.7 |
| P. punonensis XJB-7 | No effect |
| L. fusiformis XJB-17 | 143.18 ± 7.06 |
| S. lutetiensis XJB-66 | 115.75 ± 0.65 |
| L. adecarboxylata XJB-12 | 36.21 ± 0.3 |
| P. alvei XJB-14 | No effect |
| P. agglomerans XJB-62 | 153.98 ± 2.06 |
| Vitamin C | 5.87 ± 0.52 |
| № | Composition | Rt (min) | Relative Peak Area % | ||||
|---|---|---|---|---|---|---|---|
| NB | N-free | TSD | LB | MRS | |||
| 1 | Butanoic acid, 3-methyl- | 3.410 | 3.19 | 19.03 | |||
| 2 | Butanoic acid, 2-methyl- | 3.690 | 2.61 | ||||
| 3 | p-Xylene | 3.741 | 1.31 | 2.22 | |||
| 4 | Phenylethyl Alcohol | 7.139 | 0.25 | ||||
| 5 | Benzoic acid | 7.844 | 0.15 | ||||
| 6 | Benzeneacetic acid, methyl ester | 8.057 | 1.19 | ||||
| 7 | Benzeneacetic acid | 9.085 | 1.62 | ||||
| 8 | Indole | 9.756 | 0.13 | ||||
| 9 | Benzenepropanoic acid, .alpha.-hydroxy-, methyl ester | 10.775 | 0.30 | ||||
| 10 | Orcinol | 10.852 | 6.62 | ||||
| 11 | 1,3-Benzenediol, 4,5-dimethyl- | 11.412 | 11.48 | ||||
| 12 | 13-Methyltetradecanal | 11.999 | 1.13 | ||||
| 13 | 2,2′-Isopropylidenebis(5 methylfuran) | 14.343 | 11.13 | ||||
| 14 | 2-Tridecen-1-ol, (E)- | 14.403 | 0.44 | ||||
| 15 | Pyrrolo[1,2-a]pyrazine-1,4 dione, hexahydro- | 15.337 | 0.33 | ||||
| 16 | Methyl 13-methyltetradecanoate | 15.609 | 0.68 | 0.64 | |||
| 17 | Methyl 9-methyltetradecanoate | 15.609 | 0.71 | ||||
| 18 | Diethyltrisulphide | 17.206 | 2.92 | ||||
| 19 | Pyrrolo[1,2-a]pyrazine-1,4dione, hexahydro-3-(2 methylpropyl)- | 17.317 | 4.45 | ||||
| 20 | Hexadecanoic acid, methyl ester | 17.223 | 7.33 | ||||
| 21 | Pyrrolo[1,2-a]pyrazine-1,4dione, hexahydro-3-(2-methylpropyl)- | 17.512 | 1.46 | 2.41 | |||
| 22 | Dibutyl phthalate | 17.775 | 1.48 | 1.72 | 1.29 | ||
| 23 | Tetradecanamide | 18.684 | 0.55 | ||||
| 24 | 9-Octadecenamide, (Z)- | 18.684 | 0.78 | ||||
| 25 | 9-Hexadecen-1-ol, (Z)- | 18.965 | 0.26 | ||||
| 26 | 2-[2-[2-[2-[2-[2-[2-(Hydroxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]e hanol | 19.126 | 0.30 | ||||
| 27 | 1-Octadecene | 19.271 | 0.82 | ||||
| 28 | 9-Octadecenoic acid (Z)-, methyl ester | 19.517 | 1.38 | 16.05 | 0.39 | 1.17 | |
| 29 | Cis-13-Octadecenoic acid, methyl ester | 19.593 | 0.30 | ||||
| 30 | Heptadecanoic acid, 16-methyl-, methyl ester | 19.848 | 2.24 | ||||
| 31 | Methyl stearate | 19.848 | 0.29 | ||||
| 32 | Methyl 7,12-octadecadienoate | 20.112 | 0.18 | ||||
| 33 | Methyl 10-trans,12-cis octadecadienoate | 20.111 | 7.55 | 0.15 | |||
| 34 | Hexadecanamide | 20.604 | 0.65 | ||||
| 35 | 1,4,7,10,13,16-Hexaoxacyclooctadecane | 23.068 | 1.06 | ||||
| 36 | Pyrrolo[1,2-a]pyrazine-1,4dione, hexahydro-3-(phenylmethyl)- | 23.552 | 2.18 | 26.25 | 29.16 | 12.23 | |
| 37 | 9-Octadecenamide, (Z)- | 23.204 | 1.72 | ||||
| 38 | 2(1H)-Naphthalenone, octahydro-4a-methyl-7-(1-methylethyl)-, (4a.alpha.,7.beta.,8a.beta.)- | 23.824 | 0.29 | ||||
| 39 | Eicosane | 30.595 | 1.17 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litao, N.; Rustamova, N.; Paerhati, P.; Ning, H.-X.; Yili, A. Culturable Diversity and Biological Properties of Bacterial Endophytes Associated with the Medicinal Plants of Vernonia anthelmintica (L.) Willd. Appl. Sci. 2023, 13, 9797. https://doi.org/10.3390/app13179797
Litao N, Rustamova N, Paerhati P, Ning H-X, Yili A. Culturable Diversity and Biological Properties of Bacterial Endophytes Associated with the Medicinal Plants of Vernonia anthelmintica (L.) Willd. Applied Sciences. 2023; 13(17):9797. https://doi.org/10.3390/app13179797
Chicago/Turabian StyleLitao, Niu, Nigora Rustamova, Paiziliya Paerhati, Hui-Xia Ning, and Abulimiti Yili. 2023. "Culturable Diversity and Biological Properties of Bacterial Endophytes Associated with the Medicinal Plants of Vernonia anthelmintica (L.) Willd" Applied Sciences 13, no. 17: 9797. https://doi.org/10.3390/app13179797








