Prediction of Part Shrinkage for Injection Molded Crystalline Polymer via Cavity Pressure and Melt Temperature Monitoring
Abstract
:1. Introduction
2. Experimental Procedure
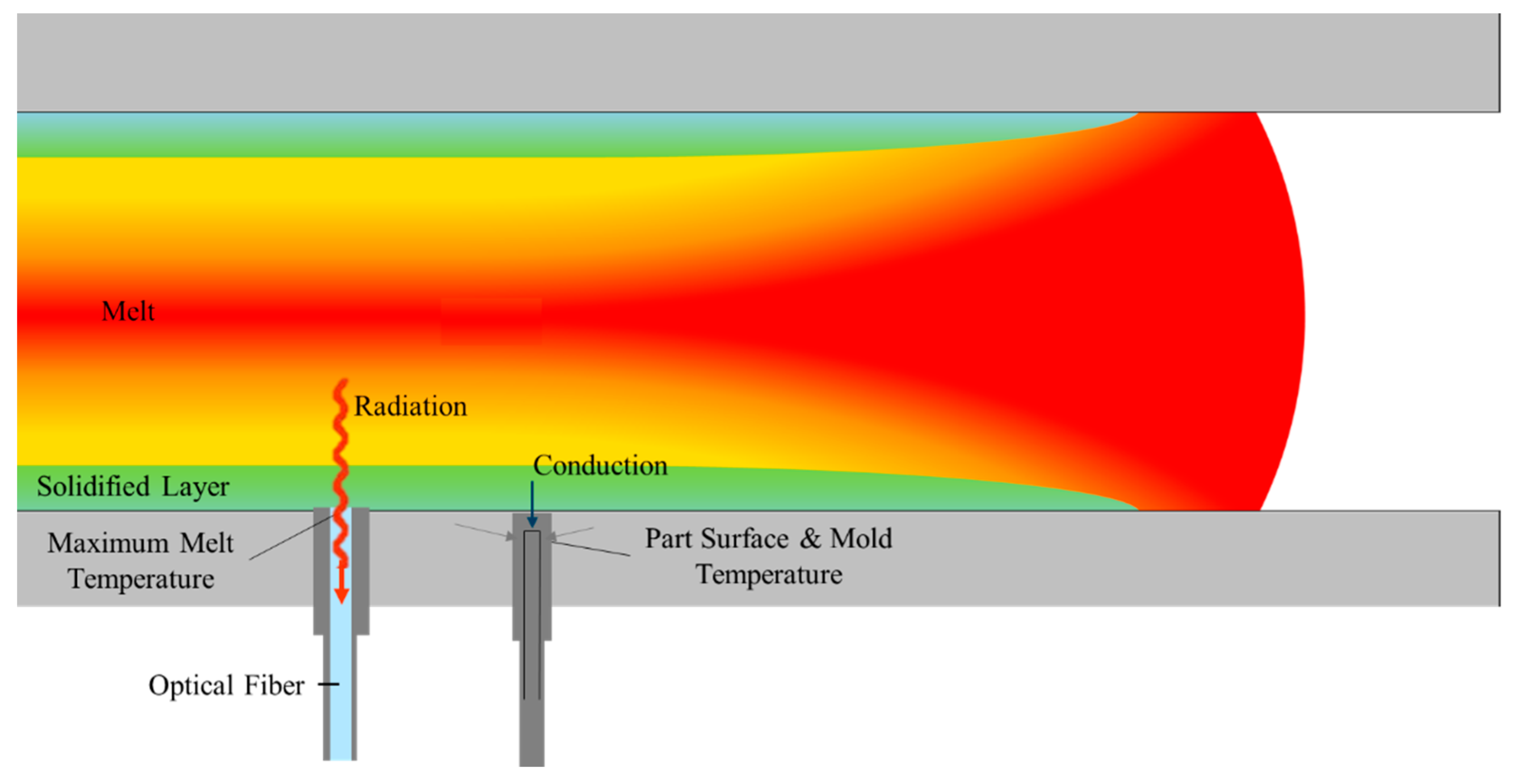
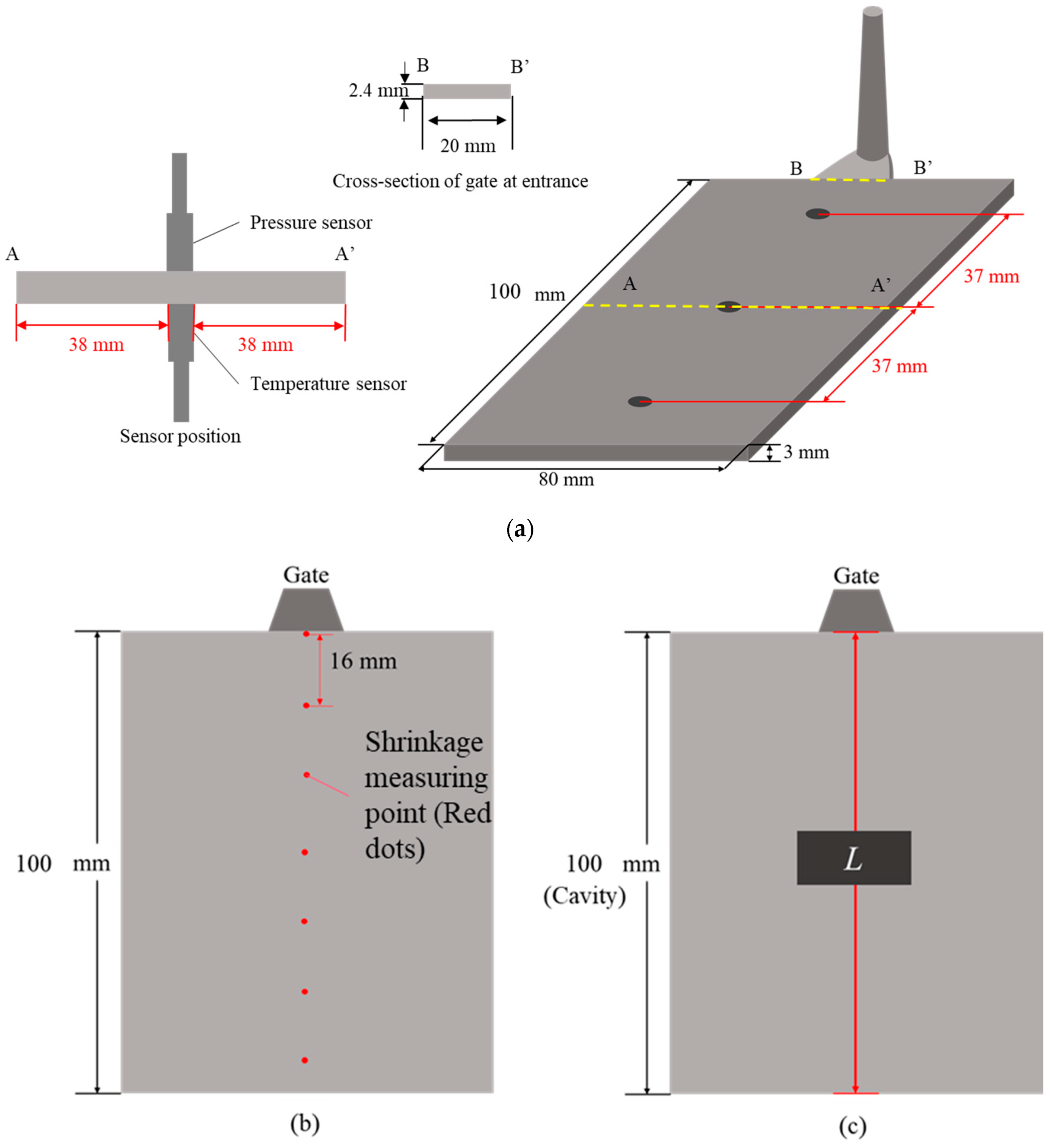
2.1. Equipment
2.2. Molding Materials Used
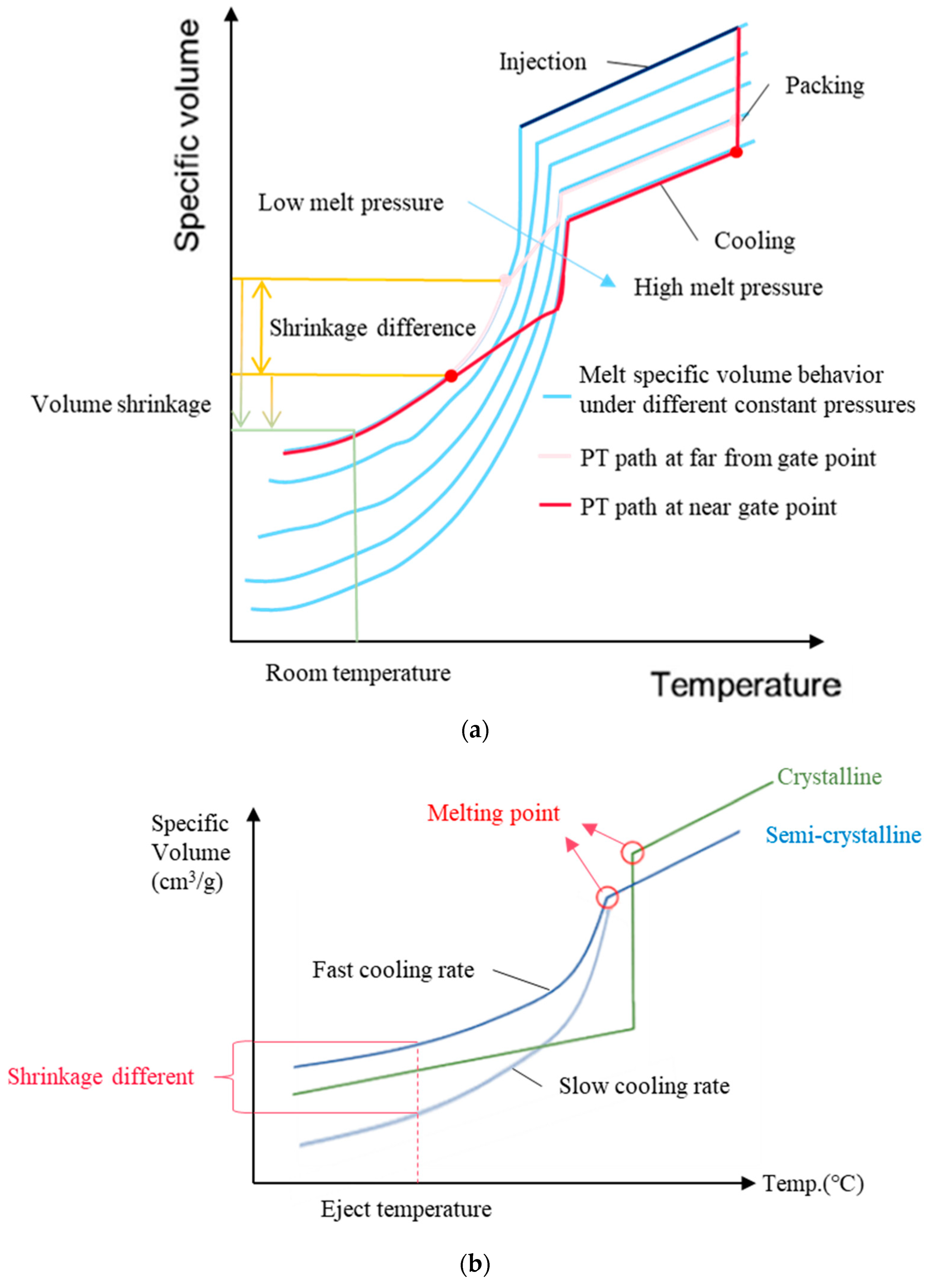
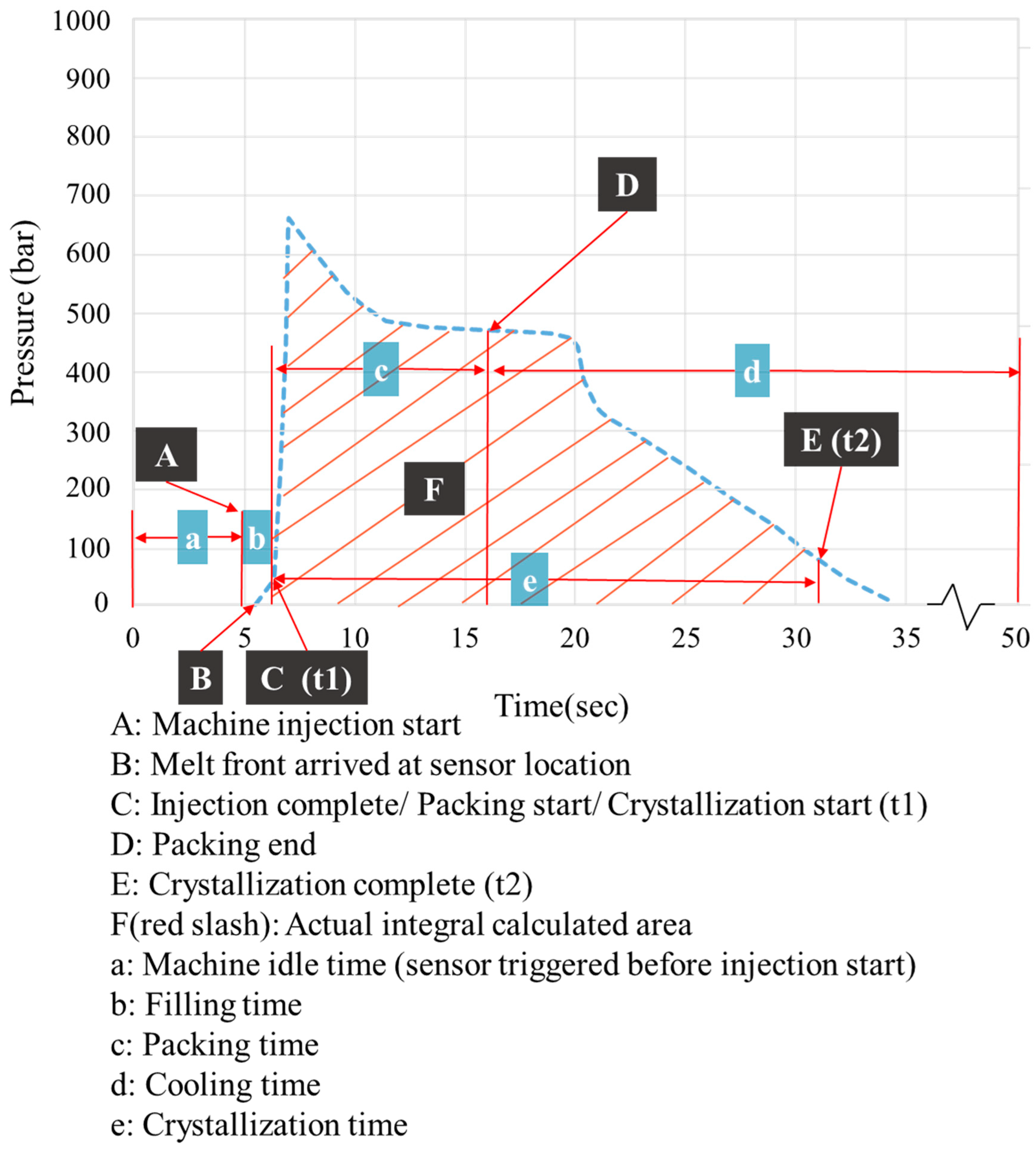
2.3. Verification and Prediction Model for the Complete Crystallization Time
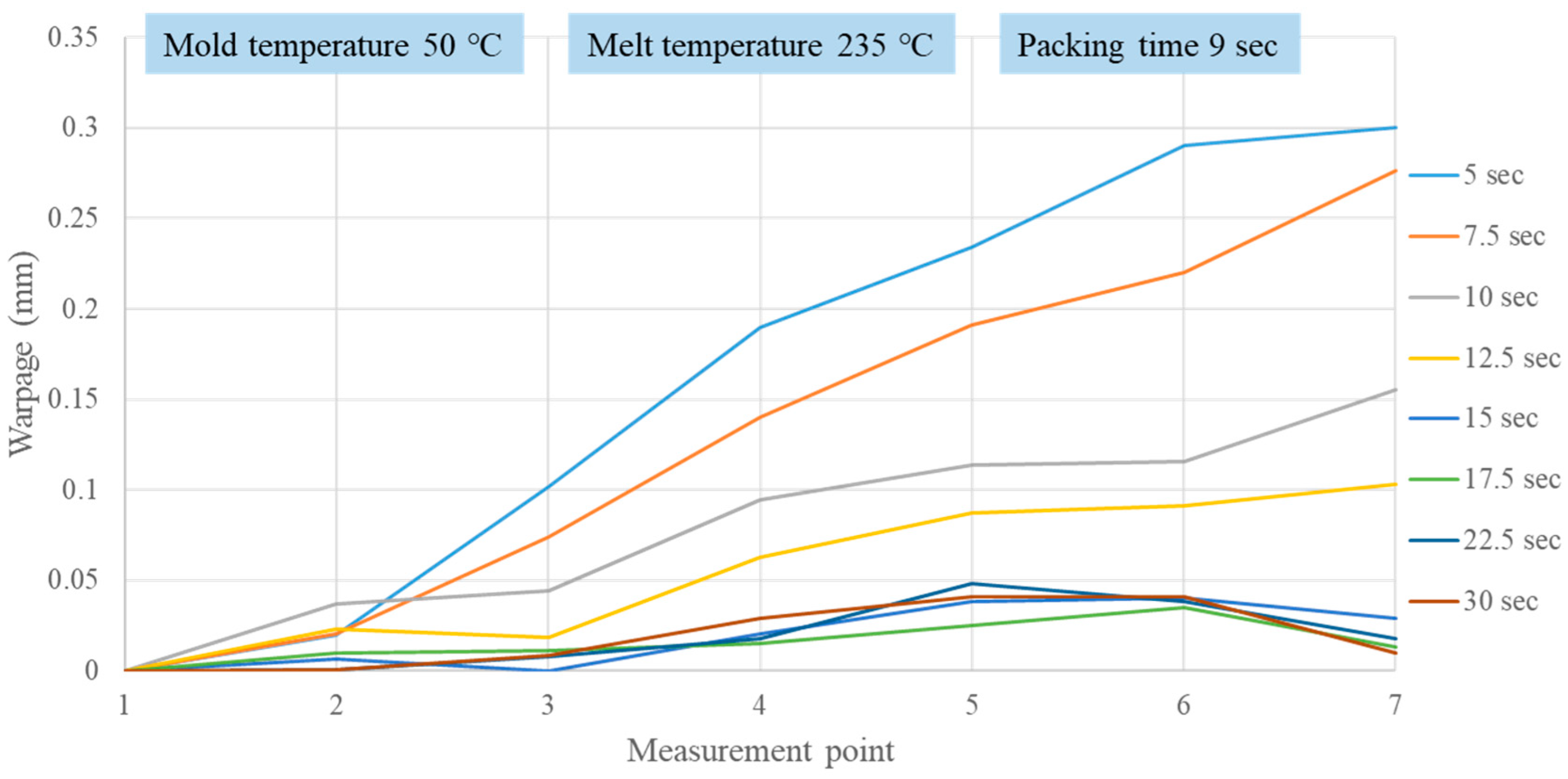
2.4. Correlating Crystallization Completion with the Extent of Part Warpage
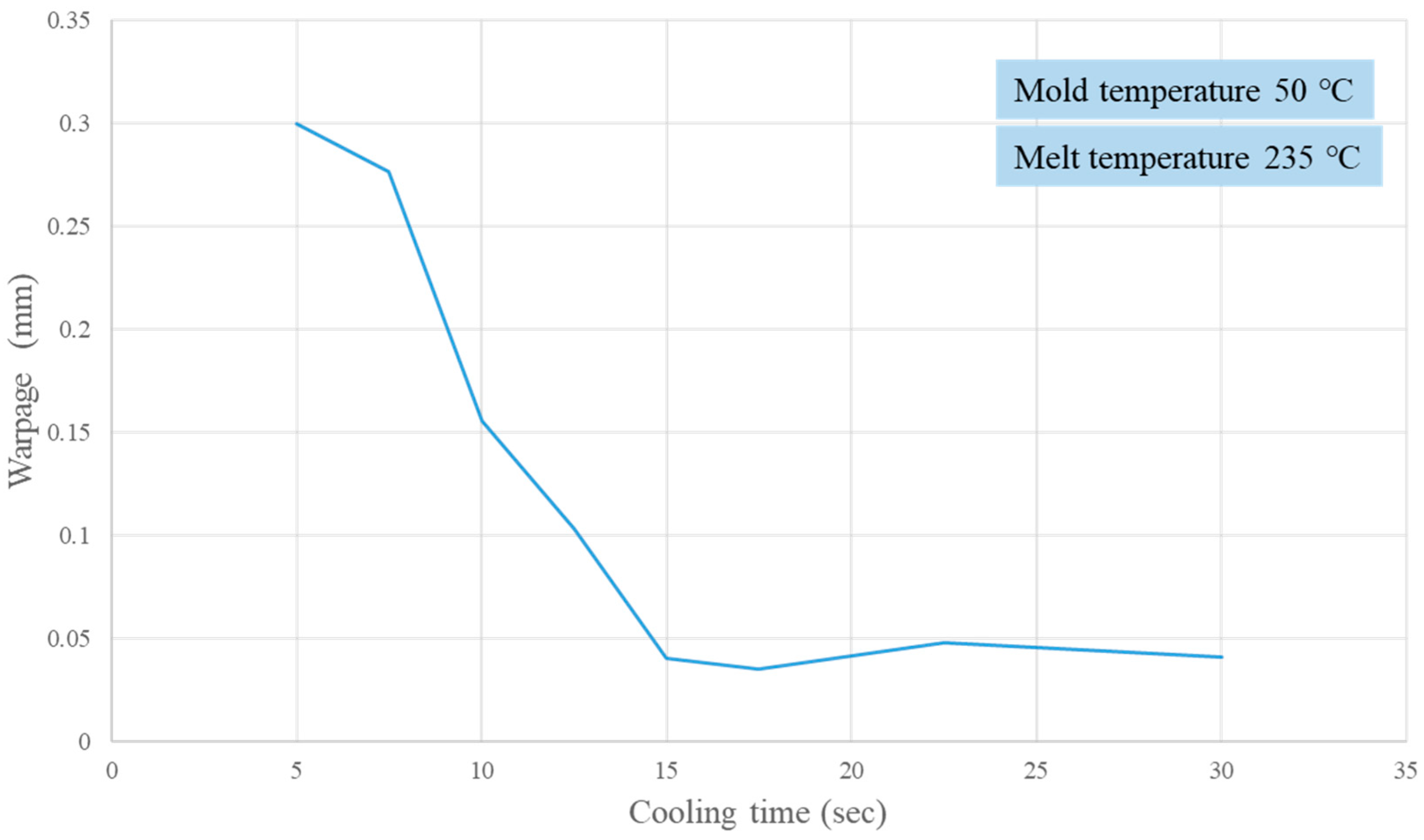
2.5. Influence of Time-Averaged Cavity Pressure on Part Warpage
2.6. Verification Experiments for Prediction Models
3. Results and Discussion
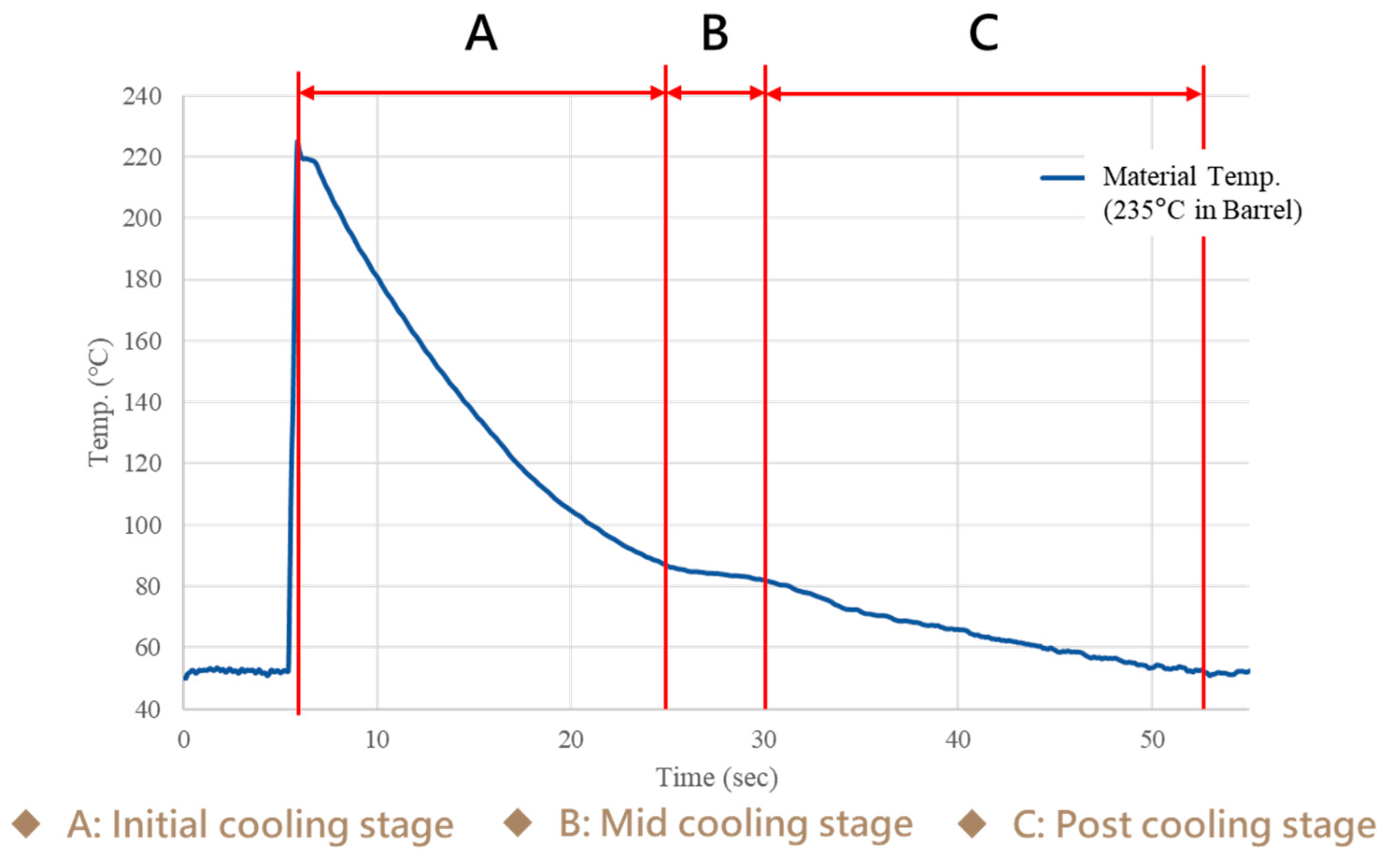
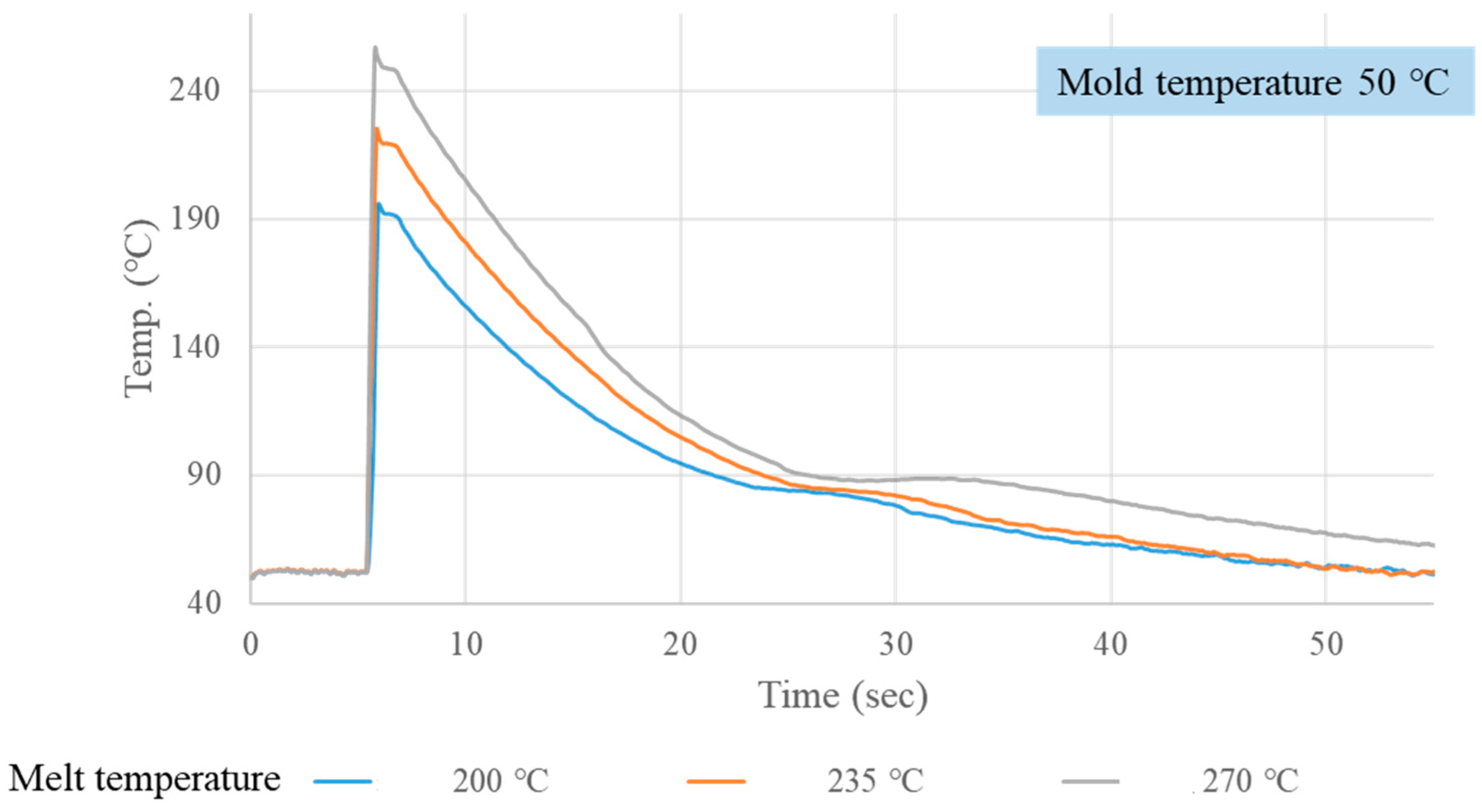
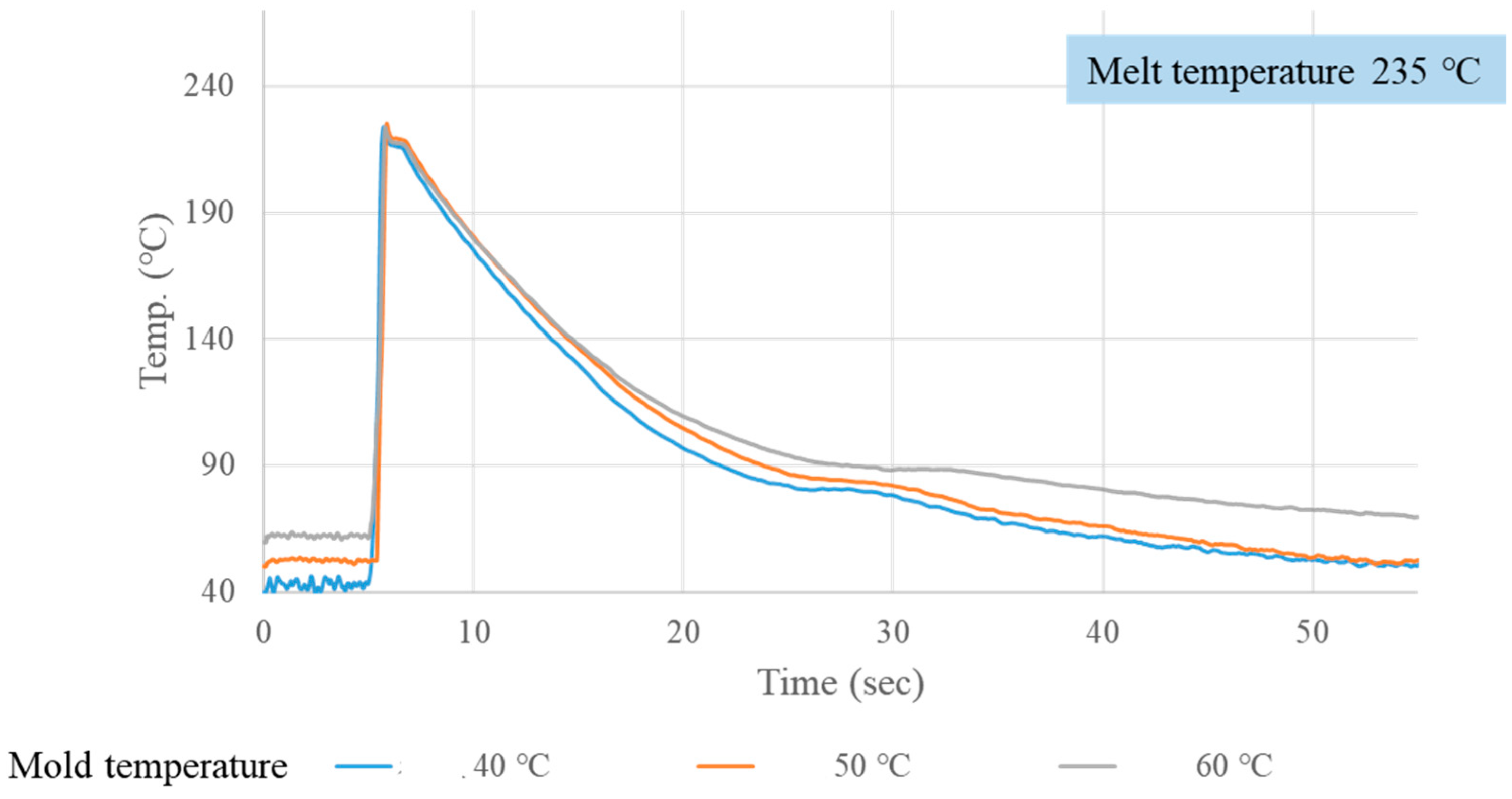
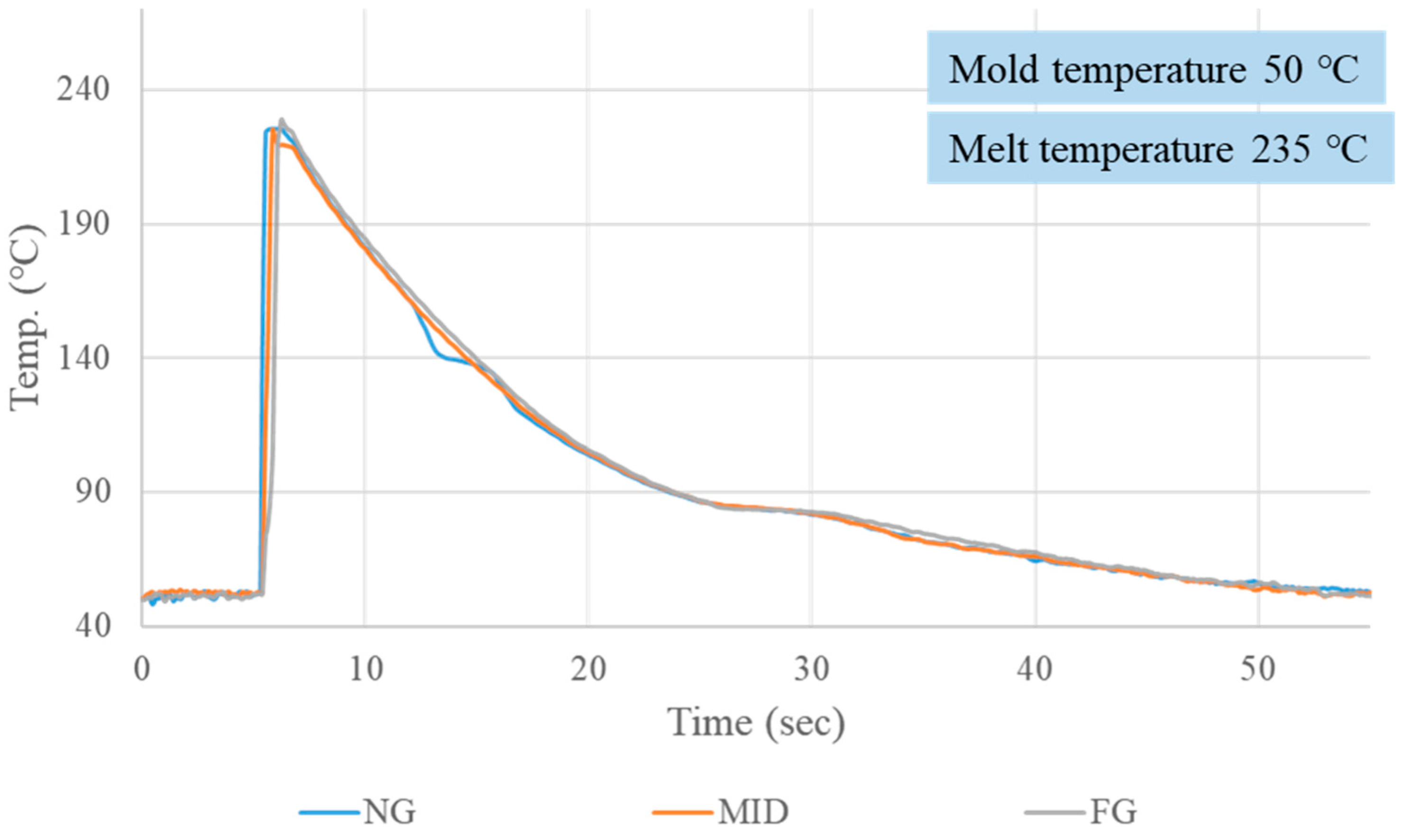
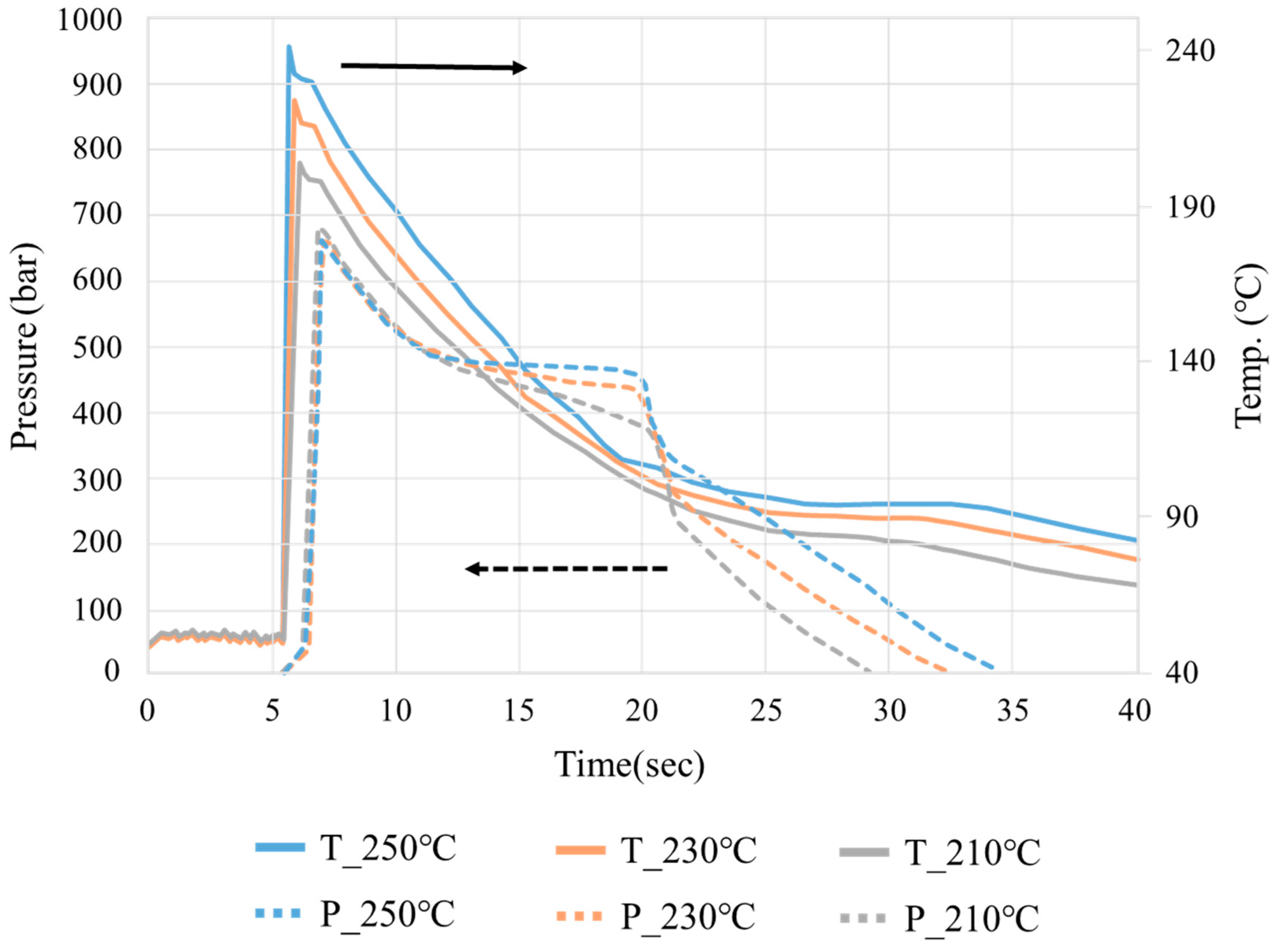
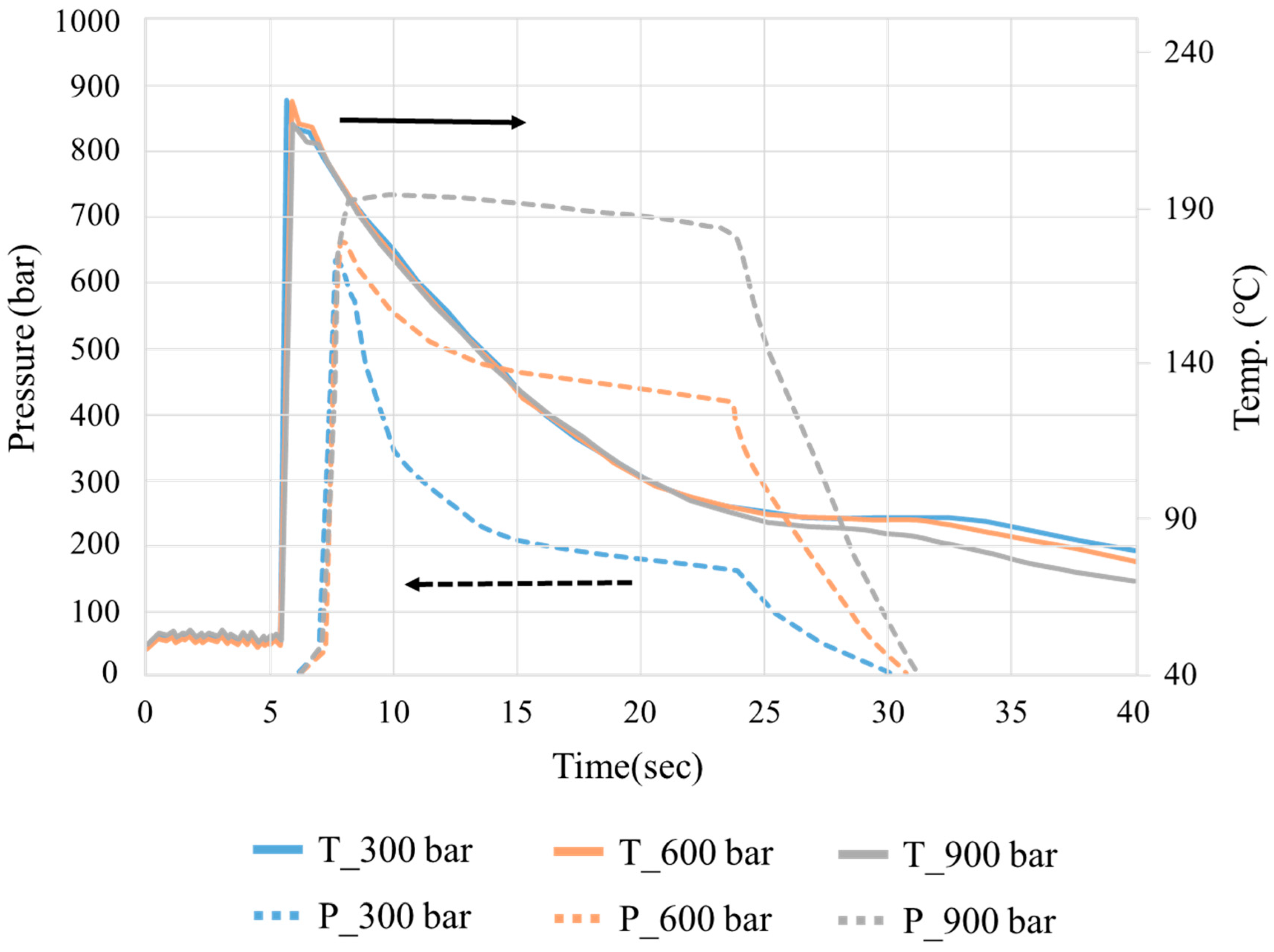
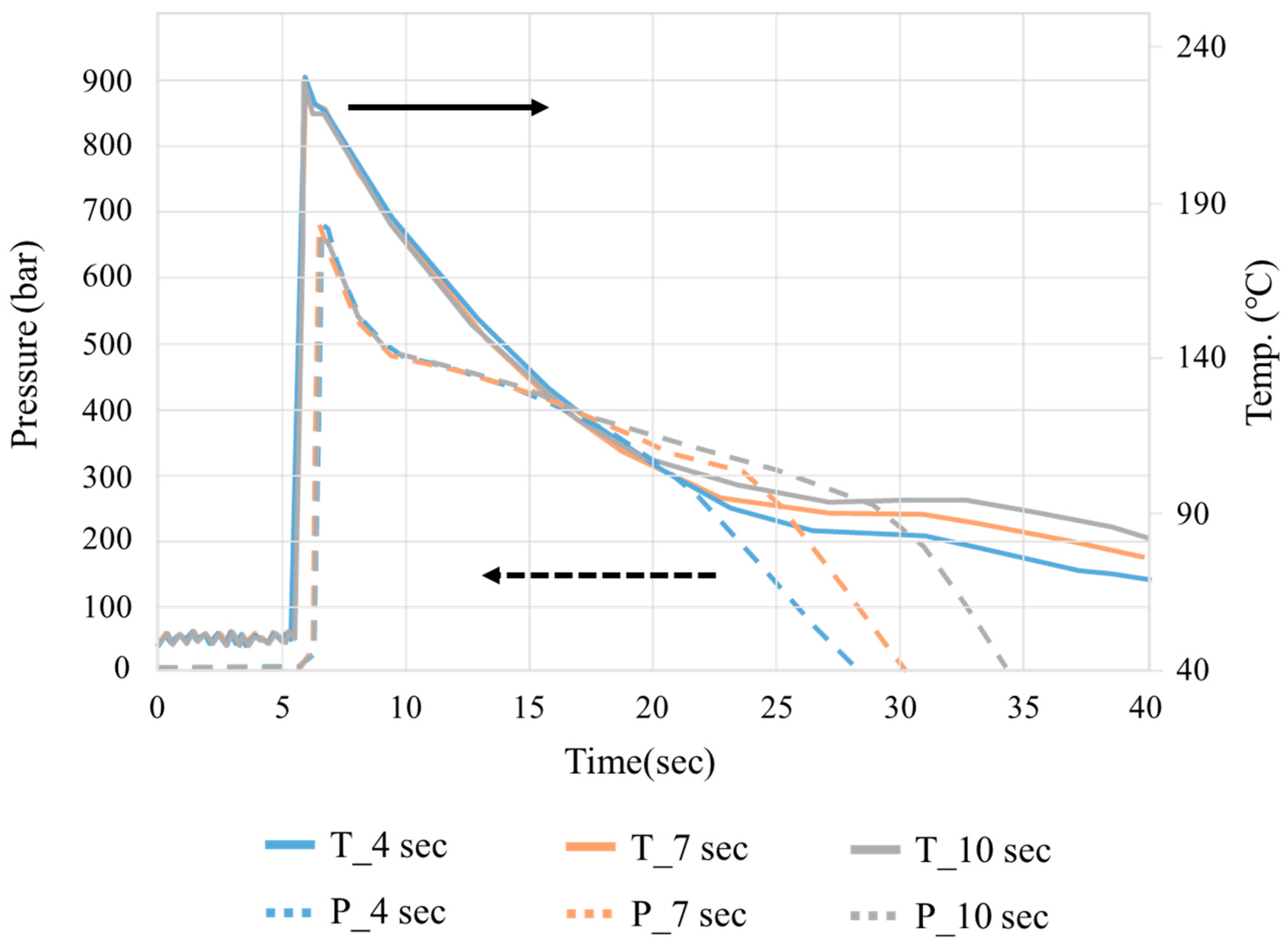
3.1. Crystallization Completion Monitoring
3.2. Verifying Crystallization Completion Time from Part Warpage
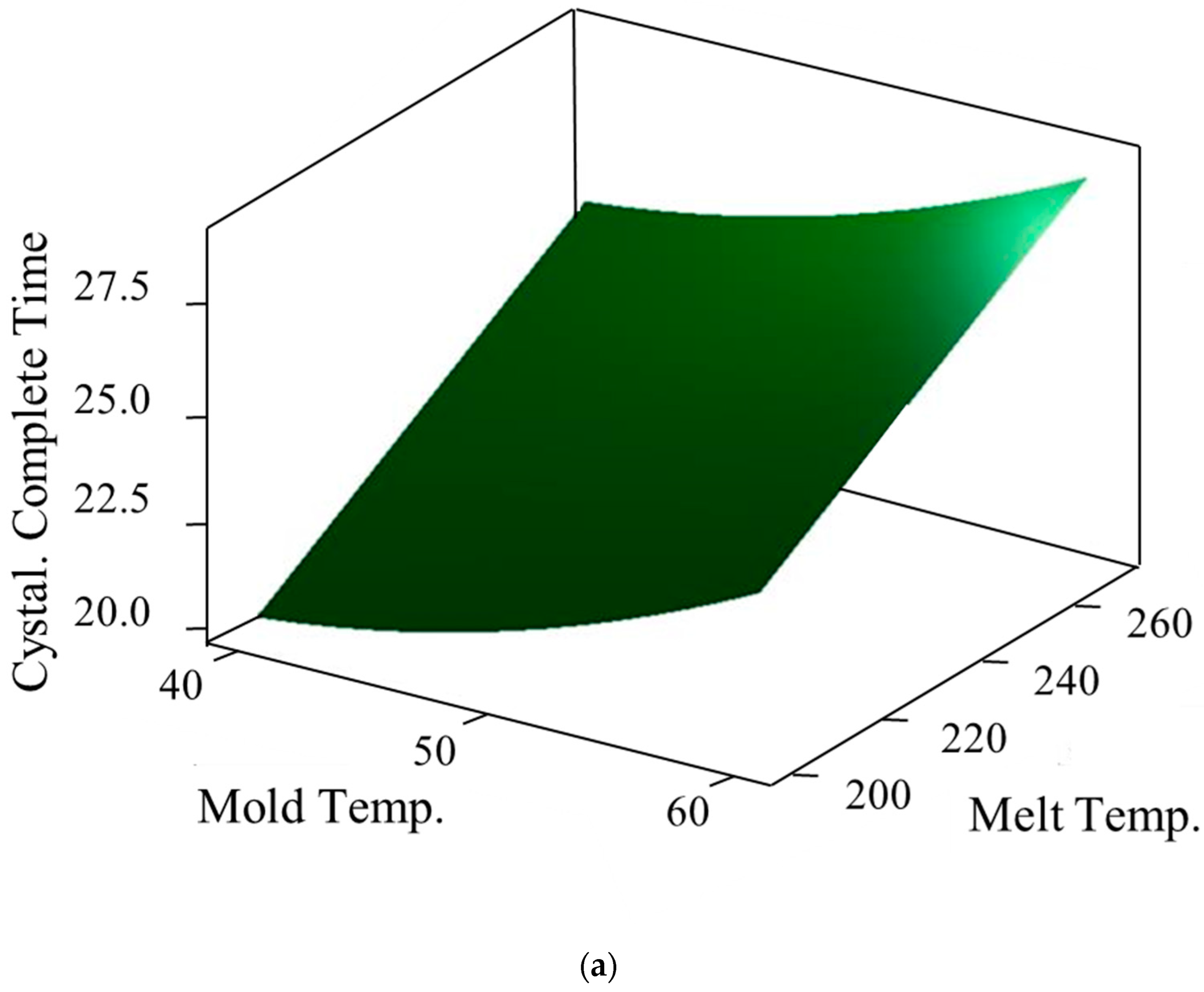
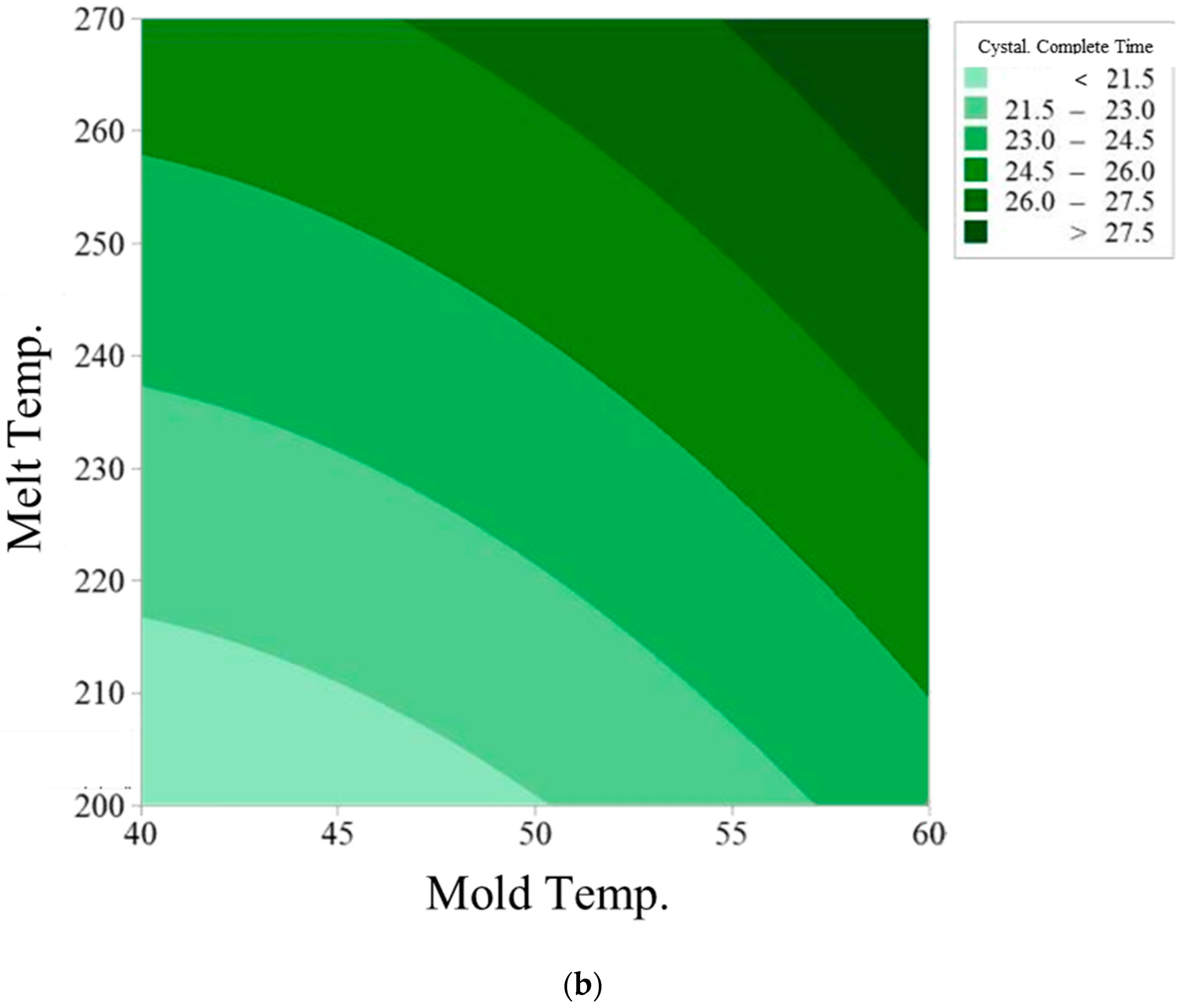
3.3. Prediction Model of Crystallization Completion Time
3.4. Prediction Model of Part Shrinkage Varying with the Time-Averaged Packing Pressure
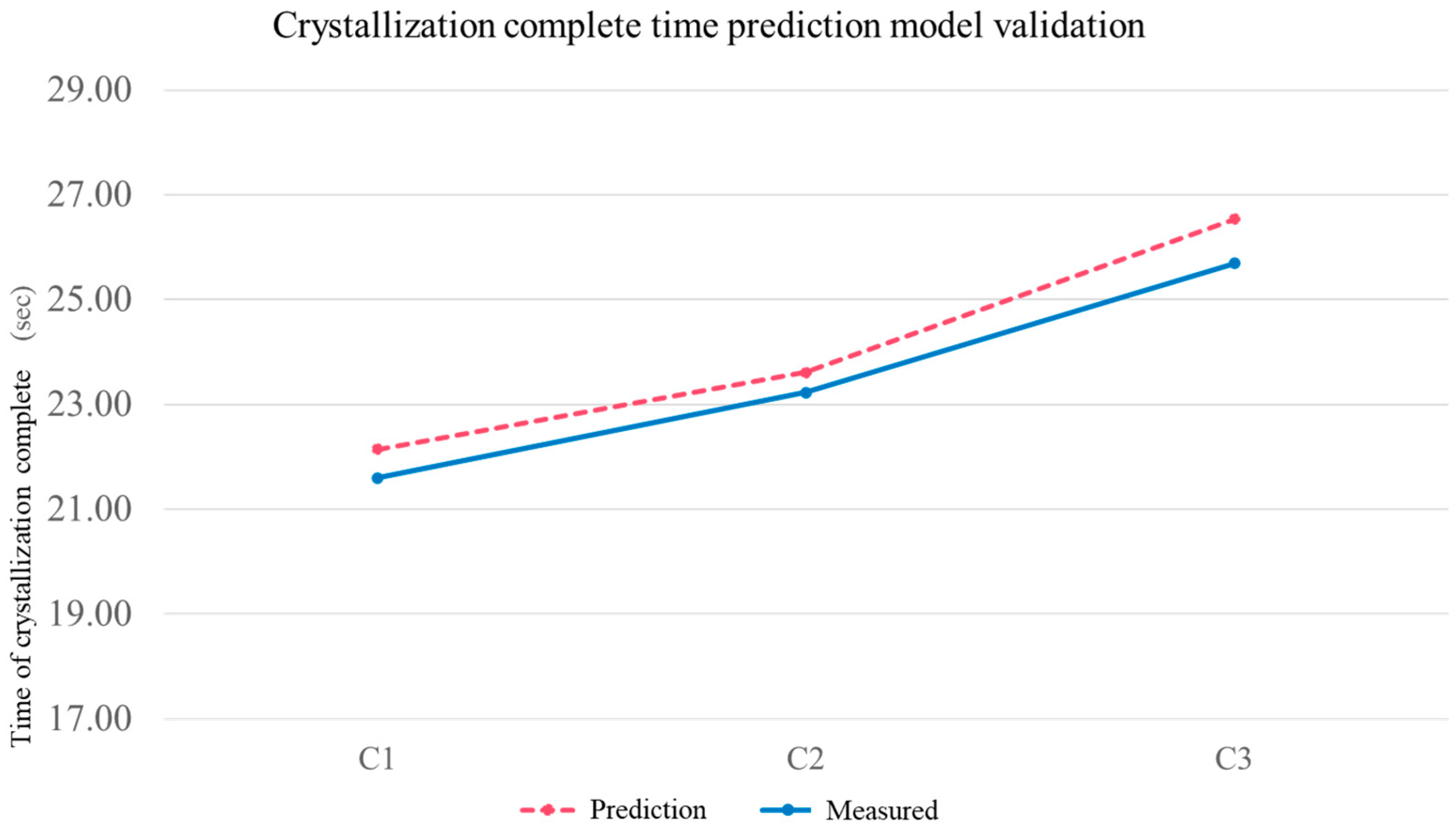
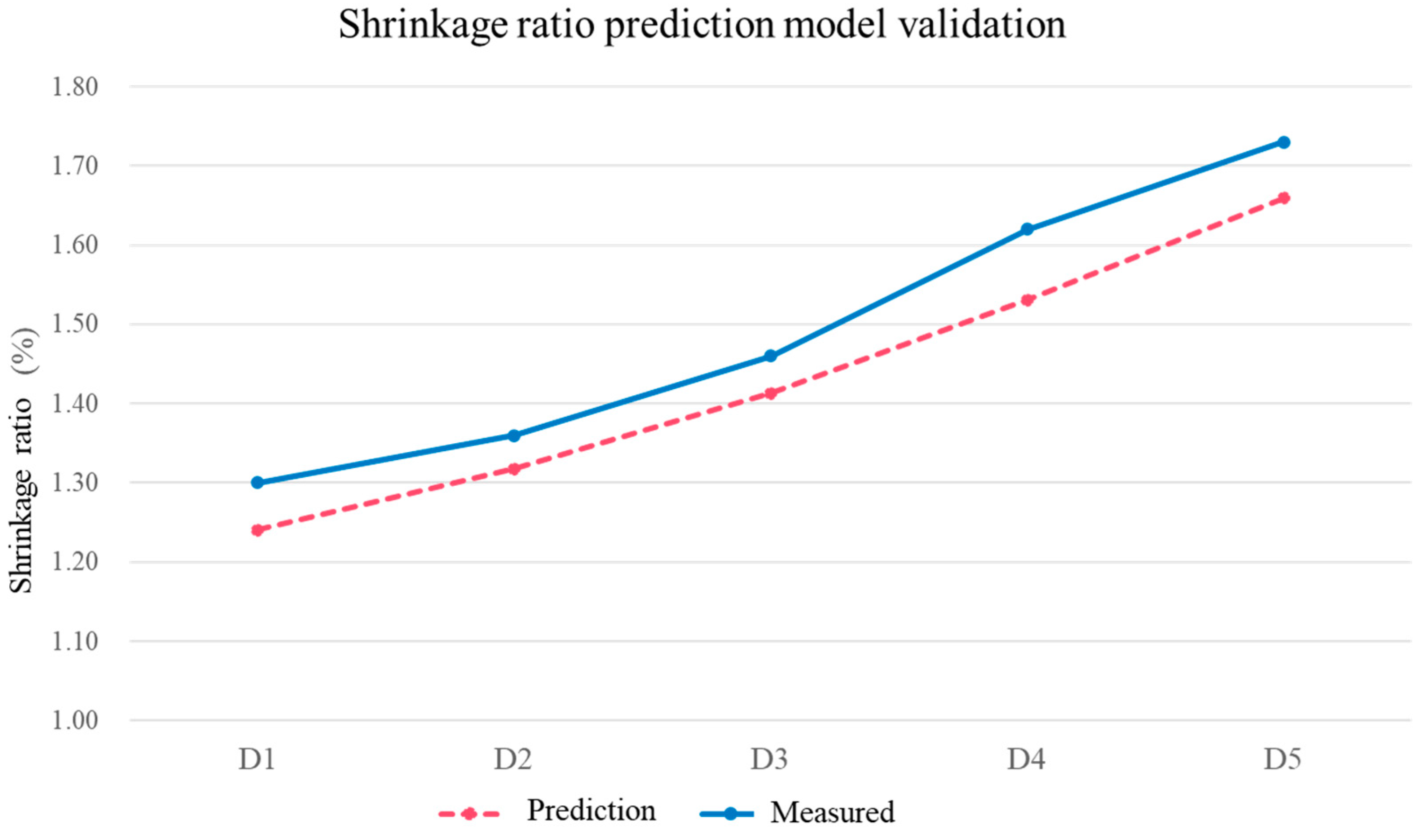
3.5. Verification Experiments
| Group | Melt Temp. (°C) | Packing Pressure (Bar) | Packing Time (S) | tcrys. (S) | PSavg (Bar) |
|---|---|---|---|---|---|
| B1 | 210 | 900 | 10 | 22.31 | 307.02 |
| B2 | 210 | 900 | 7 | 21.78 | 280.52 |
| B3 | 210 | 900 | 4 | 21.48 | 150.62 |
| B4 | 210 | 600 | 10 | 21.67 | 191.56 |
| B5 | 210 | 600 | 7 | 21.60 | 170.77 |
| B6 | 210 | 600 | 4 | 23.54 | 119.22 |
| B7 | 210 | 300 | 10 | 22.43 | 79.08 |
| B8 | 210 | 300 | 7 | 22.27 | 69.14 |
| B9 | 210 | 300 | 4 | 22.60 | 58.15 |
| B10 | 230 | 900 | 10 | 23.74 | 342.48 |
| B11 | 230 | 900 | 7 | 23.36 | 261.16 |
| B12 | 230 | 900 | 4 | 24.72 | 119.65 |
| B13 | 230 | 600 | 10 | 23.49 | 203.01 |
| B14 | 230 | 600 | 7 | 23.23 | 152.35 |
| B15 | 230 | 600 | 4 | 24.46 | 99.61 |
| B16 | 230 | 300 | 10 | 24.74 | 78.36 |
| B17 | 230 | 300 | 7 | 24.52 | 68.74 |
| B18 | 230 | 300 | 4 | 25.14 | 50.20 |
| B19 | 250 | 900 | 10 | 25.21 | 336.84 |
| B20 | 250 | 900 | 7 | 24.60 | 210.53 |
| B21 | 250 | 900 | 4 | 27.04 | 108.73 |
| B22 | 250 | 600 | 10 | 24.89 | 199.10 |
| B23 | 250 | 600 | 7 | 25.69 | 136.91 |
| B24 | 250 | 600 | 4 | 27.00 | 77.44 |
| B25 | 250 | 300 | 10 | 25.89 | 82.14 |
| B26 | 250 | 300 | 7 | 26.18 | 65.96 |
| B27 | 250 | 300 | 4 | 27.03 | 46.07 |
| Equations | Coefficients |
|---|---|
| Linear equation: | R-sq R-sq (Adj.) |
| 89.36% 89.16% | |
| Quadratic equation: | R-sq R-sq (Adj.) |
| 94.00% 93.76% | |
| Cubic equation: | R-sq R-sq (Adj.) |
| 94.00% 93.64% |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Turng, L.S. A Review of Current Developments in Process and Quality Control for Injection Molding. Adv. Polym. Technol. 2005, 24, 165–182. [Google Scholar] [CrossRef]
- Johannaber, F. Injection Molding Machines, 3rd ed.; Hanser Publishers: Munich, Germany, 1994; p. 29. [Google Scholar]
- Chang, Y.H.; Chen, S.C.; Ting, Y.H.; Feng, C.T.; Hsu, C.C. The investigation of novel dynamic packing technology for injection molded part quality control and its production stability by using real-time PVT control method. Polymer 2022, 14, 2720–2736. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Wei, T.H.; Chen, S.C.; Lou, Y.F. The Investigation on PVT Control Method Establishment for Scientific Injection Molding Parameter Setting and Its Quality Control. Polym. Eng. Sci. 2020, 60, 2895–2907. [Google Scholar] [CrossRef]
- Zoller, P.; Walsh, D.J. Standard Pressure-Volume-Temperature Data for Polymers. J Polym. Sci. A. Polym Chem. 1995, 34, 1365. [Google Scholar]
- Berry, J.M.; Brostow, W.; Hess, M.; Jacobs, E.G. P-V-T Relations in A Series of Longitudinal Polymer Liquid Crystals with Varying Mesogen Concentration. Polymer 1998, 39, 4081–4088. [Google Scholar] [CrossRef]
- Wang, J.; Xie, P.; Ding, Y.; Yang, W. On-Line Testing Equipment of P–V–T Properties of Polymers Based on An Injection Molding Machine. Polym. Test. 2009, 28, 228–234. [Google Scholar] [CrossRef]
- Wang, J.; Xie, P.; Yang, W.; Ding, Y. Online Pressure–Volume–Temperature Measurements of Polypropylene Using A Testing Mold to Simulate The Injection- Molding Process. J. Appl. Polym. Sci. 2010, 118, 200–208. [Google Scholar] [CrossRef]
- Sanchez, R.; Aisa, J.; Martinez, A.; Mercado, D. On The Relationship Between Cooling Setup and Warpage in Injection Molding. Measurement 2012, 45, 1051–1056. [Google Scholar] [CrossRef]
- Wang, J.; Hopmann, C.; Röbig, M.; Hohlweck, T.; Alms, J. Modeling of pressure-specific volume-temperature behavior of polymers considering the dependence of cooling and heating processes. Mater. Des. 2020, 196, 109110. [Google Scholar] [CrossRef]
- Bushko, W.C.; Stokes, V.K. Estimates for Material Shrinkage in Molded Parts Caused by Time-Varying Cavity Pressures. Polym. Eng. Sci. 2019, 59, 1648–1656. [Google Scholar] [CrossRef]
- Chang, T.C. Robust process control in injection molding—Process capability comparison for five switchover modes. J. Inject. Molding Technol. 2002, 6, 239–246. [Google Scholar]
- Kazmer, D.O.; Velusamy, S.; Westerdale, S.; Johnston, S.; Gao, R.X. A Comparison of Seven Filling to Packing Switchover for Injection Molding. Polym. Eng. Sci. 2010, 50, 2031–2043. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Mao, T.; Zhou, H. Monitoring and dynamic control of quality stability for injection molding process. J. Mater. Process. Tech. 2017, 249, 358–366. [Google Scholar] [CrossRef]
- Hopmann, C.; Kahve, C.; Schmitz, M. Development of a novel control strategy for a highly segmented injection mold tempering for inline part warpage control. Polym. Eng. Sci. 2020, 60, 2428–2438. [Google Scholar] [CrossRef]
- Rizvi1, S.J.A. Effect of injection molding parameters on crystallinity and mechanical properties of isotactic polypropylene. Int. J. Plast. Technol. 2017, 21, 404–426. [Google Scholar] [CrossRef]
- Le, M.C.; Belhabib, S.; Nicolazo, C.; Vachot, P.; Mousseau, P.; Sarda, A.; Deterre, R. Pressure influence on crystallization kinetics during injection molding. J. Mater. Process. Technol. 2011, 211, 1757–1763. [Google Scholar] [CrossRef]
- Gao, X.; Huang, Z.; Zhou, H.; Zhang, Y.; Liang, J. Influence of Injection Rate on Crystallization of Injection Molded ß-Nucleated Isotactic Polypropylene. Polym. Eng. Sci. 2017, 57, 172–173. [Google Scholar] [CrossRef]
- Wang, G.; Hou, S.; Cao, J.; Ding, P.; Shen, J.; Chen, J. Reinforcing and toughening isotactic polypropylene through shear-induced crystallization and β-nucleating agent induced crystallization. J. Polym. Res. 2018, 25, 233–240. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, W.; Wang, X.; Li, J.; Yan, B.; Fu, J. A novel method for predicting degrees of crystallinity in injection molding during packing stage. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2017, 233, 204–214. [Google Scholar] [CrossRef]
- Yang, B.; Miao, J.B.; Min, K.; Xia, R.; Qian, J.S.; Wang, X. Solidification Behavior of High-Density Polyethylene During Injection Molding Process: Enthalpy Transformation Method. J. Appl. Polym. Sci. 2013, 128, 1922–1929. [Google Scholar] [CrossRef]
- Yang, B.; Fu, X.R.; Yang, W.; Liang, S.P.; Hu, S.; Yang, M.B. Simulation of Phase-Change Heat Transfer During Cooling Stage of Gas-Assisted Injection Molding of High-Density Polyethylene via Enthalpy Transformation Approach. Polym. Eng. Sci. 2009, 10, 1234–1242. [Google Scholar] [CrossRef]
- Yang, B.; Fu, X.R.; Yang, W.; Huang, L.; Yang, M.B.; Feng, J.M. Numerical Prediction of Phase-Change Heat Conduction of Injection-Molded High Density Polyethylene Thick-Walled Parts Via the Enthalpy Transforming Model with Mushy Zone. Polym. Eng. Sci. 2008, 48, 1707–1717. [Google Scholar] [CrossRef]
- Futaba Web. Available online: https://mms.mtb.futaba.co.jp/en/products/resintemperature/ (accessed on 30 July 2021).
- Abeykoon, C.; Martin, P.J.; Kelly, A.L.; Brown, E.C. A review and Evaluation of Melt Temperature Sensors for Polymer Extrusion. Sens. Actuators 2012, 181, 16–27. [Google Scholar] [CrossRef]
- Maier, C. Infrared Temperature Measurement of Polymers. Polym. Eng. Sci. 1996, 36, 1502–1512. [Google Scholar] [CrossRef]
- Heidari, B.S.; Oliaei, E.; Shayesteh, H.; Davachi, S.M.; Hejazi, I.; Seyfi, J.; Bahrami, M.; Rashedi, H. Simulation of mechanical behavior and optimization of simulated injection molding process for PLA based antibacterial composite and nanocomposite bone screws using central composite design. J. Mech. Behavior Bio. Mat. 2017, 65, 160–176. [Google Scholar] [CrossRef]
- Xu, R.T.; Wang, T.H.; Huang, C.T.; Chen, P.H.; Jong, W.R.; Chen, S.C.; Hsu, D.; Chang, R.Y. Study on Machine Identification and its Effect on the RSM Optimization in Injection Molding; SPE Technical Papers: Richardson, TX, USA, 2022. [Google Scholar]
- Pantani, R.; Coccorullo, I.; Speranza, V.; Titomanlio, G. Modeling of Morphology Evolution in the Injection Molding. Prog. Polym. Sci. 2005, 30, 1185–1222. [Google Scholar] [CrossRef]
- Cai, B.L. Study on Establishment of Enthalpy Transition Method and Average Solidification Pressure for Semi-Crystalline Polymer of Injection Molding Quality Prediction Method. Ph.D. Dissertation, Chung Yuan Christian University, Taoyuan City, Taiwan, 2022. [Google Scholar]
- Oliaei, E.; Heidari, B.S.; Davachi, S.M.; Bahrami, M.; Davoodi, S.; Hejazi, I.; Seyfi, J. Warpage and shrinkage optimization of injection-molded plastic spoon parts for biodegradable polymers using Taguchi, ANOVA and artificial neural network methods. J. Mat. Sci. Technol. 2016, 32, 710–720. [Google Scholar] [CrossRef]


| Varying Process Parameters | ||
|---|---|---|
| RSM Group | Mold Temp. (°C) | Melt Temp. (°C) |
| A1 | 40 | 200 |
| A2 | 60 | 200 |
| A3 | 40 | 270 |
| A4 | 60 | 270 |
| A5 | 40 | 235 |
| A6 | 60 | 235 |
| A7 | 50 | 200 |
| A8 | 50 | 270 |
| A9 | 50 | 235 |
| A10 | 50 | 235 |
| A11 | 50 | 235 |
| A12 | 50 | 235 |
| A13 | 50 | 235 |
| Fixed process parameters | ||
| Injection speed (mm/s) | 20 | |
| Velocity/pressure switch position (mm) | 4 | |
| Packing pressure (bar) | 800 | |
| Packing time (s) | 9 | |
| Cooling time (s) | 45 | |
| Fixed Process Parameters | ||
|---|---|---|
| Injection speed | (mm/s) | 20 |
| Velocity/pressure switch position | (mm) | 4 |
| Packing pressure | (bar) | 800 |
| Packing time | (s) | 9 |
| Melt temperature | (°C) | 235 |
| Mold temperature | (°C) | 50 |
| Cooling time | (s) | 5, 7.5, 10, 12.5, 15, 17.5, 22.5, 30 |
| Group | Melt Temp. (°C) | Packing Pressure (Bar) | Packing Time (S) |
|---|---|---|---|
| B1 | 210 | 900 | 10 |
| B2 | 210 | 900 | 7 |
| B3 | 210 | 900 | 4 |
| B4 | 210 | 600 | 10 |
| B5 | 210 | 600 | 7 |
| B6 | 210 | 600 | 4 |
| B7 | 210 | 300 | 10 |
| B8 | 210 | 300 | 7 |
| B9 | 210 | 300 | 4 |
| B10 | 230 | 900 | 10 |
| B11 | 230 | 900 | 7 |
| B12 | 230 | 900 | 4 |
| B13 | 230 | 600 | 10 |
| B14 | 230 | 600 | 7 |
| B15 | 230 | 600 | 4 |
| B16 | 230 | 300 | 10 |
| B17 | 230 | 300 | 7 |
| B18 | 230 | 300 | 4 |
| B19 | 250 | 900 | 10 |
| B20 | 250 | 900 | 7 |
| B21 | 250 | 900 | 4 |
| B22 | 250 | 600 | 10 |
| B23 | 250 | 600 | 7 |
| B24 | 250 | 600 | 4 |
| B25 | 250 | 300 | 10 |
| B26 | 250 | 300 | 7 |
| B27 | 250 | 300 | 4 |
| Varying Process Parameters | |
|---|---|
| Group | Melt Temp. (°C) |
| C1 | 210 |
| C2 | 230 |
| C3 | 250 |
| Fixed process parameters | |
| Mold temperature (°C) | 50 |
| Injection speed (mm/s) | 20 |
| Velocity/pressure switch position (mm) | 4 |
| Packing pressure (bar) | 800 |
| Packing time (s) | 9 |
| Cooling time (s) | 45 |
| Varying Process Parameters | |
|---|---|
| Group | Packing Time (S) |
| D1 | 11 |
| D2 | 9 |
| D3 | 7 |
| D4 | 5 |
| D5 | 3 |
| Fixed process parameters | |
| Mold temperature (°C) | 50 |
| Melt temperature (°C) | 270 |
| Injection speed (mm/s) | 20 |
| Velocity/pressure switch position (mm) | 4 |
| Packing pressure (bar) | 800 |
| Cooling time (s) | 45 |
| Mold Temp. (°C) | Melt Temp. (°C) | Crystallization Completion Time (S) | |||
|---|---|---|---|---|---|
| NG | MID | FG | Average | ||
| 40 | 200 | 21.01 | 20.39 | 19.16 | 20.18 |
| 40 | 235 | 23.68 | 22.82 | 22.91 | 23.14 |
| 40 | 270 | 25.70 | 24.81 | 25.26 | 25.26 |
| 50 | 200 | 22.22 | 21.37 | 21.24 | 21.61 |
| 50 | 235 | 24.35 | 24.15 | 23.69 | 24.06 |
| 50 | 270 | 27.80 | 25.74 | 26.16 | 26.57 |
| 60 | 200 | 24.77 | 22.70 | 23.89 | 23.78 |
| 60 | 235 | 26.28 | 26.95 | 25.62 | 26.28 |
| 60 | 270 | 30.50 | 28.44 | 28.09 | 29.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-C.; Tsai, B.-L.; Hsieh, C.-C.; Cheng, N.-T.; Shen, E.-N.; Feng, C.-T. Prediction of Part Shrinkage for Injection Molded Crystalline Polymer via Cavity Pressure and Melt Temperature Monitoring. Appl. Sci. 2023, 13, 9884. https://doi.org/10.3390/app13179884
Chen S-C, Tsai B-L, Hsieh C-C, Cheng N-T, Shen E-N, Feng C-T. Prediction of Part Shrinkage for Injection Molded Crystalline Polymer via Cavity Pressure and Melt Temperature Monitoring. Applied Sciences. 2023; 13(17):9884. https://doi.org/10.3390/app13179884
Chicago/Turabian StyleChen, Shia-Chung, Bi-Lin Tsai, Cheng-Chang Hsieh, Nien-Tien Cheng, En-Nien Shen, and Ching-Te Feng. 2023. "Prediction of Part Shrinkage for Injection Molded Crystalline Polymer via Cavity Pressure and Melt Temperature Monitoring" Applied Sciences 13, no. 17: 9884. https://doi.org/10.3390/app13179884





