Multipotent Antiviral Effects of Deacetylated Chitosan Supplemented with Grapefruit Seed Extract against Influenza and Parainfluenza Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deacetylated Chitosan and GSE Preparation Methods
2.2. Experimental Sample Composition
2.3. Antibodies and Chemicals
2.4. Cell Culture and Viability Assay
2.5. Plaque Assay
2.6. Western Blot Analysis
2.7. qRT-PCR
2.8. Statistical Analysis
3. Results
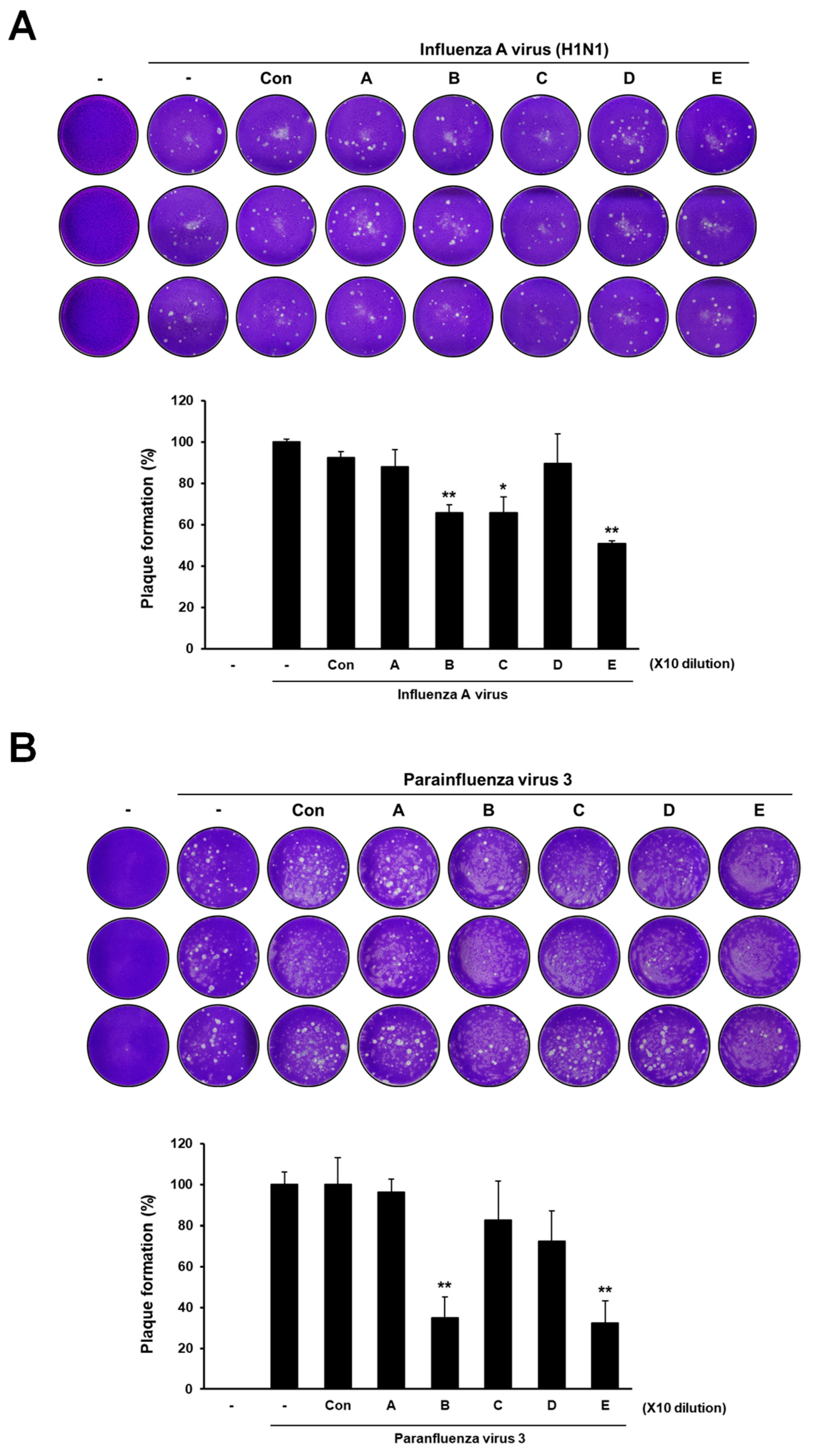
3.1. Antiviral Activities of Deacetylated Chitosan with GSE against Human Influenza A Virus and Parainfluenza Virus Type 3
3.2. Antiviral Activity of Deacetylated Chitosan in Human A549 Cells
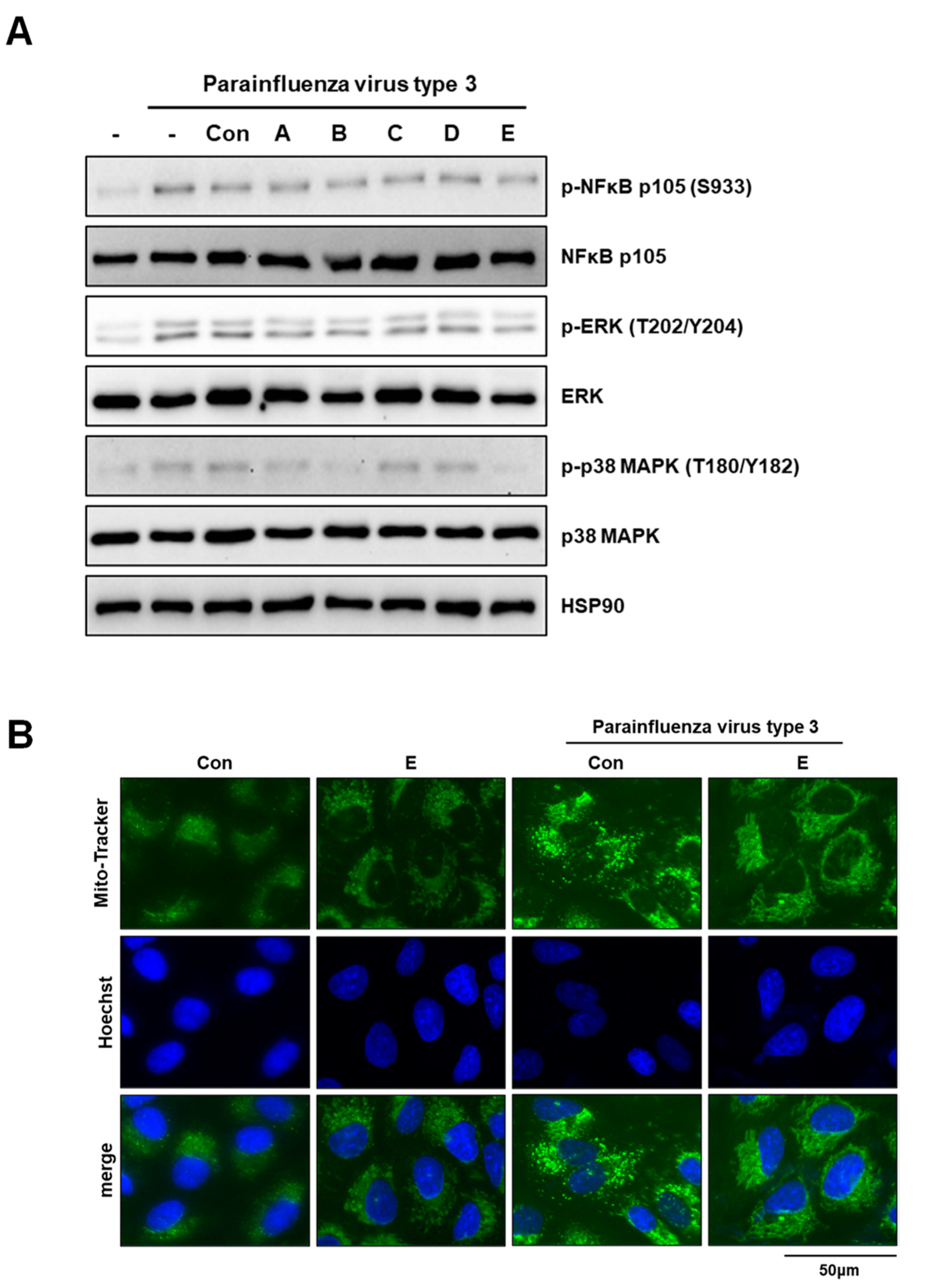
3.3. Deacetylated Chitosan Prevents Virus-Induced P38 MAPK Activation and Mitochondrial Fragmentation in A549 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medina, R.A.; García-Sastre, A. Influenza A viruses: New research developments. Nat. Rev. Microbiol. 2011, 9, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.S.; Lee, N.; Joynt, G.M.; Choi, K.W.; Cheung, J.L.K.; Yeung, A.C.M.; Lam, P.; Wong, R.; Leung, B.-W.; So, H.-Y.; et al. Clinical and virological course of infection with haemagglutinin D222G mutant strain of 2009 pandemic influenza A (H1N1) virus. J. Clin. Virol. 2011, 50, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bukreyev, A.; Thompson Catherine, I.; Watson, B.; Peeples Mark, E.; Collins Peter, L.; Pickles Raymond, J. Infection of Ciliated Cells by Human Parainfluenza Virus Type 3 in an In Vitro Model of Human Airway Epithelium. J. Virol. 2005, 79, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Battles, M.B.; McLellan, J.S. Respiratory syncytial virus entry and how to block it. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.; Lima, M.A.B.; Franco, L.O.; Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Chenite, A.; Felt-Baeyens, O.; Mayer, J.M.; Gurny, R. Pseudo-thermosetting chitosan hydrogels for biomedical application. Int. J. Pharm. 2005, 288, 197–206. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Tan, R.S.L.; Hassandarvish, P.; Chee, C.F.; Chan, L.W.; Wong, T.W. Chitosan and its derivatives as polymeric anti-viral therapeutics and potential anti-SARS-CoV-2 nanomedicine. Carbohydr. Polym. 2022, 290, 119500. [Google Scholar] [CrossRef]
- Artan, M.; Karadeniz, F.; Karagozlu, M.Z.; Kim, M.-M.; Kim, S.-K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydr. Res. 2010, 345, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Qu, D.; Wang, H.; Sun, Z.; Liu, X.; Chen, J.; Li, C.; Li, X.; Chen, Z. Intranasal Administration of Chitosan Against Influenza A (H7N9) Virus Infection in a Mouse Model. Sci. Rep. 2016, 6, 28729. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, X.; Zhang, C.; Shao, X.; Zhang, X.; Zhang, Q.; Jiang, X. Conjugating influenza a (H1N1) antigen to n-trimethylaminoethylmethacrylate chitosan nanoparticles improves the immunogenicity of the antigen after nasal administration. J. Med. Virol. 2015, 87, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, Y.; Huang, L.; Wang, S.; Hao, C.; Sun, L.; Zhang, Y.; Wang, W.; Li, C. The inhibition effects and mechanisms of sulfated chitooligosaccharides on influenza A virus in vitro and in vivo. Carbohydr. Polym. 2022, 286, 119316. [Google Scholar] [CrossRef]
- Komura, M.; Suzuki, M.; Sangsriratanakul, N.; Ito, M.; Takahashi, S.; Alam, M.S.; Ono, M.; Daio, C.; Shoham, D.; Takehara, K. Inhibitory effect of grapefruit seed extract (GSE) on avian pathogens. J. Vet. Med. Sci. 2019, 81, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Go, C.C.; Pandav, K.; Sanchez-Gonzalez, M.A.; Ferrer, G. Potential Role of Xylitol Plus Grapefruit Seed Extract Nasal Spray Solution in COVID-19: Case Series. Cureus 2020, 12, e11315. [Google Scholar] [CrossRef]
- Han, H.W.; Kwak, J.H.; Jang, T.S.; Knowles, J.C.; Kim, H.W.; Lee, H.H.; Lee, J.H. Grapefruit Seed Extract as a Natural Derived Antibacterial Substance against Multidrug-Resistant Bacteria. Antibiotics 2021, 10, 85. [Google Scholar] [CrossRef]
- Roy, S.; Zhang, W.; Biswas, D.; Ramakrishnan, R.; Rhim, J.W. Grapefruit Seed Extract-Added Functional Films and Coating for Active Packaging Applications: A Review. Molecules 2023, 28, 730. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. PTR 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytother. Res. PTR 2020, 34, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, D.; Shin, Y.C.; Lee, J.S.; Jung, J.; Ryoo, S. Low molecular weight chitooligosaccharide inhibits infection of SARS-CoV-2 in vitro. J. Appl. Microbiol. 2022, 133, 1089–1098. [Google Scholar] [CrossRef]
- Joo, S.; Baek, S.; Kang, J.; Seo, D.S.; Kwon, T.K.; Jang, Y. α-ketoglutarate suppresses immediate early gene expression in cancer cells. Biochem. Biophys. Res. Commun. 2022, 637, 144–152. [Google Scholar] [CrossRef]
- Chirkov, S.N. The antiviral activity of chitosan (review). Prikl. Biokhim. Mikrobiol. 2002, 38, 5–13. [Google Scholar] [PubMed]
- Nishimura, S.I.; Kai, H.; Shinada, K.; Yoshida, T.; Tokura, S.; Kurita, K.; Nakashima, H.; Yamamoto, N.; Uryu, T. Regioselective syntheses of sulfated polysaccharides: Specific anti-HIV-1 activity of novel chitin sulfates. Carbohydr. Res. 1998, 306, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, W.; Wang, W.; Zhang, X.; Zhao, X. The inhibitory effects and mechanisms of 3,6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018, 198, 329–338. [Google Scholar] [CrossRef]
- Ciejka, J.; Wolski, K.; Nowakowska, M.; Pyrc, K.; Szczubiałka, K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 735–742. [Google Scholar] [CrossRef]
- Milewska, A.; Ciejka, J.; Kaminski, K.; Karewicz, A.; Bielska, D.; Zeglen, S.; Karolak, W.; Nowakowska, M.; Potempa, J.; Bosch, B.J.; et al. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013, 97, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Kalathiya, U.; Padariya, M.; Mayordomo, M.; Lisowska, M.; Nicholson, J.; Singh, A.; Baginski, M.; Fahraeus, R.; Carragher, N.; Ball, K.; et al. Highly Conserved Homotrimer Cavity Formed by the SARS-CoV-2 Spike Glycoprotein: A Novel Binding Site. J. Clin. Med. 2020, 9, 1473. [Google Scholar] [CrossRef]
- Jha, P. Effect of grapefruit seed extract ratios on functional properties of corn starch-chitosan bionanocomposite films for active packaging. Int. J. Biol. Macromol. 2020, 163, 1546–1556. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Do, A.R.; Cho, S.J.; Cho, Y.Y.; Kwon, E.Y.; Choi, J.Y.; Lee, J.H.; Han, Y.; Kim, Y.S.; Piao, Z.; Shin, Y.C.; et al. Antiobesity Effects of Short-Chain Chitosan in Diet-Induced Obese Mice. J. Med. Food 2018, 21, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Gopan, G.; Jose, J.; Khot, K.B.; Bandiwadekar, A. The use of cellulose, chitosan and hyaluronic acid in transdermal therapeutic management of obesity: A review. Int. J. Biol. Macromol. 2023, 244, 125374. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef]
- Patel, P.S.; Buras, E.D.; Balasubramanyam, A. The role of the immune system in obesity and insulin resistance. J. Obes. 2013, 2013, 616193. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Ghayor, C.; Pérez Dominguez, A.; Weber, F.E. From Influenza Virus to Novel Corona Virus (SARS-CoV-2)-The Contribution of Obesity. Front. Endocrinol. 2020, 11, 556962. [Google Scholar] [CrossRef]
- Jaber, N.; Al-Remawi, M.; Al-Akayleh, F.; Al-Muhtaseb, N.; Al-Adham, I.S.I.; Collier, P.J. A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID-19. J. Appl. Microbiol. 2022, 132, 41–58. [Google Scholar] [CrossRef]
- Ding, B.; Zhang, L.; Li, Z.; Zhong, Y.; Tang, Q.; Qin, Y.; Chen, M. The Matrix Protein of Human Parainfluenza Virus Type 3 Induces Mitophagy that Suppresses Interferon Responses. Cell Host Microbe 2017, 21, 538–547.e534. [Google Scholar] [CrossRef]
- Irazoki, A.; Gordaliza-Alaguero, I.; Frank, E.; Giakoumakis, N.N.; Seco, J.; Palacín, M.; Gumà, A.; Sylow, L.; Sebastián, D.; Zorzano, A. Disruption of mitochondrial dynamics triggers muscle inflammation through interorganellar contacts and mitochondrial DNA mislocation. Nat. Commun. 2023, 14, 108. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Lee, J.S.; Kim, S.K.; Jeon, Y.J.; Moon, S.H.; Jeon, B.T.; Bahk, Y.Y.; et al. Sulfated chitosan oligosaccharides suppress LPS-induced NO production via JNK and NF-κB inactivation. Molecules 2014, 19, 18232–18247. [Google Scholar] [CrossRef]
- Liu, H.T.; Li, W.M.; Li, X.Y.; Xu, Q.S.; Liu, Q.S.; Bai, X.F.; Yu, C.; Du, Y.G. Chitosan oligosaccharides inhibit the expression of interleukin-6 in lipopolysaccharide-induced human umbilical vein endothelial cells through p38 and ERK1/2 protein kinases. Basic. Clin. Pharmacol. Toxicol. 2010, 106, 362–371. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. The improved antiviral activities of amino-modified chitosan derivatives on Newcastle virus. Drug Chem. Toxicol. 2021, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Mosafer, J.; Sabbaghi, A.H.; Badiee, A.; Dehghan, S.; Tafaghodi, M. Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 2019, 14, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ensinas, A.; Verrier, B.; Primard, C.; Cuvillier, A.; Champier, G.; Paul, S.; Delair, T. Zinc-Stabilized Chitosan-Chondroitin Sulfate Nanocomplexes for HIV-1 Infection Inhibition Application. Mol. Pharm. 2016, 13, 3279–3291. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef]



| Group | Sample Composition |
|---|---|
| A | 60% deacetylated chitosan |
| B | 85% deacetylated chitosan |
| C | GSE |
| D | 60% deacetylated chitosan + GSE |
| E | 85% deacetylated chitosan + GSE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, D.S.; Lee, J.S.; Shin, Y.C.; Jang, Y. Multipotent Antiviral Effects of Deacetylated Chitosan Supplemented with Grapefruit Seed Extract against Influenza and Parainfluenza Viruses. Appl. Sci. 2023, 13, 9938. https://doi.org/10.3390/app13179938
Seo DS, Lee JS, Shin YC, Jang Y. Multipotent Antiviral Effects of Deacetylated Chitosan Supplemented with Grapefruit Seed Extract against Influenza and Parainfluenza Viruses. Applied Sciences. 2023; 13(17):9938. https://doi.org/10.3390/app13179938
Chicago/Turabian StyleSeo, Dong Soo, Joong Su Lee, Yong Chul Shin, and Younghoon Jang. 2023. "Multipotent Antiviral Effects of Deacetylated Chitosan Supplemented with Grapefruit Seed Extract against Influenza and Parainfluenza Viruses" Applied Sciences 13, no. 17: 9938. https://doi.org/10.3390/app13179938





