Minimalized Erlangen Correction Method by Hümmer (MEK) Compared with Conventional and Minimally Invasive Correction Methods for Pectus Excavatum Single Center Experience
Abstract
:1. Introduction
2. Materials and Methods
3. Preoperative Diagnostic Tests
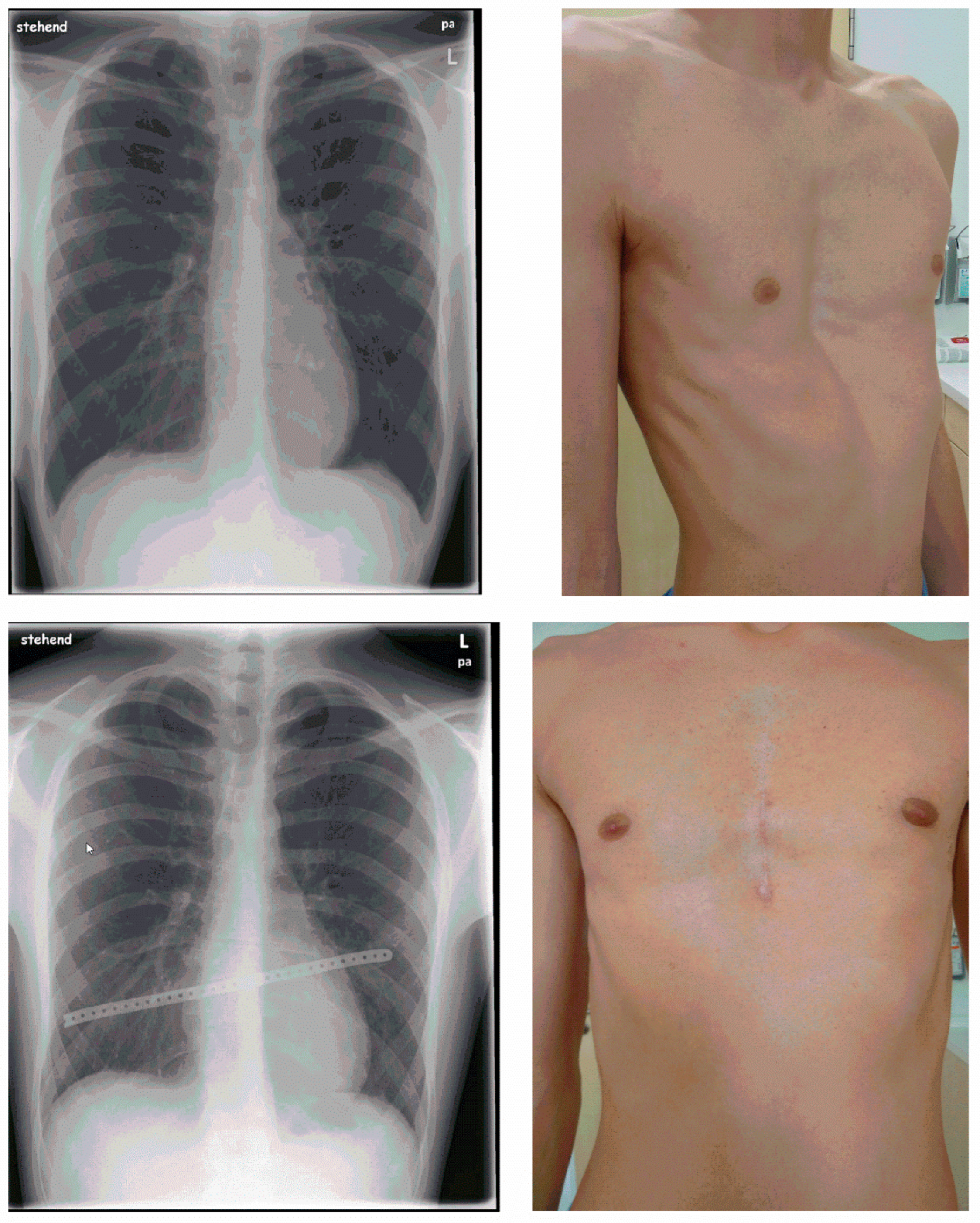
4. Technique of the Minimalized Erlangen Correction Method (MEK)
5. Perioperative and Postoperative Course
6. Patient Satisfaction
7. Statistical Analysis: Software and Tests
8. Results
9. Surgery
10. Postoperative Course
11. Metal Implant Removal
12. Patient Satisfaction
13. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creswick, H.A.; Stacey, M.W.; Kelly, R.E., Jr.; Gustin, T.; Nuss, D.; Harvey, H.; Goretsky, M.J.; Vasser, E.; Welch, J.C.; Mitchell, K.; et al. Family study of the inheritance of pectus excavatum. J. Pediatr. Surg. 2006, 41, 1699–1703. [Google Scholar] [CrossRef]
- Fokin, A.A.; Steuerwald, N.M.; Ahrens, W.A.; Allen, K.E. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin. Thorac. Cardiovasc. Surg. 2009, 21, 44–57. [Google Scholar] [CrossRef]
- Jaroszewski, D.; Notrica, D.; McMahon, L.; Steidley, D.E.; Deschamps, C. Current management of pectus excavatum: A review and update of therapy and treatment recommendations. J. Am. Board Fam. Med. 2010, 23, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.E., Jr.; Cash, T.F.; Shamberger, R.C.; Mitchell, K.K.; Mellins, R.B.; Lawson, M.L.; Oldham, K.; Azizkhan, R.G.; Hebra, A.V.; Nuss, D.; et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: Multicenter study. Pediatrics 2008, 122, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Hümmer, H.P. Die Trichterbrust Stadien- und Formgerechte Korrektur; Zuckschwerdt: München, Germany, 1985. [Google Scholar]
- Paidas, C.; Colombani, P. The Chest Wall. Principles and Practice of Pediatric Surgery; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 881–895. [Google Scholar]
- Schmüdderich, J. Ergebnisse der Funktionsdiagnostik bei Trichterbrust. Ph.D. Thesis, Medizinische Fakultät Universität Erlangen, Erlangen, Germany, 1987. [Google Scholar]
- Shamberger, R. Congenital chest wall deformities. In Pediatric Surgery, 6th ed.; Grosfeld, J.L., O’Neill, J.A., Coran, A., Fonkalsrud, E.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–2214. [Google Scholar]
- Bergmann, F.; Muensterer, O.J. Chest Wall Deformities in Children and Adolescents. Zentralblatt Fur Chir. 2022, 147, 74–82. [Google Scholar] [CrossRef]
- Abid, I.; Ewais, M.M.; Marranca, J.; Jaroszewski, D.E. Pectus Excavatum: A Review of Diagnosis and Current Treatment Options. J. Am. Osteopath. Assoc. 2017, 117, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zallen, G.S.; Glick, P.L. Miniature access pectus excavatum repair: Lessons we have learned. J. Pediatr. Surg. 2004, 39, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Koumbourlis, A.C. Pectus excavatum: Pathophysiology and clinical characteristics. Paediatr. Respir. Rev. 2009, 10, 3–6. [Google Scholar] [CrossRef]
- Hümmer, H.; Reingruber, B.; Weber, P.; Knorr, C. Indikation und Technik der “minimal-invasiven” Trichterbrustkorrektur. Chir. Allg. 2010, 4/11, 193–200. [Google Scholar]
- Rachwan, R.J.; Purpura, A.K.; Kahwash, B.M. Sudden Cardiac Arrest in a Young Patient with Severe Pectus Excavatum. Am. J. Med. Sci. 2018, 356, 570–573. [Google Scholar] [CrossRef]
- Ravitch, M.M. The Operative Treatment of Pectus Excavatum. Ann. Surg. 1949, 129, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, A.; DeBakey, M. CHŌNĒ-CHONDROSTERNON: Report of a Case and Review of the Literature. J. Thorac. Surg. 1939, 8, 469–511. [Google Scholar] [CrossRef]
- Paltia, V.; Parkkulainen, K.V.; Sulamaa, M.; Wallgren, G.R. Operative technique in funnel chest; experience in 81 cases. Acta Chir. Scand. 1959, 116, 90–98. [Google Scholar] [PubMed]
- Hegemann, G.; Schoberth, H. Operative treatment of funnel chest. Dtsch. Med. Wochenschr. 1958, Band 83, 277–282. [Google Scholar] [CrossRef]
- Pinterits, F.; Grabenwöger, F.; Dock, W.; Metz, V.; Helmer, F. Wert des Trichterbrustindex nach Hümmer zur Objektivierung des Schweregrades der Trichterbrust vor und nach chirurgischer Korrektur. Acta Chir. Austriaca 1988, 20, 8–10. [Google Scholar] [CrossRef]
- Feng, J.; Hu, T.; Liu, W.; Zhang, S.; Tang, Y.; Chen, R.; Jiang, X.; Wei, F. The biomechanical, morphologic, and histochemical properties of the costal cartilages in children with pectus excavatum. J. Pediatr. Surg. 2001, 36, 1770–1776. [Google Scholar] [CrossRef]
- Prozorovskaya, N.N.; Kozlov, E.A.; Voronov, A.V.; Verovskii, V.A.; Delvig, A.A. Characterization of costal cartilage collagen in funnel chest. Biomed. Sci. 1991, 2, 576–580. [Google Scholar]
- Serafin, J.; Swiatkowski, J.; Majkusiak, R.; Nowakowski, P. 40-year experience in surgical treatment of congenital chest deformations—Ethiopathogenesis, operative techniques and clinical results. Acta Chir. Orthop. Traumatol. Cechoslov. 2003, 70, 207–213. [Google Scholar]
- Rupprecht, H.; Hümmer, H.; Stöß, H. Zur Pathogenese der Thoraxfehlbildungen. Elektronenmikroskopiesche Untersuchungen und Spurenelementanalysen im Rippenknorpel. Z. Kinderchir. 1987, 42, 228–229. [Google Scholar]
- Behr, C.A.; Denning, N.L.; Kallis, M.P.; Maloney, C.; Soffer, S.Z.; Romano-Adesman, A.; Hong, A.R. The incidence of Marfan syndrome and cardiac anomalies in patients presenting with pectus deformities. J. Pediatr. Surg. 2019, 54, 1926–1928. [Google Scholar] [CrossRef]
- Akçali, Y.; Ceyran, H.; Hasdiraz, L. Chest wall deformities. Acta Chir. Hung. 1999, 38, 1–3. [Google Scholar] [PubMed]
- Kotzot, D.; Schwabegger, A.H. Etiology of chest wall deformities—A genetic review for the treating physician. J. Pediatr. Surg. 2009, 44, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, X.; Zhu, W.; Huang, Y.; Mou, L.; Liu, M.; Li, X.; Li, F.; Li, X.; Zhang, Y.; et al. Evidence for GAL3ST4 mutation as the potential cause of pectus excavatum. Cell Res. 2012, 22, 1712–1715. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Li, G.; Feng, Y. TINAG mutation as a genetic cause of pectus excavatum. Med. Hypotheses 2020, 137, 109557. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, A.; Ayten, A.; Oz, N.; Demircan, A. Early and long-term results of surgical repair of pectus excavatum. Asian Cardiovasc. Thorac. Ann. 2002, 10, 39–42. [Google Scholar] [CrossRef]
- Coskun, Z.K.; Turgut, H.B.; Demirsoy, S.; Cansu, A. The prevalence and effects of Pectus Excavatum and Pectus Carinatum on the respiratory function in children between 7–14 years old. Indian J. Pediatr. 2010, 77, 1017–1019. [Google Scholar] [CrossRef]
- Peterson, R.J.; Young, W.G., Jr.; Godwin, J.D.; Sabiston, D.C., Jr.; Jones, R.H. Noninvasive assessment of exercise cardiac function before and after pectus excavatum repair. J. Thorac. Cardiovasc. Surg. 1985, 90, 251–260. [Google Scholar] [CrossRef]
- Rowland, T.; Moriarty, K.; Banever, G. Effect of Pectus Excavatum Deformity on Cardiorespiratory Fitness in Adolescent Boys. Arch. Pediatr. Adolesc. Med. 2005, 159, 1069–1073. [Google Scholar] [CrossRef]
- Aronson, D.C.; Bosgraaf, R.P.; Merz, E.M.; van Steenwijk, R.P.; van Aalderen, W.M.; van Baren, R. Lung function after the minimal invasive pectus excavatum repair (Nuss procedure). World J. Surg. 2007, 31, 1518–1522. [Google Scholar] [CrossRef]
- Chen, Z.; Amos, E.B.; Luo, H.; Su, C.; Zhong, B.; Zou, J.; Lei, Y. Comparative pulmonary functional recovery after Nuss and Ravitch procedures for pectus excavatum repair: A meta-analysis. J. Cardiothorac. Surg. 2012, 7, 101. [Google Scholar] [CrossRef]
- Malek, M.H.; Berger, D.E.; Housh, T.J.; Marelich, W.D.; Coburn, J.W.; Beck, T.W. Cardiovascular function following surgical repair of pectus excavatum: A metaanalysis. Chest 2006, 130, 506–516. [Google Scholar] [CrossRef]
- Castellani, C.; Windhaber, J.; Schober, P.H.; Hoellwarth, M.E. Exercise performance testing in patients with pectus excavatum before and after Nuss procedure. Pediatr. Surg. Int. 2010, 26, 659–663. [Google Scholar] [CrossRef]
- Kowalewski, J.; Barcikowski, S.; Brocki, M. Cardiorespiratory function before and after operation for pectus excavatum: Medium-term results. Eur. J. Cardio-Thorac. Surg. 1998, 13, 275–279. [Google Scholar] [CrossRef]
- Protopapas, A.D.; Athanasiou, T. Peri-operative data on the Nuss procedure in children with pectus excavatum: Independent survey of the first 20 years’ data. J. Cardiothorac. Surg. 2008, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Dzielicki, J.; Korlacki, W.; Janicka, I.; Dzielicka, E. Difficulties and limitations in minimally invasive repair of pectus excavatum—6 years experiences with Nuss technique. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2006, 30, 801–804. [Google Scholar] [CrossRef]
- Molik, K.A.; Engum, S.A.; Rescorla, F.J.; West, K.W.; Scherer, L.R.; Grosfeld, J.L. Pectus excavatum repair: Experience with standard and minimal invasive techniques. J. Pediatr. Surg. 2001, 36, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, N.; Ruggeri, G.; Thomas, E.; Parente, G.; Di Mitri, M.; Lima, M. Long-term evaluation of patient satisfaction and quality of life in pectus excavatum repair. Ann. Pediatr. Surg. 2022, 18, 84. [Google Scholar] [CrossRef]
- Bouchard, S.; Hong, A.R.; Gilchrist, B.F.; Kuenzler, K.A. Catastrophic cardiac injuries encountered during the minimally invasive repair of pectus excavatum. Semin. Pediatr. Surg. 2009, 18, 66–72. [Google Scholar] [CrossRef]
- Hebra, A.; Kelly, R.E.; Ferro, M.M.; Yüksel, M.; Campos, J.R.M.; Nuss, D. Life-threatening complications and mortality of minimally invasive pectus surgery. J. Pediatr. Surg. 2018, 53, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Notrica, D.M. Modifications to the Nuss procedure for pectus excavatum repair: A 20-year review. Semin. Pediatr. Surg. 2018, 27, 133–150. [Google Scholar] [CrossRef]
- Kelly, R.E., Jr. Modifications and Further Development of the Original Nuss Procedure: Blessing or Curse? Eur. J. Pediatr. Surg. 2018, 28, 304–319. [Google Scholar] [CrossRef]
- Hosie, S.; Sitkiewicz, T.; Petersen, C.; Göbel, P.; Schaarschmidt, K.; Till, H.; Noatnick, M.; Winiker, H.; Hagl, C.; Schmedding, A.; et al. Minimally invasive repair of pectus excavatum—The Nuss procedure. A European multicentre experience. Eur. J. Pediatr. Surg. 2002, 12, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.E.; Penha Ada, P.; Westphal, F.L.; Silva, M.T.; Galvão, T.F. Nuss procedure for pectus excavatum repair: Critical appraisal of the evidence. Rev. Col. Bras. Cir. 2014, 41, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Jukić, M.; Mustapić, I.; Šušnjar, T.; Pogorelić, Z. Minimally Invasive Modified Nuss Procedure for Repair of Pectus Excavatum in Pediatric Patients: Single-Centre Retrospective Observational Study. Children 2021, 8, 1071. [Google Scholar] [CrossRef]
- Akhtar, M.; Razick, D.I.; Saeed, A.; Baig, O.; Kamran, R.; Ansari, U.; Sajid, Z.; Rahman, J.E. Complications and Outcomes of the Nuss Procedure in Adult Patients: A Systematic Review. Cureus 2023, 15, e35204. [Google Scholar] [CrossRef] [PubMed]
- Nuss, D.; Croitoru, D.P.; Kelly, R.E., Jr.; Goretsky, M.J.; Nuss, K.J.; Gustin, T.S. Review and discussion of the complications of minimally invasive pectus excavatum repair. Eur. J. Pediatr. Surg. 2002, 12, 230–234. [Google Scholar] [CrossRef]
- Fortmann, C.; Petersen, C. Surgery for Deformities of the Thoracic Wall: No More than Strengthening the Patient’s Self-Esteem? Eur. J. Pediatr. Surg. 2018, 28, 355–360. [Google Scholar] [CrossRef]
| Sex | |
| Male | 255 patients (84.7%) |
| Female | 46 patients (15.3%) |
| Age | 20.3 ± 9.3 years (12.8–29.4 years) |
| Body Mass Index (292 patients) | 20.3 ± 2.9 kg/m2 (12.8–29.4 kg/m2) |
| Morphology | |
| Asymmetrical | 143 patients (47.5%) |
| Symmetrical | 107 patients (35.5%) |
| Other | 51 patients (16.9%) |
| Hümmer Pectus Index | |
| 131 patients | 104 ± 13.8 (61–142) |
| 170 patients | No data |
| ECG | |
| Abnormal | 67 patients (22.3%) |
| Normal | 107 patients (35.5%) |
| No data | 127 patients (42.2%) |
| Lung function tests | |
| Abnormal | 82 patients (27.2%) |
| Normal | 142 patients (47.2%) |
| No data | 77 patients (25.6%) |
| Length of incision | 8.9 ± 1.6 cm (4.0–15.5 cm) |
| Female patients | 8.7 ± 1.5 cm |
| Male patients | 9.0 ± 1.6 cm |
| Operative procedures | |
| Chondrotomy | 301 patients (100%) |
| Retrosternal dissection | 298 patients (99.0%) |
| Tensiometry | |
| Before mobilization | 190 ± 36 N |
| After median chondrotomy | 100 ± 38 N |
| After retrosternal dissection | 36 ± 16 N |
| Number of metal implants | |
| 1 implant | 228 patients (75.7%) |
| 2 implants | 71 patients (23.6%) |
| 3 implants | 2 patients (0.7%) |
| Implant positioning | |
| Transsternal | 286 patients (95.0%) |
| Episternal | 1 patient (0.3%) |
| Trans- and intrasternal | 11 patients (3.7%) |
| Trans- and parasternal | 3 patients (1.0%) |
| Duration of operation | 89.5 ± 19.7 min (45–175 min) |
| Female patients | 100.3 ± 32.4 min |
| Male patients | 87.9 ± 17.7 min |
| Drainage | |
| Chest drains | |
| 0 | 3 patients (0.4%) |
| 1 | 297 patients (99.3%) |
| 2 | 1 patients (0.3%) |
| Redon drains | |
| 1 | 9 patients (3.0%) |
| 2 | 292 patients (97.0%) |
| Complications | |
| Pneumothorax | 54 patients (17.9%) |
| Drainage | 5 patients (1.7%) |
| Pleural effusion | 51 patients (17.0%) |
| Drainage | 9 patients (3.0%) |
| Aspiration | 6 patients (2.0%) |
| Wound healing | |
| Normal | 182 patients (62.1%) |
| Redness | 72 patients (25.9%) |
| Swelling | 9 patients (3.1%) |
| Secretion | 8 patients (2.7%) |
| Dehiscence | 2 patients (0.7%) |
| Mobilization | 3 ± 0.6 days after surgery (3–7 days) |
| Length of hospitalization | 9 ± 1.7 days after surgery (5–21 days) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denzinger, M.; Reis Wolfertstetter, P.; Sossau, D.; Hümmer, H.P.; Knorr, C. Minimalized Erlangen Correction Method by Hümmer (MEK) Compared with Conventional and Minimally Invasive Correction Methods for Pectus Excavatum Single Center Experience. Appl. Sci. 2023, 13, 10009. https://doi.org/10.3390/app131810009
Denzinger M, Reis Wolfertstetter P, Sossau D, Hümmer HP, Knorr C. Minimalized Erlangen Correction Method by Hümmer (MEK) Compared with Conventional and Minimally Invasive Correction Methods for Pectus Excavatum Single Center Experience. Applied Sciences. 2023; 13(18):10009. https://doi.org/10.3390/app131810009
Chicago/Turabian StyleDenzinger, Markus, Patricia Reis Wolfertstetter, Daniel Sossau, Hans Peter Hümmer, and Christian Knorr. 2023. "Minimalized Erlangen Correction Method by Hümmer (MEK) Compared with Conventional and Minimally Invasive Correction Methods for Pectus Excavatum Single Center Experience" Applied Sciences 13, no. 18: 10009. https://doi.org/10.3390/app131810009






