Remediation Opportunities for Arsenic-Contaminated Gold Mine Waste
Abstract
:1. Introduction
1.1. Gold Mining and Refining
1.2. Environmental Impacts
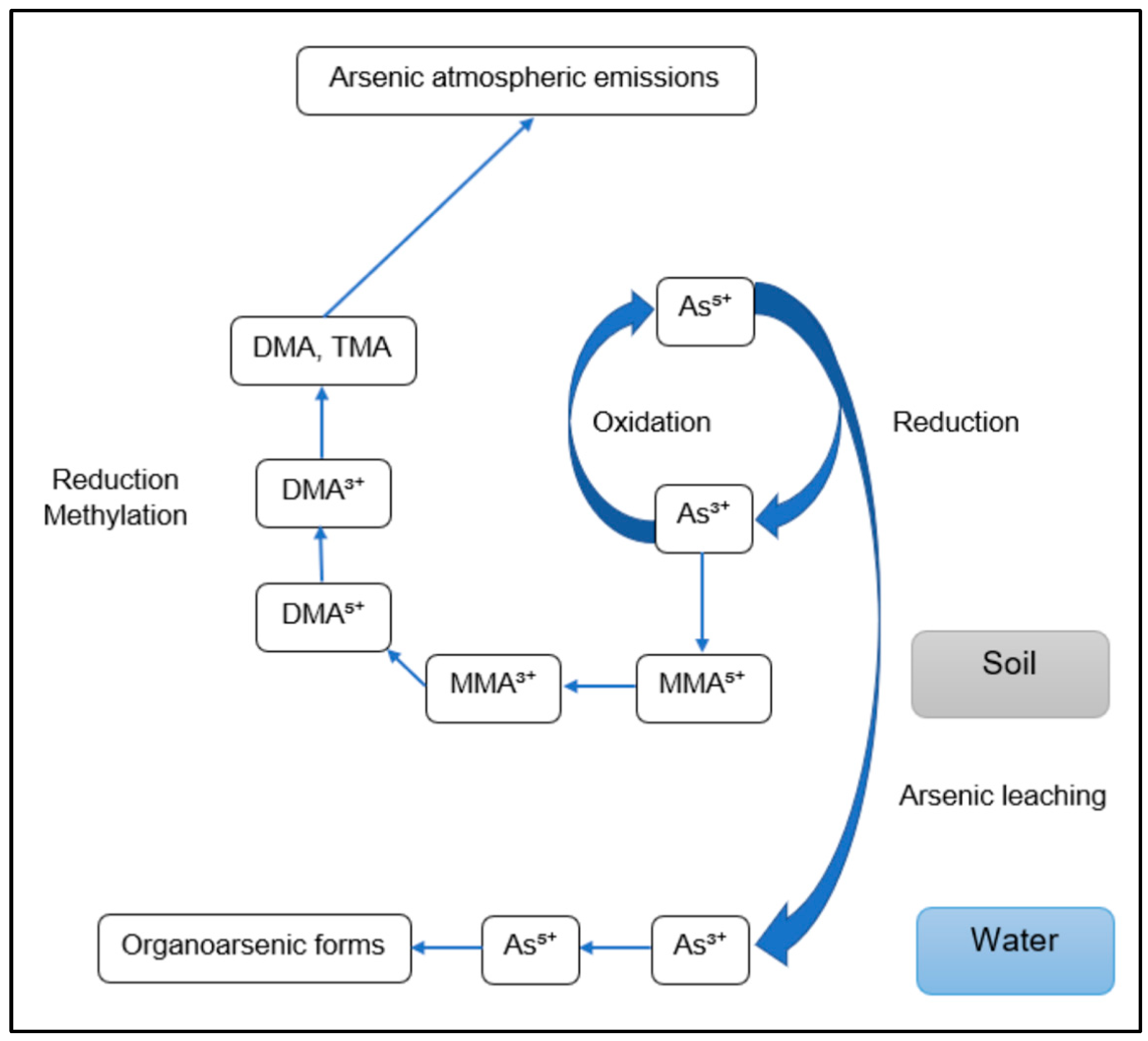
2. Arsenic Speciation and Toxicity
2.1. Speciation
2.2. Arsenic and Human Health
3. Mitigating Arsenic-Contaminated Mine Waste
3.1. Global Arsenic Concentrations
| Site | Soil Type | As (mg/kg) | Legislated Values (mg/kg) | References |
|---|---|---|---|---|
| Giant Mine, Yellowknife, Northwest Territories, Canada | Soil outcrops | 156–5760 (mean 1550) | 12 | [21,53] |
| Giant Mine, Yellowknife, Northwest Territories, Canada | Soil cores | 11–7680 (mean 177) | 12 | [21,53] |
| Nova Scotia Gold Mining District, Canada | Tailings | 200–77,000 | 12 | [52,53] |
| Meguma Terrane, Nova Scotia, Canada | Tailings | 318–76,500 | 12 | [32,53] |
| USA | Soil | 162–12,483 (median 73) | 5–20 | [65,66] |
| Morro do Ouro, Minas Gerais State, Brazil | Soil | 26–699 | [67] | |
| Delita, Cuba | Tailings | 1085–35,372 | [68] | |
| Cornwall, England | Soil | 67–4550 | 20 | [55,57] |
| Cornwall, England | Household garden soil | 23–471 (mean 262) | 20 | [56,57] |
| Cornwall, England | Household dust | 43–486 (mean 149) | 20 | [56,57] |
| Southern, Tuscany, Italy | Soil | 5–2035 | [69] | |
| Castromil Mine, Portugal | Soil | 31–6909 (mean 820) | [58] | |
| Zloty Stok Gold Mine, Poland | Soil | 860–5751 | [59] | |
| Giftkies Mine, Jáchymov, Czech Republic | Soil | 74–3697 | [60] | |
| Hillgrove Mine, New South Wales, Australia | Soil | 826–1606 | 100 | [5,70] |
| Victoria, Australia | Soil | 9–9900 | 100 | [5,49] |
| Victoria, Australia | Mine waste | 1210–22,000 | 100 | [5,63] |
| Macraes Mine, South Island, New Zealand | Mine waste | 65–98,300 | [71] | |
| Dandong, Liaoning Province, China | Soil | 1944 | [72] |
3.2. Remediation Techniques
3.3. Arsenic Remediation Techniques
3.3.1. Physical and Chemical
3.3.2. Biological
| Treatment | Process | Elements | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Physical | Backfilling (in-pit disposal) | Toxic elements | Eliminates new tailings dams/ponds. No erosion, spillages, or infrastructure failures. Deep underground disposal suppresses oxidation processes and leaves the surface for revegetation. | Groundwater pollution. Chemical and physical changes. Surface/groundwater and aquifer pollution. Mobility of toxic elements. | [4] |
| Physical | Wet and dry covering of tailings dams/ponds | Toxic elements | Limited oxidation and acidification. Mitigates wind and water erosion. Reduced permeability. | Erosion, instability, contamination, leachates, groundwater pollution, aesthetically unpleasant. | [4] |
| Physical and Chemical | Cement stabilisation/ solidification without/with chemical amendments (e.g., Fe, fly ash, and lime) | Pb, Zn, As, P, Cd | Elemental stabilisation. Reduced leachates. Immobilisation. | Long-term deterioration. Production of carbon dioxide. High energy consumption. Air pollution (cement dust). | [77,101,102,103,104,105,106] |
| Chemical | Fe-based sorbent | As | As stabilised Risk to human health decreased. pH increased to neutral (6.9). | Ecotoxicity still present. Available phosphorus significantly decreased, causing plant malnourishment. | [107] |
| Biological | Phytoremediation | As | Hyper-accumulator species identified for As in highly contaminated soils (1603 mg/kg). | Lack of hyper-accumulating species for arid to semi-arid climates. | [14,16,108] |
| Biological | Phytoremediation | Cu, As | Environmentally friendly. Stabilisation of PTEs in the rhizosphere. Removal via phytoextraction of PTEs. Cost effective. Can be applied in situ. | Lack of metallophytes and hyperaccumulating plants. Plant biomass can be reduced under toxic conditions. Long application time, possibly decades. Leachates. | [16,87,97] |
| Biological and Chemical | Phytoextraction with ethylene diaminetetraacetic acid (EDTA) | As | Increased plant shoot uptake of As. Increased organic matter, EC values, extractable As. Soluble As and As root uptake increased with high EDTA (5 mmol/kg). Root–shoot translocation factor increased. | High dose of EDTA (5 mmol/kg) inhibited plant growth. Soil pH decreased. Bioaccumulation factor decreased. Synthetic and does not biodegrade. | [109,110] |
| Biological and Chemical | Combinations of plant, organic matter, and inorganic lime | As | Organic matter + plant treatment stimulated the microbial community. Compost + plant was the optimal treatment. | Plant growth was inhibited by all treatments except for the compost amendment. | [93] |
| Biological | Phytostabilisation with biochar | Pb, Zn, Cu, Cd, As | Environmentally friendly. Stabilisation of PTEs in the rhizosphere. Cost effective. Improve soil nutrients. Biochar improved plant growth. Biochar immobilised elements. pH, TC, and TN increased. | Little immobilisation of As, Zn and Cd. Possible increase in As bioavailability. Long application time, possibly decades. Leachates. | [13,16] |
| Techniques | Characteristics | References |
|---|---|---|
| Phytoextraction | Arsenic uptake in above ground biomass. | [14,108] |
| Phytostabilisation | Immobilising As in the rhizosphere to prevent leaching into water and bioavailability. | [13,16] |
| Phytovolatilisation | Plant uptakes As through the xylem and transforms As to a less toxic and volatile form. Plant then releases As emissions. | [82,111] |
| Rhizodegradation | Microbial community in the rhizosphere degrade organic contaminants. | [111] |
| Rhizofiltration | Filtering of groundwater, surface water, and wastewater with plant roots. | [82,112] |
4. Phytoremediation
4.1. Identified Plant Species
| Plant Species | Common Name | Soil As (mg/kg) | As Accumulation | As Stabilisation | Characteristic | Reference |
|---|---|---|---|---|---|---|
| Pteris vittata | Chinese brake fern | 18–1603 | 1442–7526 mg/kg (fronds) | Hyperaccumulator | [14] | |
| Pteris vittata | Chinese brake fern | 0.47–8 | 11–64 mg/kg (fronds) | Hyperaccumulator | [14] | |
| Pteris vittata | Chinese brake fern | 500 | 6200–7600 mg/kg | Hyperaccumulator | [124] | |
| Pteris cretica | Cretan brake fern | 500 | 6200–7600 mg/kg | Hyperaccumulator | [124] | |
| Pteris longifolia | Longleaf brake | 500 | 6200–7600 mg/kg | Hyperaccumulator | [124] | |
| Pteris umbrosa | Jungle brake fern | 500 | 6200–7600 mg/kg | Hyperaccumulator | [124] | |
| Pteris vittata | Chinese brake fern | 57.1 ± 9% | Hyperaccumulator | [125] | ||
| Pteris umbrosa | Jungle brake fern | 56.8 ± 4.8% | Hyperaccumulator | [125] | ||
| Cassia alata | Ringworm bush | 1587 | ca. 25 mg/kg (shoots) | ca. 130 mg/kg (roots) | Phytostabiliser | [13] |
| Pityrogramma calomelanos | Silver fern | 135–510 | 5130–5610 mg/kg (young fronds) 2760–8000 mg/kg (old fronds) | 88–370 | Hyperaccumulator | [127] |
| Pityrogramma calomelanos | Silver fern | 20–8800 | 3820–8350 mg/kg (frond) | 88–370 mg/kg (root) | Hyperaccumulator | [128] |
| Pteris vittata | Chinese brake fern | 20–8800 | 4240–6030 mg/kg (frond) | 103–330 mg/kg (root) | Hyperaccumulator | [128] |
| Athyrium filix-femina | Lady fern | 74 ± 20.1 | 1.95 mg/kg (leaves) | 7.56 mg/kg (roots) | Metallophyte | [129] |
| Geranium robertianum | Herb Robert | 74 ± 20.1 | 1.95 mg/kg (leaves + stems) | 18.3 mg/kg (roots) | Metallophyte | [129] |
| Rhizomnium punctatum | Dotted thyme-moss | 218 ± 53.8 | 4.66 mg/kg (whole plant) | Metallophyte | [129] |
4.2. Co-Application of Soil Amendments
4.2.1. Organic Amendments
4.2.2. Phosphate and Organic Amendments
4.2.3. Lime and Organic Amendments
| Treatment | Soil Amendment | Plant | Soil As (mg/kg) | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| Organic | Hibiscus cannabinus core biochar (HB), sewage sludge biochar (SB) and chicken manure biochar (MB) (500 °C 3 h). | Cassia alata | 1587 | Plant height and root length increased for SB 3%. SB immobilised elements. HB significantly improved total C. Arsenic availability increased with SB at 3%. | Conflicting results for As mobility and solubility as well as speciation may play a role. | [13] |
| Organic | Compost (olive mill-waste + 10% cow manure) Biochar (Orchard prunings pyrolysed at 500 °C | 7490 | Soil + compost + biochar treatment had largest decrease in ecotoxicity using the Microtox method. Soil nutrients and fertility improved. | As leaching Unknown long-term effects of increased As bioavailability. | [42] | |
| Inorganic and organic | Hardwood biochar (500 °C, 3 h), iron sulphate | Populus Euramericana clone I45/51, Salix pupurea, Salix vimnalis | 297 ± 30 | Biochar + iron sulphate increased pH, EC, reduced metal(loid) concentrations in soil pore water. | Biochar + iron sulphate had no effect on plant growth. | [132] |
| Inorganic | Phosphate | Lupinus albus, Helianthus annuus, Brassica juncea, Pteris vittata | 25–2595 | No signs of phytotoxicity e.g., yellowing of leaves. Increased As bioaccumulation in roots and shoots. P. vittata and H. annuus were most successful at phytoextraction. | No significant change in biomass growth. | [16] |
| Inorganic | Phosphate | Brassica napus, Brassica juncea | 0–75 | As concentrations increased in shoots. Biomass increased. B. napus was most successful at As uptake. Improved physical and photosynthetic characteristics. | Results varied between plants. | [133] |
| Inorganic and organic | Phosphate rock, municipal solid waste and biosolid compost | Pteris vittata | 135 and 126 | Phosphate and compost increased As bioaccumulation. Plant mitigated leaching. | Field soil and spiked soil had different results. | [135] |
| Inorganic | Phosphate and lime | Pteris vittata | 361 | Lime balanced soil pH after phosphate application. | Phosphate and lime had no significant effect on As bioaccumulation. | [55] |
| Inorganic and organic | Fresh cow manure. Olive leaves/olive mill-waste mature compost. Sugar beet lime. | Brassica juncea | 86–634 | Manure had highest plant biomass production. Organic matter improved plant survival, growth, and soil conditions. | Manure introduced weeds. As uptake was low, Brassica juncea was not suited for phytoextraction | [94] |
| Inorganic and organic | Mature olive mill-waste compost Pig slurry Hydrated lime | Atriplex halimus | 664 ± 28 | Organic amendments with plants provided soil with essential nutrients, which improved the soil microbial community and were effective at phytostabilisation. Ecotoxicity decreased most notably with compost + plant. Limed soil had highest dissolved N in pore water, mainly inorganic N. | Plants did not grow well in lime amendment. | [93] |
| Inorganic and organic | Compost, pig slurry (PS), hydrated lime (HL). | Atriplex halimus | 664 ± 28 | Compost improved organic matter, total organic carbon, and soil microbial biomass -C and -N. Plant growth improved with amendments HL (59%), compost (79%) and PS (89%). As leaf concentrations decreased = phytostabilisation. | HL did not significantly decrease bioaccumulation of As in fruits compared to organic amendments after 12 months | [95] |
5. Ecotoxicological Assessment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Álvarez-Ayuso, E. Stabilization and Encapsulation of Arsenic-/Antimony-Bearing Mine Waste: Overview and Outlook of Existing Techniques. Crit. Rev. Environ. Sci. Technol. 2021, 52, 3720–3752. [Google Scholar] [CrossRef]
- Zhou, Q.; Teng, Y.; Liu, Y. A Study on Soil-Environmental Quality Criteria and Standards of Arsenic. Appl. Geochem. 2017, 77, 158–166. [Google Scholar] [CrossRef]
- Anawar, H.M.; Akter, F.; Solaiman, Z.M.; Strezov, V. Biochar: An Emerging Panacea for Remediation of Soil Contaminants from Mining, Industry and Sewage Wastes. Pedosphere 2015, 25, 654–665. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Tailings. In Mine Wastes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 205–241. [Google Scholar] [CrossRef]
- National Environment Protection Measure (Assessment of Site Contamination). Schedule B1 Guideline Investigation Levels Soil and Groundwater; The National Environment Protection Council: Canberra, Australia, 2011. [Google Scholar]
- Banwart, S. Save Our Soils. Nature 2011, 474, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of Soil Organic Carbon Caused by Functional Complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Szarek-Łukaszewska, G.; Grodzińska, K.; Niklińska, M.; Vogt, R.D. Soil Fertility and Plant Diversity Enhance Microbial Performance in Metal-Polluted Soils. Sci. Total Environ. 2012, 439, 211–219. [Google Scholar] [CrossRef]
- Singh, P.; Ghosh, A.K.; Kumar, S.; Kumar, M.; Sinha, P.K. Influence of Input Litter Quality and Quantity on Carbon Storage in Post-Mining Forest Soil after 14 Years of Reclamation. Ecol. Eng. 2022, 178, 106575. [Google Scholar] [CrossRef]
- Singh, P.; Ghosh, A.K.; Kumar, S.; Jat, S.L.; Seema, K.; Pradhan, S.N.; Kumar, M. A Molecular and Spectroscopic Approach to Reclamation of Coal Mine Soil Using Tree Species: A Case Study of Gevra Mining Area, Korba, India. J. Soil Sci. Plant Nutr. 2022, 22, 2205–2220. [Google Scholar] [CrossRef]
- Singh, P.; Ghosh, A.K.; Kumar, S. The Role of Input Litter Quality and Quantity on Soil Organic Matter Formation and Sequestration in Rehabilitated Mine Soil. Indian For. 2022, 148, 280. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Zhao, M.; Chao, Y.; Qiu, R.; Yang, Y.; Wang, S. Potential of Cassia Alata L. Coupled with Biochar for Heavy Metal Stabilization in Multi-Metal Mine Tailings. Int. J. Environ. Res. Public. Health 2018, 15, 494. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A Fern That Hyperaccumulates Arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Muehe, E.M.; Wang, T.; Kerl, C.F.; Planer-Friedrich, B.; Fendorf, S. Rice Production Threatened by Coupled Stresses of Climate and Soil Arsenic. Nat. Commun. 2019, 10, 4985. [Google Scholar] [CrossRef] [PubMed]
- Barbafieri, M.; Pedron, F.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Assisted Phytoremediation of a Multi-Contaminated Soil: Investigation on Arsenic and Lead Combined Mobilization and Removal. J. Environ. Manag. 2017, 203, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Ollson, C.J.; Smith, E.; Scheckel, K.G.; Betts, A.R.; Juhasz, A.L. Assessment of Arsenic Speciation and Bioaccessibility in Mine-Impacted Materials. J. Hazard. Mater. 2016, 313, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, B.W. Economics of Gold Deposits. In Gold Metallogeny and Exploration; Foster, R.P., Ed.; Springer: Dordrecht, The Netherlands, 1991; pp. 399–426. [Google Scholar] [CrossRef]
- Richardson, P.; Van Helten, J.-J. The Development of the South African Gold-Mining Industry, 1895–1918. Econ. Hist. Rev. 1984, 37, 319. [Google Scholar] [CrossRef]
- Marsden, J.; House, I. The Chemistry of Gold Extraction, 2nd ed.; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2006. [Google Scholar]
- Bromstad, M.J.; Wrye, L.A.; Jamieson, H.E. The Characterization, Mobility, and Persistence of Roaster-Derived Arsenic in Soils at Giant Mine, NWT. Appl. Geochem. 2017, 82, 102–118. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Walker, S.R.; Parsons, M.B. Mineralogical Characterization of Mine Waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Saba, M.; MohammadYousefi, A.; Rashchi, F.; Moghaddam, J. Diagnostic Pre-Treatment Procedure for Simultaneous Cyanide Leaching of Gold and Silver from a Refractory Gold/Silver Ore. Miner. Eng. 2011, 24, 1703–1709. [Google Scholar] [CrossRef]
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of Gold Extraction from Ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Bradham, K.D.; Scheckel, K.G.; Nelson, C.M.; Seales, P.E.; Lee, G.E.; Hughes, M.F.; Miller, B.W.; Yeow, A.; Gilmore, T.; Serda, S.M.; et al. Relative Bioavailability and Bioaccessibility and Speciation of Arsenic in Contaminated Soils. Environ. Health Perspect. 2011, 119, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Sovacool, B.K. The Precarious Political Economy of Cobalt: Balancing Prosperity, Poverty, and Brutality in Artisanal and Industrial Mining in the Democratic Republic of the Congo. Extr. Ind. Soc. 2019, 6, 915–939. [Google Scholar] [CrossRef]
- Sovacool, B.K. When Subterranean Slavery Supports Sustainability Transitions? Power, Patriarchy, and Child Labor in Artisanal Congolese Cobalt Mining. Extr. Ind. Soc. 2021, 8, 271–293. [Google Scholar] [CrossRef]
- Mountford, B.; Tuffnell, S. (Eds.) A Global History of Gold Rushes; California World History Library; University of California Press: Oakland, CA, USA, 2018. [Google Scholar]
- Reeves, K.; Frost, L.; Fahey, C. Integrating the Historiography of the Nineteenth-Century Gold Rushes: Integrating. Aust. Econ. Hist. Rev. 2010, 50, 111–128. [Google Scholar] [CrossRef]
- Craw, D.; Bowell, R.J. The Characterization of Arsenic in Mine Waste. Rev. Mineral. Geochem. 2014, 79, 473–505. [Google Scholar] [CrossRef]
- Bowell, R.J.; Craw, D. The Management of Arsenic in the Mining Industry. Rev. Mineral. Geochem. 2014, 79, 507–532. [Google Scholar] [CrossRef]
- Walker, S.R.; Parsons, M.B.; Jamieson, H.E.; Lanzirotti, A. Arsenic mineralogy of near-surface tailings and soils: Influences on arsenic mobility and bioaccessibility in the nova scotia gold mining districts. Can. Mineral. 2009, 47, 533–556. [Google Scholar] [CrossRef]
- Martin, R.; Dowling, K.; Pearce, D.C.; Florentine, S.; Bennett, J.W.; Stopic, A. Size-Dependent Characterisation of Historical Gold Mine Wastes to Examine Human Pathways of Exposure to Arsenic and Other Potentially Toxic Elements. Environ. Geochem. Health 2016, 38, 1097–1114. [Google Scholar] [CrossRef]
- Bolan, N.S.; Choppala, G.; Kunhikrishnan, A.; Park, J.; Naidu, R. Microbial Transformation of Trace Elements in Soils in Relation to Bioavailability and Remediation. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2013; pp. 1–56. [Google Scholar] [CrossRef]
- Bhattacharjee, H.; Rosen, B.P. Arsenic Metabolism in Prokaryotic and Eukaryotic Microbes. In Molecular Microbiology of Heavy Metals; Nies, D.H., Silver, S., Eds.; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2007; Volume 6, pp. 371–406. [Google Scholar] [CrossRef]
- Ravenscroft, P.; Brammer, H.; Richards, K.S. Arsenic Pollution: A Global Synthesis; RGS-IBG Book Series; Wiley-Blackwell: Chichester, UK; Malden, MA, USA, 2009. [Google Scholar]
- Yuan, C.; Lu, X.; Qin, J.; Rosen, B.P.; Le, X.C. Volatile Arsenic Species Released from Escherichia Coli Expressing the AsIII S-Adenosylmethionine Methyltransferase Gene. Environ. Sci. Technol. 2008, 42, 3201–3206. [Google Scholar] [CrossRef]
- United States, Agency for Toxic Substances and Disease Registry; Syracuse Research Corporation. Toxicological Profile for Arsenic; United States, Agency for Toxic Substances and Disease Registry: Atlanta, CA, USA, 2007. [CrossRef]
- Ali, I.; Jain, C.K. Advances in Arsenic Speciation Techniques. Int. J. Environ. Anal. Chem. 2004, 84, 947–964. [Google Scholar] [CrossRef]
- Kim, C.S.; Chi, C.; Miller, S.R.; Rosales, R.A.; Sugihara, E.S.; Akau, J.; Rytuba, J.J.; Webb, S.M. (Micro)Spectroscopic Analyses of Particle Size Dependence on Arsenic Distribution and Speciation in Mine Wastes. Environ. Sci. Technol. 2013, 47, 8164–8171. [Google Scholar] [CrossRef]
- Savage, K.S.; Tingle, T.N.; O’Day, P.A.; Waychunas, G.A.; Bird, D.K. Arsenic Speciation in Pyrite and Secondary Weathering Phases, Mother Lode Gold District, Tuolumne County, California. Appl. Geochem. 2000, 15, 1219–1244. [Google Scholar] [CrossRef]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J.C. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Toxicity of Metals and Arsenic in a Naturally Contaminated Mine Soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Romero-Freire, A.; Sierra-Aragón, M.; Ortiz-Bernad, I.; Martín-Peinado, F.J. Toxicity of Arsenic in Relation to Soil Properties: Implications to Regulatory Purposes. J. Soils Sediments 2014, 14, 968–979. [Google Scholar] [CrossRef]
- Martin, R.; Dowling, K.; Nankervis, S.; Pearce, D.; Florentine, S.; McKnight, S. In Vitro Assessment of Arsenic Mobility in Historical Mine Waste Dust Using Simulated Lung Fluid. Environ. Geochem. Health 2018, 40, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.A.; Gasparon, M.; Delbem, I.D.; Caldeira, C.L.; Freitas, E.T.F.; Ng, J.C.; Ciminelli, V.S.T. Gastric/Lung Bioaccessibility and Identification of Arsenic-Bearing Phases and Sources of Fine Surface Dust in a Gold Mining District. Sci. Total Environ. 2019, 689, 1244–1254. [Google Scholar] [CrossRef]
- Ozturk, M.; Metin, M.; Altay, V.; Bhat, R.A.; Ejaz, M.; Gul, A.; Unal, B.T.; Hasanuzzaman, M.; Nibir, L.; Nahar, K.; et al. Arsenic and Human Health: Genotoxicity, Epigenomic Effects, and Cancer Signaling. Biol. Trace Elem. Res. 2022, 200, 988–1001. [Google Scholar] [CrossRef]
- Palmer, M.J.; Jamieson, H.E.; Borčinová Radková, A.; Maitland, K.; Oliver, J.; Falck, H.; Richardson, M. Mineralogical, Geospatial, and Statistical Methods Combined to Estimate Geochemical Background of Arsenic in Soils for an Area Impacted by Legacy Mining Pollution. Sci. Total Environ. 2021, 776, 145926. [Google Scholar] [CrossRef]
- Pavilonis, B.; Grassman, J.; Johnson, G.; Diaz, Y.; Caravanos, J. Characterization and Risk of Exposure to Elements from Artisanal Gold Mining Operations in the Bolivian Andes. Environ. Res. 2017, 154, 1–9. [Google Scholar] [CrossRef]
- Hinwood, A.L.; Sim, M.R.; Jolley, D.; De Klerk, N.; Bastone, E.B.; Gerostamoulos, J.; Drummer, O.H. Exposure to Inorganic Arsenic in Soil Increases Urinary Inorganic Arsenic Concentrations of Residents Living in Old Mining Areas. Environ. Geochem. Health 2004, 26, 27–36. [Google Scholar] [CrossRef]
- Kumar, N. (Ed.) Arsenic Toxicity: Challenges and Solutions; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Pearce, D.C.; Dowling, K.; Sim, M.R. Cancer Incidence and Soil Arsenic Exposure in a Historical Gold Mining Area in Victoria, Australia: A Geospatial Analysis. J. Exp. Sci. Environ. Epidemiol. 2012, 22, 248–257. [Google Scholar] [CrossRef]
- Meunier, L.; Walker, S.R.; Wragg, J.; Parsons, M.B.; Koch, I.; Jamieson, H.E.; Reimer, K.J. Effects of Soil Composition and Mineralogy on the Bioaccessibility of Arsenic from Tailings and Soil in Gold Mine Districts of Nova Scotia. Environ. Sci. Technol. 2010, 44, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Canadian Council of Ministers of the Environment. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health—Arsenic (Inorganic) (1997); Manitoba Statutory Publication: Winnipeg, MB, Canada, 2001. [Google Scholar]
- Xu, J.; Thornton, I. Arsenic in Garden Soils and Vegetable Crops in Cornwall, England: Implications for Human Health. Environ. Geochem. Health 1985, 7, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Caille, N.; Swanwick, S.; Zhao, F.J.; McGrath, S.P. Arsenic Hyperaccumulation by Pteris vittata from Arsenic Contaminated Soils and the Effect of Liming and Phosphate Fertilisation. Environ. Pollut. 2004, 132, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Rieuwerts, J.S.; Searle, P.; Buck, R. Bioaccessible Arsenic in the Home Environment in Southwest England. Sci. Total Environ. 2006, 371, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Department for Environment, Food and Rural Affairs; Environmental Agency. SGV 1 Soil Guideline Values for Arsenic Contamination; Environmental Agency: Bristol, UK, 2002. [Google Scholar]
- Da Silva, E.F.; Zhang, C.; Serrano Pinto, L.; Patinha, C.; Reis, P. Hazard Assessment on Arsenic and Lead in Soils of Castromil Gold Mining Area, Portugal. Appl. Geochem. 2004, 19, 887–898. [Google Scholar] [CrossRef]
- Antosiewicz, D.M.; Escudĕ-Duran, C.; Wierzbowska, E.; Skłodowska, A. Indigenous Plant Species with the Potential for the Phytoremediation of Arsenic and Metals Contaminated Soil. Water Air Soil Pollut. 2008, 193, 197–210. [Google Scholar] [CrossRef]
- Drahota, P.; Filippi, M.; Ettler, V.; Rohovec, J.; Mihaljevič, M.; Šebek, O. Natural Attenuation of Arsenic in Soils near a Highly Contaminated Historical Mine Waste Dump. Sci. Total Environ. 2012, 414, 546–555. [Google Scholar] [CrossRef]
- Ahmad, W.; Alharthy, R.D.; Zubair, M.; Ahmed, M.; Hameed, A.; Rafique, S. Toxic and Heavy Metals Contamination Assessment in Soil and Water to Evaluate Human Health Risk. Sci. Rep. 2021, 11, 17006. [Google Scholar] [CrossRef]
- Rahaman, S.; Sinha, A.C.; Pati, R.; Mukhopadhyay, D. Arsenic Contamination: A Potential Hazard to the Affected Areas of West Bengal, India. Environ. Geochem. Health 2013, 35, 119–132. [Google Scholar] [CrossRef]
- Martin, R.; Dowling, K.; Pearce, D.C.; Florentine, S.; McKnight, S.; Stelcer, E.; Cohen, D.D.; Stopic, A.; Bennett, J.W. Trace Metal Content in Inhalable Particulate Matter (PM2.5–10 and PM2.5) Collected from Historical Mine Waste Deposits Using a Laboratory-Based Approach. Environ. Geochem. Health 2017, 39, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Dowling, K.; Florentine, S. Controlled Burn and Immediate Mobilization of Potentially Toxic Elements in Soil, from a Legacy Mine Site in Central Victoria, Australia. Sci. Total Environ. 2018, 616–617, 1022–1034. [Google Scholar] [CrossRef]
- Whitacre, S.; Basta, N.; Stevens, B.; Hanley, V.; Anderson, R.; Scheckel, K. Modification of an Existing in Vitro Method to Predict Relative Bioavailable Arsenic in Soils. Chemosphere 2017, 180, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Minnesota Department of Health. Environmental Health Information Arsenic; Minnesota Department of Health: Minnesota, MN, USA, 2007. [Google Scholar]
- Dos Santos, J.V.; De Melo Rangel, W.; Azarias Guimarães, A.; Duque Jaramillo, P.M.; Rufini, M.; Marra, L.M.; Varón López, M.; Pereira Da Silva, M.A.; Fonsêca Sousa Soares, C.R.; De Souza Moreira, F.M. Soil Biological Attributes in Arsenic-Contaminated Gold Mining Sites after Revegetation. Ecotoxicology 2013, 22, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Toujaguez, R.; Ono, F.B.; Martins, V.; Cabrera, P.P.; Blanco, A.V.; Bundschuh, J.; Guilherme, L.R.G. Arsenic Bioaccessibility in Gold Mine Tailings of Delita, Cuba. J. Hazard. Mater. 2013, 262, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Baroni, F.; Boscagli, A.; Di Lella, L.A.; Protano, G.; Riccobono, F. Arsenic in Soil and Vegetation of Contaminated Areas in Southern Tuscany (Italy). J. Geochem. Explor. 2004, 81, 1–14. [Google Scholar] [CrossRef]
- Wilson, S.C.; Leech, C.D.; Butler, L.; Lisle, L.; Ashley, P.M.; Lockwood, P.V. Effects of Nutrient and Lime Additions in Mine Site Rehabilitation Strategies on the Accumulation of Antimony and Arsenic by Native Australian Plants. J. Hazard. Mater. 2013, 261, 801–807. [Google Scholar] [CrossRef]
- Mains, D.; Craw, D. Composition and Mineralogy of Historic Gold Processing Residues, East Otago, New Zealand. N. Z. J. Geol. Geophys. 2005, 48, 641–647. [Google Scholar] [CrossRef]
- Yin, Y.; Luo, X.; Guan, X.; Zhao, J.; Tan, Y.; Shi, X.; Luo, M.; Han, X. Arsenic Release from Soil Induced by Microorganisms and Environmental Factors. Int. J. Environ. Res. Public. Health 2022, 19, 4512. [Google Scholar] [CrossRef]
- Göhre, V.; Paszkowski, U. Contribution of the Arbuscular Mycorrhizal Symbiosis to Heavy Metal Phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef]
- Khan, A.G.; Kuek, C.; Chaudhry, T.M.; Khoo, C.S.; Hayes, W.J. Role of Plants, Mycorrhizae and Phytochelators in Heavy Metal Contaminated Land Remediation. Chemosphere 2000, 41, 197–207. [Google Scholar] [CrossRef]
- Yıldız, T.D. Waste Management Costs (WMC) of Mining Companies in Turkey: Can Waste Recovery Help Meeting These Costs? Resour. Policy 2020, 68, 101706. [Google Scholar] [CrossRef]
- Begum, S.; Rath, S.K.; Rath, C.C. Applications of Microbial Communities for the Remediation of Industrial and Mining Toxic Metal Waste: A Review. Geomicrobiol. J. 2022, 39, 282–293. [Google Scholar] [CrossRef]
- Desogus, P.; Manca, P.P.; Orrù, G.; Zucca, A. Stabilization–Solidification Treatment of Mine Tailings Using Portland Cement, Potassium Dihydrogen Phosphate and Ferric Chloride Hexahydrate. Miner. Eng. 2013, 45, 47–54. [Google Scholar] [CrossRef]
- Garcia-Troncoso, N.; Baykara, H.; Cornejo, M.H.; Riofrio, A.; Tinoco-Hidalgo, M.; Flores-Rada, J. Comparative Mechanical Properties of Conventional Concrete Mixture and Concrete Incorporating Mining Tailings Sands. Case Stud. Constr. Mater. 2022, 16, e01031. [Google Scholar] [CrossRef]
- Kamaludin, N.H.; Jalaludin, J.; Mohd Tamrin, S.B.; Md Akim, A.; Martiana, T.; Widajati, N. Exposure to Silica, Arsenic, and Chromium (VI) in Cement Workers: A Probability Health Risk Assessment. Aerosol Air Qual. Res. 2020, 20, 2347–2370. [Google Scholar] [CrossRef]
- Ledda, C.; Rapisarda, V.; Bracci, M.; Proietti, L.; Zuccarello, M.; Fallico, R.; Fiore, M.; Ferrante, M. Professional Exposure to Basaltic Rock Dust: Assessment by the Vibrio Fischeri Ecotoxicological Test. J. Occup. Med. Toxicol. 2013, 8, 23. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W. Arsenic Transformations in the soil-rhizosphere-Plant System: Fundamentals and Potential Application to Phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- Vithanage, M.; Dabrowska, B.B.; Mukherjee, A.B.; Sandhi, A.; Bhattacharya, P. Arsenic Uptake by Plants and Possible Phytoremediation Applications: A Brief Overview. Environ. Chem. Lett. 2012, 10, 217–224. [Google Scholar] [CrossRef]
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular Mycorrhizal Fungi in Phytoremediation of Contaminated Areas by Trace Elements: Mechanisms and Major Benefits of Their Applications. World J. Microbiol. Biotechnol. 2015, 31, 1655–1664. [Google Scholar] [CrossRef]
- Lombi, E.; Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Phytoremediation of Heavy Metal-Contaminated Soils: Natural Hyperaccumulation versus Chemically Enhanced Phytoextraction. J. Environ. Qual. 2001, 30, 1919–1926. [Google Scholar] [CrossRef]
- Schneider, J.; Stürmer, S.L.; Guilherme, L.R.G.; De Souza Moreira, F.M.; Soares, C.R.F.D.S. Arbuscular Mycorrhizal Fungi in Arsenic-Contaminated Areas in Brazil. J. Hazard. Mater. 2013, 262, 1105–1115. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Won, S.; Ha, M.-G.; Nguyen, D.D.; Kang, H.Y. Bioleaching for Environmental Remediation of Toxic Metals and Metalloids: A Review on Soils, Sediments, and Mine Tailings. Chemosphere 2021, 282, 131108. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W.; Zhang, H.; Nurmi, J.; Štipek, K.; Fischerova, Z.; Schweiger, P.; Köllensperger, G.; Ma, L.Q.; Stingeder, G. Rhizosphere Characteristics of the Arsenic Hyperaccumulator Pteris vittata L. and Monitoring of Phytoremoval Efficiency. Environ. Sci. Technol. 2003, 37, 5008–5014. [Google Scholar] [CrossRef]
- Gomes, M.P.; Andrade, M.L.; Nascentes, C.C.; Scotti, M.R. Arsenic Root Sequestration by a Tropical Woody Legume as Affected by Arbuscular Mycorrhizal Fungi and Organic Matter: Implications for Land Reclamation. Water Air Soil Pollut. 2014, 225, 1919. [Google Scholar] [CrossRef]
- Haslmayr, H.-P.; Meißner, S.; Langella, F.; Baumgarten, A.; Geletneky, J. Establishing Best Practice for Microbially Aided Phytoremediation. Environ. Sci. Pollut. Res. 2014, 21, 6765–6774. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Phytoextraction of Mine Wastes—Options and Impossibilities. Geochemistry 2005, 65, 29–42. [Google Scholar] [CrossRef]
- Archer, M.J.G.; Caldwell, R.A. Response of Six Australian Plant Species to Heavy Metal Contamination at an Abandoned Mine Site. Water Air Soil Pollut. 2004, 157, 257–267. [Google Scholar] [CrossRef]
- Vocciante, M.; Caretta, A.; Bua, L.; Bagatin, R.; Franchi, E.; Petruzzelli, G.; Ferro, S. Enhancements in Phytoremediation Technology: Environmental Assessment Including Different Options of Biomass Disposal and Comparison with a Consolidated Approach. J. Environ. Manag. 2019, 237, 560–568. [Google Scholar] [CrossRef]
- Pardo, T.; Clemente, R.; Epelde, L.; Garbisu, C.; Bernal, M.P. Evaluation of the Phytostabilisation Efficiency in a Trace Elements Contaminated Soil Using Soil Health Indicators. J. Hazard. Mater. 2014, 268, 68–76. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Bernal, M.P. Uptake of Heavy Metals and As by Brassica Juncea Grown in a Contaminated Soil in Aznalcóllar (Spain): The Effect of Soil Amendments. Environ. Pollut. 2005, 138, 46–58. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Pardo, T.; Martínez-Fernández, D.; Bernal, M.P. The Use of a Halophytic Plant Species and Organic Amendments for the Remediation of a Trace Elements-Contaminated Soil under Semi-Arid Conditions. J. Hazard. Mater. 2012, 223–224, 63–71. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, S.; Thangavel, P.; Li, Q.; Zheng, H.; Bai, J.; Qiu, R. Phytostabilization Potential of Jatropha Curcas L. in Polymetallic Acid Mine Tailings. Int. J. Phytoremed. 2011, 13, 788–804. [Google Scholar] [CrossRef]
- Ancheta, M.H.; Quimado, M.; Tiburan Jr, C.; Doronila, A.; Fernando, E. Copper and Arsenic Accumulation of Pityrogramma Calomelanos, Nephrolepis Biserrata, and Cynodon Dactylon in Cu- and Au- Mine Tailings. J. Degraded Min. Lands Manag. 2020, 7, 2201–2208. [Google Scholar] [CrossRef]
- Koller, C.E.; Patrick, J.W.; Rose, R.J.; Offler, C.E.; MacFarlane, G.R. Pteris umbrosa R. Br. as an Arsenic Hyperaccumulator: Accumulation, Partitioning and Comparison with the Established as Hyperaccumulator Pteris vittata. Chemosphere 2007, 66, 1256–1263. [Google Scholar] [CrossRef]
- Spooren, J.; Binnemans, K.; Björkmalm, J.; Breemersch, K.; Dams, Y.; Folens, K.; González-Moya, M.; Horckmans, L.; Komnitsas, K.; Kurylak, W.; et al. Near-Zero-Waste Processing of Low-Grade, Complex Primary Ores and Secondary Raw Materials in Europe: Technology Development Trends. Resour. Conserv. Recycl. 2020, 160, 104919. [Google Scholar] [CrossRef]
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-Thinking Mining Waste through an Integrative Approach Led by Circular Economy Aspirations. Minerals 2019, 9, 286. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 Emissions from Cement Production. Earth Syst. Sci. Data. 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, K.; Wang, Y.; Li, X.; Zhang, Q.; Liu, Y. In-Situ Stabilization/Solidification of Lead/Zinc Mine Tailings by Cemented Paste Backfill Modified with Low-Carbon Bentonite Alternative. J. Mater. Res. Technol. 2022, 17, 1200–1210. [Google Scholar] [CrossRef]
- Du, Y.-J.; Wei, M.-L.; Reddy, K.R.; Liu, Z.-P.; Jin, F. Effect of Acid Rain pH on Leaching Behavior of Cement Stabilized Lead-Contaminated Soil. J. Hazard. Mater. 2014, 271, 131–140. [Google Scholar] [CrossRef]
- Kim, C.S.; Wilson, K.M.; Rytuba, J.J. Particle-Size Dependence on Metal(Loid) Distributions in Mine Wastes: Implications for Water Contamination and Human Exposure. Appl. Geochem. 2011, 26, 484–495. [Google Scholar] [CrossRef]
- Yoon, I.-H.; Moon, D.H.; Kim, K.-W.; Lee, K.-Y.; Lee, J.-H.; Kim, M.G. Mechanism for the Stabilization/Solidification of Arsenic-Contaminated Soils with Portland Cement and Cement Kiln Dust. J. Environ. Manag. 2010, 91, 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; An, N.; Ma, W.; Ara, G.; Ali, K.; Kamanova, S.; Zuo, X.; Han, M.; Ren, X.; Xing, L. Chronic Cement Dust Load Induce Novel Damages in Foliage and Buds of Malus Domestica. Sci. Rep. 2020, 10, 12186. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Jeong, B.; Nam, K. Evaluation of the Effectiveness of in Situ Stabilization in the Field Aged Arsenic-Contaminated Soil: Chemical Extractability and Biological Response. J. Hazard. Mater. 2019, 367, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Ma, L.Q. Effects of Arsenic on Concentration and Distribution of Nutrients in the Fronds of the Arsenic Hyperaccumulator Pteris vittata L. Environ. Pollut. 2005, 135, 333–340. [Google Scholar] [CrossRef]
- Abbas, M.H.H.; Abdelhafez, A.A. Role of EDTA in Arsenic Mobilization and Its Uptake by Maize Grown on an As-Polluted Soil. Chemosphere 2013, 90, 588–594. [Google Scholar] [CrossRef]
- Makarova, A.; Nikulina, E.; Avdeenkova, T.; Pishaeva, K. The Improved Phytoextraction of Heavy Metals and the Growth of Trifolium repens L.: The Role of K2HEDP and Plant Growth Regulators Alone and in Combination. Sustainability 2021, 13, 2432. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Rhizofiltration of Combined Arsenic-Fluoride or Lead-Fluoride Polluted Water Using Common Aquatic Plants and Use of the ‘Clean’ Water for Alleviating Combined Xenobiotic Toxicity in a Sensitive Rice Variety. Environ. Pollut. 2022, 304, 119128. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Datta, R.; Quispe, M.A.; Sarkar, D. Greenhouse Study on the Phytoremediation Potential of Vetiver Grass, Chrysopogon zizanioides L., in Arsenic-Contaminated Soils. Bull. Environ. Contam. Toxicol. 2011, 86, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Huang, K.; Cai, H.-H.; Li, J.; Zhai, D.-L.; Dai, Z.-C.; Du, D.-L. Exploring the Potential of Naturalized Plants for Phytoremediation of Heavy Metal Contamination. Int. J. Environ. Res. 2017, 11, 515–521. [Google Scholar] [CrossRef]
- Klaber, N.S.; Barker, A.V. Accumulation of Phosphorus and Arsenic in Two Perennial Grasses for Soil Remediation. Commun. Soil Sci. Plant Anal. 2014, 45, 810–818. [Google Scholar] [CrossRef]
- Warren, G.P.; Alloway, B.J.; Lepp, N.W.; Singh, B.; Bochereau, F.J.M.; Penny, C. Field Trials to Assess the Uptake of Arsenic by Vegetables from Contaminated Soils and Soil Remediation with Iron Oxides. Sci. Total Environ. 2003, 311, 19–33. [Google Scholar] [CrossRef]
- Bradham, K.D.; Diamond, G.L.; Burgess, M.; Juhasz, A.; Klotzbach, J.M.; Maddaloni, M.; Nelson, C.; Scheckel, K.; Serda, S.M.; Stifelman, M.; et al. In Vivo and in Vitro Methods for Evaluating Soil Arsenic Bioavailability: Relevant to Human Health Risk Assessment. J. Toxicol. Environ. Health Part B 2018, 21, 83–114. [Google Scholar] [CrossRef]
- DeSisto, S.L.; Jamieson, H.E.; Parsons, M.B. Subsurface Variations in Arsenic Mineralogy and Geochemistry Following Long-Term Weathering of Gold Mine Tailings. Appl. Geochem. 2016, 73, 81–97. [Google Scholar] [CrossRef]
- Bondada, B.R.; Ma, L.Q. Tolerance of Heavy Metals in Vascular Plants: Arsenic Hyperaccumulation by Chinese Brake Fern (Pteris vittata L.). In Pteridology in the New Millennium; Springer Netherlands: Dordrecht, The Netherlands, 2003; pp. 397–420. [Google Scholar]
- Corzo Remigio, A.; Chaney, R.L.; Baker, A.J.M.; Edraki, M.; Erskine, P.D.; Echevarria, G.; Van Der Ent, A. Phytoextraction of High Value Elements and Contaminants from Mining and Mineral Wastes: Opportunities and Limitations. Plant Soil 2020, 449, 11–37. [Google Scholar] [CrossRef]
- García-Salgado, S.; García-Casillas, D.; Quijano-Nieto, M.A.; Bonilla-Simón, M.M. Arsenic and Heavy Metal Uptake and Accumulation in Native Plant Species from Soils Polluted by Mining Activities. Water Air Soil Pollut. 2012, 223, 559–572. [Google Scholar] [CrossRef]
- Srivastava, M.; Ma, L.Q.; Santos, J.A.G. Three New Arsenic Hyperaccumulating Ferns. Sci. Total Environ. 2006, 364, 24–31. [Google Scholar] [CrossRef]
- Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Arsenic Hyperaccumulation by Different Fern Species. New Phytol. 2002, 156, 27–31. [Google Scholar] [CrossRef]
- Koller, C.E.; Patrick, J.W.; Rose, R.J.; Offler, C.E.; MacFarlane, G.R. Arsenic and Heavy Metal Accumulation by Pteris vittata L. and P. umbrosa R. Br. Bull. Environ. Contam. Toxicol. 2008, 80, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-S.; Rouhan, G.; Amoroso, V.B.; Chiou, W.-L. Molecular Phylogeny and Biogeography of the Fern Genus Pteris (Pteridaceae). Ann. Bot. 2014, 114, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, K.; Visoottiviseth, P.; Sridokchan, W.; Goessler, W. Arsenic Species in an Arsenic Hyperaccumulating Fern, Pityrogramma Calomelanos: A Potential Phytoremediator of Arsenic-Contaminated Soils. Sci. Total Environ. 2002, 284, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Visoottiviseth, P.; Francesconi, K.; Sridokchan, W. The Potential of Thai Indigenous Plant Species for the Phytoremediation of Arsenic Contaminated Land. Environ. Pollut. 2002, 118, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Madejón, P.; Lepp, N.W. Arsenic in Soils and Plants of Woodland Regenerated on an Arsenic-Contaminated Substrate: A Sustainable Natural Remediation? Sci. Total Environ. 2007, 379, 256–262. [Google Scholar] [CrossRef]
- Dike, C.C.; Hakeem, I.G.; Rani, A.; Surapaneni, A.; Khudur, L.; Shah, K.; Ball, A.S. The Co-Application of Biochar with Bioremediation for the Removal of Petroleum Hydrocarbons from Contaminated Soil. Sci. Total Environ. 2022, 849, 157753. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Chen, S.; Wang, R.; Xu, P.; Cheng, M.; Zhang, C.; Xue, W. Biochar Facilitated the Phytoremediation of Cadmium Contaminated Sediments: Metal Behavior, Plant Toxicity, and Microbial Activity. Sci. Total Environ. 2019, 666, 1126–1133. [Google Scholar] [CrossRef]
- Simiele, M.; Lebrun, M.; Miard, F.; Trupiano, D.; Poupart, P.; Forestier, O.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted Phytoremediation of a Former Mine Soil Using Biochar and Iron Sulphate: Effects on As Soil Immobilization and Accumulation in Three Salicaceae Species. Sci. Total Environ. 2020, 710, 136203. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Fatimah, A.; Shahid, M.; Javed, M.T.; Wang, H.; Ok, Y.S.; Bashir, S.; Murtaza, B.; Saqib, Z.A.; et al. Phosphate-Assisted Phytoremediation of Arsenic by Brassica napus and Brassica juncea: Morphological and Physiological Response. Int. J. Phytoremediation 2017, 19, 670–678. [Google Scholar] [CrossRef]
- Walker, S.R.; Jamieson, H.E.; Lanzirotti, A.; Andrade, C.F.; Hall, G.E.M. The Speciation of Arsenic in Iron Oxides in Mine Wastes from the Giant Gold Mine, N.W.T.: Application of Synchrotron Micro-XRD and Micro-XANES at the Grain Scale. Can. Mineral. 2005, 43, 1205–1224. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Shiralipour, A. Effects of Compost and Phosphate Amendments on Arsenic Mobility in Soils and Arsenic Uptake by the Hyperaccumulator, Pteris vittata L. Environ. Pollut. 2003, 126, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Khudur, L.S.; Shahsavari, E.; Miranda, A.F.; Morrison, P.D.; Nugegoda, D.; Ball, A.S. Evaluating the Efficacy of Bioremediating a Diesel-Contaminated Soil Using Ecotoxicological and Bacterial Community Indices. Environ. Sci. Pollut. Res. 2015, 22, 14809–14819. [Google Scholar] [CrossRef] [PubMed]
- Panzarino, O.; Hyršl, P.; Dobeš, P.; Vojtek, L.; Vernile, P.; Bari, G.; Terzano, R.; Spagnuolo, M.; De Lillo, E. Rank-Based Biomarker Index to Assess Cadmium Ecotoxicity on the Earthworm Eisenia Andrei. Chemosphere 2016, 145, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.J.; Adetutu, E.M.; Makadia, T.H.; Ball, A.S. Microbial Community and Ecotoxicity Analysis of Bioremediated, Weathered Hydrocarbon-Contaminated Soil. Soil Res. 2011, 49, 261. [Google Scholar] [CrossRef]
- Van Coller-Myburgh, C.; Van Rensburg, L.; Maboeta, M. Utilizing Earthworm and Microbial Assays to Assess the Ecotoxicity of Chromium Mine Wastes. Appl. Soil Ecol. 2014, 83, 258–265. [Google Scholar] [CrossRef]
- ISO 17512-1:2008; Soil Quality—Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behaviour—Part 1: Test with Earthworms (Eisenia Fetida and Eisenia Andrei). International Organization for Standardization: Geneva, Switzerland, 2008.
- Organization for Economic Cooperation and Development. Test No. 207: Earthworm, Acute Toxicity Tests; Organization for Economic Cooperation and Development Publishing: Paris, France, 1984. [Google Scholar] [CrossRef]
- Aelion, C.M.; Davis, H.T.; McDermott, S.; Lawson, A.B. Soil Metal Concentrations and Toxicity: Associations with Distances to Industrial Facilities and Implications for Human Health. Sci. Total Environ. 2009, 407, 2216–2223. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the Ecotoxicity of Model Nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef]
- Khudur, L.S.; Shahsavari, E.; Webster, G.T.; Nugegoda, D.; Ball, A.S. The Impact of Lead Co-Contamination on Ecotoxicity and the Bacterial Community during the Bioremediation of Total Petroleum Hydrocarbon-Contaminated Soils. Environ. Pollut. 2019, 253, 939–948. [Google Scholar] [CrossRef]
- Schreck, E.; Foucault, Y.; Geret, F.; Pradere, P.; Dumat, C. Influence of Soil Ageing on Bioavailability and Ecotoxicity of Lead Carried by Process Waste Metallic Ultrafine Particles. Chemosphere 2011, 85, 1555–1562. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Assessing the Microbial Detoxification of Chemically Contaminated Water and Soil Using a Toxicity Test with a Luminescent Marine Bacterium. 2004. Available online: https://webstore.ansi.org/standards/astm/astmd566096 (accessed on 5 March 2022).
- Doherty, F.G. A Review of the Microtox® Toxicity Test System for Assessing the Toxicity of Sediments and Soils. Water Qual. Res. J. 2001, 36, 475–518. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besedin, J.A.; Khudur, L.S.; Netherway, P.; Ball, A.S. Remediation Opportunities for Arsenic-Contaminated Gold Mine Waste. Appl. Sci. 2023, 13, 10208. https://doi.org/10.3390/app131810208
Besedin JA, Khudur LS, Netherway P, Ball AS. Remediation Opportunities for Arsenic-Contaminated Gold Mine Waste. Applied Sciences. 2023; 13(18):10208. https://doi.org/10.3390/app131810208
Chicago/Turabian StyleBesedin, Julie A., Leadin S. Khudur, Pacian Netherway, and Andrew S. Ball. 2023. "Remediation Opportunities for Arsenic-Contaminated Gold Mine Waste" Applied Sciences 13, no. 18: 10208. https://doi.org/10.3390/app131810208








