Encapsulation of α-Lipoic Acid in Halloysite Nanotubes
Abstract
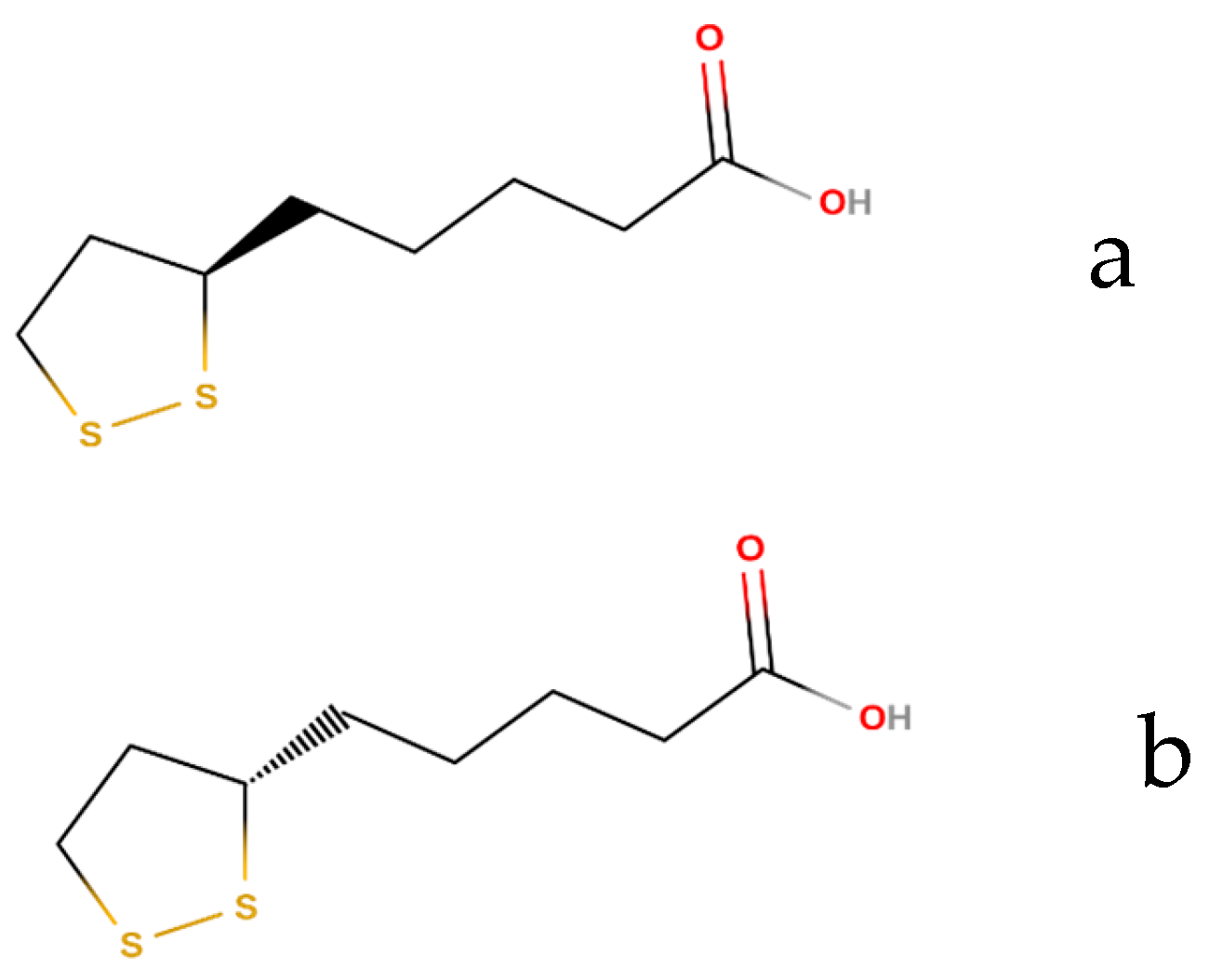
:1. Introduction
2. Materials and Methods
2.1. Reagents
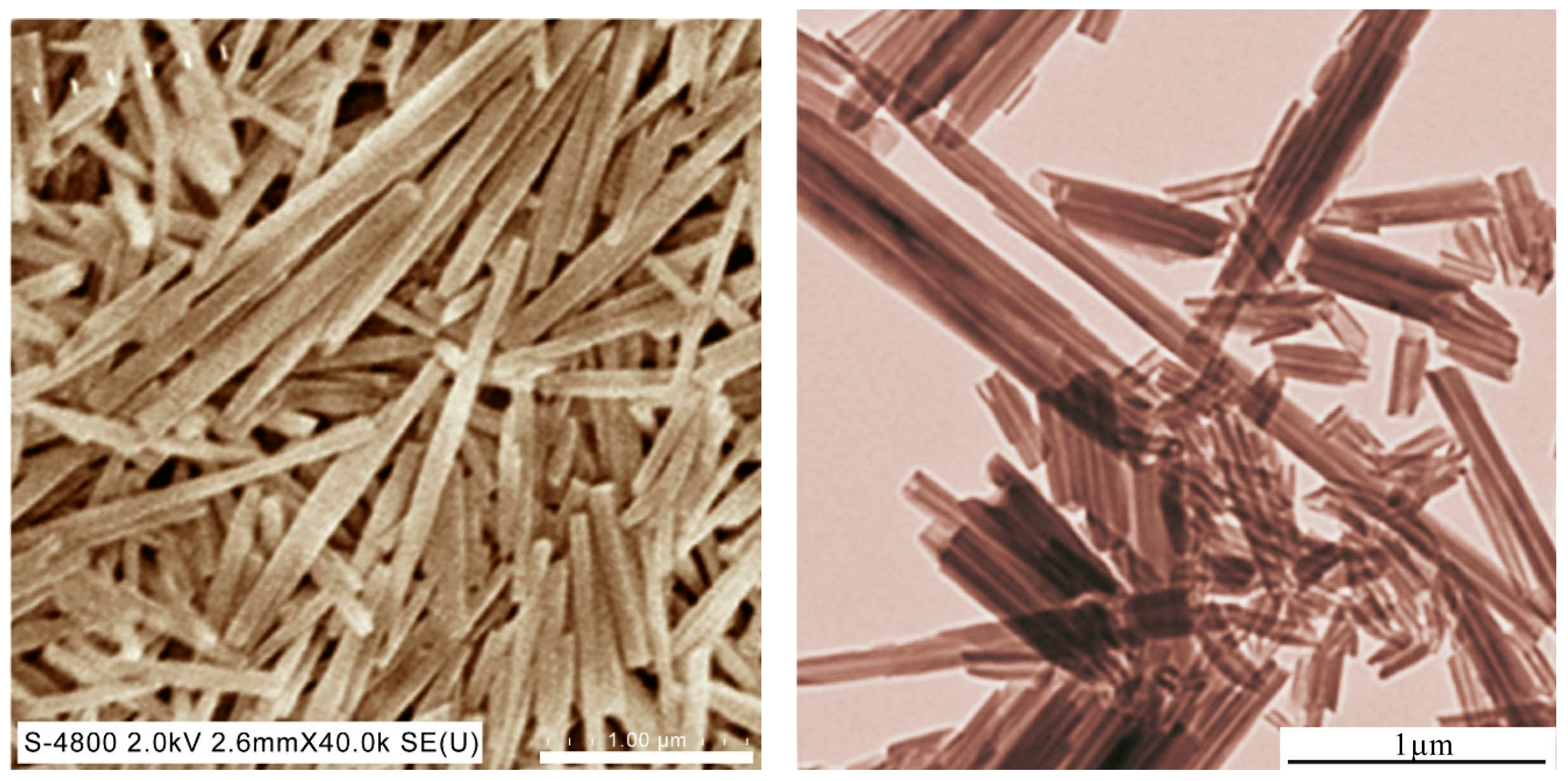
2.2. Loading Halloysite Nanotubes
2.3. Methodology for Determining the Concentration of α-Lipoic Acid
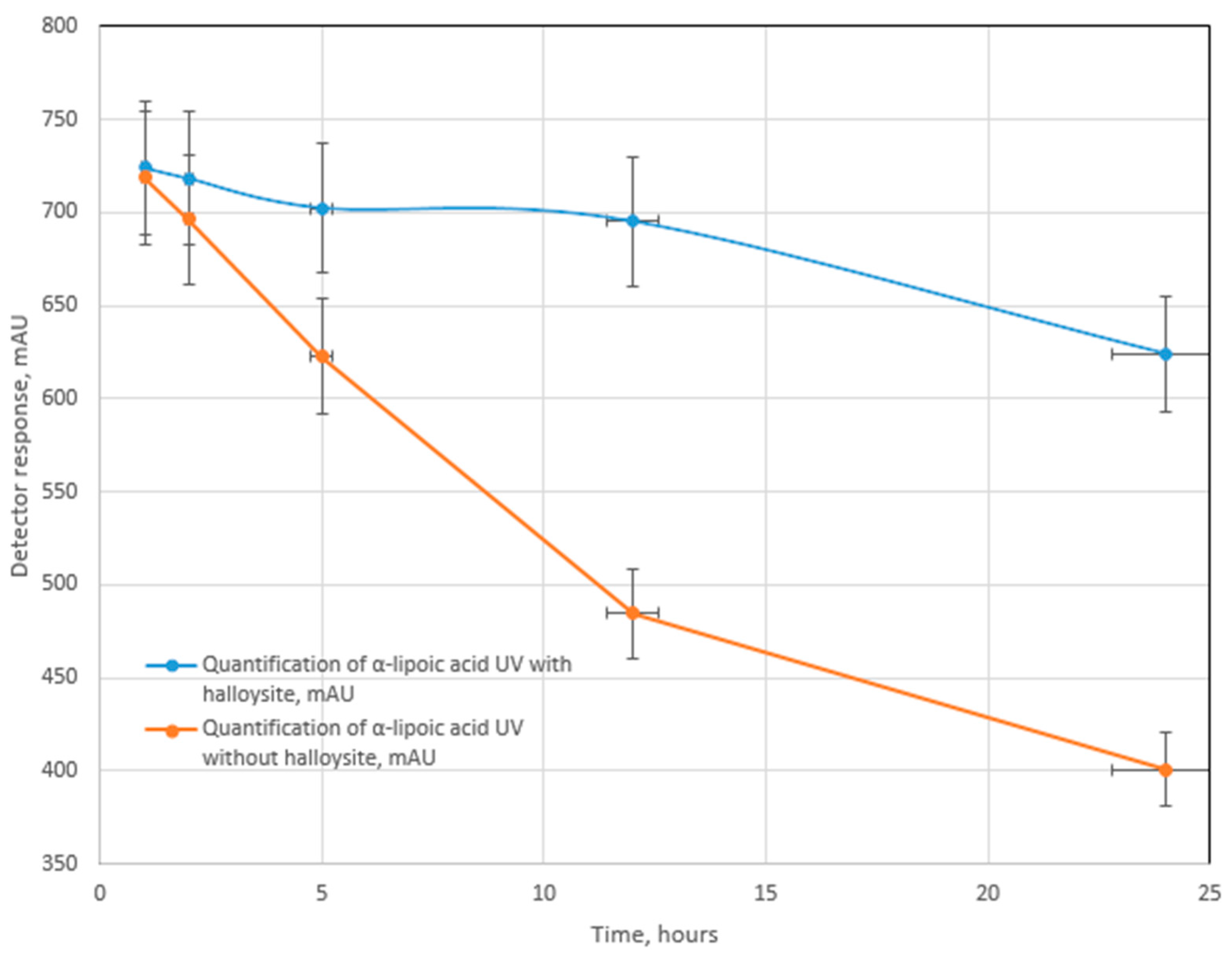
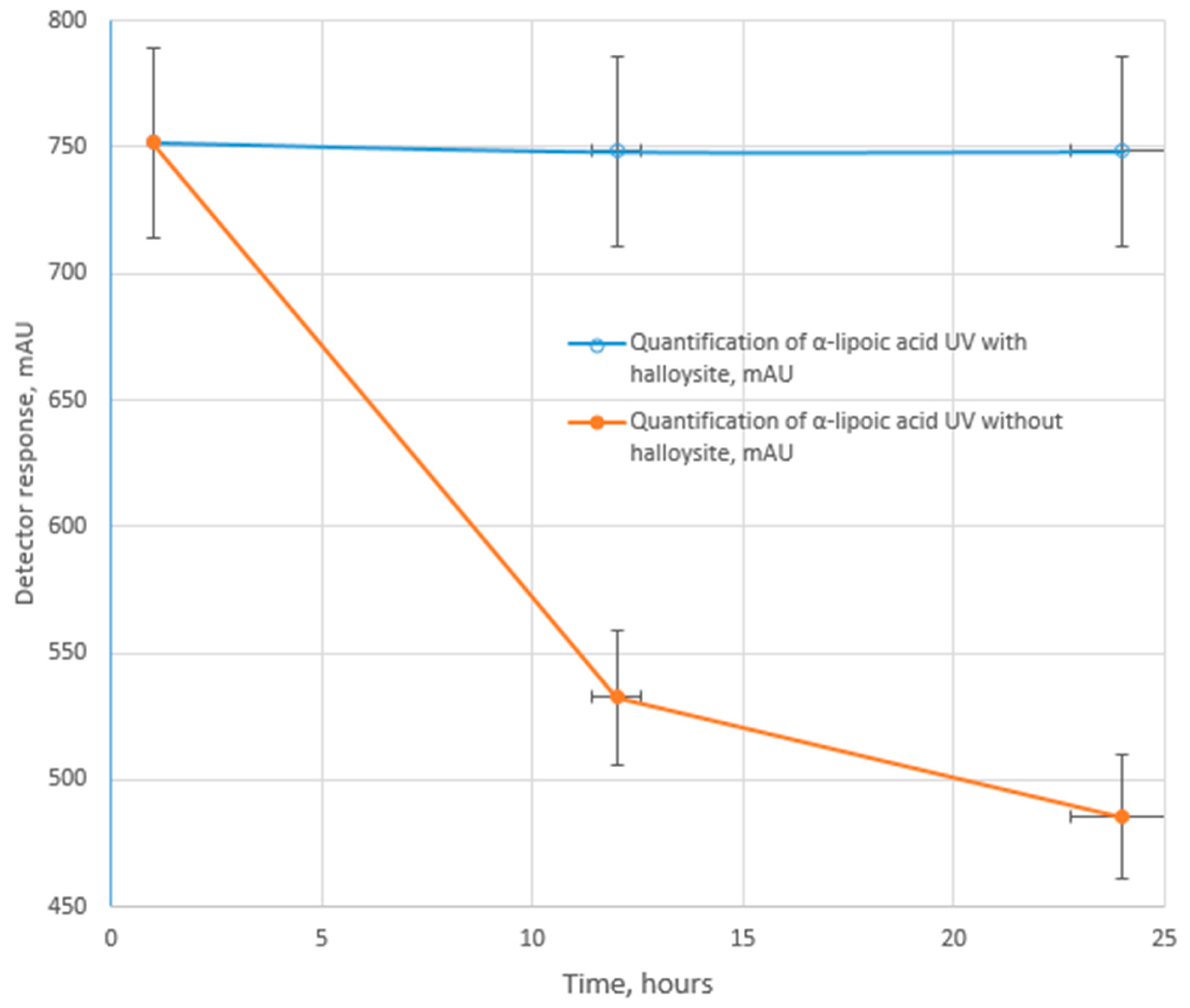
2.4. Testing for UV-, Photo-, and Thermal Stability
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bast, A.; Haenen, G.R.M.M. Lipoic Acid: A Multifunctional Antioxidant. BioFactors 2003, 17, 207–213. [Google Scholar] [CrossRef]
- Moura, F.; De Andrade, K.; Farias Dos Santos, J.; Fonseca Goulart, M. Lipoic Acid: Its Antioxidant and Anti-Inflammatory Role and Clinical Applications. Curr. Top. Med. Chem. 2015, 15, 458–483. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.L.; Crișan, S.; Bârcă, M.; Ciobanu, A.-M.; Varlas, V.N.; Pop, C.; Pali, M.-A.; Cauni, D.; Ozon, E.A.; Udeanu, D.; et al. Evaluation of Dissolution Profiles of a Newly Developed Solid Oral Immediate-Release Formula Containing Alpha-Lipoic Acid. Processes 2021, 9, 176. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K.; Bishen, S.M.; Mishra, H.; Khurana, A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev. Bras. Farmacogn. 2023, 33, 272–287. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wu, Y.; Hong, M.; Yun, Z.; Li, T.; Jiang, Y. α-Lipoic Acid Treatment Alleviates Postharvest Pericarp Browning of Litchi Fruit by Regulating Antioxidant Ability and Energy Metabolism. Postharvest Biol. Technol. 2021, 180, 111629. [Google Scholar] [CrossRef]
- Srivastava, S.; Dhaneshwar, S.; Kawathekar, N. Stress Degradation Studies and Development of Validated Stability Indicating Densitometric Method for Estimation of Alpha Lipoic Acid in Bulk and Capsule Dosage Form. Acta Chromatogr. 2023, 35, 180–186. [Google Scholar] [CrossRef]
- Ruktanonchai, U.; Bejrapha, P.; Sakulkhu, U.; Opanasopit, P.; Bunyapraphatsara, N.; Junyaprasert, V.; Puttipipatkhachorn, S. Physicochemical Characteristics, Cytotoxicity, and Antioxidant Activity of Three Lipid Nanoparticulate Formulations of Alpha-Lipoic Acid. AAPS PharmSciTech 2009, 10, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Dima, Ş.; Dima, C.; Iordăchescu, G. Encapsulation of Functional Lipophilic Food and Drug Biocomponents. Food Eng. Rev. 2015, 7, 417–438. [Google Scholar] [CrossRef]
- Lei, X.; Zhou, Y.; Liu, X.; Kong, L.; Liao, L.; Li, Y.; Liu, M.; Tian, L.; Rao, W.; Lv, G. Effective PH-Responsive Nanocarrier Based on the Anisotropic Surfaces of Halloysite Nanotubes for Controlled Drug Release. Appl. Clay Sci. 2023, 232, 106799. [Google Scholar] [CrossRef]
- Calvino, M.M.; Lazzara, G.; Cavallaro, G.; Milioto, S. Inclusion Complexes of Triblock L35 Copolymer and Hydroxyl Propyl Cyclodextrins: A Physico-Chemical Study. New J. Chem. 2022, 46, 6114–6120. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Seo, T.-R.; Lim, S.-T. Preparation of Aqueous Dispersion of β-Carotene Nano-Composites through Complex Formation with Starch Dextrin. Food Hydrocoll. 2013, 33, 256–263. [Google Scholar] [CrossRef]
- Li, Y.-X.; Park, E.Y.; Lim, S.-T. Stabilization of Alpha-Lipoic Acid by Complex Formation with Octenylsuccinylated High Amylose Starch. Food Chem. 2018, 242, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, J.; Zhou, X.; Xia, Q. Physicochemical Characterization, Identification and Improved Photo-Stability of Alpha-Lipoic Acid-Loaded Nanostructured Lipid Carrier. Drug Dev. Ind. Pharm. 2014, 40, 201–210. [Google Scholar] [CrossRef]
- Cavallaro, G.; Chiappisi, L.; Pasbakhsh, P.; Gradzielski, M.; Lazzara, G. A Structural Comparison of Halloysite Nanotubes of Different Origin by Small-Angle Neutron Scattering (SANS) and Electric Birefringence. Appl. Clay Sci. 2018, 160, 71–80. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of Properties of Various Halloysites Relevant to Their Use as Nanotubes and Microfibre Fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Durgut, E.; Cinar, M.; Terzi, M.; Kursun Unver, I.; Yildirim, Y.; Ozdemir, O. Evaluation of Different Dispersants on the Dispersion/Sedimentation Behavior of Halloysite, Kaolinite, and Quartz Suspensions in the Enrichment of Halloysite Ore by Mechanical Dispersion. Minerals 2022, 12, 1426. [Google Scholar] [CrossRef]
- Calvino, M.M.; Cavallaro, G.; Lisuzzo, L.; Milioto, S.; Lazzara, G. Separation of Halloysite/Kaolinite Mixtures in Water Controlled by Sucrose Addition: The Influence of the Attractive Forces on the Sedimentation Behavior. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128530. [Google Scholar] [CrossRef]
- Liao, J.; Wang, H.; Liu, N.; Yang, H. Functionally Modified Halloysite Nanotubes for Personalized Bioapplications. Adv. Colloid Interface Sci. 2023, 311, 102812. [Google Scholar] [CrossRef]
- Glotov, A.; Novikov, A.; Stavitskaya, A.; Nedolivko, V.; Kopitsyn, D.; Kuchierskaya, A.; Ivanov, E.; Stytsenko, V.; Vinokurov, V.; Lvov, Y. Nanoreactors Based on Hydrophobized Tubular Aluminosilicates Decorated with Ruthenium: Highly Active and Stable Catalysts for Aromatics Hydrogenation. Catal. Today 2021, 378, 33–42. [Google Scholar] [CrossRef]
- Farinmade, A.; Ojo, O.F.; Trout, J.; He, J.; John, V.; Blake, D.A.; Lvov, Y.M.; Zhang, D.; Nguyen, D.; Bose, A. Targeted and Stimulus-Responsive Delivery of Surfactant to the Oil–Water Interface for Applications in Oil Spill Remediation. ACS Appl. Mater. Interfaces 2020, 12, 1840–1849. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes Filled with MgO for Paper Reinforcement and Deacidification. Appl. Clay Sci. 2021, 213, 106231. [Google Scholar] [CrossRef]
- Lvov, Y.; Aerov, A.; Fakhrullin, R. Clay Nanotube Encapsulation for Functional Biocomposites. Adv. Colloid Interface Sci. 2014, 207, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Dzamukova, M.R.; Naumenko, E.A.; Lvov, Y.M.; Fakhrullin, R.F. Enzyme-Activated Intracellular Drug Delivery with Tubule Clay Nanoformulation. Sci. Rep. 2015, 5, 10560. [Google Scholar] [CrossRef]
- Qin, Y.; Su, W.; Meng, G.; Cui, L.; Wu, J.; Yang, S.; Liu, Z.; Liu, J.; Guo, X. Polymer-Modified Halloysite Nanotubes with High Adhesion and UV-Shielding Properties for Chlopyrifos Application on Cotton Leaves. Appl. Clay Sci. 2023, 234, 106811. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Nanocomposites Based on Halloysite Nanotubes and Sulphated Galactan from Red Seaweed Gloiopeltis: Properties and Delivery Capacity of Sodium Diclofenac. Int. J. Biol. Macromol. 2023, 234, 123645. [Google Scholar] [CrossRef]
- Caruso, M.R.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes/Keratin Composites for Wool Treatment. Appl. Clay Sci. 2023, 238, 106930. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Parisi, F.; Lazzara, G. Halloysite Nanotubes Loaded with Calcium Hydroxide: Alkaline Fillers for the Deacidification of Waterlogged Archeological Woods. ACS Appl. Mater. Interfaces 2018, 10, 27355–27364. [Google Scholar] [CrossRef] [PubMed]
- Yendluri, R.; Lvov, Y.; de Villiers, M.M.; Vinokurov, V.; Naumenko, E.; Tarasova, E.; Fakhrullin, R. Paclitaxel Encapsulated in Halloysite Clay Nanotubes for Intestinal and Intracellular Delivery. J. Pharm. Sci. 2017, 106, 3131–3139. [Google Scholar] [CrossRef]
- Kryuchkova, M.; Danilushkina, A.; Lvov, Y.; Fakhrullin, R. Evaluation of Toxicity of Nanoclays and Graphene Oxide in Vivo: A Paramecium caudatum Study. Environ. Sci. Nano 2016, 3, 442–452. [Google Scholar] [CrossRef]
- Rozhina, E.; Batasheva, S.; Miftakhova, R.; Yan, X.; Vikulina, A.; Volodkin, D.; Fakhrullin, R. Comparative Cytotoxicity of Kaolinite, Halloysite, Multiwalled Carbon Nanotubes and Graphene Oxide. Appl. Clay Sci. 2021, 205, 106041. [Google Scholar] [CrossRef]
- Wang, X.; Gong, J.; Rong, R.; Gui, Z.; Hu, T.; Xu, X. Halloysite Nanotubes-Induced Al Accumulation and Fibrotic Response in Lung of Mice after 30-Day Repeated Oral Administration. J. Agric. Food Chem. 2018, 66, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Caruso, M.R.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Keratin/Alginate Hybrid Hydrogels Filled with Halloysite Clay Nanotubes for Protective Treatment of Human Hair. Int. J. Biol. Macromol. 2022, 222, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.; Fakhrullina, G.; Fakhrullin, R.; Lvov, Y. Self-Assembly of Clay Nanotubes on Hair Surface for Medical and Cosmetic Formulations. Nanoscale 2018, 10, 18205–18216. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, E.; Fakhrullin, R. Halloysite Nanoclay/Biopolymers Composite Materials in Tissue Engineering. Biotechnol. J. 2019, 14, 1900055. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.M.; Deasy, P.B.; Ziaka, E.; Claffey, N. Formulation and Preliminary in Vivo Dog Studies of a Novel Drug Delivery System for the Treatment of Periodontitis. Int. J. Pharm. 2004, 274, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Pasbakhsh, P.; Milioto, S.; Lazzara, G. Why Does Vacuum Drive to the Loading of Halloysite Nanotubes? The Key Role of Water Confinement. J. Colloid Interface Sci. 2019, 547, 361–369. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes Filled with Salicylic Acid and Sodium Diclofenac: Effects of Vacuum Pumping on Loading and Release Properties. J. Nanostructure Chem. 2021, 11, 663–673. [Google Scholar] [CrossRef]
- Tan, D.; Yuan, P.; Annabi-Bergaya, F.; Liu, D.; Wang, L.; Liu, H.; He, H. Loading and in Vitro Release of Ibuprofen in Tubular Halloysite. Appl. Clay Sci. 2014, 96, 50–55. [Google Scholar] [CrossRef]
- Ambrogi, V.; Latterini, L.; Nocchetti, M.; Pagano, C.; Ricci, M. Montmorillonite as an Agent for Drug Photostability. J. Mater. Chem. 2012, 22, 22743–22749. [Google Scholar] [CrossRef]
- Da Rocha, M.C.; Braz, E.M.D.A.; Honório, L.M.C.; Trigueiro, P.; Fonseca, M.G.; Silva-Filho, E.C.; Carrasco, S.M.; Polo, M.S.; Iborra, C.V.; Osajima, J.A. Understanding the Effect of UV Light in Systems Containing Clay Minerals and Tetracycline. Appl. Clay Sci. 2019, 183, 105311. [Google Scholar] [CrossRef]
- Ambrogi, V.; Nocchetti, M.; Latterini, L. Promethazine–Montmorillonite Inclusion Complex To Enhance Drug Photostability. Langmuir 2014, 30, 14612–14620. [Google Scholar] [CrossRef]
- Yendluri, R.; Otto, D.P.; De Villiers, M.M.; Vinokurov, V.; Lvov, Y.M. Application of Halloysite Clay Nanotubes as a Pharmaceutical Excipient. Int. J. Pharm. 2017, 521, 267–273. [Google Scholar] [CrossRef]
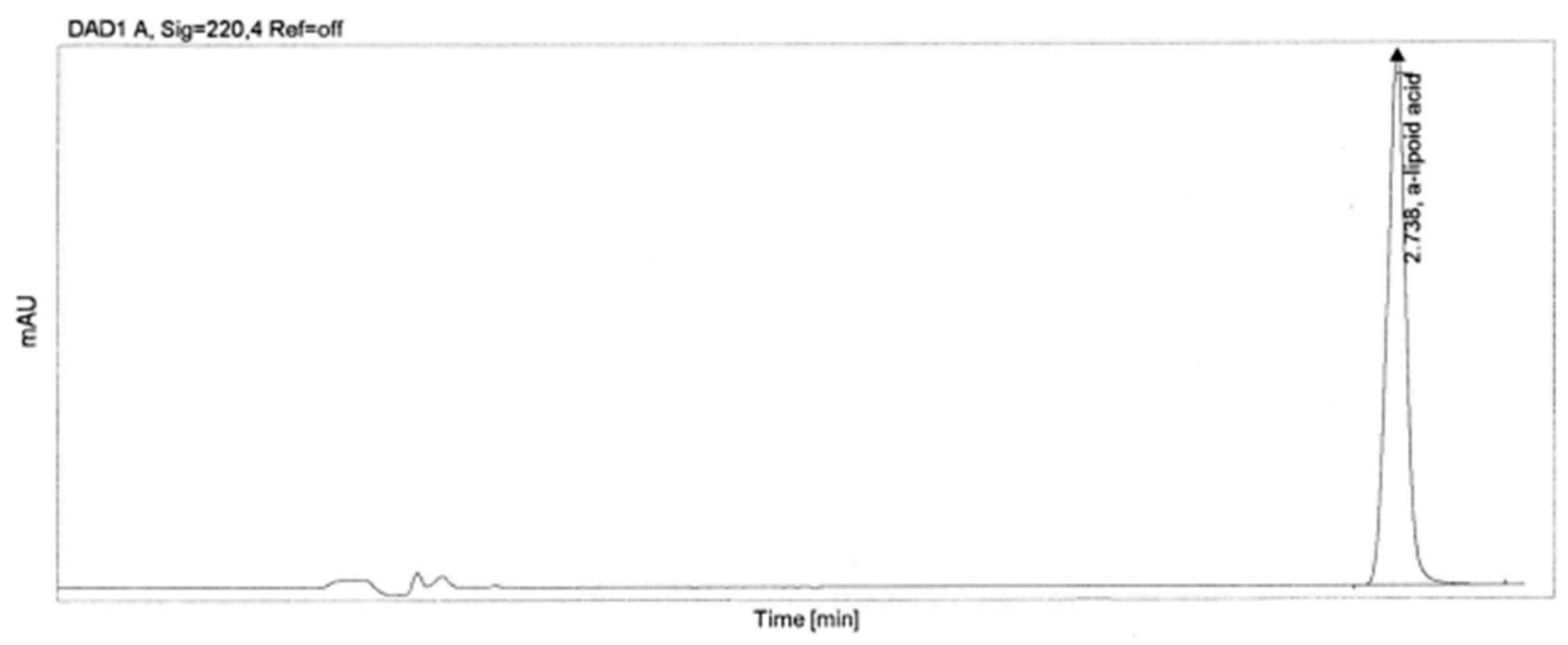

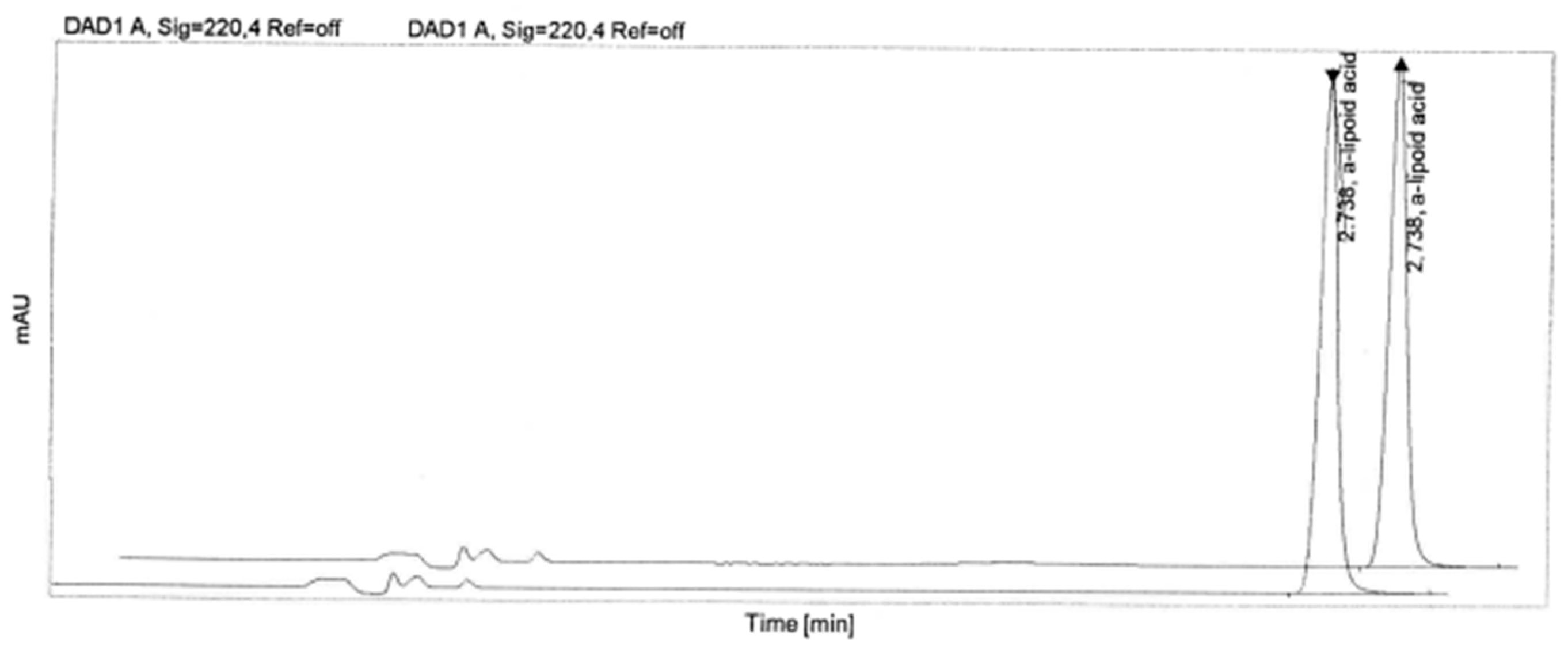
| Sample | RT (min) | Area | Peak Height | Tail Factor | Theoretical Plates EP |
|---|---|---|---|---|---|
| α-lipoic acid | 2.738 | 718.83 | 270.829 | 1.0 | 25,769 |
| Sample | Sample | RT (min) | Area | Peak Height | Tail Factor | Theoretical Plates EP |
|---|---|---|---|---|---|---|
| photostability test of α-lipoic acid | 1st injection | 2.736 | 606.63 | 224.664 | 1.0 | 25,569 |
| 2nd injection | 2.736 | 607.53 | 225.021 | 1.1 | 25,580 | |
| Mean | 2.736 | 607.08 | 1.1 | 25,574 | ||
| RSD | 0.00 | 0.10 | ||||
| photostability test of α-lipoic acid with HNTs | 1st injection | 2.738 | 727.31 | 273.213 | 1.0 | 25,732 |
| 2nd injection | 2.738 | 728.72 | 273.507 | 1.0 | 25,672 | |
| Mean | 2.738 | 728.02 | 1.0 | 25,702 | ||
| RSD | 0.01 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnyk, A.; Chyhyrynets, O.; Lazzara, G. Encapsulation of α-Lipoic Acid in Halloysite Nanotubes. Appl. Sci. 2023, 13, 10214. https://doi.org/10.3390/app131810214
Melnyk A, Chyhyrynets O, Lazzara G. Encapsulation of α-Lipoic Acid in Halloysite Nanotubes. Applied Sciences. 2023; 13(18):10214. https://doi.org/10.3390/app131810214
Chicago/Turabian StyleMelnyk, Andrii, Olena Chyhyrynets, and Giuseppe Lazzara. 2023. "Encapsulation of α-Lipoic Acid in Halloysite Nanotubes" Applied Sciences 13, no. 18: 10214. https://doi.org/10.3390/app131810214






