Abstract
The purpose of this study was to analyze the stability of new emulsions prepared on the basis of modified fats with varying amounts of hydroxypropylmethylcellulose. Selected techniques for evaluating the stability of emulsion systems, such as Turbiscan analysis, and the evaluation of microstructure, texture, particle size and viscosity were used. The evaluation of the above-mentioned studied parameters allowed us to indicate differentiated stability and their properties. The most desired results in terms of stability evaluation were determined for the emulsion prepared on the basis of modified mutton tallow with hemp seed oil in a ratio 3:1 (by weight) containing 0.6% (by weight) of hydroxypropylmethylcellulose. In addition, as the hemp seed oil content in the interesterified fat phase increased, a decrease in the stability of the emulsion systems was noted. Mentioned emulsions were characterized with the lowest value of TSI. Moreover, for those emulsions, the smallest increment in the droplet diameter values of systems after 31 days was recorded. The prepared model formulation can serve as a starting material for new emulsion chemical, cosmetic or food systems.
1. Introduction
The desirable quality attributes of emulsion products are derived mainly from their composition and the processes they have undergone [1,2]. These attributes are refined as early as when the research and development stage of the final product starts [1]. The quality attributes of emulsion products are the result of complex interactions and include molecular, microscopic, macroscopic, colloidal and organoleptic structures and characteristics [1]. Therefore, it is considered difficult to produce an emulsion product that exhibits high stability, is safe and meets consumer demands [3]. Depending on the application of the product, expectations for its final quality characteristics may vary, while the most important determinants of an emulsion product quality include stability, rheological properties and appearance [4,5].
The term stability refers to the ability of an emulsion to counteract phase separation. The slower destabilization changes occur, the more stable the emulsion is [6]. Thermodynamically, emulsions are not considered as stable systems. The dispersed and dispersion phases tend to separate with time [6]. There are several types of mechanisms of emulsion instability [5]. The most common of them include gravity separation (creaming and sedimentation), flocculation, coalescence, Ostwald ripening and phase inversion [7,8]. In fact, when observing emulsion destabilization rarely can only one mechanism be distinguished; usually at least two types of instabilities occur at the same time [9]. A significant role with regard to the stability of emulsion systems is played by the properties of the dispersed phase droplets, i.e., their size and polydispersity, charge, concentration, and the interactions between them [5,10]. The characteristics of dispersed phase droplets affect not only the stability of emulsion systems, but also sensory properties, which are particularly important for the consumers of cosmetic and food products [11]. Moreover, the stability of emulsions also depends directly on the components of the emulsion product, i.e., the type of phases, their mutual solubility, the type of emulsifier or the presence of other additives [10]. Substances that modulate textural attributes by altering the rheological properties of systems and, at the same time, allow an increase in the durability of emulsion systems, are stabilizers acting as texture modifiers [12]. There are two mechanisms of action of texture modifiers. These consist in increasing the viscosity of the continuous phase (viscosity modifiers) or in creating a gel structure in this phase (gelling agents) [5,12]. The use of viscosity modifiers, as well as gelling substances, aims at limiting (paralyzing) the movement of dispersed phase droplets, which slows down the dynamics of changes occurring in the emulsion system, while giving the final product certain rheological properties [5]. One of the viscosity modifiers is hydroxypropylmethylcellulose. This compound is commonly used in the food, pharmaceutical and cosmetic industries [13,14,15,16] as an emulsifier, as well as a texture modifier in emulsion systems [14]. HPMC significantly increases the viscosity of the aqueous phase of emulsion systems, and improves their rheological properties, effectively protecting them from flocculation and creaminess processes [17]. In addition, as indicated by Alizadeh et al. (2019) [18], HPMC exhibits health-promoting effects, as, among other things, it lowers blood cholesterol levels.
The aim of the presented work was to design model emulsion systems based on enzyme-modified fats for shaping the quality of target products in the food, cosmetic and pharmaceutical industries. It was expected that we would produce stable emulsion products containing modified fat with sufficient amount of emulsifiers and varying concentrations of hydroxypropylmethylcellulose. The derivation of such systems is different from that of a typical emulsion system. According to Shao et al. [19] the common strategies for improving emulsion stability are the modification of emulsifiers, use of stabilizers, and optimization of manufacturing techniques. In this paper, we focused on the first two approaches. We used emulsions in which the fat phase was an enzymatically interesterified fat containing, in parallel, a sufficient amount of mono- and diacylglycerols that could stabilize the system. The effectiveness of the enzymatic modification of the fat blends in the production of certain amount of mono- and diacylglycerols sufficient to stabilize emulsion system has been confirmed in our previous study [20]. The amount of emulsifiers formed, using the addition of water to the enzyme preparation (during enzymatic modification) in the range of 1.00–1.25%, proved sufficient to produce an emulsion with high stability. The introduction into the system and the determination of the lowest amount of hydroxypropylmethylcellulose allowed us to obtain a satisfactory texture and viscosity; thus, the stability of the emulsion was also an important point of this work.
2. Materials and Methods
2.1. Material
Unrefined cold-pressed hemp seed oil (Oleofarm, Wroclaw, Poland) and mutton tallow (Meat-Farm Radoslaw Luczak, Stefanowo, Poland) were used as fat raw materials in the study.
Before the target enzymatic modification of mutton tallow, a bleaching process was carried out using bleaching earth (HRT Poland, Kolobrzeg, Poland). The catalyst used in the enzymatic modification of fats (mutton tallow with hemp seed oil) was lipase from Rhizomucor miehei immobilized on immobead 150, ≥300 U g−1 (Sigma Aldrich, Saint Louis, MO, USA).
To produce emulsion products based on enzymatically modified fats, hydroxypropylmethylcellulose (Midland, MI, USA) was used as a texture modifier. The commercial preparation Euxyl K712 (Schülke & Mayr GmbH, Norderstedt, Germany), which is an aqueous solution of sodium benzoate and potassium sorbate, was used as a preservative in the emulsions.
2.2. Methods
2.2.1. Enzymatic Modification of Fat Blends (MAG and DAG formation) and Fats—Brief Characteristics
Fat blends T-1 to T-5 (Table 1) were subjected to partial hydrolysis during an enzymatic interesterification reaction using immobilized lipase from Rhizomucor miehei (5.0% (w/w) in relation to the fat blend mass) in a 250 mL Erlenmeyer flask. The reaction was performed in a shaker equipped with a thermostatic water bath (SWB 22N, Labo Play, Bytom, Poland). In order to obtain an increased amount of DAG and MAG, water was introduced into the system at a rate of 1.1% (w/w) in relation to the fat blend mass. Reaction time (6 h) was measured from the time the enzyme and water were added to the pre-heated (60 °C) fat blends. The reaction was carried out at the aforementioned temperature, at a shaking speed of 200 rpm. The process was stopped by filtering out the enzyme.

Table 1.
Characteristics of the modified fat blends.
Mutton tallow was bleached before the enzymatic interesterification with bleaching earth in a round-bottom flask equipped with a condenser, at the following conditions: 2% w/w of bleaching earth, process temperature: 80 °C, process duration: 1 h.
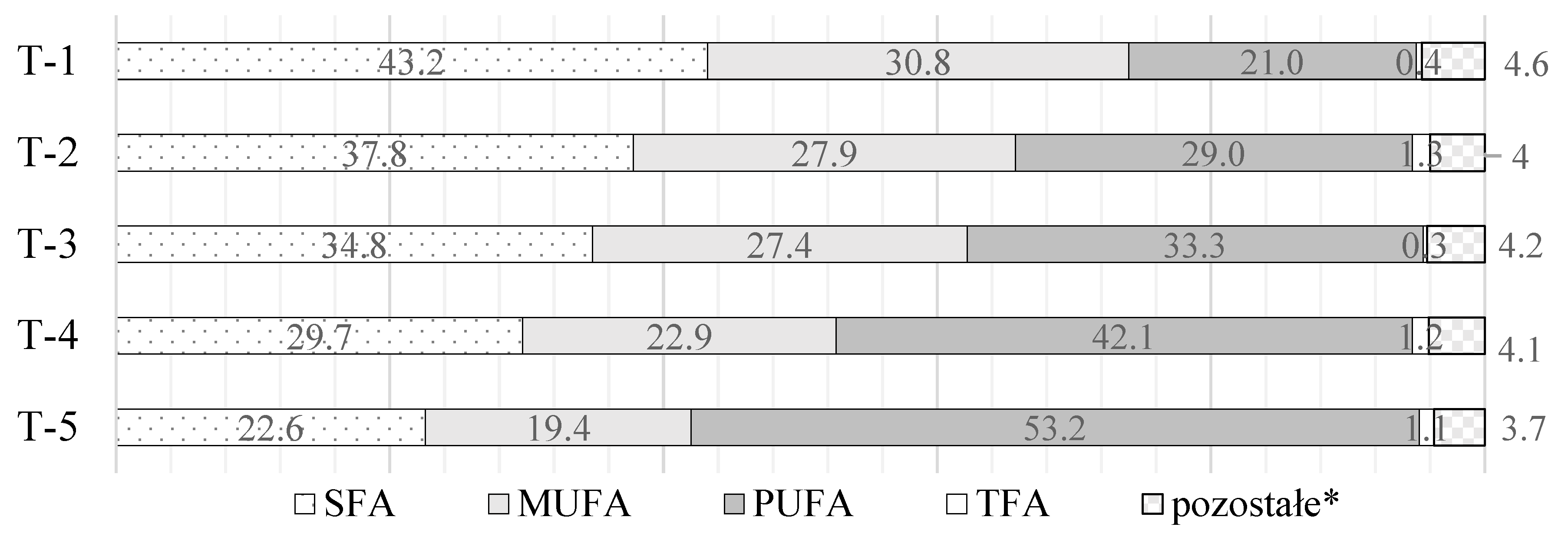
Brief characteristics of the modified fat blends are presented below (Table 1, Figure 1). MAG accounted for less than 1.5% (w/w) of the modified fat blends. The content of DAG and MAG was determined using gel permeation chromatography. A chromatograph—Agilent 1100 series (Agilent Technologies, Wilmington, DE, USA) equipped with a refractive index detector (RID) and two Phenogel columns—300 × 7.80 mm, 5 μm (100 Å) and 300 × 7.80 mm, 5 μm (50 Å) (Phenomenex, Utrecht, The Netherlands)—were used. As sample solvent as well as mobile-phase (isocratic flow (1.0 mL/min)) tetrahydrofuran (THF) stabilized with butylated hydroxytoluene was used.
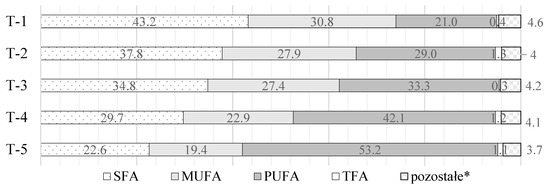
Figure 1.
Share (%) of individual fatty acid groups in fat blends T-1–T-5 (* as other unidentified fatty acids were marked).
2.2.2. Emulsion Preparation
Emulsions were prepared using mechanical homogenization (homogenizer—rotor-stator type, ULTRA-TURRAX T18, dispersing tool S18G-19G (IKA, Shanghai, China); 4 min; rotation speed 18,500 rpm) of the aqueous and fat phases. The fat phases (30% w/w) were enzymatically modified fat blends with manufactured emulsifiers (mono- and diacylglycerols); the aqueous phase (70% w/w) was an aqueous solution of texture modifier (HPMC) with a concentration of 0.6 or 0.8 or 1.0% w/w. The phases were heated to 50–55 °C before combining. Once the emulsions had cooled to ambient temperature, a preservative was added (quantum satis—as much as necessary). Emulsions were made according to the specifications below (Table 1 and Table 2).

Table 2.
Emulsion composition.
2.2.3. Evaluation of Processes Occurring in Emulsions during Storage
A Turbiscan Lab Expert (Formulaction, L’Union, France) using multiple light scattering (MLS) was used to analyze destabilization processes occurring in the produced emulsion systems. The light source was a diode emitting radiation in the near-infrared range (λ = 880 nm). The device was equipped with two synchronized detectors receiving transmitted (T, or transmitted) and backscattered (BS, or backscattered) light through the sample. The identification and quantitative analysis of destabilization processes in emulsion systems is made possible by changes in the intensity of the T and BS signals, which depend on the size of the dispersed phase droplets and their volume contribution [21]. During the measurement, the samples were scanned with the moving optical head of the camera in their entire height (about 40–42 mm). The scans were performed for a period of 30 ± 1 days, at intervals of several days. The measurement and storage temperature of the samples was 25 °C.
TSI (Turbiscan stability index) was calculated according to the following formula [22]:
where
scani (h)—average BS value for each time (i) measurement,
scani−1 (h)—average BS value for each time (i − 1) measurement,
H—the total height of the sample.
TSI is a statistical parameter whose value is the sum of all processes occurring in the sample [23]. This parameter, therefore, makes it possible to assess the kinetics of destabilization changes occurring in the system. It takes values from 0 (for highly stable systems) to 100 (for extremely unstable systems) [24]. According to the information provided by the manufacturer of the Turbiscan device in the Application Note (2019) [25], dispersions can be divided into 5 categories depending on the TSI value obtained. The category descriptions proposed by Domian et al. (2020) [26] we used in the manuscript (Table 3).

Table 3.
Categories of system stability based on TSI values [25,26].
2.2.4. Testing the Droplet Diameter of Emulsion Systems
Droplet diameter was calculated using the relationships described by the formulas below [27]:
where
BS—intensity of backscattered light,
λ*—the average path length of photons,
d—the average droplet diameter of the dispersed phase,
Φ—volume fraction of the dispersed phase,
g and Qs—optical parameters derived from Mie theory.
All the parameters described above were calculated using TurbiSoft 2.0 software (Turbiscan Lab Expert).
2.2.5. Texture Study of Emulsion Systems
Texture parameters of the emulsion systems were determined with a Texture Analyzer CT3 texturometer (Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA) using a 25.4 mm diameter nylon sphere-shaped probe. The measurement was performed on samples placed in identical cylindrical dishes measuring 70 × 50 mm. Emulsions were penetrated once to a depth of 10 mm at a probe travel rate (in both directions) of 2 mm/s. Measurements were carried out at 20 °C, 48 h after emulsion formation in triplicate, and the results are presented as mean ± SD. Hardness and adhesion force, which were defined as the maximum and minimum of the force peak (g) for the compression cycle [28], respectively, were determined as texture parameters of the emulsion systems. A higher hardness is coupled with a higher-firmness and less-spreadable sample [29].
2.2.6. Dynamic Viscosity Study of Emulsion Systems
The dynamic viscosity of the emulsion systems was determined using a Brookfield DV-III Ultra viscosity meter, model HA (Brookfield Engineering Laboratories, Stoughton, MA, USA), equipped with a Helipath system. Analyses were performed at 20 °C using a T-C spindle (No. 93) and variable speeds of 2, 5, 10 and 15 rpm. The test was performed 48 h after emulsion formation in triplicate, and results are presented as mean ± SD.
3. Results and Discussion
Emulsion quality is a concept that consists of many physical and chemical interactions that indicate that an emulsion changes its appearance. To prepare a stable system, appropriate emulsifiers or stabilizers must be used. Often, the use of a given emulsifier is more beneficial to the stability of an emulsion by using it with a suitable thickener (stabilizer). The selection of these two components often allows one to obtain a long-term emulsion (with high stability). In the presented work, the stability and physicochemical properties of emulsions based on modified fats, where the thickener was hydroxypropylmethylcellulose (HMPC), were evaluated.
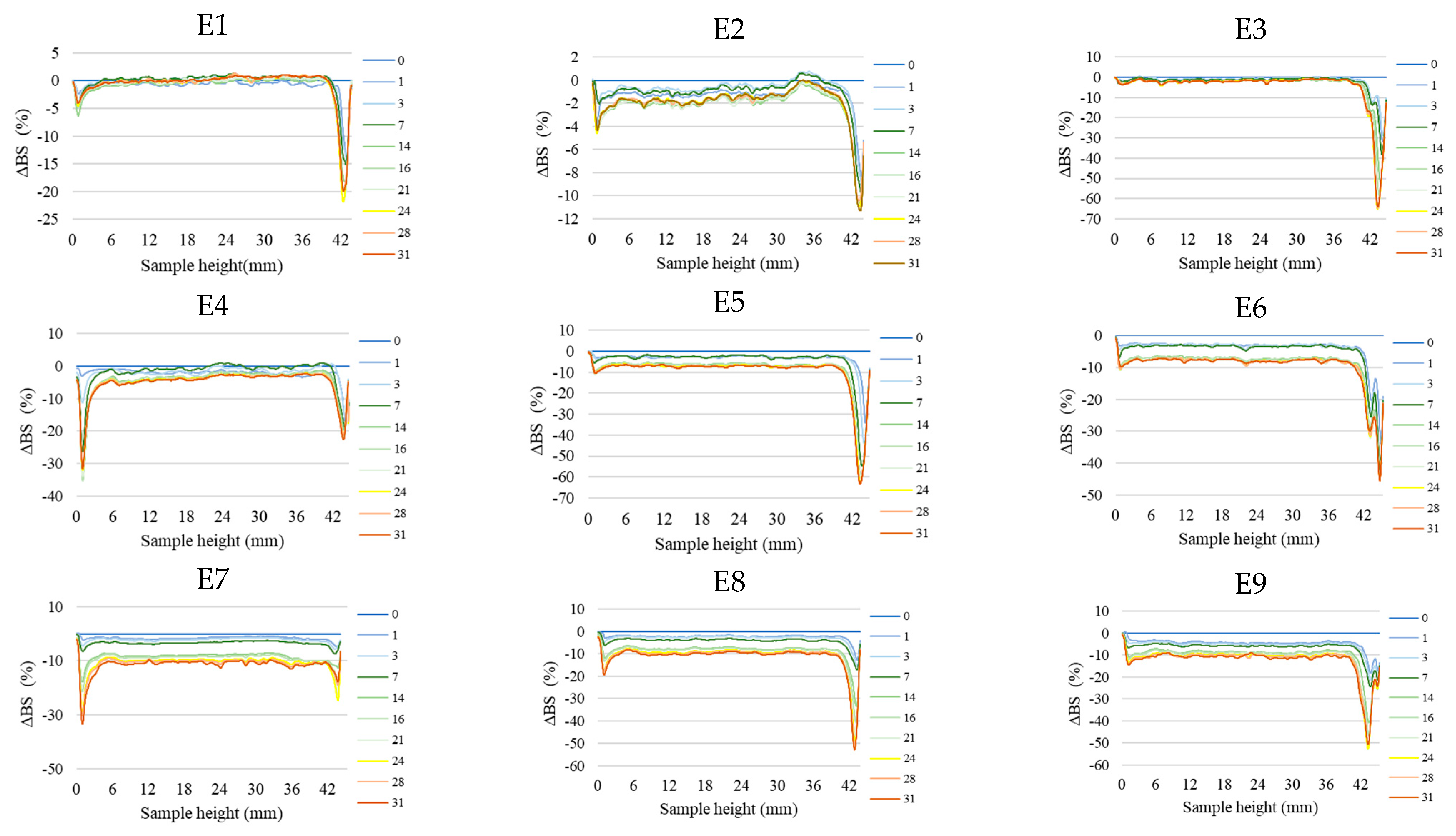
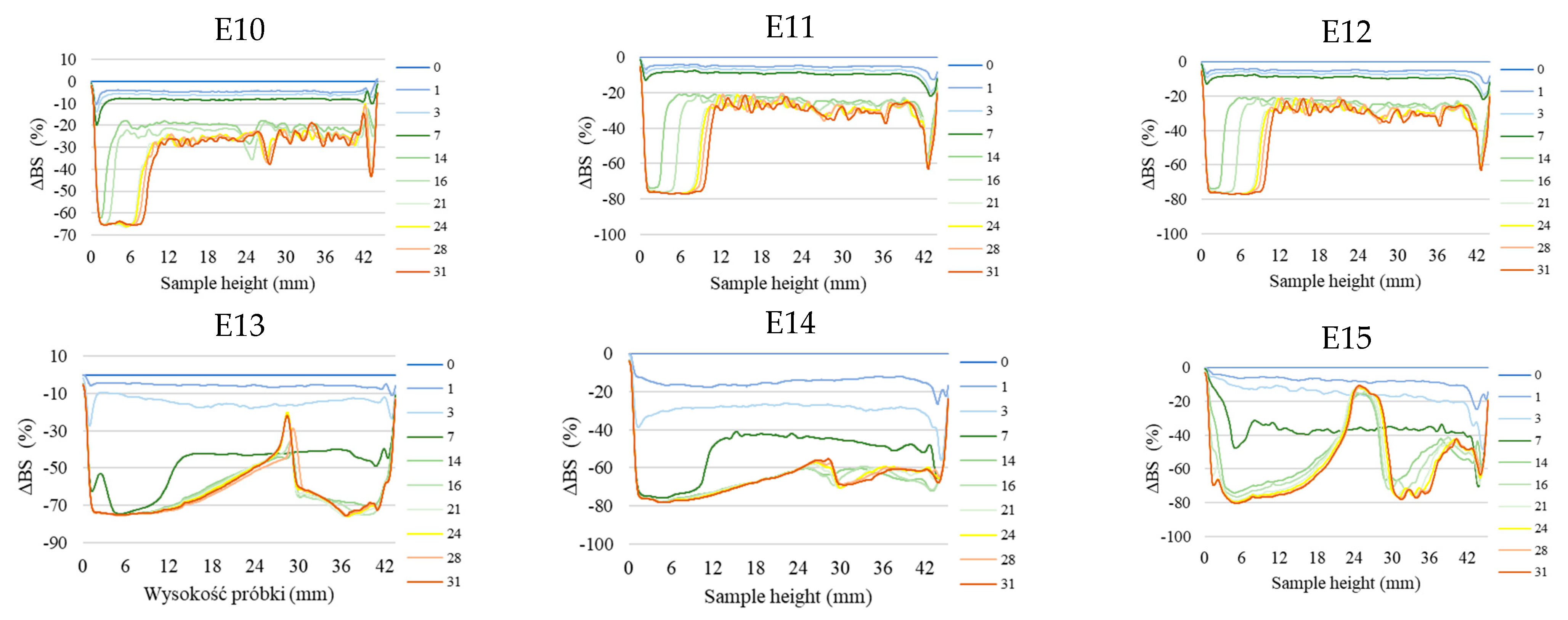
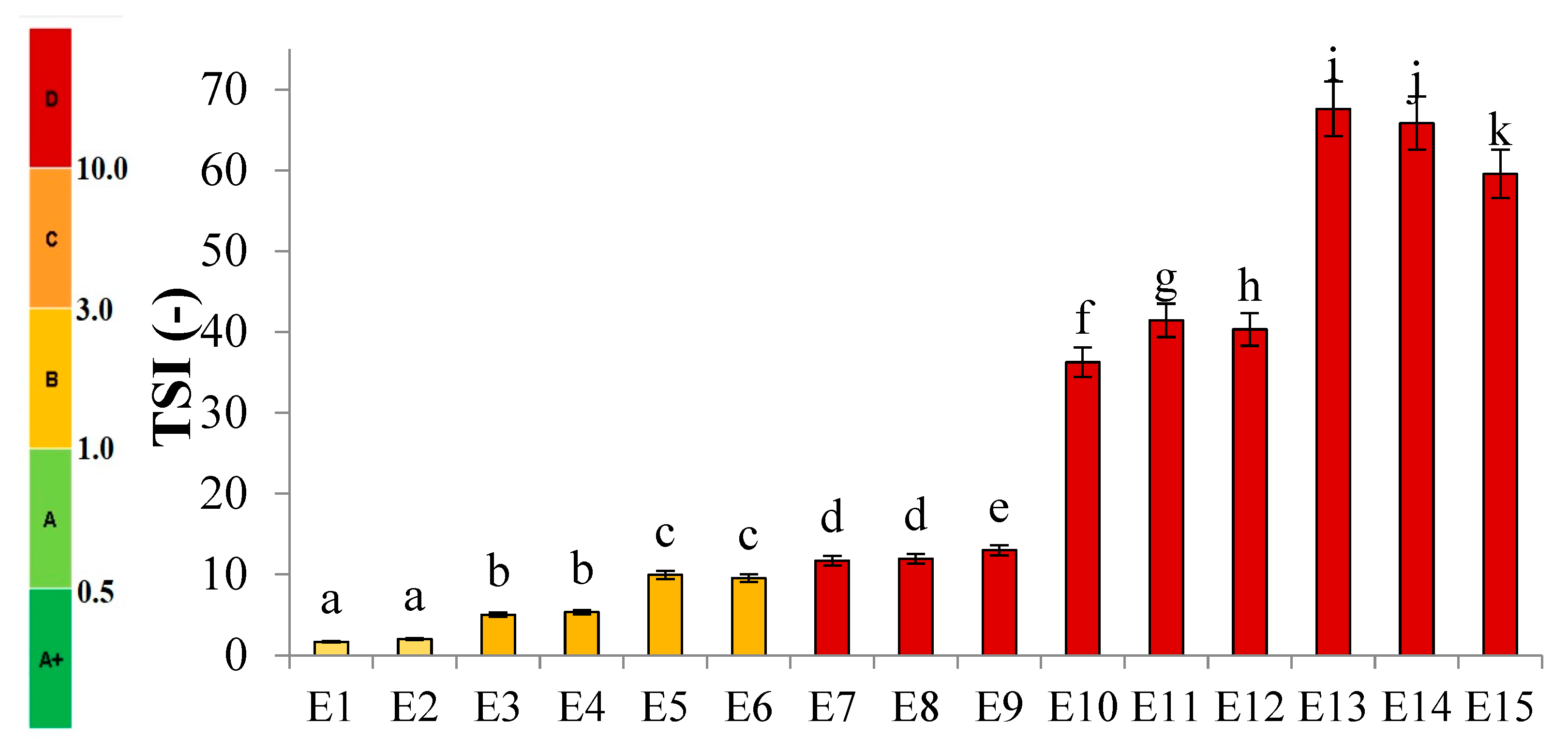
Figure 2 shows the profiles of backscattered light intensity as a function of sample height in the reference mode for emulsions E1–E15. The emulsions differed in their type of fatty phase and HPMC concentration. Using comparative analysis of ΔBS profiles from successive scans for all emulsions during 31 days of storage, it was found that the most advanced destabilization changes were characterized by systems E13–E15, whose ΔBS curves showed significant fluctuations across the height of the measurement dishes. They indicated significant dynamics in terms of the process of flocculation and coalescence, as well as creaminess. All of the above-mentioned processes had started already in the first days after the production of these systems. In general, after 7–14 days of storage, these emulsions were completely stratified (Figure 2). Analyzing the backscattered light intensity profiles over time for the E10–E12 systems, it was found that the ΔBS records were characteristic of the occurrence of changes in the average droplet size or their aggregation, thereby progressing flocculation or coalescence-type processes. It was also found that a gravitational separation process was present in these systems, which resulted in the migration of fat phase droplets into the upper parts of the vials and the formation of a clear layer in the lower parts of the vials. Unlike the E13–E15 emulsions, these changes did not lead to complete phase separation after 31 days of storage. All of the E10–E15 emulsions discussed were characterized by very-high-stability coefficient values, which ranged from 36.3 to 67.6. This classified these systems in category D, which confirmed their high instability [26] (Figure 3).
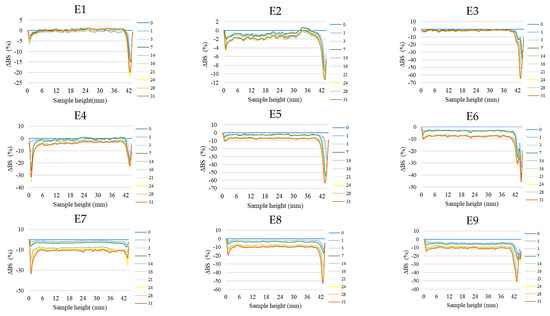
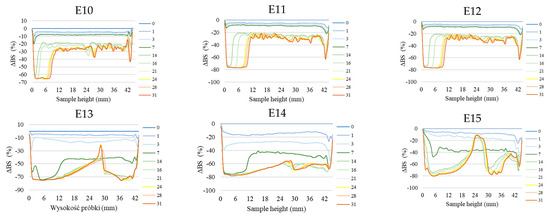
Figure 2.
Profiles of changes in backscattered light intensity as a function of sample height for E1–E15 emulsions when stored for 31 days at 25 °C.
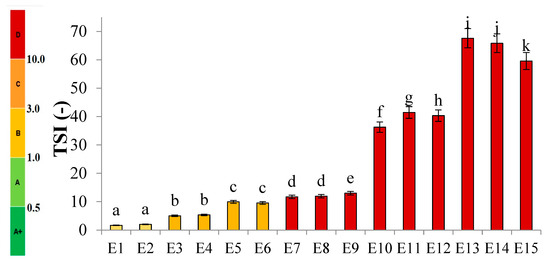
Figure 3.
TSI values for E1–E15 systems when stored for 31 days at 25 °C. a–k—different letters indicate statistically significant differences between the averages for TSI values (p ≤ 0.05).
A decrease in the concentration of the dispersed phase in the lower parts of the vials and a simultaneous increase in the upper parts of the vessels due to gravitational migration processes were also observed for systems E4–E9 (Figure 2). The extent of these processes after the storage period was much less than for the above-discussed systems E10–E15. The curves showing ΔBS for the E4–E9 emulsion systems in the part of the graphs corresponding to the middle zone of the measurement vessels did not overlap. This unambiguously indicated the processes leading to an increase in the average droplet size of the dispersed phase, corresponding to the processes of flocculation and coalescence. It was observed that the average values of changes in the intensity of backscattered light in the middle part of the graphs were larger for systems E7–E9 (9.7–10.7%) than for E4–E6 (3.5–8.0%). This kind of observation leads to the conclusion that emulsions with a higher proportion of hemp seed oil in the fatty phase lost their stability faster. The stability factor values determined after 31 days of storage for the E4–E9 systems were in the range of 5.0–13.0, which classified these systems into categories C or D, i.e., systems with poor or unsatisfactory stability (Figure 3).
Among HPMC-stabilized emulsions, the least-advanced destabilization processes were observed for systems E1–E3 (Figure 2). The course of ΔBS curves from successive scans did not coincide in the parts of the graphs corresponding to the lower or upper part of the measurement vessels. This indicated an early stage in the process of migration of dispersed phase droplets into the upper parts of the vials. The average ΔBS values of those recorded in the middle part of the graphs did not exceed 2.0%. This confirmed that flocculation and coalescence processes did not occur for these systems [30]. Analyzing the obtained stability index values for the aforementioned systems, it was found that two emulsions (E1 and E2) qualified for category B, indicating their satisfactory stability (Figure 3). The calculated TSI coefficient values for these systems (<2.0), statistically, were not significantly different from each other (p > 0.05), although they were significantly lower (p ≤ 0.05) than the values obtained for the other systems. On the other hand, the TSI value determined for emulsion E3, which contained the highest HPMC concentration analyzed (1.0 % w/w), was 5.0, indicating a significantly lower stability of this system (category C) compared to systems E16 and E17.
Analyzing the discussed results of stability evaluation of the presented fifteen emulsion systems, a clear dependence of the advancement of destabilization changes on the type of fat phase used was observed. As the hemp seed oil content in the esterified fat phase increased, a decrease in the stability of the emulsion systems was noted (E1–E3 > E4–E6 > E7–E9 > E10–E12 > E13–E15). On the other hand, in the case of the next variable used—HPMC concentration—no single, consistent relationship was noted for all systems. Based on the TSI values obtained, in the case of systems E1–E3 and E7–E9, it can be concluded that the use of lower HPMC concentrations (0.6 and 0.8% w/w) was correlated with a statistically significantly (p ≤ 0.05) increased stability of these systems. However, in the case of emulsions E4–E6 and E10–E12, only the lowest concentration used (0.6% w/w) of this texture modifier was associated with statistically significantly (p ≤ 0.05) reduced values of the TSI parameter.
The above-presented results are consistent with those obtained by Paximada et al. (2016) [16], who showed that HPMC is an ineffective viscosity modifier due to the fact that it forms a network that poorly stabilizes the dispersed phase droplets of an emulsion. On the other hand, the results obtained from the evaluation of the stability of systems produced on the basis of modified fats show that the effectiveness of this thickener depends on both the composition of the fat phase and the type of emulsifier used. The ΔBS results for the E1 and E2 systems indicate that it is possible to produce emulsion systems containing HPMC that show good stability for at least 31 days.
Performing a visual assessment of the changes occurring in the emulsions, no destabilizing changes were observed for the E1–E9 systems. These changes, especially for emulsions E7–E9, invisible to the naked eye, were registered by measuring the intensity of backscattered light (category D). On the other hand, from the image of emulsions E10–E12, the appearance of a clear layer in the lower part of the vials was clearly visible (Figure 4). On the other hand, in the middle and upper part of the vials for the aforementioned emulsions, significant-sized fat phase droplets visible to the naked eye were observed. Based on these observations, it was concluded that the processes of creaminess and coalescence that occurred in these systems after the storage period were at an advanced stage. The visual evaluation of emulsions E13–E15 was unfavorable. The photo clearly shows aging changes and stratification of the emulsion system, as well as inhomogeneity of the fatty phase deposited in the upper part of the vial (3).

Figure 4.
Photograph of the E1–E15 systems taken after 31 days of storage at 25 °C.
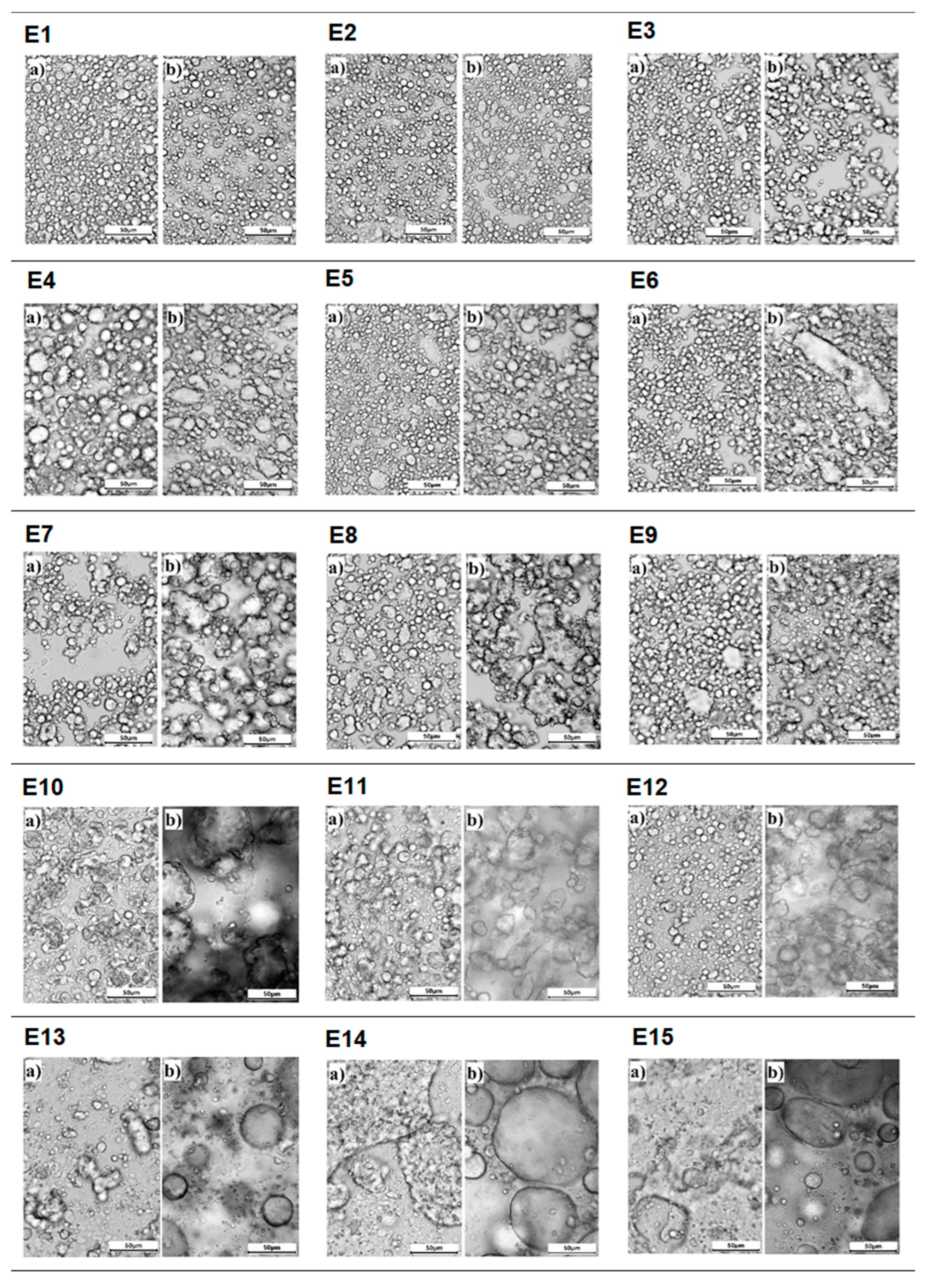
Photographs showing the microstructure of the E1–E15 emulsion systems, taken 24 h after their manufacture and after 31 days of storage, are shown in Figure 5. The E13–E15 emulsions, 24 h after their manufacture, were characterized by unevenly distributed droplets forming larger clusters. According to the authors [21], such an image confirms the inefficient homogenization of these systems. It can be assumed that the insufficient homogenization of the systems may be related to the incorrect selection of homogenization parameters but also to the type of fat phase used. The presented emulsions had a modified fat phase with a predominance of hemp seed oil, and perhaps the higher proportion of hemp seed oil negatively affected the final efficiency of MAG and DAG manufactured. These systems, after the storage period, were characterized by polydisperse droplets of considerable size. On the other hand, for the E3–E12 systems, droplets of different sizes were visible 24 h after their formation, which certainly resulted in the appearance of significant changes in the microscopic image for these emulsions after 31 days of storage. After this time, they were characterized by the appearance of unevenly distributed clusters of droplets of larger sizes compared to those observed for the systems a day after their manufacture. Based on these observations, the main processes that occurred in these systems (E3–E12) during storage were flocculation and coalescence. Of these emulsions, the most unfavorable microstructure changes, consisting of a significant increase in the diameter of the dispersed phase droplets, were characterized by systems E10–E12. In contrast, for emulsions E3–E9, the observed changes were much less pronounced and the increase in droplet size was smaller than for systems E10–E12. The most favorable microstructure image, characterized by dispersed phase droplets of a similar diameter, evenly distributed throughout the volume of the samples, was observed for systems E1 and E2. It was noted that after the storage period, no clear changes were registered in the microstructure image of these systems. This suggests that the dynamics of changes in the size and distribution of droplets in the scattering phase were extremely slow.
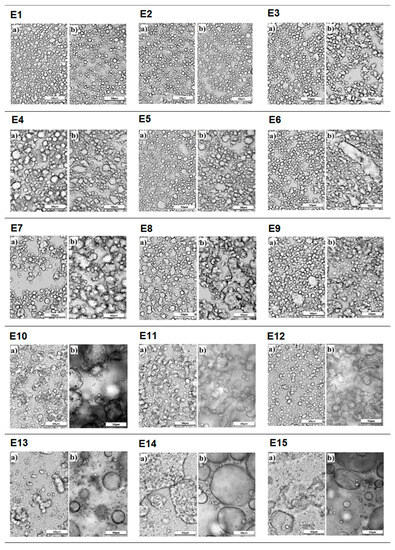
Figure 5.
Microstructure of E1–E15 systems (a) 24 h after their manufacture and (b) after 31 days of refrigerated storage (2–7 °C) (G × 400).
Analyzing the changes in the microstructure of systems E1–E15, it was found that it was mainly dependent on the type of fat phase of the emulsion. It was observed that the higher the hemp seed oil content in the enzyme-modified fat constituting the dispersed phase, the more imperfections the microstructure showed. This trend was also observed in the work of Kowalska et al., 2015 [31], where the size of dispersed phase droplets of emulsions containing a variable hemp seed oil content as the fat phase was studied. It was noted that systems containing a higher amount of this oil were generally characterized by larger droplets with high polydispersity.
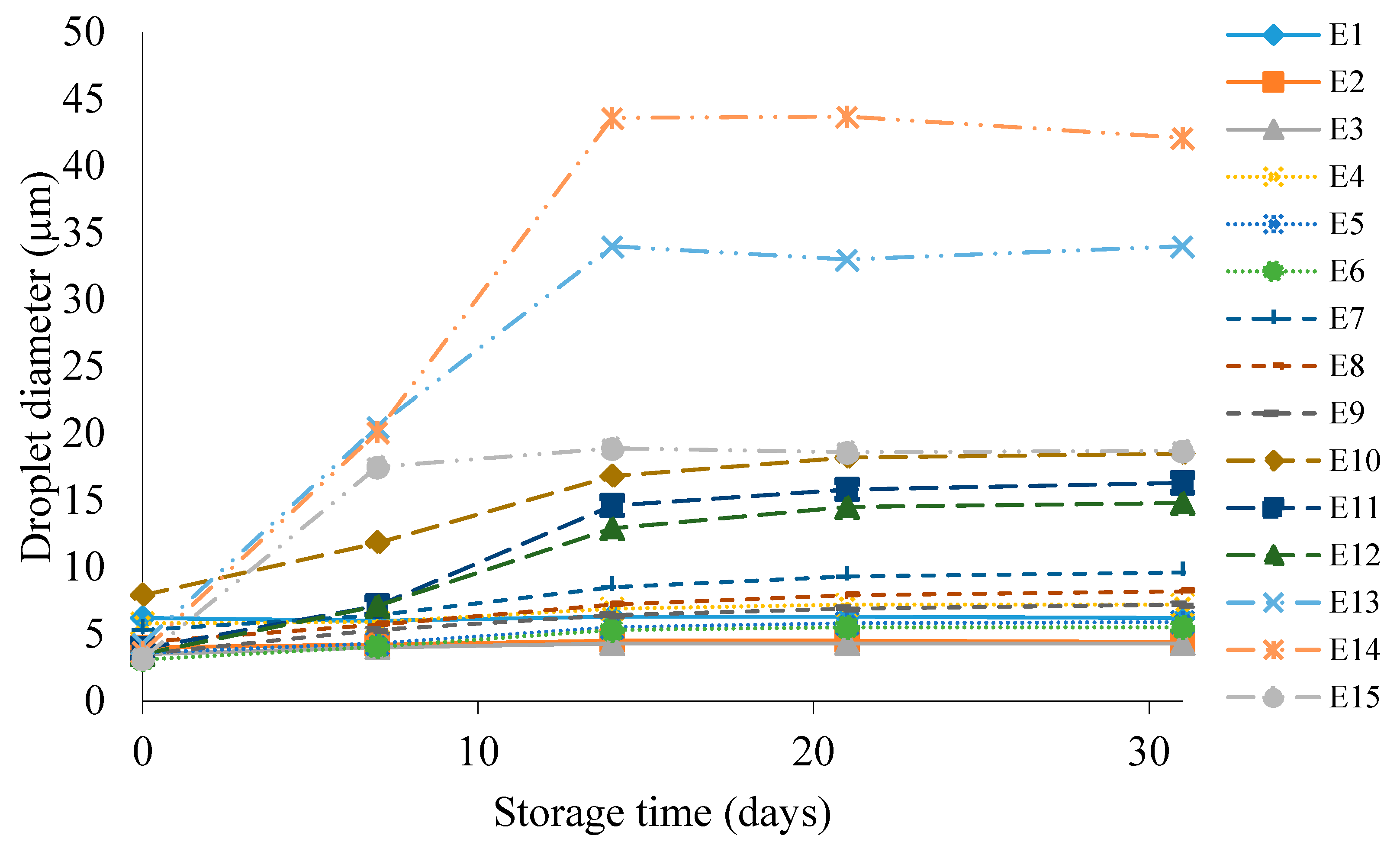
Figure 6 shows the droplet diameter of the dispersed phase of E1–E15 emulsions during their storage for 31 days at 25 °C. It was observed that for emulsions containing the same fatty phases, as the concentration of HPMC in the system increased, the determined droplet diameter was smaller (E3 < E2 < E1, E6 < E5 < E4, etc.). This relationship was also maintained for each individual droplet diameter measurement during the storage period. The lowest values of the dispersed phase droplet diameter immediately after emulsion formation, falling within the range of 3.1–3.5 µm, were recorded for systems containing 1.0% w/w HPMC, i.e., E3, E6, E9, E12, E15. In contrast, the highest values for the same time point, in the range of 4.3–7.9 µm, were recorded for systems E1, E4, E7, E10, E13, i.e., emulsions that were stabilized with 0.6% w/w HPMC additive. The increase in the dispersed phase droplet diameter of the tested systems during the 31-day storage period depended much more on the type of fat phase used. As the proportion of hemp seed oil in the fatty phase increased, a greater increase in droplet diameter after the storage period was recorded. By far, the largest increment was characterized by the E10–E15 systems, for which the droplet diameter increased in the range of 11.3 µm for E12 to 38.4 µm for E14, after 31 days of storage of these emulsions. On the other hand, the smallest increment in the droplet diameter values of systems after preparation and stored for 31 days was recorded for emulsions E1, E2 and E3, which were 0.0, 0.4 and 0.8 µm, respectively.
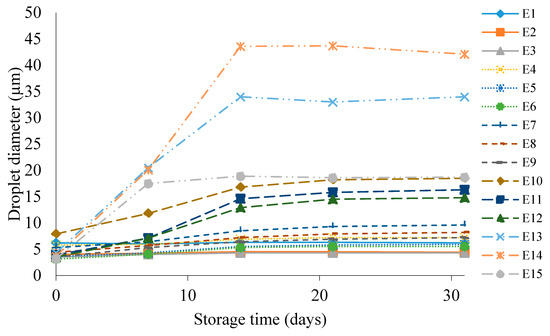
Figure 6.
Dispersed-phase droplet diameter of E1–E15 systems when stored for 31 days at 25 °C.
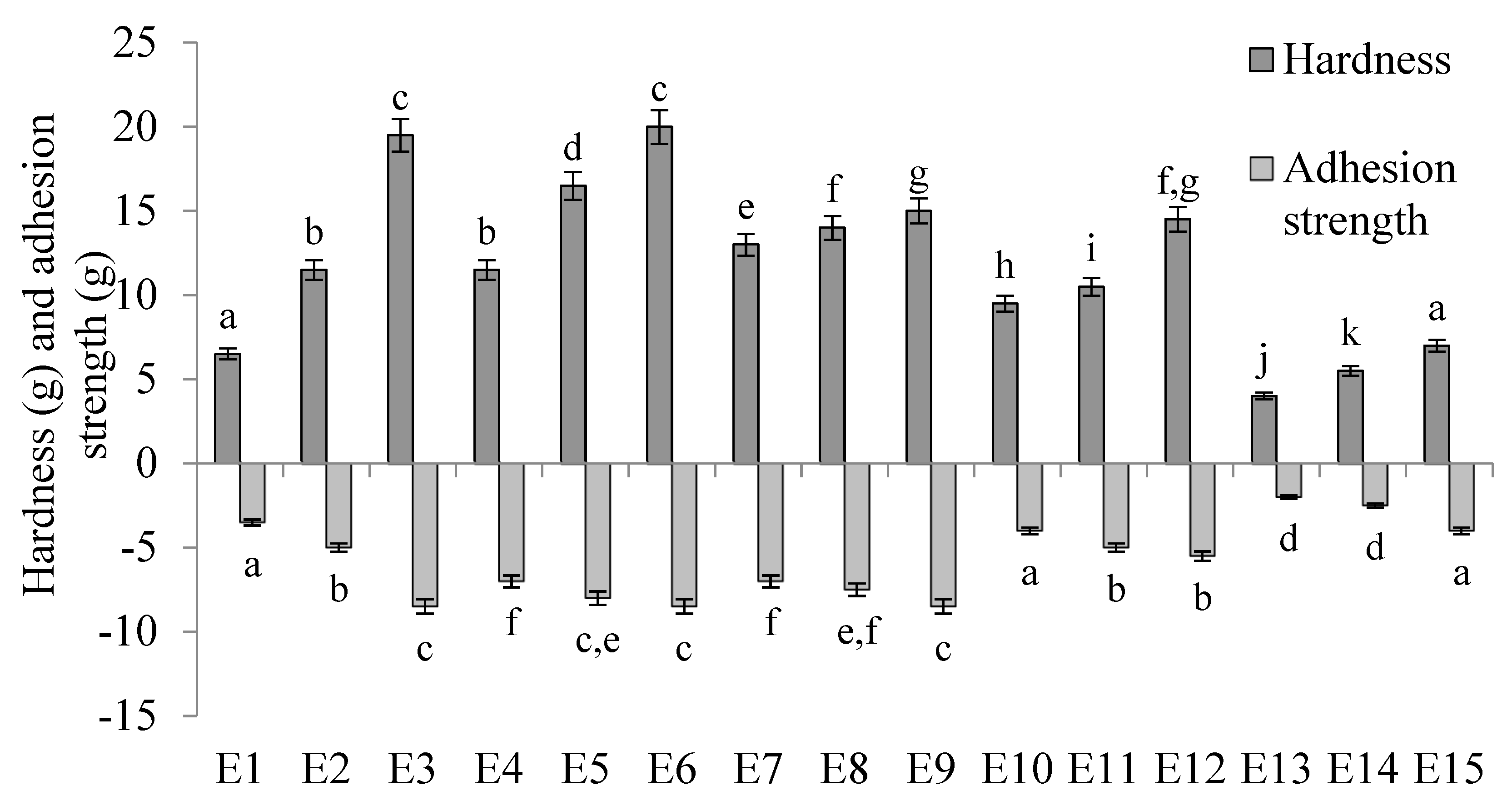
The hardness and adhesion strength values of the E1–E15 systems are shown in Figure 7. The hardness of the emulsion systems produced was significantly dependent on the concentration of texture modifier used. For systems containing the same fatty phases, i.e., E1–E3, E4–E6, etc., statistically significantly (p ≤ 0.05) higher hardness values were recorded as the HPMC content increased. The type of fat phase used in the systems also affected their hardness, although there was no apparent correlation between the ratio of fats in the fat phase and the results obtained for this parameter. Considering emulsions containing 0.6 and 0.8% w/w HPMC, the obtained results of the hardness parameter were statistically significantly (p ≤ 0.05) different for each of the fat phases used. Among emulsions containing 0.6% w/w HPMC, emulsion E7, containing fatty phase T-3, had the highest values. In contrast, among emulsions containing 0.8% w/w HPMC, the highest values were recorded for system E5, containing phase T-2. For systems containing 1.0% w/w HPMC, the hardness values obtained for systems containing the T-1 and T-2 phases (E3 and E6) were statistically significantly (p ≤ 0.05) higher than the values obtained for other systems with the same content of texture modifier, but were not significantly (p > 0.05) different from each other. Similarly, the hardness values of the systems containing T-3 and T-4 fat (E9 and E12) were not statistically significantly (p > 0.05) different from each other, but were significantly (p ≤ 0.05) higher than the values determined for E3 and E6 and significantly (p ≤ 0.05) lower than the value determined for the E15 system.
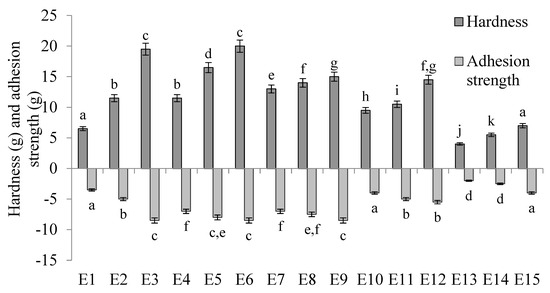
Figure 7.
Hardness and adhesion strength of E1–E15 systems determined 48 h after their manufacture. a–k—different letters indicate statistically significant differences between the averages for individual hardness parameters (p ≤ 0.05).
Analyzing the adhesion force determined for the tested systems, it was found that the values of this parameter depended on the HPMC concentration used. In general, as the concentration of HPMC in the systems increased, higher values of adhesion force were recorded. Yet, only in the case of emulsions containing a mixture of T-1 (i.e., E1–E3) as the fatty phase, each of the stabilizer concentrations used resulted in adhesion strength values that were statistically significantly (p ≤ 0.05) different from each other. For each emulsion containing the other fat phases (T-2, T-3, T-4, T-5), values were recorded that were not statistically significantly different from each other when the differences in the amount of HPMC introduced into these systems were equal to 0.2% w/w (i.e., 0.6 and 0.8% w/w or 0.8 and 1.0% w/w). The type of fat phase used in the systems also showed an effect on adhesion strength, although there was no apparent correlation between the ratio of fats in the fat phase (vegetable oil to animal fat) and the values obtained for this parameter.
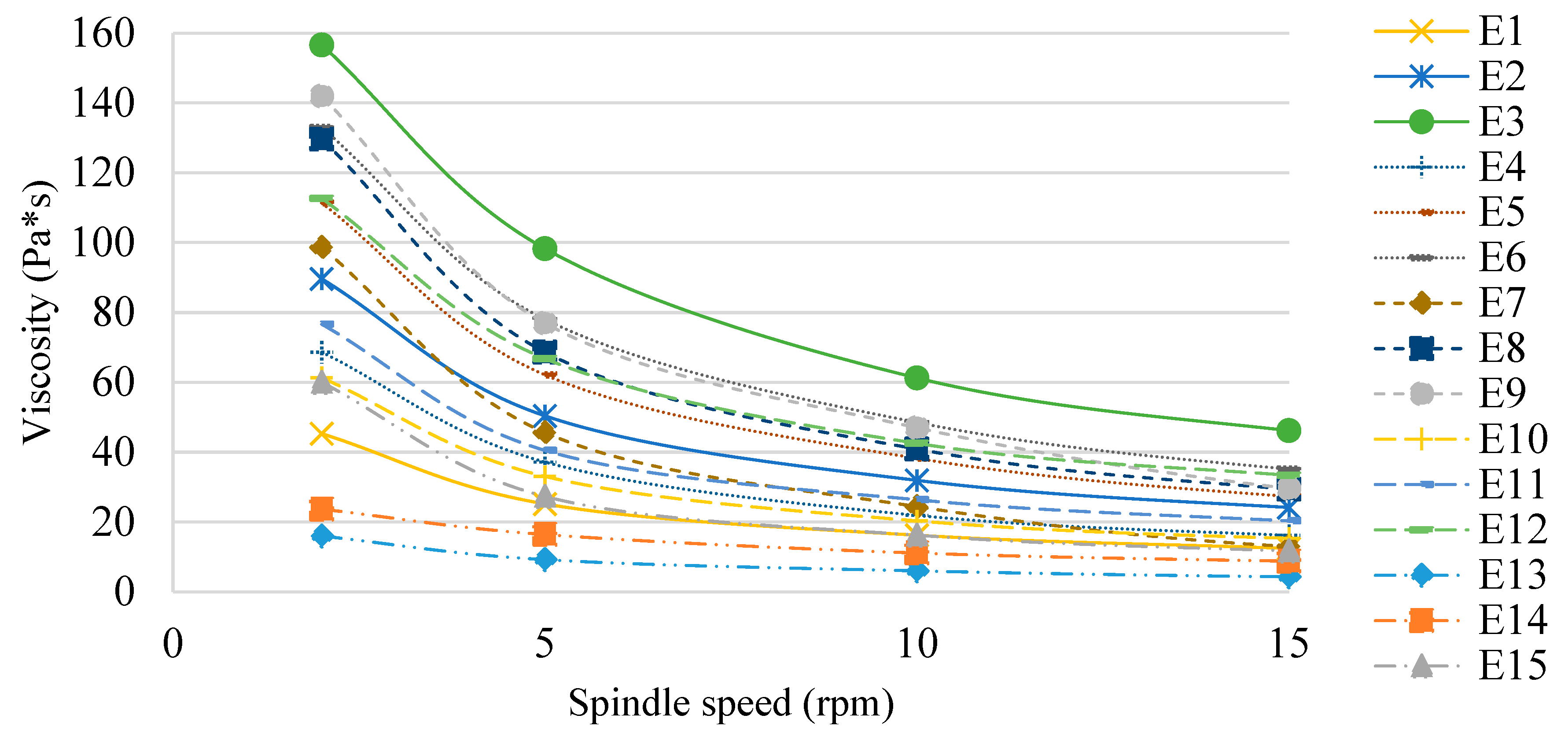
The determined viscosity values of the E1–E15 systems 48 h after their manufacture are shown in Figure 8. The type of fat phase used affected the viscosity of the emulsion systems tested, although it was not correlated with the ratio of mutton tallow and hemp seed oil in the modified mixtures constituting the fat phases. The effect of the HPMC concentration on the viscosity of the emulsion systems was also observed. Regardless of the fatty phase used, the viscosity of the systems for each spindle speed used reached higher values as the concentration of the texture modifier increased (E1 < E2 < E3, E4 < E5 < E6, etc.). Larger differences in viscosity were recorded at the lowest spindle speed analyzed (2 rpm). On the other hand, at the highest speed (15 rpm), differences in viscosity values due to HPMC concentration were much smaller. All analyzed systems containing hydroxypropylmethylcellulose as a texture modifier exhibited the characteristics of shear-thinning (pseudoplastic) non-niutonic liquids [32]. Similar results were obtained in their study by Espert et al. (2020) [33], who analyzed emulsion cocoa creams stabilized with HPMC.
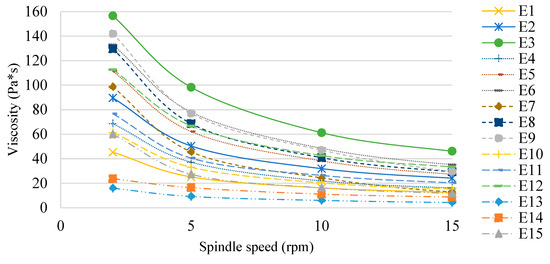
Figure 8.
Viscosity of E1–E15 systems determined 48 h after their manufacture.
4. Summary and Conclusions
Summarizing the analysis of the results obtained for emulsion systems stabilized with hydroxypropylmethylcellulose, it was found that they were characterized by varying stability and properties. The obtained values of the determined parameters were influenced both by the type of fatty phase of the emulsion used and the amount of HPMC introduced. Of all the emulsions analyzed, the systems that were characterized by the far-least advanced destabilization processes were E1 and E2. These formulations were the only ones to qualify for category B (satisfactory system stability). They also showed the most favorable microstructures and the smallest differences in droplet diameter values after storage. On the other hand, limiting it to the best-formed emulsion taking into account its stability, and at the same time not overlooking economic aspects (HPMC content), emulsion E1 should be pointed as the most favorable. With the introduction of 0.6 g of HPMC, the system was characterized by the required stability. Therefore, a higher addition of this thickener is not advisable. In the opinion of the authors, the production of emulsions on the basis of the above-mentioned fat and the determination of the amount of a given thickener allowed us to indicate a certain base (emulsion model system), which can be used especially in such industries as pharmaceuticals, cosmetics and food, especially since the proposed product is based mostly on natural ingredients. It seems that such systems remain free of any allergens. In addition, it should be noted that the proposed technique for obtaining the new fats used for emulsion is compatible with sustainable development due to the use of biocatalysts in the process of modifying fats. This is equivalent to the absence of waste for this technique.
Author Contributions
Conceptualization, M.K. and M.W.; methodology, M.K. and M.W.; software, M.W.; validation, M.W. formal analysis, M.K.; investigation, M.W.; resources, M.K. and M.W.; data curation, M.W.; writing—original draft preparation, M.W. and M.K.; supervision, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Nor applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Kazimierz Pulaski University of Technology and Humanities in Radom, for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Domian, E.; Mańko-Jurkowska, D.; Górska, A. Heat-induced gelation, rheology and stability of oil-in-water emulsions prepared with patatin-rich potato protein. Food Bioprod. Process. 2023, 139, 144–156. [Google Scholar]
- Yamashita, Y.; Miyahara, R.; Sakamoto, K. Emulsion and emulsification technology. In Cosmetic Science and Technology—Theoretical Principles and Applications; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 489–506. [Google Scholar]
- Taslikh, M.; Mollakhalili-Meybodi, N.; Alizadeh, A.M.; Mousavi, M.M.; Nayebzadeh, K.; Mortazavian, A.M. Mayonnaise main ingredients influence on its structure as an emulsion. J. Food Sci. Technol. 2022, 59, 2108–2116. [Google Scholar] [PubMed]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [PubMed]
- Dickinson, E. Hydrocolloids and emulsion stability. In Handbook of Hydrocolloids, 2nd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Tadros, T.F. Emulsion formation, stability, and rheology. In Emulsion Formation and Stability; Tadros, T.F., Ed.; Wiley-VCH Verlag GmBH & Co, KGaA: Weinheim, Germany, 2013; pp. 1–75. [Google Scholar]
- Kowalska, M.; Zbikowska, A.; Smiechowski, K.; Marciniak-Lukasiak, K. Wpływ ilości lecytyny słonecznikowej i czasu homogenizacji na stabilność emulsji spożywczej zawierającej olej z orzechów włoskich. Żywność Nauka Technologia Jakość 2014, 21, 78–91. [Google Scholar] [CrossRef]
- Cerimedo, M.S.; Iriart, C.H.; Candal, R.J.; Herrera, M.L. Stability of emulsions formulated with high concentrations of sodium caseinate and trehalose. Food Res. Int. 2010, 43, 1482–1493. [Google Scholar]
- Georgieva, D.; Schmitt, V.; Leal-Calderon, F.; Langevin, D. On the possible role of surface elasticity in emulsion stability. Langmuir 2009, 25, 5565–5573. [Google Scholar]
- Håkansson, A. Emulsion formation by homogenization: Current understanding and future perspectives. Annu. Rev. Food Sci. Technol. 2019, 10, 239–258. [Google Scholar]
- Zhu, Y.; Gao, H.; Liu, W.; Zou, L.; McClements, D.J. A review of the rheological properties of dilute and concentrated food emulsions. J. Texture Stud. 2020, 51, 45–55. [Google Scholar]
- Schulz, M.B.; Daniels, R. Hydroxypropylmethylcellulose (HPMC) as emulsifier for submicron emulsions: Influence of molecular weight and substitution type on the droplet size after high-pressure homogenization. Eur. J. Pharm. Biopharm. 2000, 49, 231–236. [Google Scholar]
- Daniels, R.; Knie, U. Galenics of dermal products–vehicles, properties and drug release. JDDG J. Dtsch. Dermatol. Ges. 2007, 5, 367–383. [Google Scholar]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Paximada, P.; Tsouko, E.; Kopsahelis, N.; Koutinas, A.A.; Mandala, I. Bacterial cellulose as stabilizer of o/w emulsions. Food Hydrocoll. 2016, 53, 225–232. [Google Scholar]
- Zhang, M.; Yang, B.; Liu, W.; Li, S. Influence of hydroxypropyl methylcellulose, methylcellulose, gelatin, poloxamer 407 and poloxamer 188 on the formation and stability of soybean oil-in-water emulsions. Asian J. Pharm. 2017, 12, 521–531. [Google Scholar]
- Alizadeh, L.; Abdolmaleki, K.; Nayebzadeh, K.; Bahmaei, M. Characterization of sodium caseinate/Hydroxypropyl methylcellulose concentrated emulsions: Effect of mixing ratio, concentration and wax addition. Int. J. Biol. Macromol. 2019, 128, 796–803. [Google Scholar] [PubMed]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [PubMed]
- Kowalska, M.; Woźniak, M.; Krzton-Maziopa, A.; Tavernier, S.; Pazdur, Ł.; Żbikowska, A. Development of the emulsions containing modified fats formed via enzymatic interesterification catalyzed by specific lipase with various amount of water. J. Dispers. Sci. Technol. 2019, 40, 192–205. [Google Scholar]
- Liu, J.; Huang, X.; Lu, L.; Li, M.; Xu, J.; Deng, H. Turbiscan Lab® Expert analysis of the biological demulsification of a water-in-oil emulsion by two biodemulsifiers. J. Hazard. Mater. 2011, 190, 214–221. [Google Scholar]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT-Food Sci. Technol. 2016, 66, 590–597. [Google Scholar]
- Wiśniewska, M. Influences of polyacrylic acid adsorption and temperature on the alumina suspension stability. Powder Technol. 2010, 198, 258–266. [Google Scholar]
- Nastaj, M.; Terpiłowski, K.; Sołowiej, B.G. The effect of native and polymerised whey protein isolate addition on surface and microstructural properties of processed cheeses and their meltability determined by Turbiscan. Int. J. Food Sci. Technol. 2020, 55, 2179–2187. [Google Scholar]
- Application Note Formulaction. Turbiscan Stability Scale. The Stability Criteria and Correlation to Visual Observation. 2019. Available online: https://formulaction.com/wp-content/uploads/2022/08/TS_06_TSI-Scale-and-correlation-with-visual-observation.pdf (accessed on 31 July 2023).
- Domian, E.; Marzec, A.; Kowalska, H. Assessing the effectiveness of colloidal microcrystalline cellulose as a suspending agent for black and white liquid dyes. Int. J. Food Sci. Technol. 2020, 56, 2504–2515. [Google Scholar]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Lapčíková, B.; Lapčík, L.; Valenta, T.; Kučerová, T. Functional and quality profile evaluation of butters, spreadable fats, and shortenings available from Czech market. Foods 2022, 11, 3437. [Google Scholar] [CrossRef] [PubMed]
- Brookfield Ametek. Texture Analysis Application Note: Moisturizing Cream Spreadability. Available online: https://www.brookfieldengineering.com/brookfield-university/learning-center/application-notes/texture-applications/personal-care-products/moisturizing-cream-spreadability (accessed on 31 July 2023).
- Silva, A.C.; González-Mira, E.; García, M.L.; Egea, M.A.; Fonseca, J.; Silva, R.; Santos, D.; Souto, E.B.; Ferreira, D. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. B Biointerfaces 2011, 86, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Ziomek, M.; Żbikowska, A. Stability of cosmetic emulsion containing different amount of hemp oil. Int. J. Cosmet. Sci. 2015, 37, 408–416. [Google Scholar] [CrossRef]
- Wei, W.; Pengyu, W.; Li, K.; Jimiao, D.; Kunyi, W.; Jing, G. Prediction of the apparent viscosity of non-Newtonian water-in-crude oil emulsions. Pet. Explor. Dev. 2013, 40, 130–133. [Google Scholar]
- Espert, M.; Salvador, A.; Sanz, T.; Hernández, M.J. Cellulose ether emulsions as fat source in cocoa creams: Thermorheological properties (flow and viscoelasticity). LWT 2020, 117, 108640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).