Mitigating Carbon Emissions: The Impact of Peat Moss Feeding on CH4 and CO2 Emissions during Pig Slurry Storage
Abstract
:1. Introduction
2. Materials and Methods
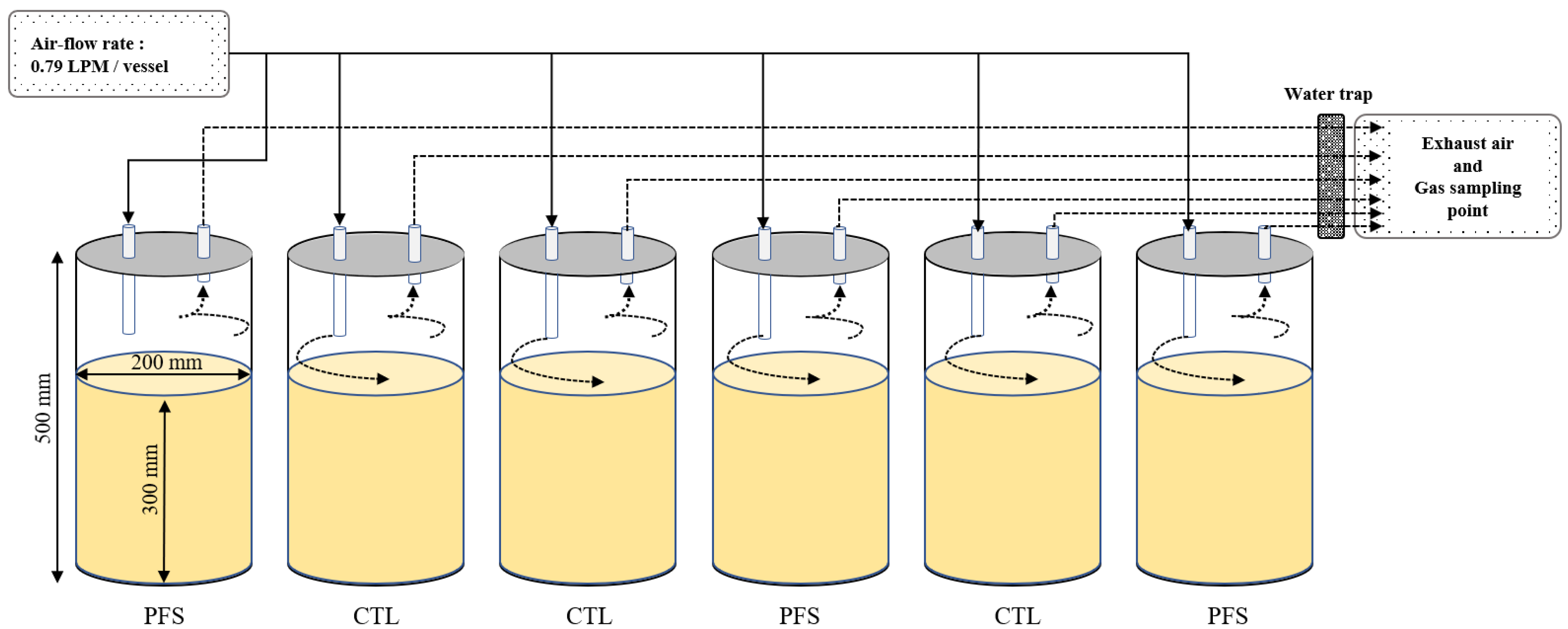
2.1. Experimental Design for Gas Emissions
2.2. Animals and Housing Conditions
2.3. Physico-Chemical Analysis
2.4. Analysis of Methane (CH4) and Carbon Dioxide (CO2) Emissions
- CE = Target gas concentration of exhausted air (mL·m−3).
- V = Headspace ventilation rate (m3·min−1).
- MW = Molecular weight of target gas (g·mol−1).
- TE = Exhaust air temperature (°C).
- CA = Target gas concentration of ambient air (mL·m−3).
- TA = Ambient air temperature (°C).
2.5. Statistical Analysis
3. Results and Discussion
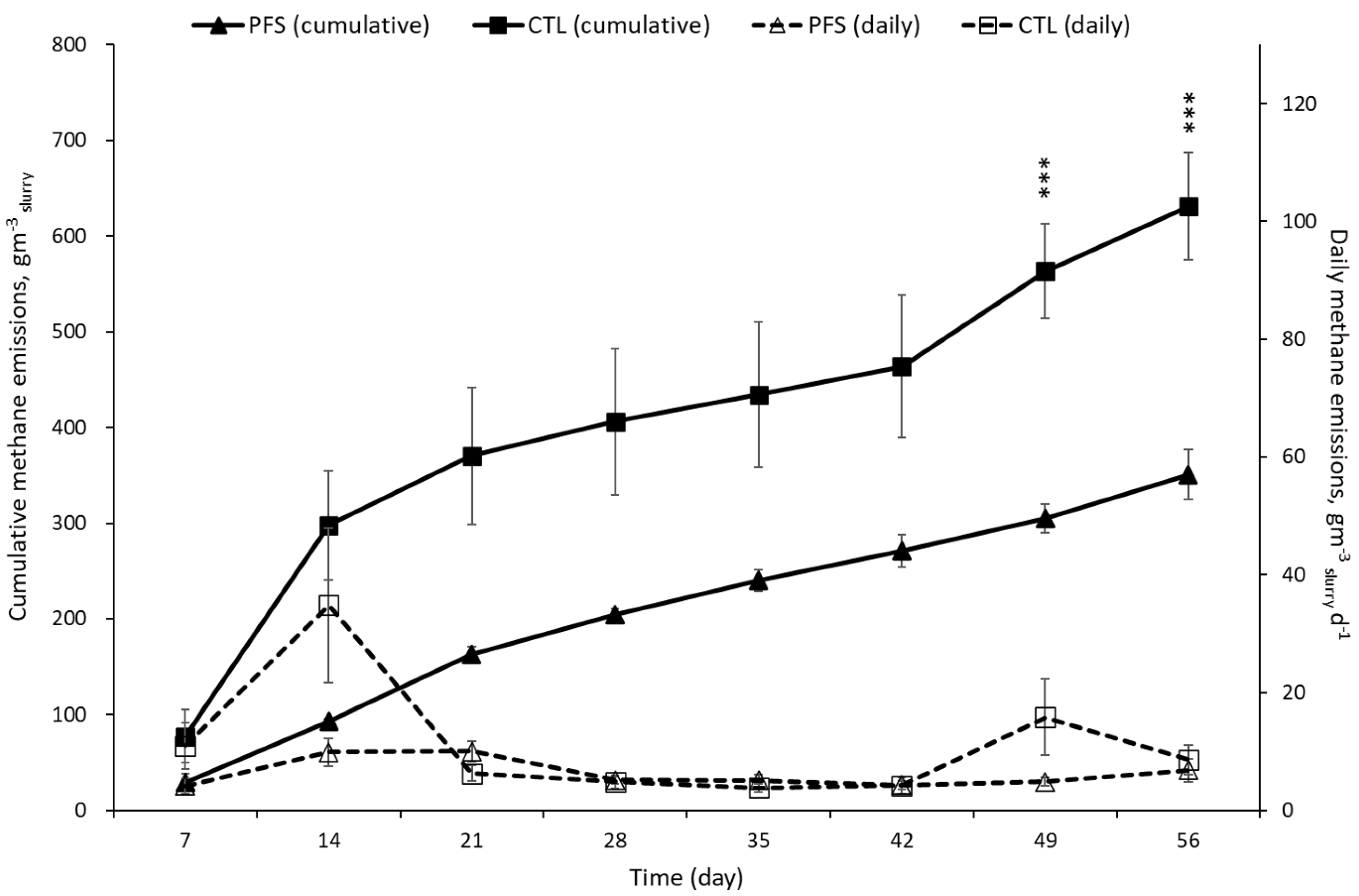
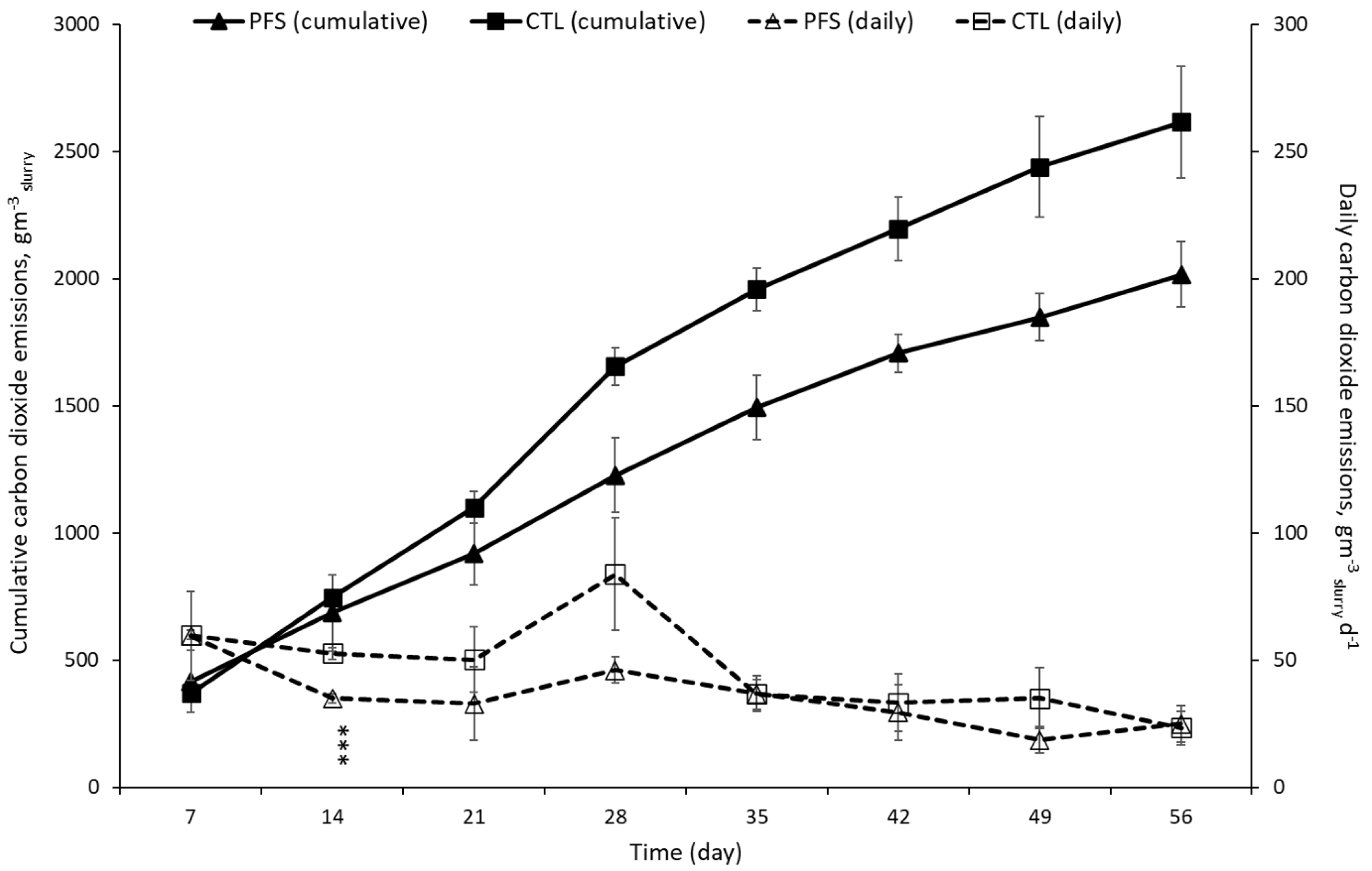
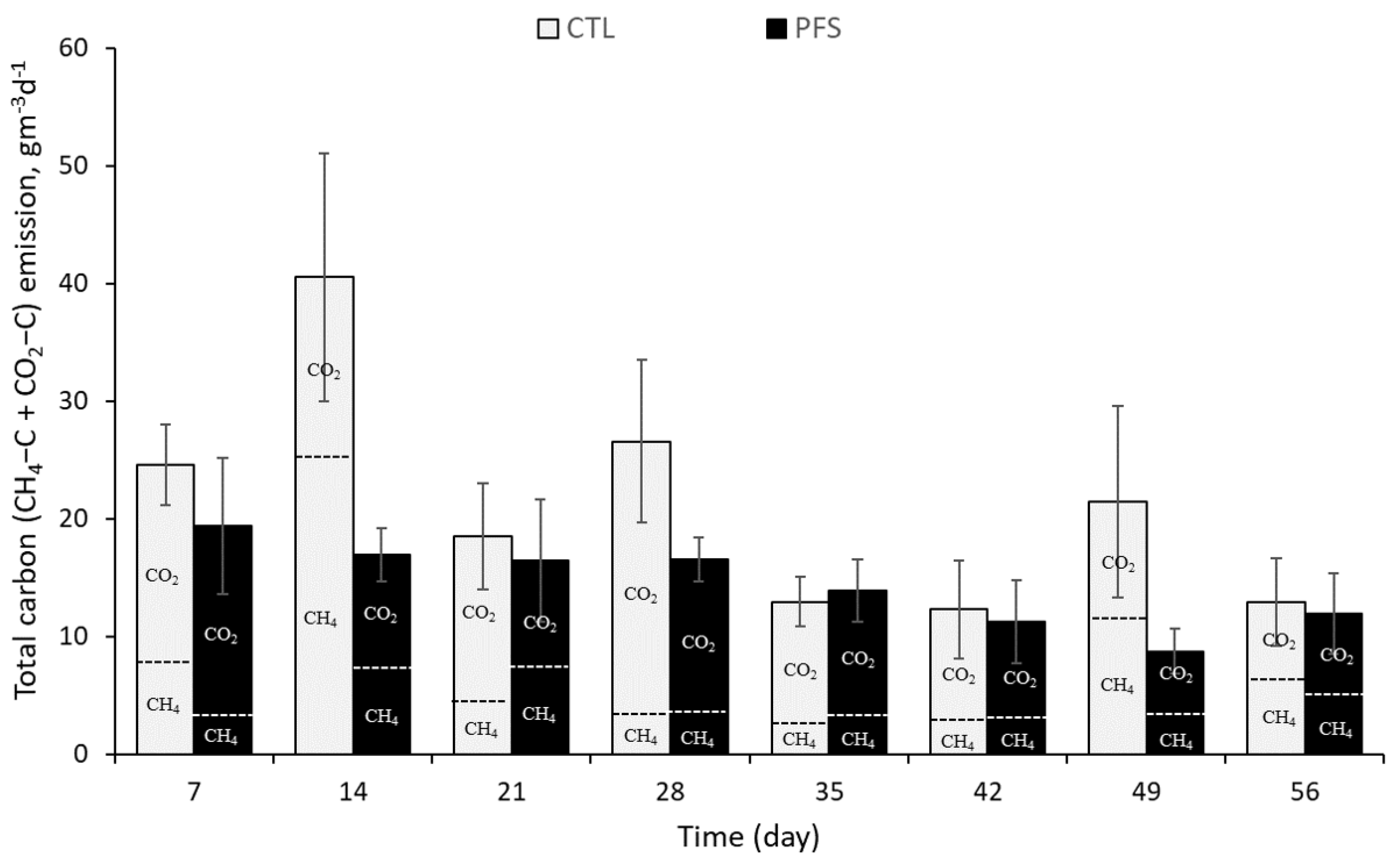
3.1. CH4 and CO2 Emissions during Storage of Pig Slurry
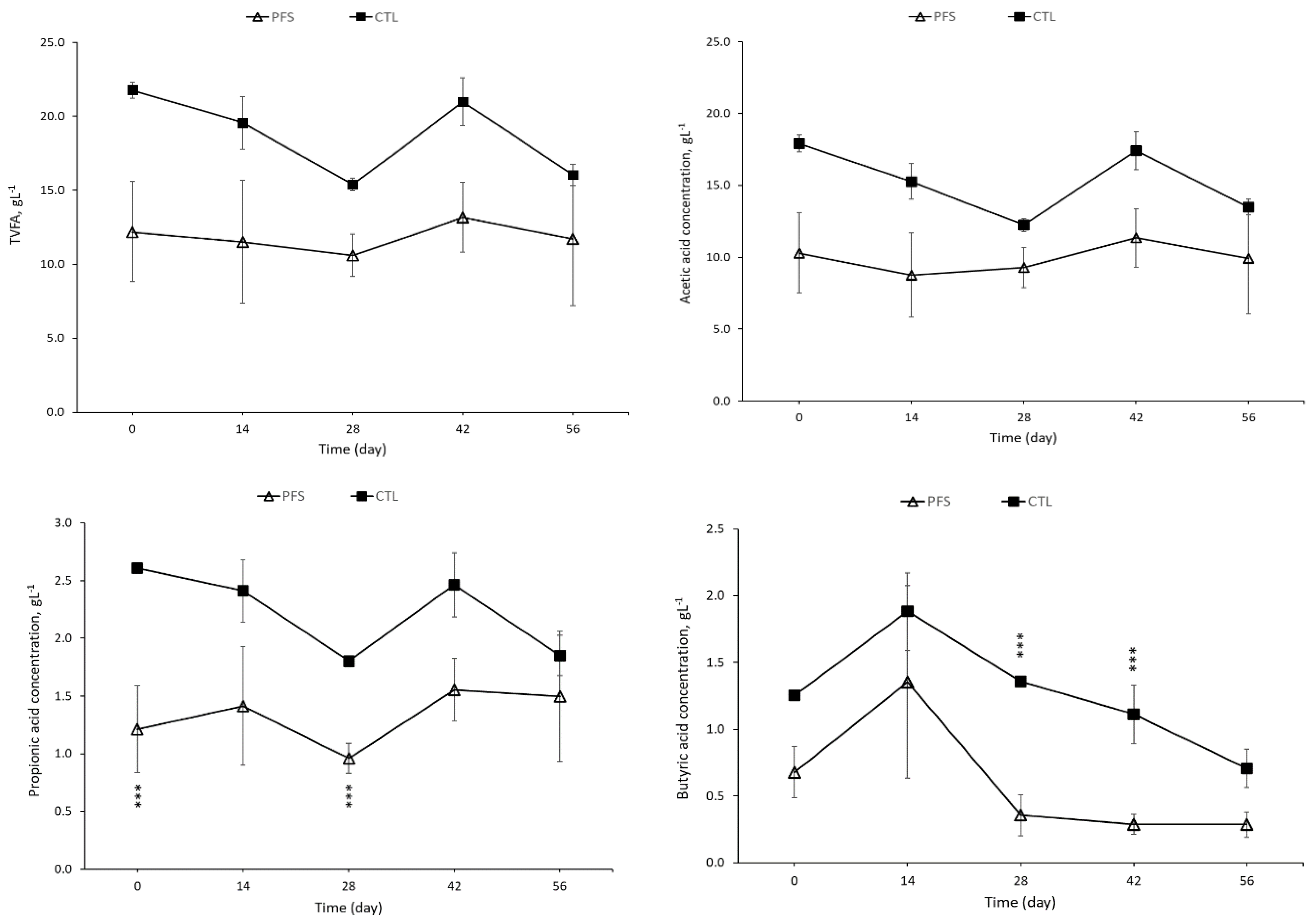
3.2. Concentration of Volatile Fatty Acid (VFA) in Pig Slurry
3.3. Changes in CH4 Carbon (CH4-C), CO2 Carbon (CO2-C), and Carbon Dioxide Equivalent (CO2-eq.) Emissions during Storage of Pig Slurry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Climate and Clean Air Coalition, United Nations Environment Programme (UNEP). Global Methane Assessment: Benefits and Costs of Mitigating Methane Emissions; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Iyer, G.; Ou, Y.; Edmonds, J.; Fawcett, A.A.; Hultman, N.; McFarland, J.; Fuhrman, J.; Waldhoff, S.; McJeon, H. Ratcheting of climate pledges needed to limit peak global warming. Nat. Clim. Chang. 2022, 12, 1129–1135. [Google Scholar] [CrossRef]
- Whitehead, T.R.; Cotta, M.A. Phylogenetic diversity of methanogenic archaea in swine waste storage pits. FEMS Microbiol. Lett. 1999, 179, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, H.G.; Sommer, S.G.; Smith, K.; Matsunaka, T.; Ong, H.K. Guidelines for sustainable manure management in Asian livestock production systems. In Guidelines for Sustainable Manure Management in Asian Livestock Production Systems; International Atomic Energy Agency: Vienna, Austria, 2008; pp. 1–118. [Google Scholar]
- Wi, J.; Lee, S.; Kim, E.; Lee, M.; Koziel, J.A.; Ahn, H. Evaluation of semi-continuous pit manure recharge system performance on mitigation of ammonia and hydrogen sulfide emissions from a swine finishing barn. Atmosphere 2019, 10, 170. [Google Scholar] [CrossRef]
- Harrison, J.; Smith, D. Manure Storage, Process Improvement for Animal Feeding Operations; Cooperative Extension Service, Utah State University: Logan, UT, USA, 2004. [Google Scholar]
- Li, Y.; Liu, H.; Li, G.; Luo, W.; Sun, Y. Manure digestate storage under different conditions: Chemical characteristics and contaminant residuals. Sci. Total Environ. 2018, 639, 19–25. [Google Scholar] [CrossRef]
- Mostafa, E.; Selders, A.; Buescher, W. Aeration of pig slurry affects ammonia and greenhouse gases emissions. Int. J. Environ. Sci. Technol. 2019, 16, 7327–7338. [Google Scholar] [CrossRef]
- Jang, Y.N.; Hwang, O.; Jung, M.W.; Ahn, B.K.; Kim, H.; Jo, G.; Yun, Y.M. Comprehensive analysis of microbial dynamics linked with the reduction of odorous compounds in a full-scale swine manure pit recharge system with recirculation of aerobically treated liquid fertilizer. Sci. Total Environ. 2021, 777, 146122. [Google Scholar] [CrossRef]
- Gavrilova, O.; Leip, A.; Dong, H.; MacDonald, J.D.; Gomez Bravo, C.A.; Amon, B.; Barahona Rosales, R.; Prado, A.D.; de Lima, M.A.; Oyhantcabal, W.; et al. Emmisions from Livestock and Manure Management. 2019. Available online: https://www.alice.cnptia.embrapa.br/alice/bitstream/doc/1119362/1/Limaemissionslivestock2019.pdf (accessed on 10 July 2023).
- Park, K.H.; Thompson, A.G.; Marinier, M.; Clark, K.; Wagner-Riddle, C. Greenhouse gas emissions from stored liquid swine manure in a cold climate. Atmos. Environ. 2006, 40, 618–627. [Google Scholar] [CrossRef]
- Misselbrook, T.; Hunt, J.; Perazzolo, F.; Provolo, G. Greenhouse gas and ammonia emissions from slurry storage: Impacts of temperature and potential mitigation through covering (pig slurry) or acidification (cattle slurry). J. Environ. Qual. 2016, 45, 1520–1530. [Google Scholar] [CrossRef]
- Martinez, J.; Guiziou, F.; Peu, P.; Gueutier, V. Influence of treatment techniques for pig slurry on methane emissions during subsequent storage. Biosyst. Eng. 2003, 85, 347–354. [Google Scholar] [CrossRef]
- Petersen, S.O.; Dorno, N.; Lindholst, S.; Feilberg, A.; Eriksen, J. Emissions of CH4, N2O, NH3 and odorants from pig slurry during winter and summer storage. Nutr. Cycl. Agroecosyst. 2013, 95, 103–113. [Google Scholar] [CrossRef]
- Dinuccio, E.; Berg, W.; Balsari, P. Gaseous emissions from the storage of untreated slurries and the fractions obtained after mechanical separation. Atmos. Environ. 2008, 42, 2448–2459. [Google Scholar] [CrossRef]
- Moset, V.; Cambra-López, M.; Estellés, F.; Torres, A.G.; Cerisuelo, A. Evolution of chemical composition and gas emissions from aged pig slurry during outdoor storage with and without prior solid separation. Biosyst. Eng. 2012, 111, 2–10. [Google Scholar] [CrossRef]
- Petersen, S.O.; Højberg, O.; Poulsen, M.; Schwab, C.; Eriksen, J. Methanogenic community changes, and emissions of methane and other gases, during storage of acidified and untreated pig slurry. J. Appl. Microbiol. 2014, 117, 160–172. [Google Scholar] [CrossRef]
- Philippe, F.X.; Nicks, B. Review on greenhouse gas emissions from pig houses: Production of carbon dioxide, methane and nitrous oxide by animals and manure. Agric. Ecosyst. Environ. 2015, 199, 10–25. [Google Scholar] [CrossRef]
- Petersen, S.O.; Miller, D.N. Perspective Greenhouse gas mitigation by covers on livestock slurry tanks and lagoons? J. Sci. Food Agric. 2006, 86, 1407–1411. [Google Scholar] [CrossRef]
- Dalby, F.R.; Hafner, S.D.; Petersen, S.O.; VanderZaag, A.C.; Habtewold, J.; Dunfield, K.; Chantigny, M.H.; Sommer, S.G. Understanding methane emission from stored animal manure: A review to guide model development. J. Environ. Qual. 2021, 50, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Blodau, C.; Deppe, M. Humic acid addition lowers methane release in peats of the Mer Bleue bog, Canada. Soil Biol. Biochem. 2012, 52, 96–98. [Google Scholar] [CrossRef]
- Shah, S.B.; Kolar, P. Evaluation of additive for reducing gaseous emissions from swine waste. Agric. Eng. Int. CIGR J. 2012, 14, 10–20. [Google Scholar]
- Liang, T.; Elmaadawy, K.; Liu, B.; Hu, J.; Hou, H.; Yang, J. Anaerobic fermentation of waste activated sludge for volatile fatty acid production: Recent updates of pretreatment methods and the potential effect of humic and nutrients substances. Process Saf. Environ. Prot. 2021, 145, 321–339. [Google Scholar] [CrossRef]
- Trckova, M.; Matlova, L.; Hudcova, H.; Faldyna, M.; Zraly, Z.; Dvorska, L.; Beran, V.; Pavlik, I. Peat as a feed supplement for animals: A literature review. Vet. Med. 2005, 50, 361. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Cervantes, F.J. The role of humic substances in mitigating greenhouse gases emissions: Current knowledge and research gaps. Sci. Total Environ. 2021, 750, 141677. [Google Scholar] [CrossRef]
- Yap, S.D.; Astals, S.; Lu, Y.; Peces, M.; Jensen, P.D.; Batstone, D.J.; Tait, S. Humic acid inhibition of hydrolysis and methanogenesis with different anaerobic inocula. Waste Manag. 2018, 80, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Azman, S.; Khadem, A.F.; Plugge, C.M.; Stams, A.J.; Bec, S.; Zeeman, G. Effect of humic acid on anaerobic digestion of cellulose and xylan in completely stirred tank reactors: Inhibitory effect, mitigation of the inhibition and the dynamics of the microbial communities. Appl. Microbiol. Biotechnol. 2017, 101, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.V.; van Lier, J.B.; Zeeman, G. Humic acid-like and fulvic acid-like inhibition on the hydrolysis of cellulose and tributyrin. Bioenergy Res. 2015, 8, 821–831. [Google Scholar] [CrossRef]
- Hyun-Sang, S.; Ki-Hoon, K. Chemical and spectroscopic characterization of peat moss and its different humic fractions (Humin, Humic acid and fulvic acid). J. Soil Groundw. Environ. 2004, 9, 42–51. [Google Scholar]
- Kocabağli, N.; Alp, M.; Acar, N.; Kahraman, R. The effects of dietary humate supplementation on broiler growth and carcass yield. Poult. Sci. 2002, 81, 227–230. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.J.; Yoo, J.S.; Kim, H.J.; Cho, J.H.; Kim, I.H. Effects of supplemental humic substances on growth performance, blood characteristics and meat quality in finishing pigs. Livest. Sci. 2008, 117, 270–274. [Google Scholar] [CrossRef]
- Ji, F.; McGlone, J.J.; Kim, S.W. Effects of dietary humic substances on pig growth performance, carcass characteristics, and ammonia emission. J. Anim. Sci. 2006, 84, 2482–2490. [Google Scholar] [CrossRef]
- Fuchs, B.; Orda, J.; Preś, J.; Muchowicz, M. The effect of feeding piglets up to the 100th day of their life with peat preparation on their growth and physiological and biochemical indices. Arch. Vet. Pol. 1995, 35, 97–107. [Google Scholar]
- Ozturk, E.; Ocak, N.; Turan, A.; Erener, G.; Altop, A.; Cankaya, S. Performance, carcass, gastrointestinal tract and meat quality traits, and selected blood parameters of broilers fed diets supplemented with humic substances. J. Sci. Food Agric. 2012, 92, 59–65. [Google Scholar] [CrossRef]
- Ozturk, E.; Ocak, N.; Coskun, I.; Turhan, S.; Erener, G. Effects of humic substances supplementation provided through drinking water on performance, carcass traits and meat quality of broilers. J. Anim. Physiol. Anim. Nutr. 2010, 94, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Alagawany, M.; Abd El-Hack, M.E.; Saeed, M.; Arain, M.A.; Elnesr, S.S. Humic acid as a feed additive in poultry diets: A review. Iran. J. Vet. Res. 2019, 20, 167. [Google Scholar] [PubMed]
- Arif, M.; Rehman, A.; Abd El-Hack, M.E.; Saeed, M.; Khan, F.; Akhtar, M.; Swelum, A.A.; Saadeldin, I.M.; Alowaimer, A.N. Growth, carcass traits, cecal microbial counts, and blood chemistry of meat-type quail fed diets supplemented with humic acid and black cumin seeds. Asian-Australas. J. Anim. Sci. 2018, 31, 1930. [Google Scholar] [CrossRef] [PubMed]
- Chastain, J.P.; Camberato, J.J.; Albrecht, J.E.; Adams, J. Swine manure production and nutrient content. In South Carolina Confined Animal Manure Managers Certification Program; Clemson University: Clemson, SC, USA, 1999; pp. 1–17. [Google Scholar]
- Haug, R.T. The Practical Handbook of Compost Engineering; Routledge: London, UK, 2018. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater (2540B, 2540E); APHA: Washington, DC, USA, 1992. [Google Scholar]
- Lee, Y.J.; Kim, D.H.; Shin, J.H.; Lee, S.H.; Lee, J.H.; Ahn, H.K. Effect of peat moss diet supplementation on NH3, H2S, and CH4 emissions from sows. In Proceedings of the 19th Asian-Australasian Association of Animal Production (AAAP) Animal Science Congress. Animal Production for Human & Nature, ICC Jeju, Jeju, Republic of Korea, 23–26 August 2022. [Google Scholar]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZaag, A. Ammonia and greenhouse gas emissions from slurry storage-A review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Husted, S. An open chamber technique for determination of methane emission from stored livestock manure. Atmos. Environ. 1993, 27, 1635–1642. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Zhao, W.; Yang, T.; Zhang, X.; Xie, X.; Cui, H.; Wei, Z. Effect of precursors combined with bacteria communities on the formation of humic substances during different materials composting. Bioresour. Technol. 2017, 226, 191–199. [Google Scholar] [CrossRef]
- Guo, X.X.; Liu, H.T.; Wu, S.B. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Pascual, I.; Azcona, I.; Aguirreolea, J.; Morales, F.; Corpas, F.J.; Palma, J.M.; Rellan-Alvarez, R.; Sanchez-Diaz, M. Growth, yield, and fruit quality of pepper plants amended with two sanitized sewage sludges. J. Agric. Food Chem. 2010, 58, 6951–6959. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Le, P.D.; Chim, C.; Le, N.D.; Fievez, V. Potential to mitigate ammonia emission from slurry by increasing dietary fermentable fiber through inclusion of tropical byproducts in practical diets for growing pigs. Asian-Australas. J. Anim. Sci. 2019, 32, 574. [Google Scholar] [CrossRef]
- Canh, T.T.; Aarnink, A.J.A.; Verstegen, M.W.A.; Schrama, J.W. Influence of dietary factors on the pH and ammonia emission of slurry from growing-finishing pigs. J. Anim. Sci. 1998, 76, 1123–1130. [Google Scholar] [CrossRef]
- Conn, K.L.; Topp, E.; Lazarovits, G. Factors influencing the concentration of volatile fatty acids, ammonia, and other nutrients in stored liquid pig manure. J. Environ. Qual. 2007, 36, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Biological degradation and greenhouse gas emissions during pre-storage of liquid animal manure. J. Environ. Qual. 2004, 33, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G.; Petersen, S.O.; Sørensen, P.; Poulsen, H.D.; Møller, H.B. Methane and carbon dioxide emissions and nitrogen turnover during liquid manure storage. Nutr. Cycl. Agroecosyst. 2007, 78, 27–36. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Synthesis Report (Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change); Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014. [Google Scholar]
| Parameters | CTL | PFS |
|---|---|---|
| pH | 7.8 ± 0.0 n.s. | 8.1 ± 0.0 n.s. |
| Total solid (TS, g·kg−1 slurry) | 127.5 ± 1.7 n.s. | 133.5 ± 3.8 n.s. |
| Volatile solid (VS, g·kg−1 slurry) | 103.4 ± 1.7 n.s. | 105.5 ± 3.9 n.s. |
| Carbon (C, g·kg−1 slurry) | 57.4 ± 0.9 n.s. | 58.6 ± 2.2 n.s. |
| Total volatile fatty acid (TVFA, g kg−1 slurry) | 24.0 ± 0.6 n.s. | 13.8 ± 3.5 n.s. |
| Humic Substances | Raw Pig Slurry | Peat Moss | |
|---|---|---|---|
| CTL | PFS | ||
| g·kg−1 slurry | g·kg−1 slurry | % TS | |
| Humic acid | 0.08 ± 0.0 a | 0.18 ± 0.0 b | 6.43 |
| Fulvic acid | 7.8 ± 0.1 a | 14.4 ± 0.2 b | 8.03 |
| Humin | 54.3 ± 0.9 a | 60.8 ± 1.0 b | 26.5 |
| Period | Methane (CH4) | Carbon Dioxide (CO2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daily Emissions | Cumulative Emissions | Daily Emissions | Cumulative Emissions | |||||||||
| PFS | CTL | p-Value | PFS | CTL | p-Value | PFS | CTL | p-Value | PFS | CTL | p-Value | |
| g·kg−1 VS·d−1 | g·kg−1 VS | g·kg−1 VS·d−1 | g·kg−1 VS | |||||||||
| 1st period (0–7 d) | 0.14 ± 0.05 | 0.16 ± 0.06 | 0.829 | 0.97 ± 0.32 a | 1.09 ± 0.39 a | 0.862 | 0.84 ± 0.25 | 0.84 ± 0.03 | 0.992 | 5.85 ± 1.73 a | 5.88 ± 0.18 a | 0.991 |
| 2nd period (8–14 d) | 0.15 ± 0.04 | 0.54 ± 0.21 | 0.203 | 2.05 ± 0.06 ab | 4.90 ± 1.15 ab | 0.180 | 0.55 ± 0.03 | 0.82 ± 0.04 | 0.009 | 9.68 ± 1.91 ab | 11.64 ± 0.26 ab | 0.453 |
| 3rd period (15–28 d) | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.629 | 3.00 ± 0.11 bc | 5.72 ± 1.25 ab | 0.216 | 0.55 ± 0.06 | 1.00 ± 0.26 | 0.250 | 17.36 ± 2.75 abc | 25.63 ± 3.97 abc | 0.235 |
| 4th period (29–42 d) | 0.05 ± 0.01 | 0.05 ± 0.02 | 1.000 | 3.69 ± 0.05 cd | 6.40 ± 1.17 ab | 0.199 | 0.34 ± 0.13 | 0.38 ± 0.13 | 0.832 | 22.11 ± 1.93 bc | 31.01 ± 5.02 bc | 0.248 |
| 5th period (43–56 d) | 0.08 ± 0.02 | 0.10 ± 0.03 | 0.601 | 4.75 ± 0.32 d | 7.74 ± 1.13 b | 0.106 | 0.27 ± 0.08 | 0.26 ± 0.07 | 0.925 | 25.94 ± 2.82 c | 34.61 ± 5.35 c | 0.307 |
| Parameter | Cumulative CO2-eq. Emissions | ||
|---|---|---|---|
| CH4-C | CO2-C | Total | |
| g·CO2-eq.·m−3 | g·CO2-eq.·m−3 | g·CO2-eq.·m−3 | |
| PFS | 7423 ± 601 a | 541 ± 34 | 7963 ± 634 a |
| CTL | 13,205 ± 1164 b | 717 ± 61 | 13,922 ± 1224 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Ahn, H. Mitigating Carbon Emissions: The Impact of Peat Moss Feeding on CH4 and CO2 Emissions during Pig Slurry Storage. Appl. Sci. 2023, 13, 10492. https://doi.org/10.3390/app131810492
Lee J, Ahn H. Mitigating Carbon Emissions: The Impact of Peat Moss Feeding on CH4 and CO2 Emissions during Pig Slurry Storage. Applied Sciences. 2023; 13(18):10492. https://doi.org/10.3390/app131810492
Chicago/Turabian StyleLee, Joonhee, and Heekwon Ahn. 2023. "Mitigating Carbon Emissions: The Impact of Peat Moss Feeding on CH4 and CO2 Emissions during Pig Slurry Storage" Applied Sciences 13, no. 18: 10492. https://doi.org/10.3390/app131810492
APA StyleLee, J., & Ahn, H. (2023). Mitigating Carbon Emissions: The Impact of Peat Moss Feeding on CH4 and CO2 Emissions during Pig Slurry Storage. Applied Sciences, 13(18), 10492. https://doi.org/10.3390/app131810492







