Magnetic Resonance Roadmap in Detecting and Staging Endometriosis: Usual and Unusual Localizations
Abstract
1. Introduction
2. Indications for MRI
2.1. Procedure and Patient Preparation
2.2. Technical Requirements and Protocols of Acquisition
3. Localization
3.1. Superficial Peritoneal Lesions (SUP)
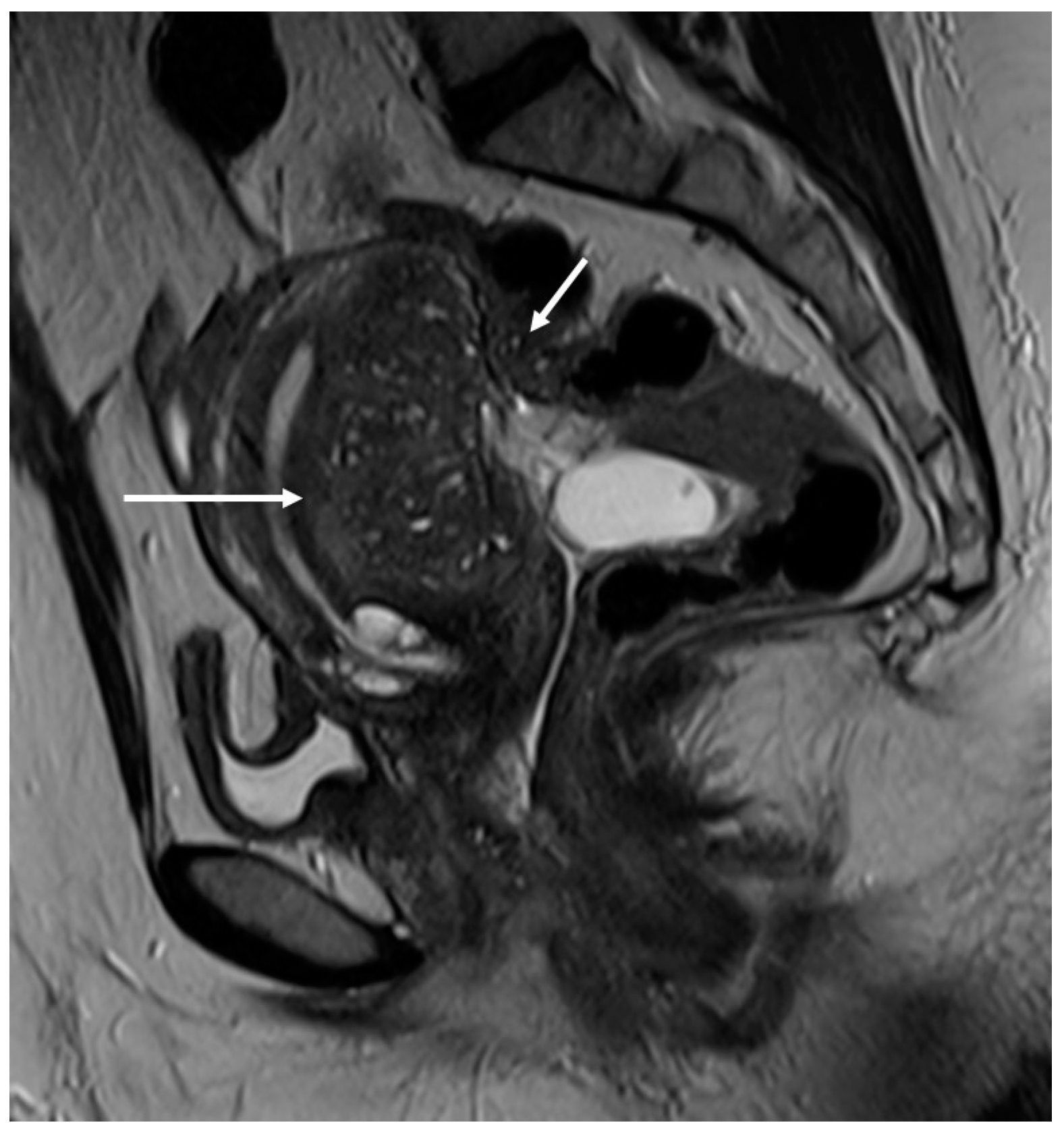
3.2. Deep Infiltrating Endometriosis (DIE)
- -
- Right and left anterolateral compartments: distal round ligament;
- -
- Anterocentral: proximal round ligament and bladder;
- -
- Right and left mediolateral: parametrium, ureter, uterine artery and pelvic wall (external iliac and/or obturator vessels);
- -
- Mediocentral: torus, proximal uterosacral ligaments, posterior vaginal fornix, rectovaginal septum, anterior mesorectum and external adenomyosis;
- -
- Right and left posterolateral: distal uterosacral ligaments, sacro-rectal-genital septum and pelvic wall (sacral roots, sciatic nerve, internal iliac vessels);
- -
- Posterocentral: rectum and rectosigmoid junction.
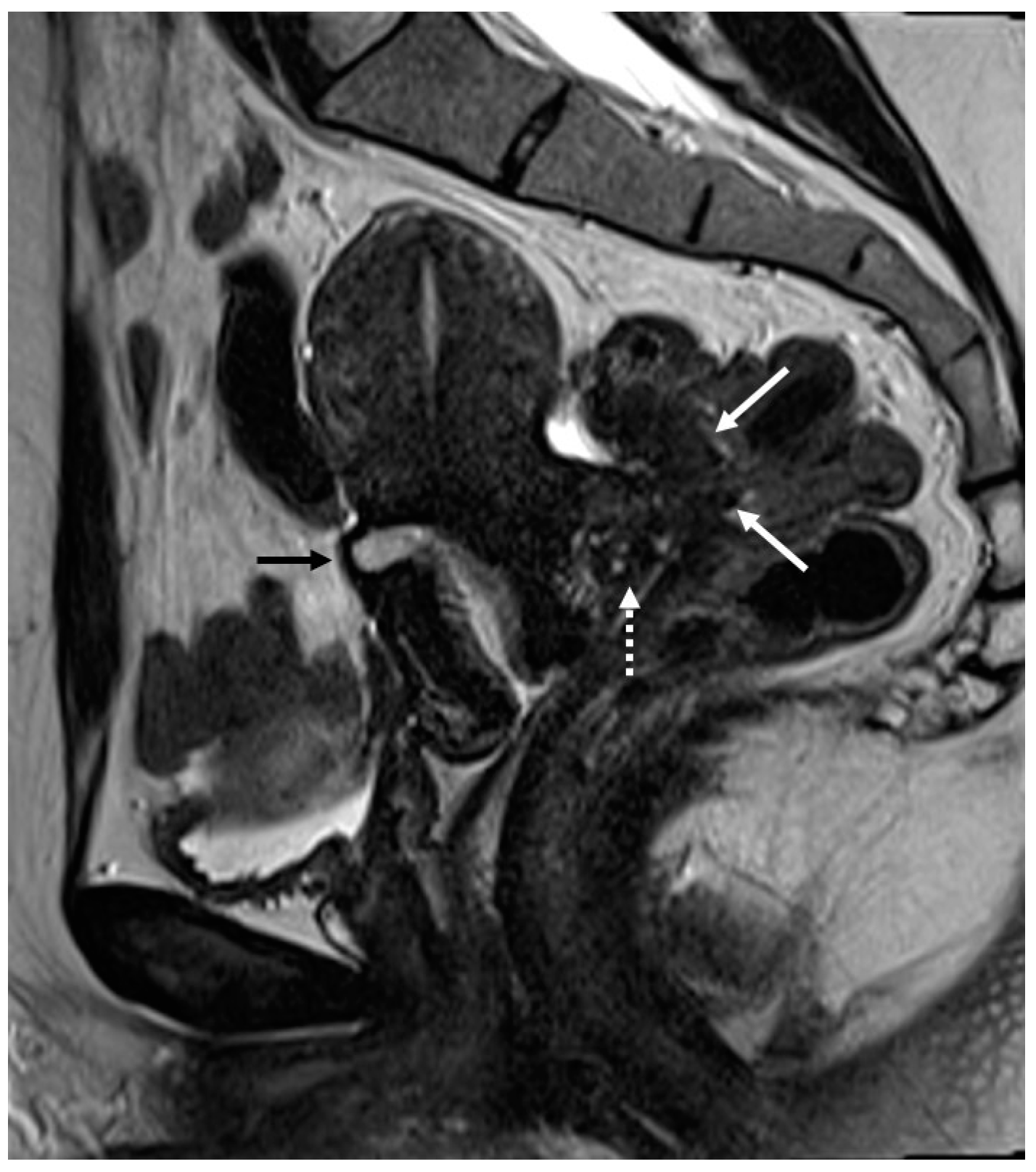
3.2.1. Bladder and Urinary Tract
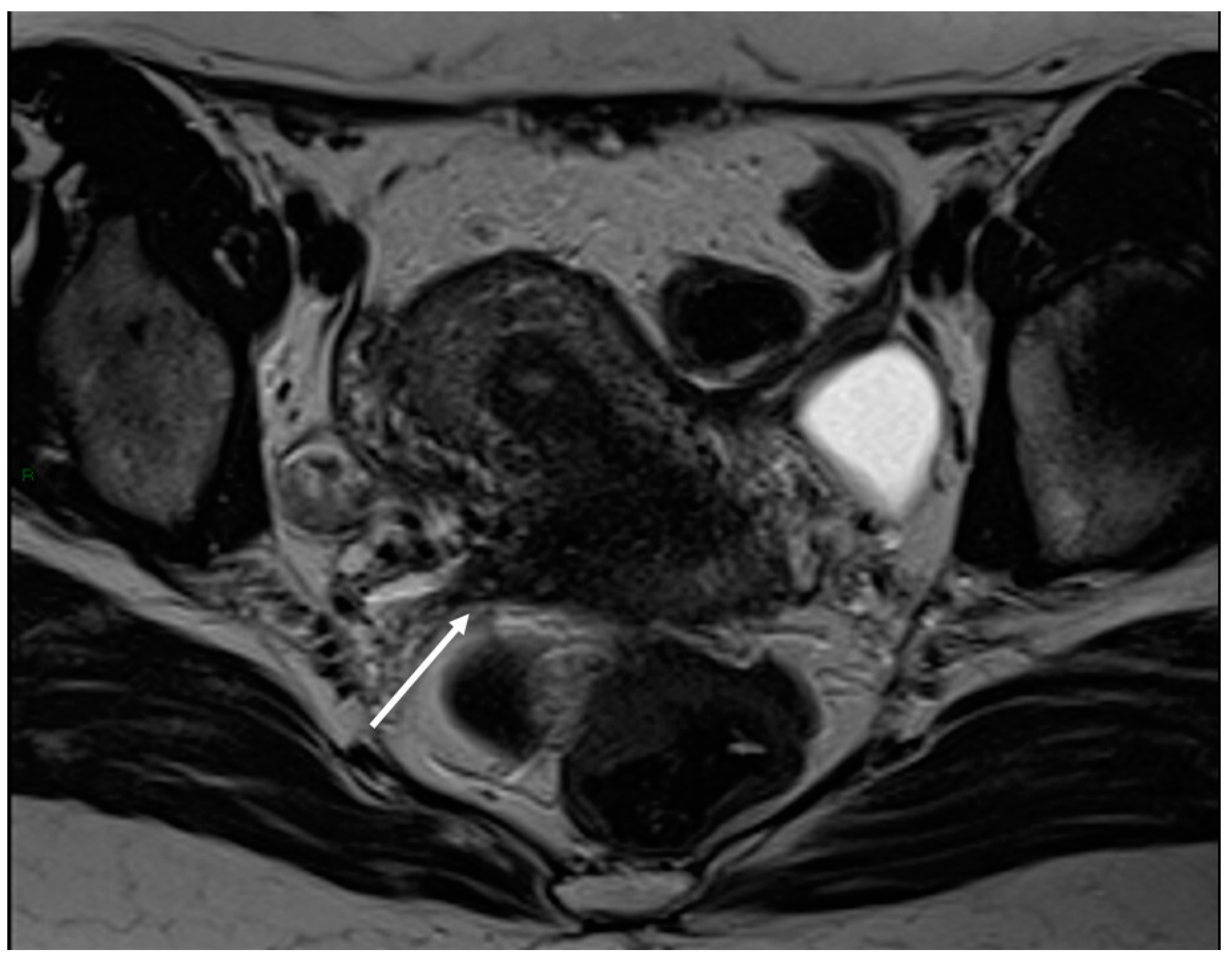
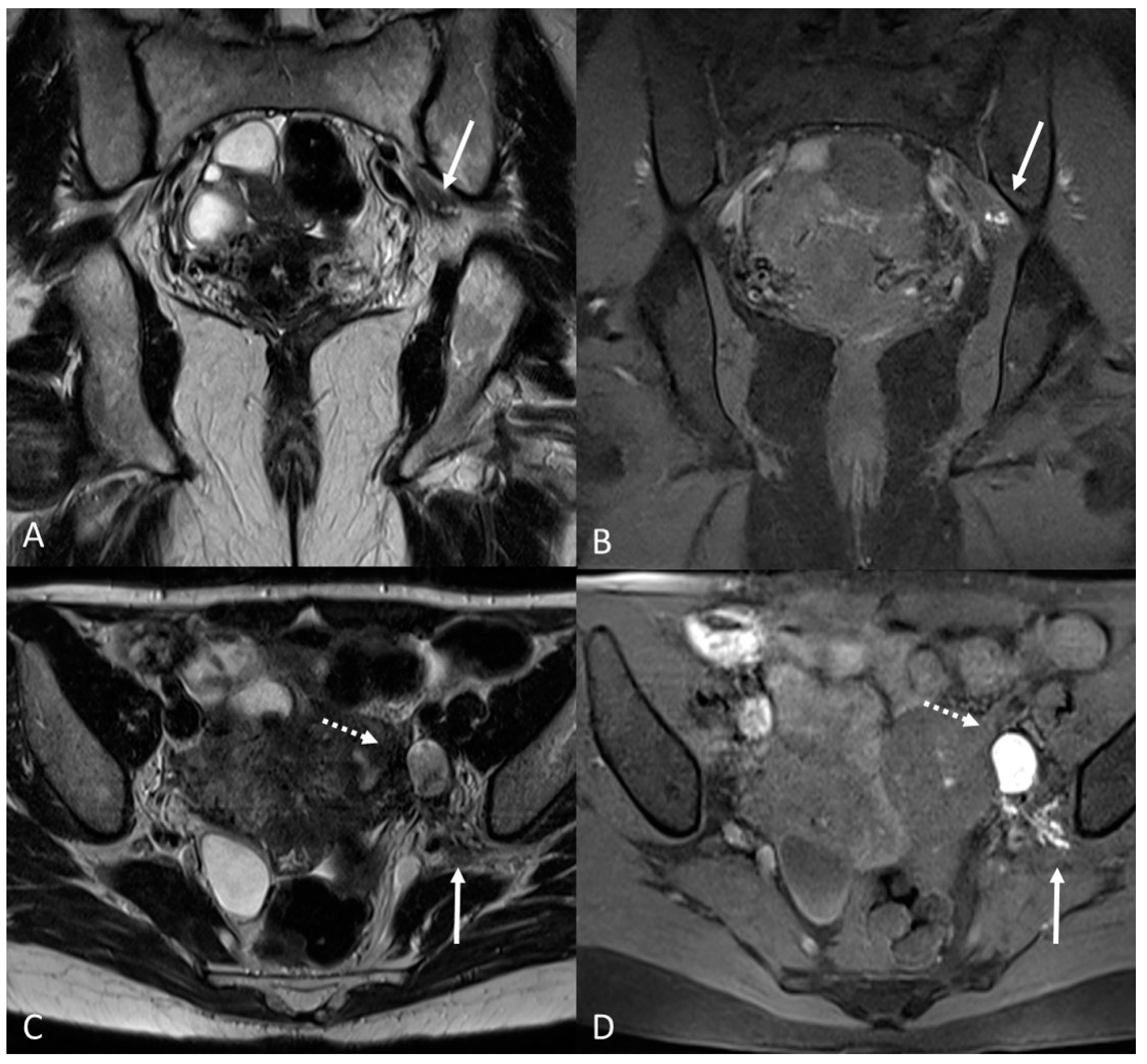
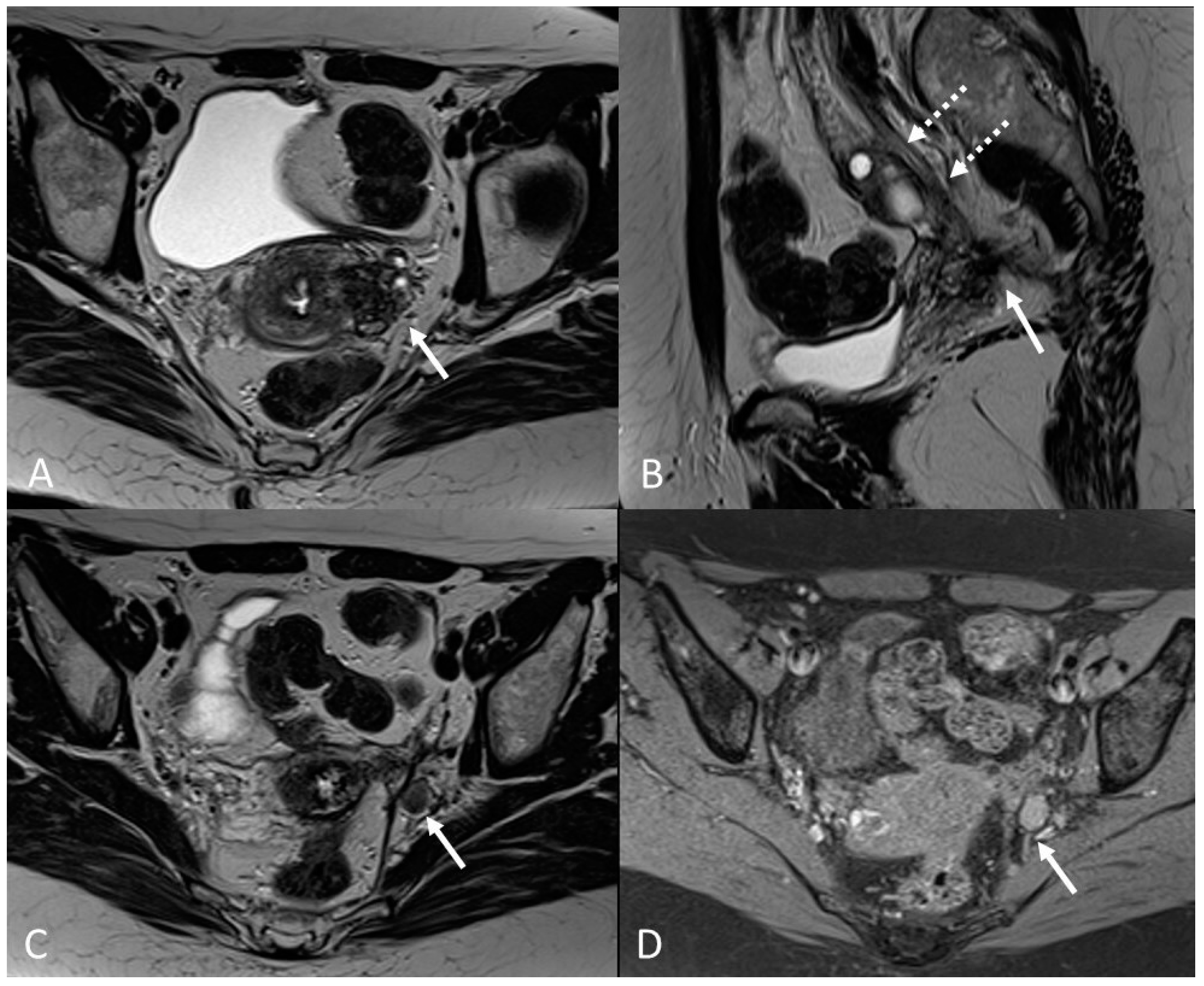
3.2.2. Round Ligaments
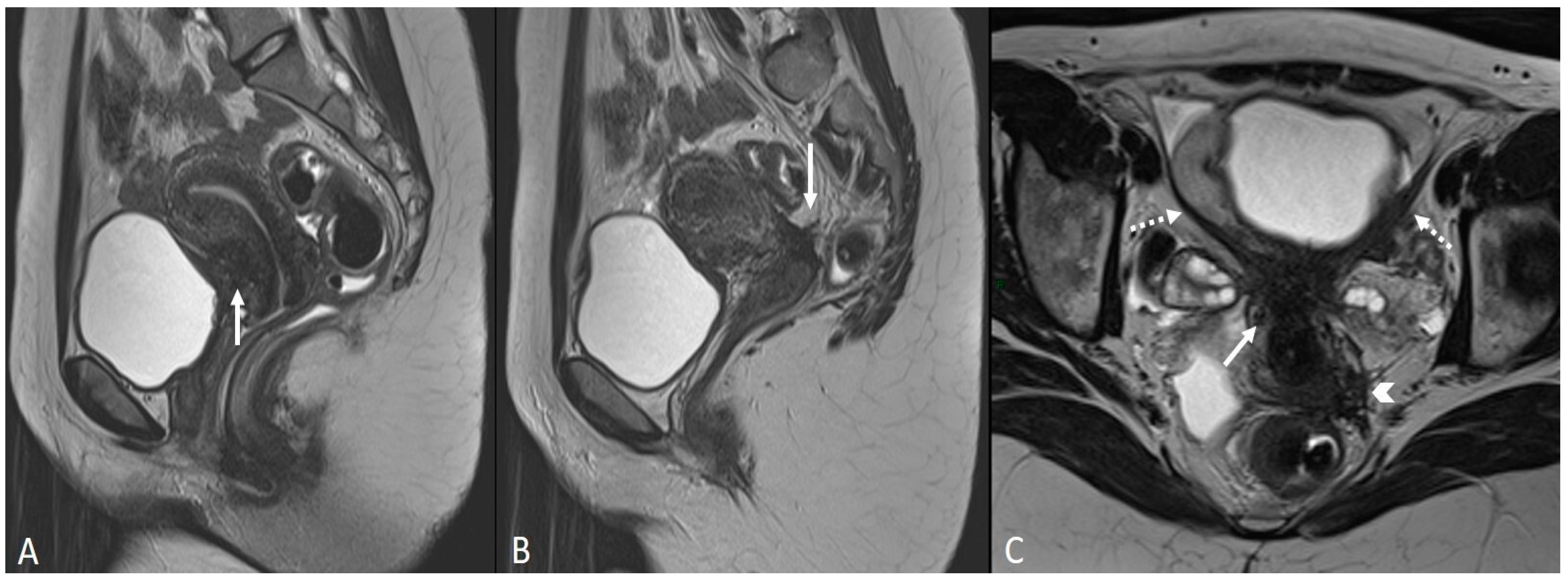
3.2.3. Torus Uterinus and Sacrouterine Ligaments
3.2.4. Rectovaginal Septum
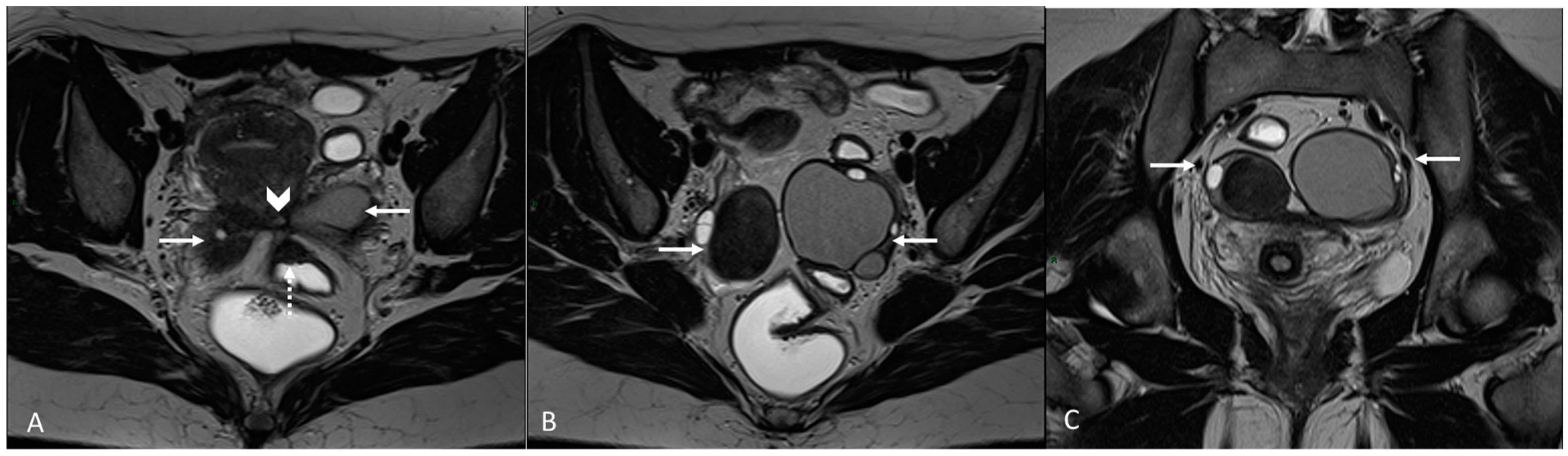
3.2.5. Rectosigmoid Colon
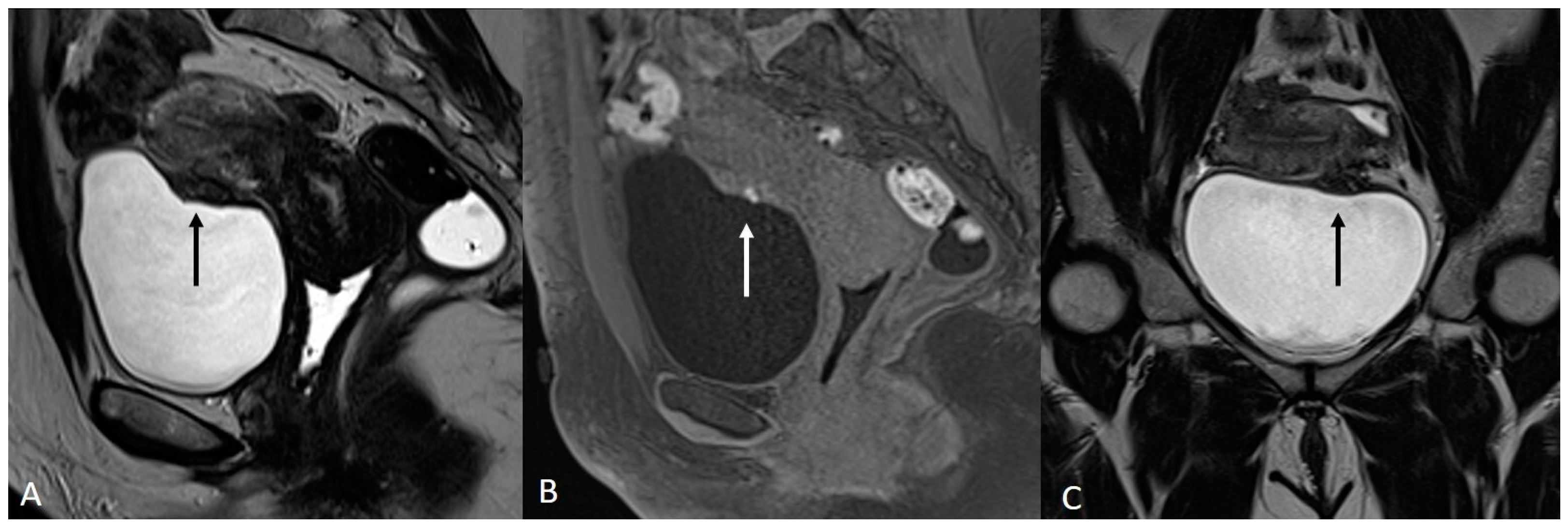
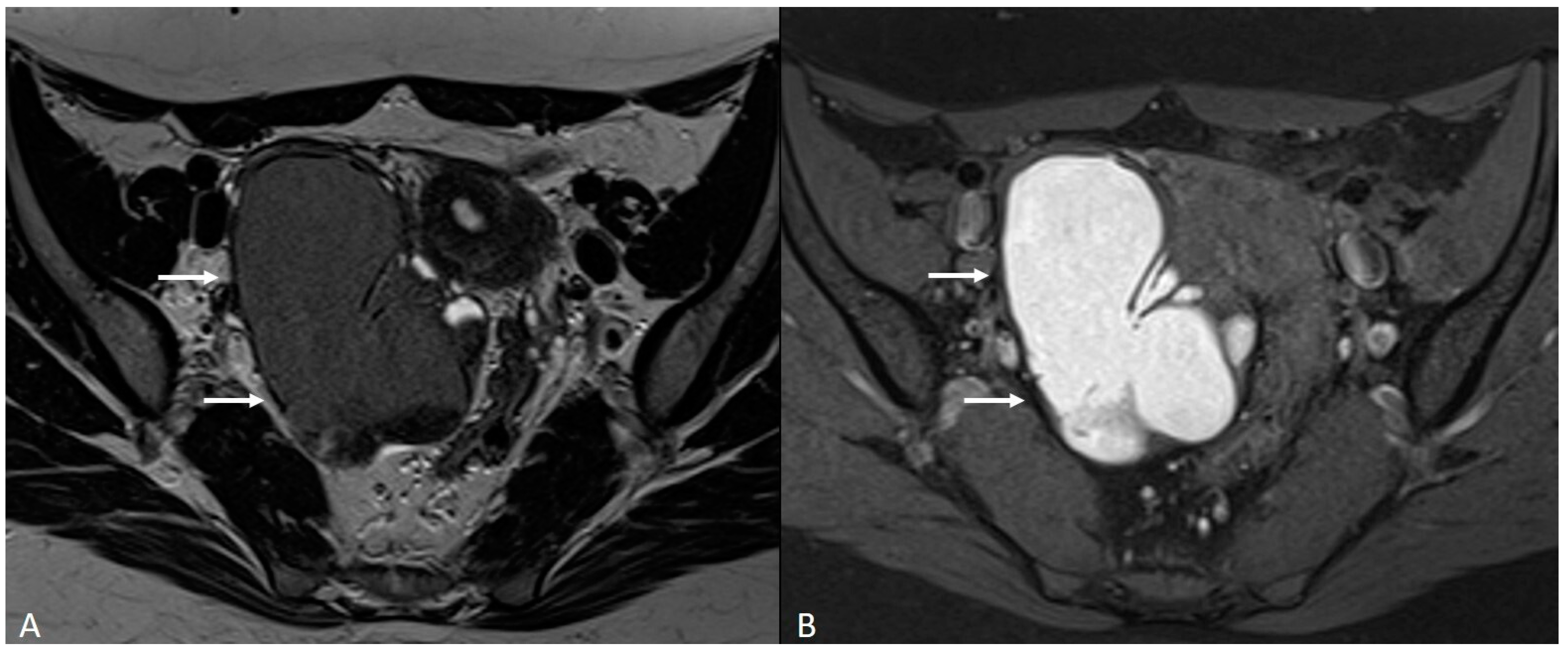
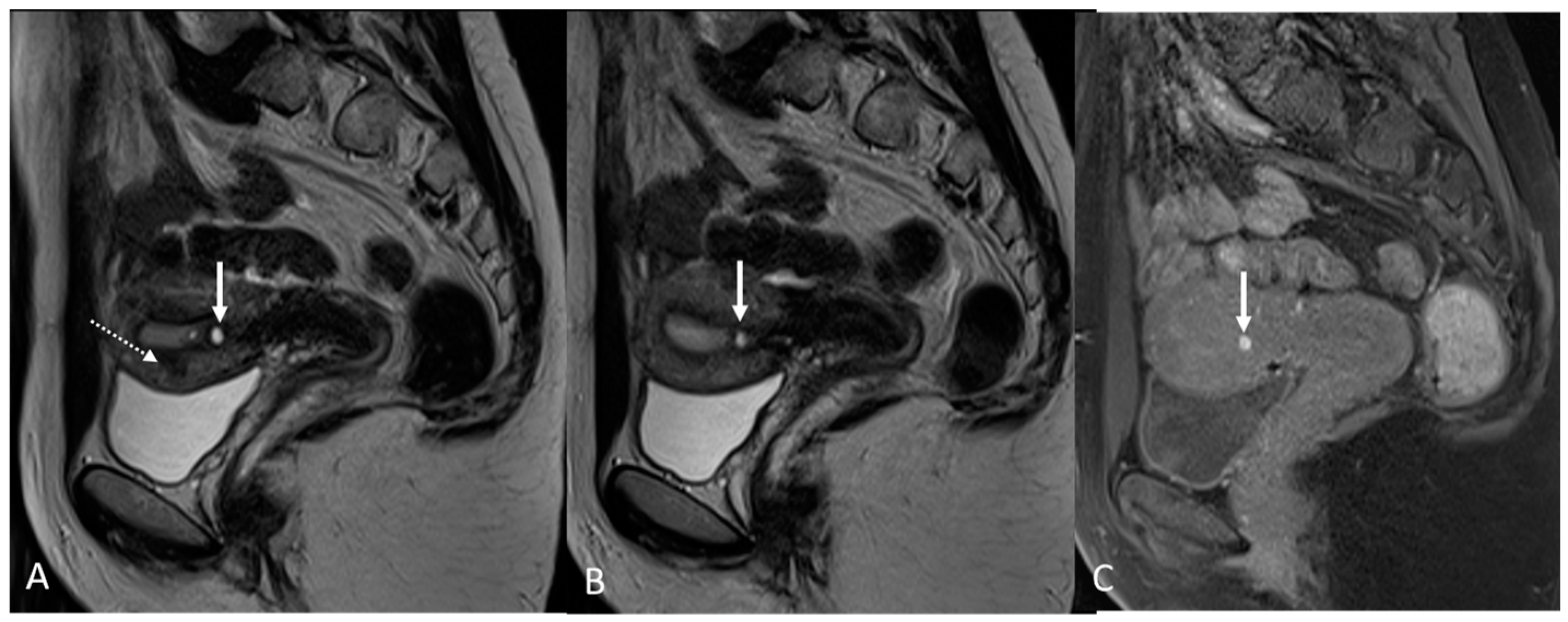
4. Ovarian Endometrioma
5. Adenomyosis
- -
- The parameter known as “ratiomax” corresponds to the ratio between JZmax and the total thickness of the myometrium, with a value exceeding 40% typically considered an acceptable diagnostic parameter [68];
- -
- The parameter called “JZ differential” (JZdiff) measures the maximum and minimum thickness difference between the anterior and posterior uterine walls, where a value greater than 5 mm is indicative [68].
6. Atypical Site
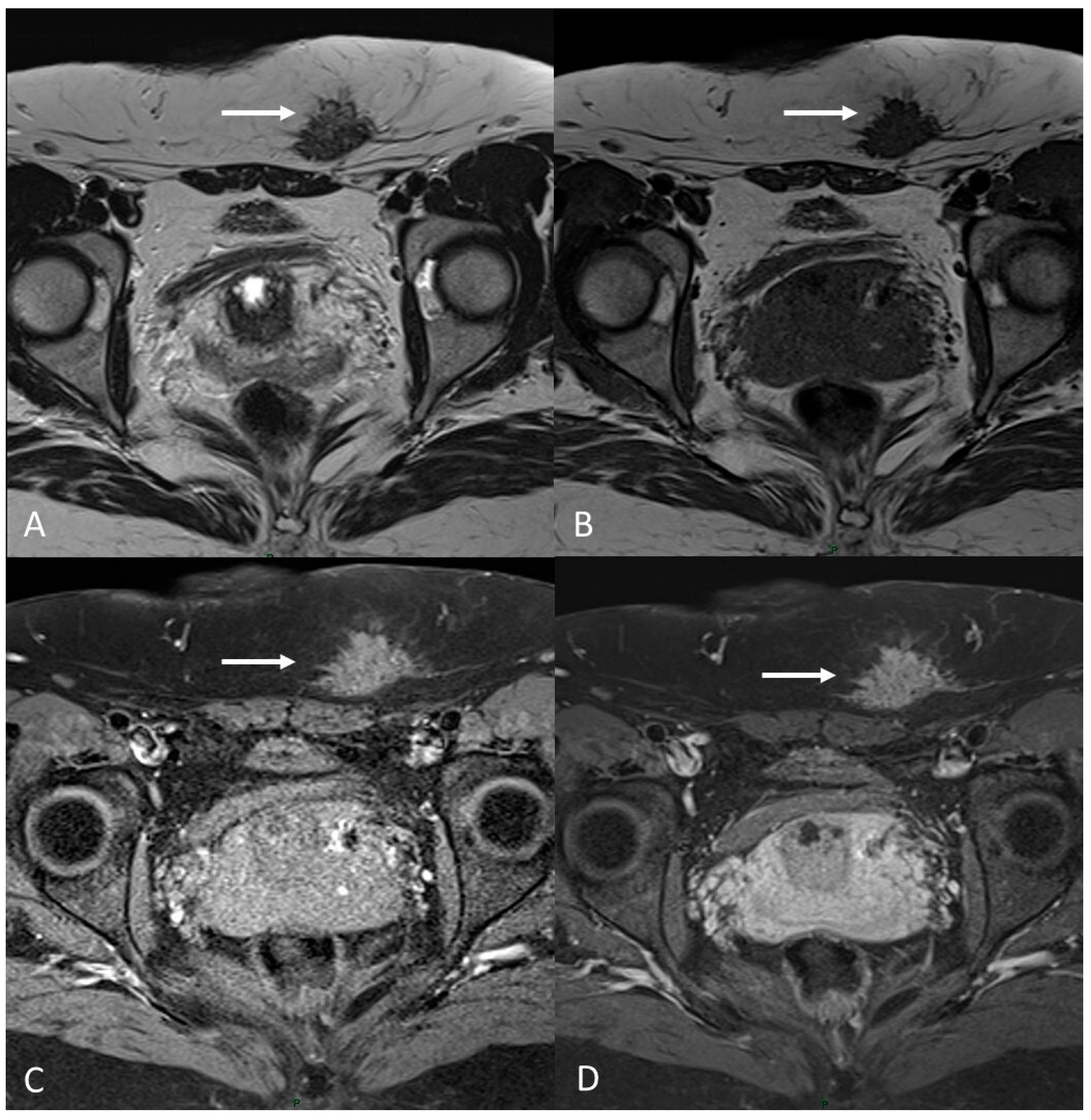
6.1. Soft Tissues Localization
6.2. Gastrointestinal Localization
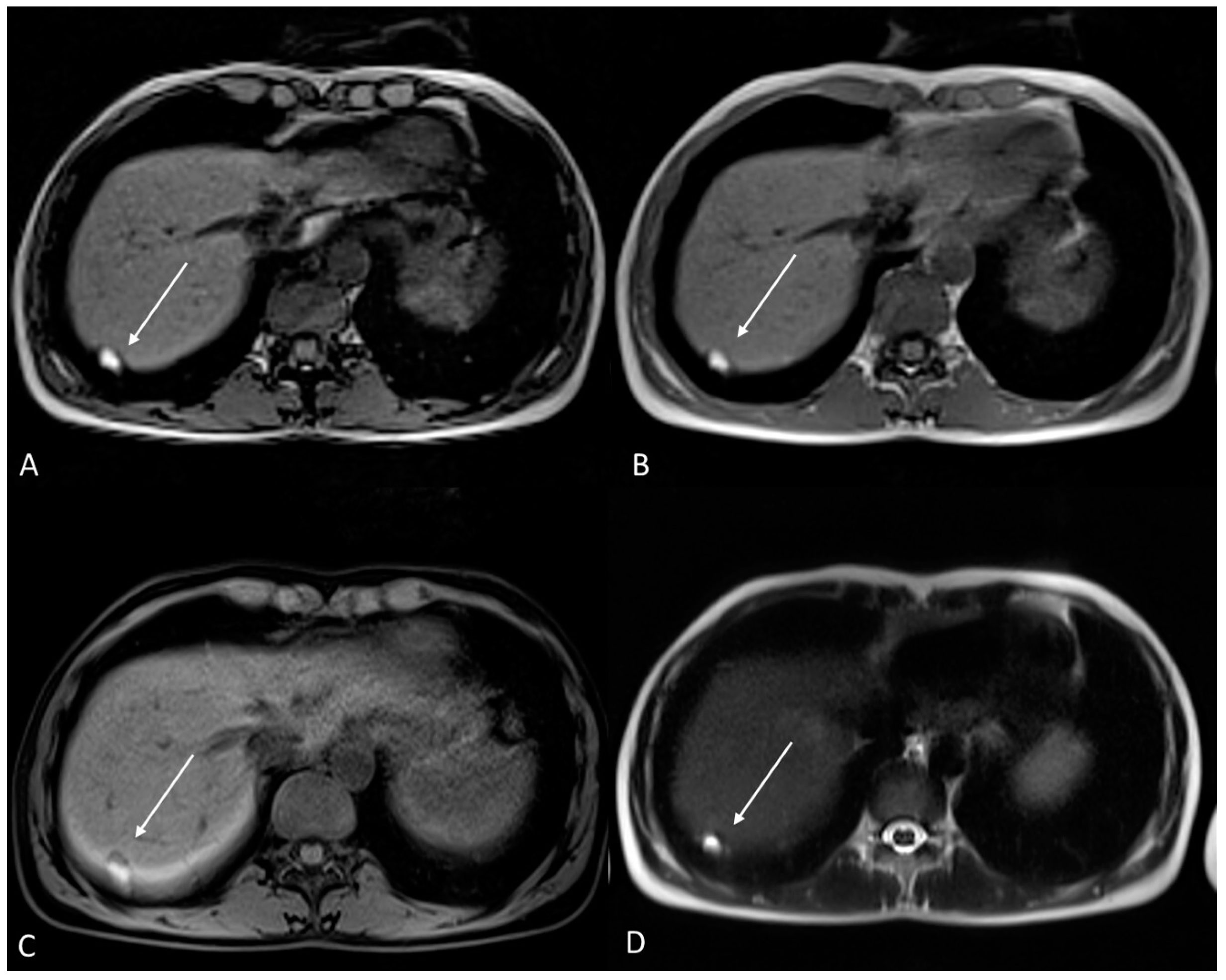
6.3. Thoracic Endometriosis
6.4. Neural Involvement
7. Post-Operative Imaging
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horne, A.W.; Missmer, S.A. Pathophysiology, Diagnosis, and Management of Endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of Endometriosis: The Genetic/Epigenetic Theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Amro, B.; Ramirez Aristondo, M.E.; Alsuwaidi, S.; Almaamari, B.; Hakim, Z.; Tahlak, M.; Wattiez, A.; Koninckx, P.R. New Understanding of Diagnosis, Treatment and Prevention of Endometriosis. Int. J. Environ. Res. Public Health 2022, 19, 6725. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis Is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Yu, E.H.; Joo, J.K. Commentary on the New 2022 European Society of Human Reproduction and Embryology (ESHRE) Endometriosis Guidelines. Clin. Exp. Reprod. Med. 2022, 49, 219–224. [Google Scholar] [CrossRef]
- Bazot, M.; Darai, E.; Hourani, R.; Thomassin, I.; Cortez, A.; Uzan, S.; Buy, J.-N. Deep Pelvic Endometriosis: MR Imaging for Diagnosis and Prediction of Extension of Disease. Radiology 2004, 232, 379–389. [Google Scholar] [CrossRef]
- Zanardi, R.; Del Frate, C.; Zuiani, C.; Bazzocchi, M. Staging of Pelvic Endometriosis Based on MRI Findings versus Laparoscopic Classification according to the American Fertility Society. Abdom. Imaging 2003, 28, 733–742. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, D.Y.; Moon, D.H.; Lee, S. Percutaneous Cryoablation of Multiple Pulmonary Endometriosis. J. Chest Surg. 2021, 54, 75–78. [Google Scholar] [CrossRef]
- Welch, B.T.; Ehman, E.C.; VanBuren, W.M.; Cope, A.G.; Welch, T.L.; Woodrum, D.A.; Kurup, A.N.; Burnett, T.L. Percutaneous Cryoablation of Abdominal Wall Endometriosis: The Mayo Clinic Approach. Abdom. Radiol. 2020, 45, 1813–1817. [Google Scholar] [CrossRef]
- Bazot, M.; Bharwani, N.; Huchon, C.; Kinkel, K.; Cunha, T.M.; Guerra, A.; Manganaro, L.; Buñesch, L.; Kido, A.; Togashi, K.; et al. European Society of Urogenital Radiology (ESUR) Guidelines: MR Imaging of Pelvic Endometriosis. Eur. Radiol. 2017, 27, 2765–2775. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Thomassin, I.; Hourani, R.; Cortez, A.; Darai, E. Diagnostic Accuracy of Transvaginal Sonography for Deep Pelvic Endometriosis. Ultrasound Obstet. Gynecol. Off. 2004, 24, 180–185. [Google Scholar] [CrossRef]
- Mais, V.; Guerriero, S.; Ajossa, S.; Angiolucci, M.; Paoletti, A.M.; Melis, G.B. The Efficiency of Transvaginal Ultrasonography in the Diagnosis of Endometrioma. Fertil. Steril. 1993, 60, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Chamié, L.P.; Blasbalg, R.; Pereira, R.M.A.; Warmbrand, G.; Serafini, P.C. Findings of Pelvic Endometriosis at Transvaginal US, MR Imaging, and Laparoscopy. Radiographics 2011, 31, E77–E100. [Google Scholar] [CrossRef]
- Guerriero, S.; Ajossa, S.; Orozco, R.; Perniciano, M.; Jurado, M.; Melis, G.B.; Alcazar, J.L. Accuracy of Transvaginal Ultrasound for Diagnosis of Deep Endometriosis in the Rectosigmoid: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2016, 47, 281–289. [Google Scholar] [CrossRef]
- Kinkel, K.; Chapron, C.; Balleyguier, C.; Fritel, X.; Dubuisson, J.B.; Moreau, J.F. Magnetic Resonance Imaging Characteristics of Deep Endometriosis. Hum. Reprod. 1999, 14, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging Modalities for the Non-Invasive Diagnosis of Endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef] [PubMed]
- Ciggaar, I.A.; Henneman, O.D.F.; Oei, S.A.; Vanhooymissen, I.J.; Blikkendaal, M.D.; Bipat, S. Bowel Preparation in MRI for Detection of Endometriosis: Comparison of the Effect of an Enema, no Additional Medication and Intravenous Butylscopolamine on Image Quality. Eur. J. Radiol. 2022, 149, 110222. [Google Scholar] [CrossRef]
- Yang, R.K.; Roth, C.G.; Ward, R.J.; de Jesus, J.O.; Mitchell, D.G. Optimizing Abdominal MR Imaging: Approaches to Common Problems. Radiographics 2010, 30, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, A.; Binkert, C.A.; Koh, D.-M.; Hergan, K.; von Weymarn, C.; Graf, N.; Patak, M.A.; Roos, J.E.; Horstmann, M.; Kos, S.; et al. Evaluation of the Anti-Peristaltic Effect of Glucagon and Hyoscine on the Small Bowel: Comparison of Intravenous and Intramuscular Drug Administration. Eur. Radiol. 2012, 22, 1186–1194. [Google Scholar] [CrossRef]
- Fiaschetti, V.; Crusco, S.; Meschini, A.; Cama, V.; Di Vito, L.; Marziali, M.; Piccione, E.; Calabria, F.; Simonetti, G. Deeply Infiltrating Endometriosis: Evaluation of Retro-Cervical Space on MRI after Vaginal Opacification. Eur. J. Radiol. 2012, 81, 3638–3645. [Google Scholar] [CrossRef] [PubMed]
- Tamai, K.; Togashi, K.; Ito, T.; Morisawa, N.; Fujiwara, T.; Koyama, T. MR Imaging Findings of Adenomyosis: Correlation with Histopathologic Features and Diagnostic Pitfalls. Radiographics 2005, 25, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Hottat, N.; Larrousse, C.; Anaf, V.; Noël, J.-C.; Matos, C.; Absil, J.; Metens, T. Endometriosis: Contribution of 3.0-T Pelvic MR Imaging in Preoperative Assessment–Initial Results. Radiology 2009, 253, 126–134. [Google Scholar] [CrossRef] [PubMed]
- McCauley, T.R.; McCarthy, S.; Lange, R. Pelvic Phased Array Coil: Image Quality Assessment for Spin-Echo MR Imaging. Magn. Reson. Imaging 1992, 10, 513–522. [Google Scholar] [CrossRef]
- Bazot, M.; Gasner, A.; Ballester, M.; Daraï, E. Value of Thin-Section Oblique Axial T2-Weighted Magnetic Resonance Images to Assess Uterosacral Ligament Endometriosis. Hum. Reprod. 2011, 26, 346–353. [Google Scholar] [CrossRef]
- Cornfeld, D.M.; Israel, G.; McCarthy, S.M.; Weinreb, J.C. Pelvic Imaging Using a T1W Fat-Suppressed Three-Dimensional Dual Echo Dixon Technique at 3T. J. Magn. Reson. Imaging 2008, 28, 121–127. [Google Scholar] [CrossRef]
- Togashi, K.; Nishimura, K.; Kimura, I.; Tsuda, Y.; Yamashita, K.; Shibata, T.; Nakano, Y.; Konishi, J.; Konishi, I.; Mori, T. Endometrial Cysts: Diagnosis with MR Imaging. Radiology 1991, 180, 73–78. [Google Scholar] [CrossRef]
- Suzuki, S.; Yasumoto, M.; Matsumoto, R.; Andoh, A. MR Findings of Ruptured Endometrial Cyst: Comparison with Tubo-Ovarian Abscess. Eur. J. Radiol. 2012, 81, 3631–3637. [Google Scholar] [CrossRef]
- Onbas, O.; Kantarci, M.; Alper, F.; Kumtepe, Y.; Durur, I.; Ingec, M.; Gursan, N.; Okur, A. Nodular Endometriosis: Dynamic MR Imaging. Abdom. Imaging 2007, 32, 451–456. [Google Scholar] [CrossRef]
- Tanaka, Y.O.; Yoshizako, T.; Nishida, M.; Yamaguchi, M.; Sugimura, K.; Itai, Y. Ovarian Carcinoma in Patients with Endometriosis: MR Imaging Findings. AJR Am. J. Roentgenol. 2000, 175, 1423–1430. [Google Scholar] [CrossRef]
- Takahashi, N.; Yoshino, O.; Maeda, E.; Naganawa, S.; Harada, M.; Koga, K.; Hiraike, O.; Nakamura, M.; Tabuchi, T.; Hori, M.; et al. Usefulness of T2 Star-Weighted Imaging in Ovarian Cysts and Tumors. J. Obstet. Gynaecol. Res. 2016, 42, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Sivajohan, B.; Elgendi, M.; Menon, C.; Allaire, C.; Yong, P.; Bedaiwy, M.A. Clinical Use of Artificial Intelligence in Endometriosis: A Scoping Review. NPJ Digit. Med. 2022, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xie, H.; Lin, J.; Wang, Y.; Yin, Y. Diagnosis and Nursing Intervention of Gynecological Ovarian Endometriosis with Magnetic Resonance Imaging under Artificial Intelligence Algorithm. Comput. Intell. Neurosci. 2022, 2022, 3123310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nicholes, K.; Shih, I.-M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.; Härmä, K.; Reid, S.; Rao, T.; Lo, G.; Yang, N.; Karia, S.; Lee, E.; Borok, N. Endometriosis: A Multimodal Imaging Review. Eur. J. Radiol. 2023, 158, 110610. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Reis, F.M.; Santulli, P.; Marcellin, L.; Borghese, B.; Lafay-Pillet, M.-C.; Chapron, C. Superficial Peritoneal Endometriosis: Clinical Characteristics of 203 Confirmed Cases and 1292 Endometriosis-Free Controls. Reprod. Sci. 2020, 27, 309–315. [Google Scholar] [CrossRef]
- Rolla, E. Endometriosis: Advances and Controversies in Classification, Pathogenesis, Diagnosis, and Treatment. F1000Research 2019, 8, F1000 Faculty Rev-529. [Google Scholar] [CrossRef]
- Rousset, P.; Florin, M.; Bharwani, N.; Touboul, C.; Monroc, M.; Golfier, F.; Nougaret, S.; Thomassin-Naggara, I.; ENDOVALIRM Group. Deep Pelvic Infiltrating Endometriosis: MRI Consensus Lexicon and Compartment-Based Approach from the ENDOVALIRM Group. Diagn. Interv. Imaging 2023, 104, 95–112. [Google Scholar] [CrossRef]
- Rousset, P.; Bischoff, E.; Charlot, M.; Grangeon, F.; Dubernard, G.; Paparel, P.; Lega, J.-C.; Golfier, F. Bladder Endometriosis: Preoperative MRI Analysis with Assessment of Extension to Ureteral Orifices. Diagn. Interv. Imaging 2021, 102, 255–263. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef]
- Sherman, A.K.; MacLachlan, L.S. A Review of Urinary Tract Endometriosis. Curr. Urol. Rep. 2022, 23, 219–223. [Google Scholar] [CrossRef]
- Kaniewska, M.; Gołofit, P.; Heubner, M.; Maake, C.; Kubik-Huch, R.A. Suspensory Ligaments of the Female Genital Organs: MRI Evaluation with Intraoperative Correlation. Radiographics 2018, 38, 2195–2211. [Google Scholar] [CrossRef]
- Del Frate, C.; Girometti, R.; Pittino, M.; Del Frate, G.; Bazzocchi, M.; Zuiani, C. Deep Retroperitoneal Pelvic Endometriosis: MR Imaging Appearance with Laparoscopic Correlation. Radiographics 2006, 26, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Gerges, B.; Li, W.; Leonardi, M.; Mol, B.W.; Condous, G. Meta-Analysis and Systematic Review to Determine the Optimal Imaging Modality for the Detection of Uterosacral Ligaments/Torus Uterinus, Rectovaginal Septum and Vaginal Deep Endometriosis. Hum. Reprod. Open 2021, 2021, hoab041. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Sergenti, G.; Buggio, L.; Frattaruolo, M.P.; Dridi, D.; Berlanda, N. Advances in the Medical Management of Bowel Endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Kermarrec, E.; Bendifallah, S.; Daraï, E. MRI of Intestinal Endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Cranney, R.; Condous, G.; Reid, S. An Update on the Diagnosis, Surgical Management, and Fertility Outcomes for Women with Endometrioma. Acta Obstet. Gynecol. Scand. 2017, 96, 633–643. [Google Scholar] [CrossRef]
- Cecchino, G.N.; García-Velasco, J.A. Endometrioma, Fertility, and Assisted Reproductive Treatments: Connecting the Dots. Curr. Opin. Obstet. Gynecol. 2018, 30, 223–228. [Google Scholar] [CrossRef]
- Kaponis, A.; Taniguchi, F.; Azuma, Y.; Deura, I.; Vitsas, C.; Decavalas, G.O.; Harada, T. Current Treatment of Endometrioma. Obstet. Gynecol. Surv. 2015, 70, 183–195. [Google Scholar] [CrossRef]
- Kido, A.; Himoto, Y.; Moribata, Y.; Kurata, Y.; Nakamoto, Y. MRI in the Diagnosis of Endometriosis and Related Diseases. Korean J. Radiol. 2022, 23, 426–445. [Google Scholar] [CrossRef]
- Dias, J.L.; Veloso Gomes, F.; Lucas, R.; Cunha, T.M. The Shading Sign: Is It Exclusive of Endometriomas? Abdom. Imaging 2015, 40, 2566–2572. [Google Scholar] [CrossRef]
- Wolfman, W.; Thurston, J.; Yeung, G.; Glanc, P. Guideline No. 404: Initial Investigation and Management of Benign Ovarian Masses. J. Obstet. Gynaecol. Can. 2020, 42, 1040–1050.e1. [Google Scholar] [CrossRef]
- Singla, V.; Dawadi, K.; Singh, T.; Prabhakar, N.; Srinivasan, R.; Suri, V.; Khandelwal, N. Multiparametric MRI Evaluation of Complex Ovarian Masses. Curr. Probl. Diagn. Radiol. 2021, 50, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Forstner, R.; Thomassin-Naggara, I.; Cunha, T.M.; Kinkel, K.; Masselli, G.; Kubik-Huch, R.; Spencer, J.A.; Rockall, A. ESUR Recommendations for MR Imaging of the Sonographically Indeterminate Adnexal Mass: An Update. Eur. Radiol. 2017, 27, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, E.A.; Maturen, K.E.; Rockall, A.; Reinhold, C.; Addley, H.; Jha, P.; Bharwani, N.; Thomassin-Naggara, I. Ovary: MRI Characterisation and O-RADS MRI. Br. J. Radiol. 2021, 94, 20210157. [Google Scholar] [CrossRef]
- Biggs, W.S.; Marks, S.T. Diagnosis and Management of Adnexal Masses. Am. Fam. Physician 2016, 93, 676–681. [Google Scholar] [PubMed]
- Modesitt, S.C.; Pavlik, E.J.; Ueland, F.R.; DePriest, P.D.; Kryscio, R.J.; van Nagell, J.R. Risk of Malignancy in Unilocular Ovarian Cystic Tumors Less than 10 Centimeters in Diameter. Obstet. Gynecol. 2003, 102, 594–599. [Google Scholar] [CrossRef]
- Gershenson, D.M. Management of Borderline Ovarian Tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 49–59. [Google Scholar] [CrossRef]
- Dason, E.S.; Maxim, M.; Sanders, A.; Papillon-Smith, J.; Ng, D.; Chan, C.; Sobel, M. Guideline No. 437: Diagnosis and Management of Adenomyosis. J. Obstet. Gynaecol. Can. 2023, 45, 417–429.e1. [Google Scholar] [CrossRef]
- Vannuccini, S.; Petraglia, F. Recent Advances in Understanding and Managing Adenomyosis. F1000Research 2019, 8, F1000 Faculty Rev-283. [Google Scholar] [CrossRef] [PubMed]
- Schrager, S.; Yogendran, L.; Marquez, C.M.; Sadowski, E.A. Adenomyosis: Diagnosis and Management. Am. Fam. Physician 2022, 105, 33–38. [Google Scholar] [PubMed]
- Kobayashi, H.; Matsubara, S. A Classification Proposal for Adenomyosis Based on Magnetic Resonance Imaging. Gynecol. Obstet. Investig. 2020, 85, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lacheta, J. Uterine Adenomyosis: Pathogenesis, Diagnostics, Symptomatology and Treatment. Ceska Gynekol. 2019, 84, 240–246. [Google Scholar] [PubMed]
- Bourdon, M.; Santulli, P.; Marcellin, L.; Maignien, C.; Maitrot-Mantelet, L.; Bordonne, C.; Plu Bureau, G.; Chapron, C. Adenomyosis: An Update Regarding Its Diagnosis and Clinical Features. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bazot, M.; Tsatoumas, M.; Munro, M.G.; Reinhold, C. MRI of Adenomyosis: Where Are We Today? Can. Assoc. Radiol. J. 2023, 74, 58–68. [Google Scholar] [CrossRef]
- Celli, V.; Dolciami, M.; Ninkova, R.; Ercolani, G.; Rizzo, S.; Porpora, M.G.; Catalano, C.; Manganaro, L. MRI and Adenomyosis: What Can Radiologists Evaluate? Int. J. Environ. Res. Public Health 2022, 19, 5840. [Google Scholar] [CrossRef]
- Nougaret, S.; Sbarra, M.; Robbins, J. Imaging Spectrum of Benign Uterine Disease and Treatment Options. Radiol. Clin. N. Am. 2020, 58, 239–256. [Google Scholar] [CrossRef]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.-W.; Just, P.-A.; Noël, J.-C.; et al. Diagnosing Adenomyosis: An Integrated Clinical and Imaging Approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef]
- Bordonné, C.; Puntonet, J.; Maitrot-Mantelet, L.; Bourdon, M.; Marcellin, L.; Dion, E.; Plu-Bureau, G.; Santulli, P.; Chapron, C. Imaging for Evaluation of Endometriosis and Adenomyosis. Minerva Obstet. Gynecol. 2021, 73, 290–303. [Google Scholar] [CrossRef]
- Song, S.E.; Sung, D.J.; Park, B.J.; Kim, M.J.; Cho, S.B.; Kim, K.A. MR Imaging Features of Uterine Adenomyomas. Abdom. Imaging 2011, 36, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Ozsarlak, O.; Schepens, E.; de Schepper, A.M.; Deckers, F.; Parizel, P.M.; Campo, R. Transient Uterine Contraction Mimicking Adenomyosis on MRI. Eur. Radiol. 1998, 8, 54–56. [Google Scholar] [CrossRef]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Daraï, E. Role of Transvaginal Sonography and Magnetic Resonance Imaging in the Diagnosis of Uterine Adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef]
- Manta, L.; Suciu, N.; Constantin, A.; Toader, O.; Popa, F. Focal Adenomyosis (Intramural Endometriotic Cyst) in a very Young Patient—Differential Diagnosis with Uterine Fibromatosis. J. Med. Life 2016, 9, 180–182. [Google Scholar] [PubMed]
- Takeuchi, M.; Matsuzaki, K.; Harada, M. MR Manifestations of Uterine Polypoid Adenomyoma. Abdom. Imaging 2015, 40, 480–487. [Google Scholar] [CrossRef]
- Andres, M.P.; Arcoverde, F.V.L.; Souza, C.C.C.; Fernandes, L.F.C.; Abrão, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef]
- Chamié, L.P.; Ribeiro, D.M.F.R.; Tiferes, D.A.; de Macedo Neto, A.C.; Serafini, P.C. Atypical Sites of Deeply Infiltrative Endometriosis: Clinical Characteristics and Imaging Findings. Radiographics 2018, 38, 309–328. [Google Scholar] [CrossRef]
- Raffi, L.; Suresh, R.; McCalmont, T.H.; Twigg, A.R. Cutaneous Endometriosis. Int. J. Women Dermatol. 2019, 5, 384–386. [Google Scholar] [CrossRef]
- Youssef, A.T. The Ultrasound of Subcutaneous Extrapelvic Endometriosis. J. Ultrason. 2020, 20, e176–e180. [Google Scholar] [CrossRef]
- Dalkalitsis, A.; Salta, S.; Tsakiridis, I.; Dagklis, T.; Kalogiannidis, I.; Mamopoulos, A.; Daniilidis, A.; Athanasiadis, A.; Navrozoglou, I.; Paschopoulos, M.; et al. Inguinal Endometriosis: A Systematic Review. Taiwan. J. Obstet. Gynecol. 2022, 61, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, R.; Castorina, S.; Castorina, E.G.; Panarello, A.; Antoci, S.A.M. Thrombosis of Iliac Vessels, a Rare Complication of Endometriosis: Case Report and Review of Literature. J. Adv. Res. 2017, 8, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, M.; Chen, K.; Fong, Y.F. A Rare Case of Endometriosis Invading External Iliac Vein Causing Deep Vein Thrombosis. Am. J. Obstet. Gynecol. 2019, 220, 113–114. [Google Scholar] [CrossRef]
- Ferrero, S.; Stabilini, C.; Barra, F.; Clarizia, R.; Roviglione, G.; Ceccaroni, M. Bowel Resection for Intestinal Endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Lindheim, S.R.; Backhus, L.; Vu, M.; Vang, N.; Nezhat, A.; Nezhat, C. Thoracic Endometriosis Syndrome: A Review of Diagnosis and Management. JSLS 2019, 23, e2019.00029. [Google Scholar] [CrossRef]
- Elefante, C.; Brancati, G.E.; Oragvelidze, E.; Lattanzi, L.; Maremmani, I.; Perugi, G. Psychiatric Symptoms in Patients with Cerebral Endometriosis: A Case Report and Literature Review. J. Clin. Med. 2022, 11, 7212. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of Endometriosis: A Review with Comparison of 8 Guidelines. BMC Women Health 2021, 21, 397. [Google Scholar] [CrossRef]
- Bonavina, G.; Taylor, H.S. Endometriosis-Associated Infertility: From Pathophysiology to Tailored Treatment. Front. Endocrinol. 2022, 13, 1020827. [Google Scholar] [CrossRef]
- Guerra, A.; Daraï, E.; Osório, F.; Setúbal, A.; Bendifallah, S.; Loureiro, A.; Thomassin-Naggara, I. Imaging of Postoperative Endometriosis. Diagn. Interv. Imaging 2019, 100, 607–618. [Google Scholar] [CrossRef]
- Chamié, L.P.; Ribeiro, D.M.F.R.; Ribeiro, G.M.P.A.R.; Serafini, P.C. Postoperative Imaging Findings after Laparoscopic Surgery for Deeply Infiltrating Endometriosis. Abdom. Radiol. 2020, 45, 1847–1865. [Google Scholar] [CrossRef]
- Tang, X.; Ling, R.; Gong, J.; Mei, D.; Luo, Y.; Li, M.; Xu, J.; Ma, L. Deep Infiltrating Endometriosis MR Imaging with Surgical Correlation. Quant. Imaging Med. Surg. 2018, 8, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Darici, E.; Salama, M.; Bokor, A.; Oral, E.; Dauser, B.; Hudelist, G. Different Segmental Resection Techniques and Postoperative Complications in Patients with Colorectal Endometriosis: A Systematic Review. ACTA Obstet. Gynecol. Scand. 2022, 101, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Burnett, T.L.; Feldman, M.K.; Huang, J.Q. The Role of Imaging as a Guide to the Surgical Treatment of Endometriosis. Abdom. Radiol. 2020, 45, 1840–1846. [Google Scholar] [CrossRef]
- Surgical Therapy of Ovarian Endometrioma: Recurrence and Pregnancy Rates—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25392619/ (accessed on 19 July 2023).
- Singh, S.S.; Gude, K.; Perdeaux, E.; Gattrell, W.T.; Becker, C.M. Surgical Outcomes in Patients with Endometriosis: A Systematic Review. J. Obstet. Gynaecol. Can. 2020, 42, 881–888.e11. [Google Scholar] [CrossRef] [PubMed]




| Localization | MRI Sign | MRI Findings | |
|---|---|---|---|
| Ovary | Ovarian endometrioma | T1 high signal multiplicity | Multiple high signal cyst on T1WI |
| T2 shading | Marked gradation or shading on T2WI | ||
| T2 dark spot sign | Well-defined markedly hypointense foci within the cyst on T2WI | ||
| Deep infiltrating endometriosis | Posterior cul-de-sac-obliteration | Hypointense nodular lesions (T1/T2WI) | |
| Soft tissue thickening with irregular, indistinct or stellate margins (T1/T2WI) | |||
| Elevated posterior vaginal fornix on T1/T2WI | |||
| Intestinal /rectum tethering towards uterus on T2WI | |||
| Fibrotic plaques or nodules covering the serosal surface of the uterus on T2WI | |||
| Retrouterine fibrous mass on T2WI | |||
| Torus uterinus | T2WI low-intensity thickening or mass with regular/irregular margins | ||
| Ovary | Kissing ovary | Bilateral ovaries located on top of the uterus | |
| Round ligament | Thickened and irregular with a nodular appearance | ||
| Rectosigmoid colon | Mushroom cap | Focal thickening of the rectal wall | |
| Bladder vescico-uterine space | Hypointense nodules on T1/T2WI | ||
| Extra pelvic endometriosis | Abdominal wall | Hypointense solid mass on T2WI with/Without hyperintense hemorrhagic cyst on T1WI | |
| Diaphragmatic | Hyperintense nodules on T1WI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccolo, C.L.; Cea, L.; Sbarra, M.; De Nicola, A.M.; De Cicco Nardone, C.; Faiella, E.; Grasso, R.F.; Beomonte Zobel, B. Magnetic Resonance Roadmap in Detecting and Staging Endometriosis: Usual and Unusual Localizations. Appl. Sci. 2023, 13, 10509. https://doi.org/10.3390/app131810509
Piccolo CL, Cea L, Sbarra M, De Nicola AM, De Cicco Nardone C, Faiella E, Grasso RF, Beomonte Zobel B. Magnetic Resonance Roadmap in Detecting and Staging Endometriosis: Usual and Unusual Localizations. Applied Sciences. 2023; 13(18):10509. https://doi.org/10.3390/app131810509
Chicago/Turabian StylePiccolo, Claudia Lucia, Laura Cea, Martina Sbarra, Anna Maria De Nicola, Carlo De Cicco Nardone, Eliodoro Faiella, Rosario Francesco Grasso, and Bruno Beomonte Zobel. 2023. "Magnetic Resonance Roadmap in Detecting and Staging Endometriosis: Usual and Unusual Localizations" Applied Sciences 13, no. 18: 10509. https://doi.org/10.3390/app131810509
APA StylePiccolo, C. L., Cea, L., Sbarra, M., De Nicola, A. M., De Cicco Nardone, C., Faiella, E., Grasso, R. F., & Beomonte Zobel, B. (2023). Magnetic Resonance Roadmap in Detecting and Staging Endometriosis: Usual and Unusual Localizations. Applied Sciences, 13(18), 10509. https://doi.org/10.3390/app131810509








