Abstract
The objective of this study was to analyze the perception of visual verticality (VV) in subjects with Fibromyalgia Syndrome (FMS) and to correlate this with the symptoms of the disease and balance capacity. A cross-sectional study including 54 patients (51 female) was conducted. The evaluation of visual verticality was carried out with a virtual reality device by calculating the Mean Absolute Error (MAE) of degrees deviation in two tests: the Subjective Visual Vertical (SVV) test for the contribution of the vestibular system to the perception of verticality and the Rod and Frame test (RFT) for the contribution of the visual system. In total, 16 subjects (29.6%) presented good VV perception, 6 subjects (11.1%) presented an exclusive alteration of the SVV test, 19 subjects (35.2%) presented an exclusive alteration of the RFT and 13 subjects (24.1%) showed alteration in the two tests. The MAE in the SVV test showed medium correlations with several variables such as the Fibromyalgia Impact Questionnaire (FIQ) (Rho = 0.399, p = 0.003), the Pain Catastrophizing Scale (PCS) (Rho = 0.417, p = 0.002), the Dizziness Handicap Inventory (DHI) (Rho = 0.376, p = 0.005), and the Activities-Specific Balance Confidence Scale (ABC-16) (Rho = −0.367, p = 0.006). The MAE in the RFT showed medium correlations with the Instability Support Reduced (Rho = 0.327, p = 0.016) and Instability Gait Eyes Open (Rho = 0.312, p = 0.022) subscales of the JAEN (Joined Assessment of Equilibrium and Neuro-motor) Scale. Conclusions: Around 70% of the subjects with FMS showed some alteration in the perception of VV; 60% of these patients presented visual system dependence. The SVV test correlates with the impact and health status of FMS, and the RFT correlates with the alteration in the dynamic balance.
1. Introduction
Fibromyalgia Syndrome (FMS) is a highly disabling chronic condition for the affected patients, the main manifestation of which is generalized and persistent musculoskeletal pain [1]. FMS is accompanied by multiple symptoms, including fatigue, morning stiffness, sleep disorders, altered mood, cognitive difficulties, sensory hypersensitivity, headaches, migraines, or balance disturbances [2,3], which, together with pain underlying the chronic disease, cause a substantial worsening of the perceived quality of life, as well as a functional deterioration of the activities of daily living. Likewise, this loss of function has been shown to be highly associated with work disability in some of these patients [4].
FMS affects 2.1% of the general population worldwide, with a greater presence among women (4:1) and a higher prevalence in Europe (2.64%) than in the American continent (2.41%) and individuals from the eastern Pacific (1.62%) [5]. FMS is considered the third most common musculoskeletal condition in terms of prevalence, after low back pain (14.8%) and osteoarthritis (10.2%) [5,6]. However, with regard to the functionality of the patient, FMS is considered the second musculoskeletal disease with the greatest functional impairment, second only to rheumatoid arthritis [5].
The literature shows a wide variety of potential pathophysiological factors to respond to the origin of FMS, betting on a multifactorial origin [7]. Genetic predisposition, the presence of environmental triggers, and alterations in the modulation and processing of sensory information are the pathophysiological mechanisms with the greatest evidence [2,7]. However, the view most shared by the scientific community is that the phenomenon of central sensitization (CS) is the main underlying mechanism and perpetuation factor of the FMS symptomatology [2,3,7]. CS has also been proposed as a possible reason for medically unexplained symptoms [8], among which is chronic dizziness. This association is since vestibular, visual, and somatosensory information processing can be affected by the mechanisms of CS perpetuation [9].
The perception of chronic dizziness is one of the most reported complaints by patients with FMS, being manifested in about 64% of the cases [10]. Visually induced vertigo has been suggested to be one of the mechanisms responsible for the central dizziness disorder of FMS [10]. Instability or staggering, clumsiness while moving or carrying out activities of daily living, tinnitus, photosensitivity, “mental fog”, migraine, as well as the vegetative courtship response such as nausea or stomach upset, are other complaints that are referred to frequently by those who have this disease [10,11].
The perception of verticality is essential in all conditions in which an individual has to stabilize, such as when standing, walking, and in most motor activities [12,13]. The most used methods to analyze the perception of visual verticality (VV) in clinical practice and research is the Subjective Visual Vertical (SVV) test [14], which provides information on the contribution of the vestibular system to the perception of VV. Likewise, the Rod and Frame Test (RFT) [15] assesses the contribution of the visual system to the perception of VV at the same time that it classifies the subjects as visually dependent or independent. Recent studies have reported that FMS patients appear to have poor vestibular and visual scores in balances tests and showed that static balance and gait during unsteadiness conditions with open or closed eyes are significantly worse in FMS patients and older adults than in healthy subjects [16].
The use of new technologies has grown in recent years, covering wide fields of medical sciences and especially in the fields of neurology [17], otorhinolaryngology [18], and rehabilitation [19]. The applications developed have been diverse, including artificial intelligence and virtual reality used in the Neuropsychological and Cognitive-Behavioral Assessment of Neurodegenerative Disease [20]. Evaluate MedTech consensus forecasts find that the MedTech market will achieve sales of USD 594.5bn by 2024, growing at a rate of 5.6% per year between 2017 and 2024. Neurology is again forecast to be the fastest-growing device area, with sales expected to rise to USD 15.8bn in 2024, representing 9.1% market growth per year between 2017 and 2024 [21].
The development of virtual reality instruments has allowed the measurement of the perception of VV with greater precision as well as a better standardization of the evaluation procedures [22]. The new devices based on virtual reality also offer the possibility of investigating the relationships between the variables that characterize FMS, delving into the pathophysiology of the disease. Although, as previously reported, an alteration in postural balance has been found in subjects with FMS, it remains to be determined to what extent the alteration in the perception of verticality is involved in this process. It is also not clear whether this altered perception of verticality correlates with other variables such as central sensitization, the impact of the disease, confidence in body balance, or the ability of patients to maintain static or dynamic balance.
However, and despite the importance of the perception of verticality on motor control, no study has been dedicated to the analysis of this important function in patients with Fibromyalgia. For this reason, the main objective of this study was to analyze the perception of VV in subjects with FMS and the relationships of this capacity with the signs and symptoms of the disease. Specifically, this work aimed to characterize patients with FMS based on the disorder of perception of verticality and to analyze if this dysfunction was related to the presence of falls, the impact of the disease, confidence in balance, as well as with static and dynamic postural balance.
2. Materials and Methods
2.1. Study Design
A cross-sectional study was conducted in a sample of patients diagnosed with FMS, developed in accordance with the guidelines for the communication of observational studies established in the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) Statement. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee for Biomedical Research of Andalusia PEIBA (protocol code: EPSF1). Written informed consent was obtained from all participants.
2.2. Participants
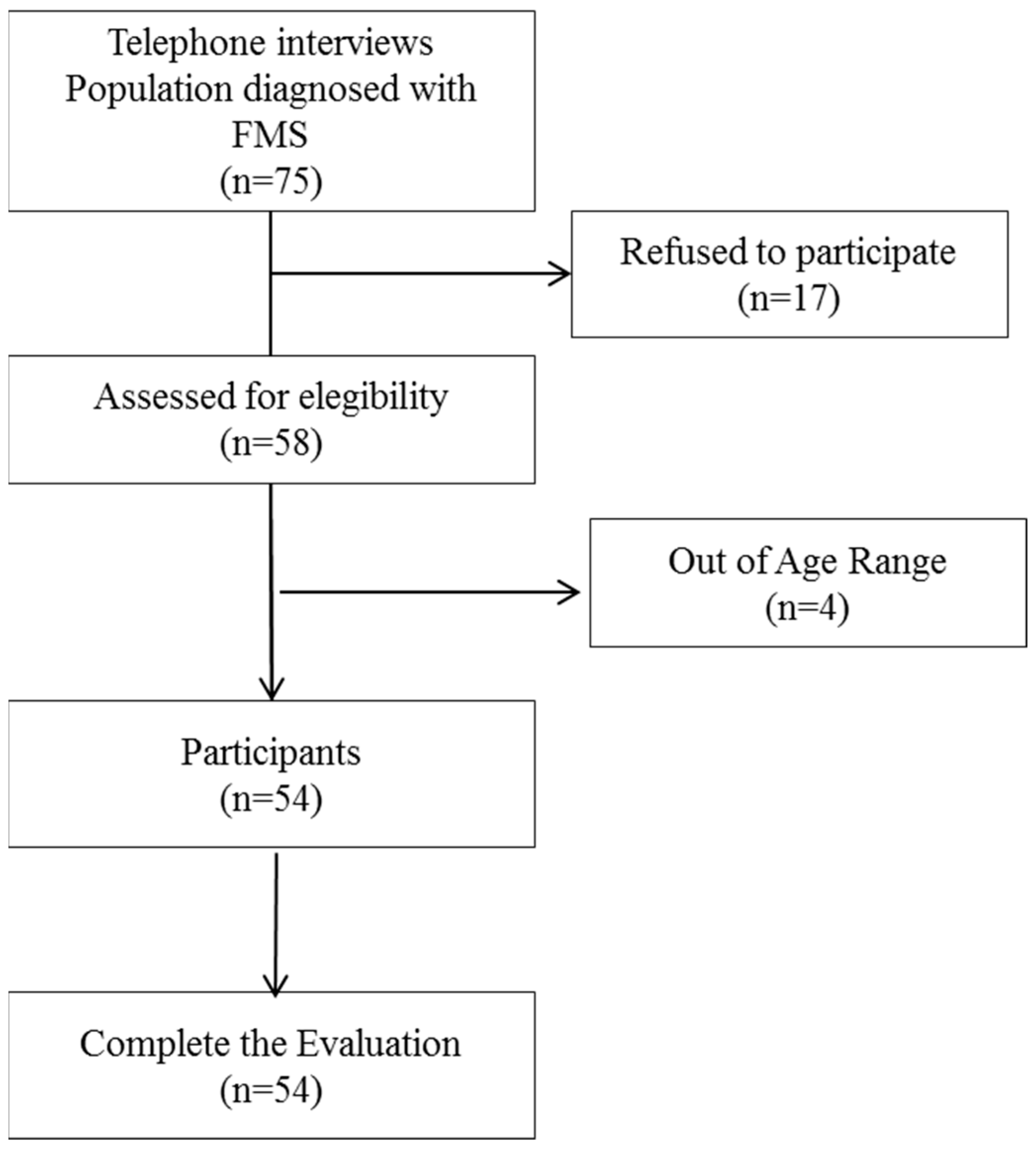
Participants were selected from the Fibromyalgia Association of Jaen (AFIXA) over a period of 6 months (April to September 2022). Telephone contact was made with 75 possible participants who received detailed information about the characteristics of the study and their participation in it; of these, 54 agreed to participate and were evaluated. A flowchart is presented in Figure 1.
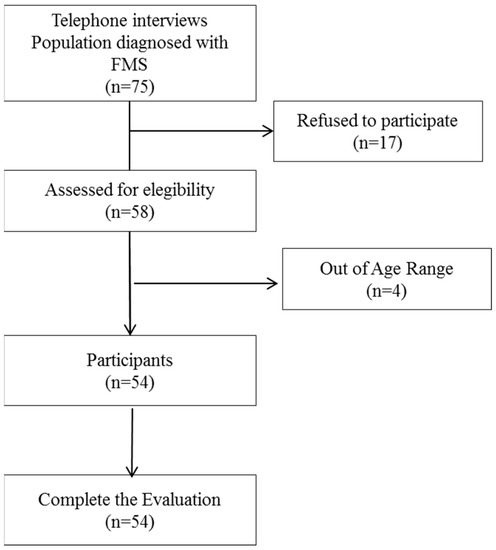
Figure 1.
Flowchart. FMS, Fibromyalgia Syndrome.
The inclusion criteria were (1) being aged between 18 and 70 years and (2) having a diagnosis of FMS according to the 2016 American College of Rheumatology (ACR) criteria. Exclusion criteria were as follows: (1) cognitive impairment impacting ability to fill out the scales and questionnaires, (2) musculoskeletal surgical intervention in the preceding 6 months and/or acute traumatic pathology to the inferior limb(s), and/or (3) musculoskeletal disease with deformity of the inferior limbs. Data collection was carried out on the premises of the University of Jaen.
2.3. Sample Size Calculation
To calculate the sample size, the Statistical Program MedCalc® Statistical Software version 20.110 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org) was used. For an expected correlation coefficient of 0.4, working with a confidence level of 95% and a statistical power of 80%, a total of 46 study subjects was necessary. Finally, 54 subjects were included to improve the reliability of the estimate.
2.4. Measurements
Demographic and anthropometric data such as gender, age, education level, occupation, weight, height, and body mass index (BMI) were collected.
2.4.1. Primary Outcome Measures
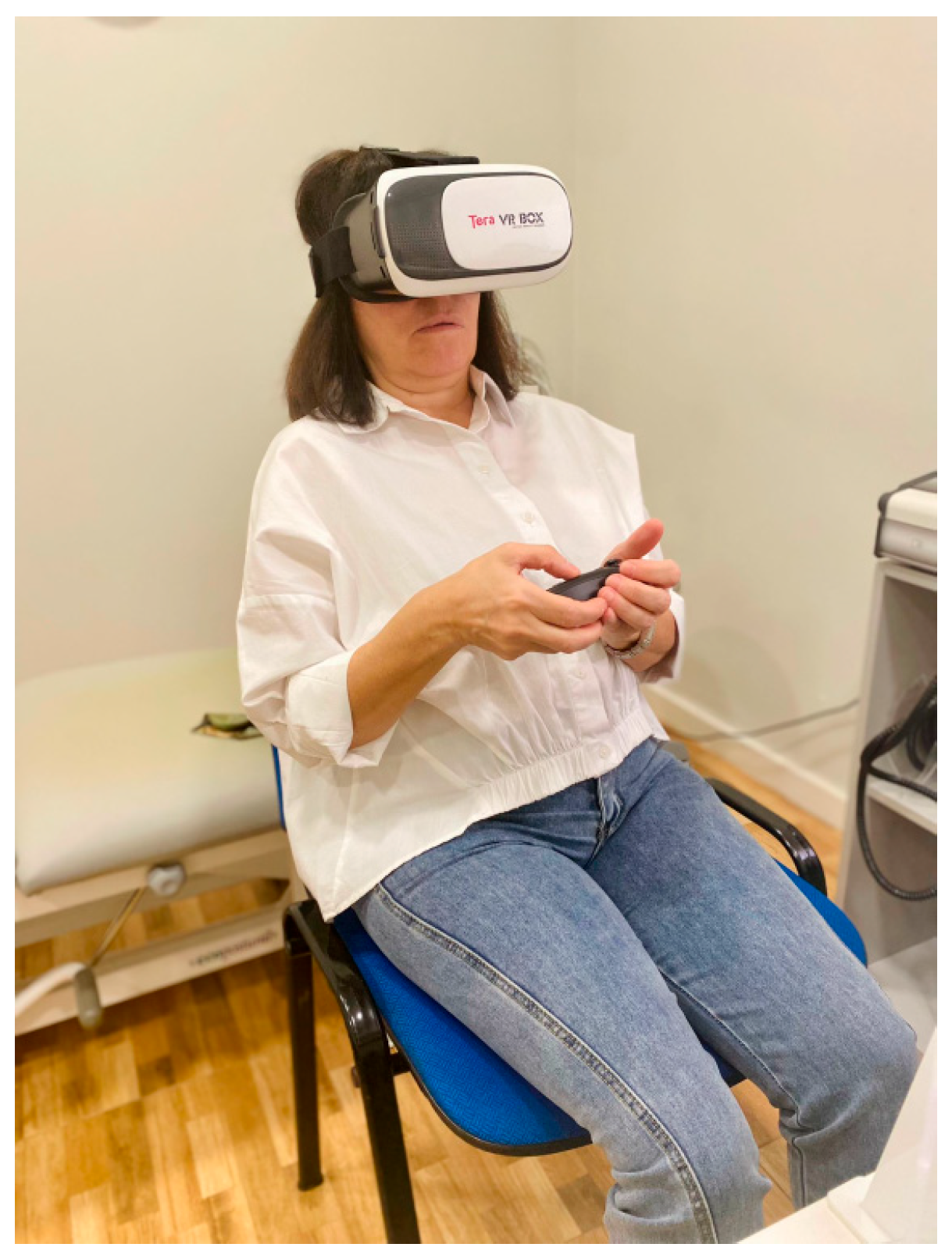
The evaluation of visual verticality was carried out with a virtual reality device. The device was previously validated and showed good reliability (Intraclass Correlation Coefficient (ICC) = 0.85; 95% confidence interval (CI) = 0.75–0.92) [23,24]. The VV measurement tool uses a Google Cardboard-compatible headset, a smartphone, and a Bluetooth joystick. An app implemented using the Google VR software development kit displays a stereoscopic view, and the head movements are captured by the smartphone’s built-in orientation sensors [23]. Here, a web application on a desktop computer was used by the evaluator in order to launch the tests on the mobile device and collect the outcomes. The subject to be evaluated was seated with their back straight, their feet parallel to the floor, and without resting their arms on the chair (Figure 2).
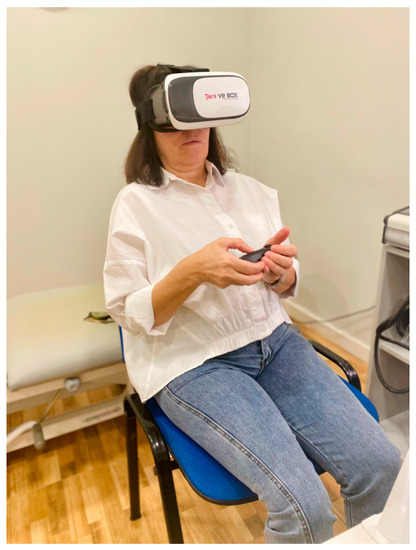
Figure 2.
Patient position.
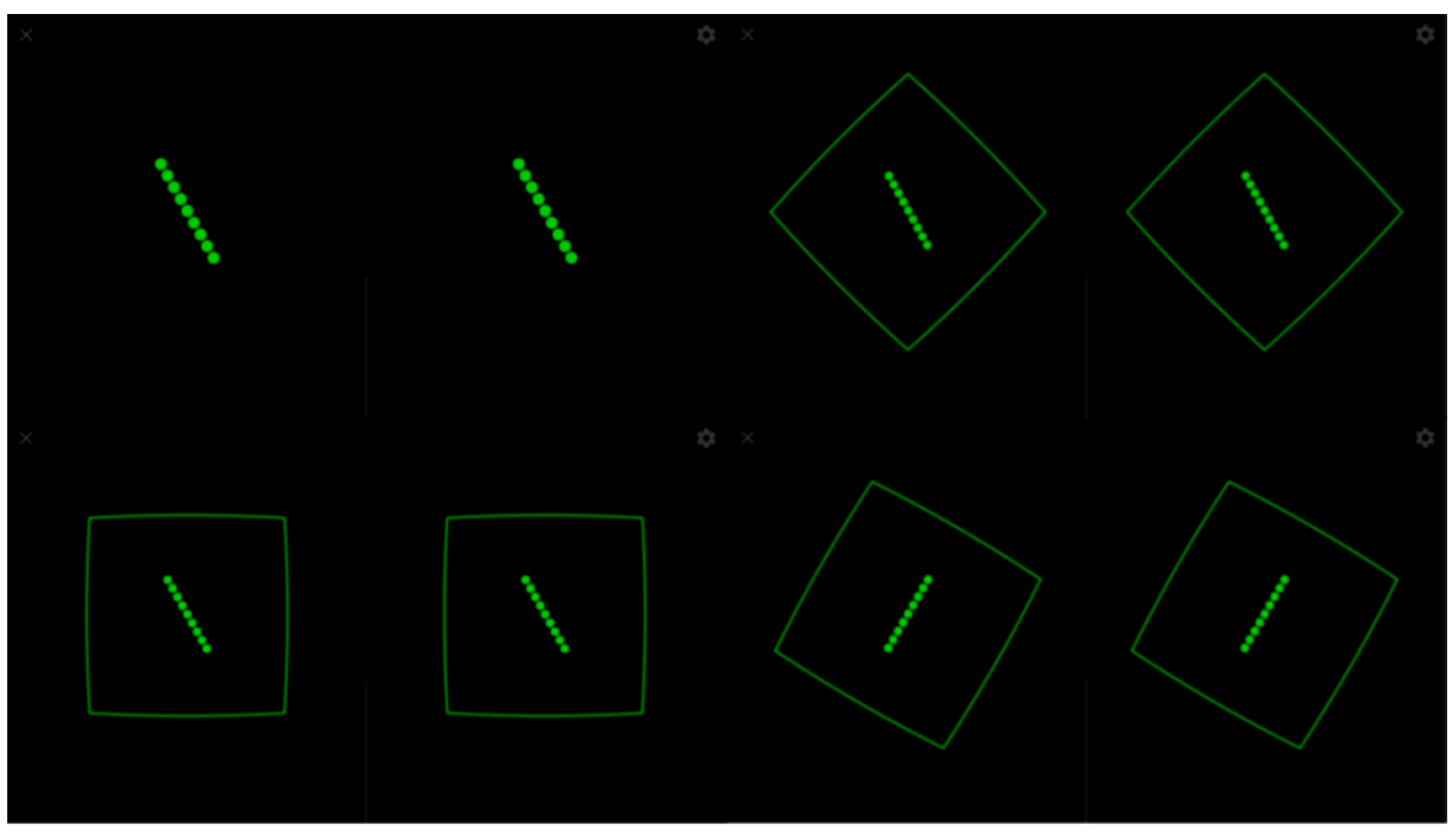
For the SVV measurement, an experiment consisting of a green line on a black background without visual references was launched (Figure 3). In line with generally accepted recommendations, 6 trials were performed in which the line appeared with an initial slope of 45°, 30°, and 15° alternately to the right and left. The subject was asked to place the green line in the vertical direction using the joystick without a time limit and pressing the trigger when they were sure that they had achieved a correct alignment. In the case of the RFT, the subject was instructed to place the green line in the vertical direction, which started from an inclination of 30° to the right and left alternately, which was projected within a frame that presented four random positions: neutral at 0° and inclinations of 15°, 30°, and 45° to the right and left, conducting a total of 14 trials (Figure 3). In both cases, the alignment error was calculated through the Mean Absolute Error (MAE), that is, the arithmetic mean of the error committed in each unsigned trial (regardless of whether the deviation was right or left). Thus, the MAE-SVV and the MAE-RFT were obtained. Deviations from the MAE-SVV of ±2.5 degrees [25] and of ±4.5 degrees for MAE-RFT [26] were established as normal values.
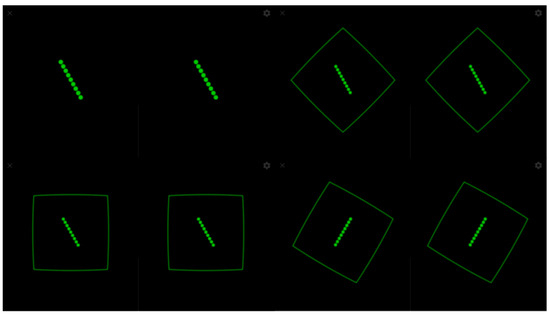
Figure 3.
SVV and RFT experiment.
2.4.2. Patient-Reported Outcome Measures (PROMs)
The number of falls suffered in the last 3 months was recorded by asking participants to answer the question, “How many falls have you suffered in the last 3 months?”, with a fall described as an “unexpected event that causes the person to fall to the ground against his or her will” [27].
To record the severity of the disease, the Fibromyalgia Impact Questionnaire (FIQ) [28] was used, with the total scores of which ranging from 0 to 100 points. The FIQ consists of 3 categories according to the level of affectation: mild vital affectation (<50 points); moderate vital affectation (50–75 points); severe vital affectation with impossibility to carry out a job or function (>75 points).
The Numerical Pain Rating Scale (NPRS) [29] was used to measure participants’ pain intensity, the scores of which range from 0 (absence of pain) to 10 (the most intense pain perceived.
The level of central sensitization was determined through total scores (0–100 points) of the Central Sensitization Inventory [30].
The level of catastrophism was assessed with the Pain Catastrophizing Scale (PCS) [31]. It consists of 13 items, with the total score ranging from 0 to 52 points, and three subscales: Pain Catastrophizing Scale Rumination, Magnification, and Hopelessness.
Fear of movement was analyzed through the Tampa Scale of Kinesiophobia (TSK) [32]. It consists of 11 items, with the total scores ranging from 11 to 44 points.
The disability perceived by vertigo or dizziness was analyzed though the Dizziness Handicap Inventory (DHI) [33]. It consists of 25 items divided into three separate subscales: the emotional (DHI-E), the functional (DHI-F), and the Physical (DHI-P) subscales. Total DHI scores range from 0 to 100 points.
The level of confidence in balance was assessed with the Activities-specific Balance Confidence Scale (ABC-16) [34]. It consists of 16 items with a scale of ascending answers from not at all confident (0%) to completely safe (100%) and a total score ranging between 0 and 100%.
Fear of falling was analyzed with the Falls Efficacy Scale International (FES-I) [35]. It consists of 16 items, with a total score ranging from 16 to 64 points. Higher scores indicate a greater fear of falling.
The level of fatigue was determined through the Fatigue Severity Scale (FSS) [36]. It consists of 9 items that analyze aspects of physical and mental fatigue, with scores ranging from 9 to 63 points, where scores ≥ 36 indicate severe fatigue.
In all cases, higher scores indicate a higher level of the corresponding variable.
2.4.3. Static and Dynamic Balance Measures
A platform with a resistive pressure sensor with a surface of 400 × 400 mm and an acquisition frequency of 40 Hz and FreeStep© Standard 3.0 software (Sensor Medica, Rome, Italy) was used to measure static balance. The procedure is described in Romero-Franco et al., 2013 [37]. The posturographic parameters were the Sway Area, the mean velocity of the displacement of the center of pressure, the dispersion parameters in the medial–lateral and posterior–anterior directions, and the mean medial–lateral and posterior–anterior oscillation.
The functional assessment of balance was carried out through the Joint Assessment of Equilibrium and Neuro-motor Scale (JAEN Scale) [16]. The screening tool consists of 20 balance tests with five response alternatives ranging from no balance problem (0 points) to a complete or total balance problem (4 points). It has four subscales of balance impairment (instability during head movement, instability when support is reduced, instability during gait with eyes open, and instability standing and walking with eyes closed), and a total score of 0 to 80 points, where a higher score indicates a higher degree of balance disorder.
2.5. Data Analysis
Data were described by means and standard deviations for continuous variables and frequencies and percentages for categorical variables. The Kolmogorov–Smirnov test was used to analyze the normal distribution of continuous variables, while the verification of homoscedasticity was carried out with the Levene test. The relationship between the variables was verified with Spearman’s Rho non-parametric Correlation Coefficient. We worked with a confidence level of 95% (alpha error less than 5%). Data were analyzed using the Statistical Program for Social Sciences Version 24.0 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY, USA: IBM Corp.).
3. Results
Of the 54 subjects who met the inclusion criteria, 51 were women, and the subjects had a mean age of 53 years (SD = 6). The sociodemographic characteristics of the sample are shown in Table 1. The static SVV test measure showed a mean MAE of 2.41 (SD = 1.36), while the RFT showed a mean MAE of 10.82 (SD = 10.16). Taking the normal values of both tests as reference, 19 subjects (35.2%) presented an altered SVV, while 32 subjects (59.3%) presented an elevated RFT. In total, 16 subjects (29.6%) presented good visual verticality measured with both tests, 6 subjects (11.1%) presented an exclusive alteration in the SVV test, 19 subjects (35.2%) presented an exclusive alteration in the RFT, and 13 subjects (24.1%) showed high values in the two tests. In other words, 38 subjects with FMS (70.4%) presented some alteration in visual verticality perception.

Table 1.
Sociodemographic characteristics of the sample.
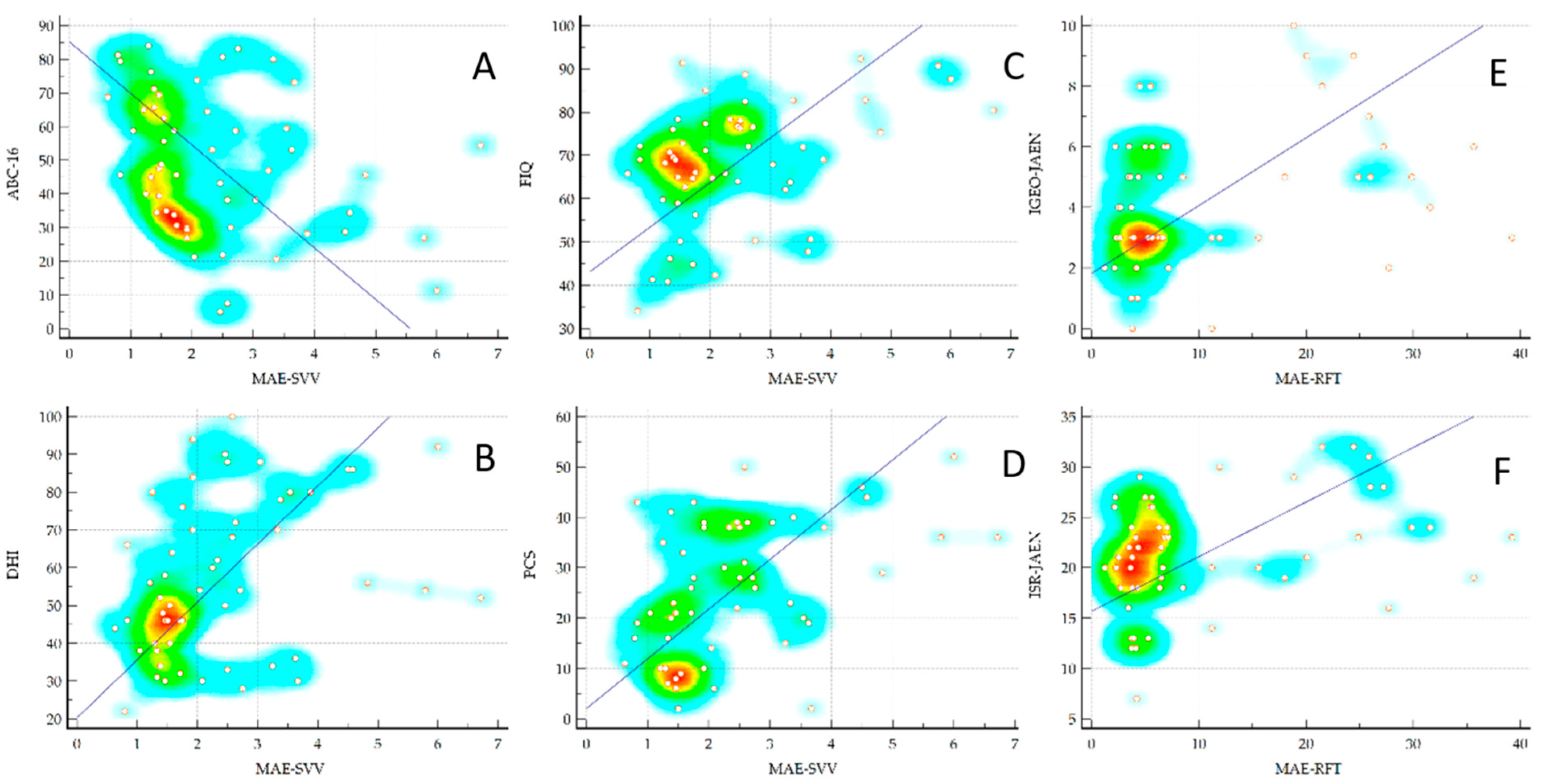
Table 2 shows the correlations between the MAE measured with the visual verticality perception tests and the PROMs. As can be seen, the MAE-SVV test showed medium but statistically significant correlations with numerous PROMs such as FIQ, PCS, DHI, and ABC-16 (Figure 4). However, the MAE-RFT values did not present any significant correlation. Table 3 shows the correlations between the MAE measured with the VV perception tests and the static and dynamic balance measures. As can be seen, the MAE-RFT showed medium but statistically significant correlations with the JAEN subscales when the base of support was reduced and during walking with eyes open (Figure 4), and a low but significant correlation with the mean of the anterior–posterior position of the CoP in the test with eyes open of the static posturography. However, the values of the SVV test did not present any significant correlation.

Table 2.
Spearman correlations between visual verticality perception test and Patient-Reported Outcome Measures.
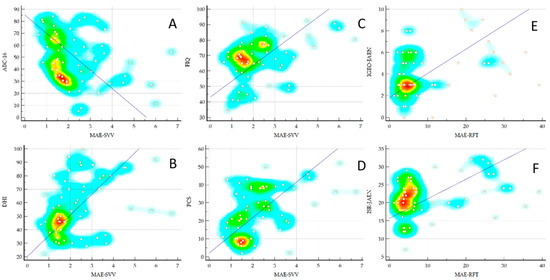
Figure 4.
Correlations between the MAE-SVV and the MAE-RFT with ABC (A), DHI (B), FIQ (C), and PCS (D), IGEO-JAEN (E) and ISR-JAEN (F), respectively. MAE, Mean Absolute Error in degrees; SVV, Subjective Visual Vertical test; RFT, Rod and Frame Test; ABC, Activities-Specific Balance Confidence Scale; DHI, Dizziness Handicap Inventory; FIQ, Fibromyalgia Impact Questionnaire; PCS, Pain Catastrophizing Scale; IGEO-JAEN, Instability Gait Eyes Open-Joint Assessment of Equilibrium and Neuro-motor Scale; ISR, Instability Support Reduced.

Table 3.
Spearman Correlations between visual verticality perception tests and static and dynamic balance measures.
4. Discussion
This work aimed to analyze the possible alteration in the perception of VV in subjects with FMS and its relationship with the signs and symptoms of the disease measured with PROMs and functional balance tests. Our results show that the most common disorder of verticality is present in 60% of these subjects and affects visual dependence measured with the RFT. However, only 35% seem to have altered verticality perception measured with the SVV test, which rather indicates a vestibular type alteration. Meanwhile, alteration in the SVV test appears to correlate with the PROM measuring function, dizziness, and balance confidence, and the RFT results appears to correlate with imbalance during challenging body positions and eyes-open gait. To the best of our knowledge, this is the first study to analyze this alteration and these relationships in subjects with FMS.
Research shows that SVV measurements are altered in both neurological [38] and vestibular patients [39], and are typically altered in peripheral vestibular disorders, especially in the acute phase, after which, the compensation mechanisms of the Central Nervous System manage to correct the deviation. The average deviation in our patients in the SVV test was 2.4º, just at the limit of what is considered a pathological deviation, which is compatible with a possible chronic and compensated vestibular problem, in which approximately one-third of the subjects would exceed the threshold of 2.5º of deviation that is considered pathological. It could be speculated that FMS is related to a possible initial peripheral vestibular disorder, although the SVV test is also altered in central disorders such as headache [40], which is presumed to have an underlying central sensitization mechanism similar to that of FMS. Against the hypothesis of central sensitization as an underlying factor is the fact that the CSI score was one of the few that did not show a correlation with the MAE-SVV.
With respect to RFT, the evidence shows high levels of visual dependence measured with this test in several groups of patients [41,42]. Regarding the main finding of our study, the alteration in the RFT values in the sample of patients with FMS could be related to a greater susceptibility in this test of the female [43] and older [44] subjects. This is relevant because we know that Fibromyalgia is a syndrome that more characteristically affects women and that it produces effects similar to aging, and even greater effects are seen when visuo-vestibular participation in maintaining body balance is analyzed [16]. On the other hand, a recent study [41] found that 41.5% of subjects with dizziness had visual dependency as measured using the RFT. Although a correlation with headache intensity could not be found in this study, 22% of subjects with dizziness had visual dependency and complained of headache, showing a certain relationship between these health problems. FMS frequently occurs with other pain conditions such as headaches (59.01%) or chronic migraines (28.8%) [45]. These pain conditions have been shown to affect disorders related to the vestibular aspect [46]. This further supports the need for the evaluation of the vestibular component in the pathophysiology of FMS [10].
Our study showed that the alteration in the RFT correlated with greater body instability. This does not have to mean that patients with FMS have an alteration similar to peripheral vestibular disorder. In fact, the study by Nair MA et al. [47] found that subjects with poor balance scores had significantly greater visual dependence, indicating that reliance on visual cues can affect balance control when this was measured in control subjects but not in a group of patients with Benign Paroxysmal Positional Vertigo (BPPV).
On the other hand, our results are in line with the literature that states that greater visual dependence could be a response to proprioceptive deficits [48]. Alterations in the somatosensory system have been extensively studied in FMS [49,50]. This approach supports the fact that patients in our study with altered RFT scores showed impaired static postural control measured with posturography and instability during reduced base support and during walking, measured with the static balance of the JAEN scale. A correlation was found between the visual dependence measured with the RFT and the anterior–posterior oscillation of the CoP, which has also been observed in adult and young subjects with Cerebral Palsy (CP) [51]. In our study, verticality measurements were performed in a sitting position. The RFT does not seem to be affected by the subject’s position at the time of recording, but the verticality estimation error seems to increase with muscle fatigue in healthy subjects [52]. However, in our study with patients with FMS, we did not find a correlation between the severity of fatigue and MAE-RFT, although we did find a correlation with the MAE-SVV test. It can be interpreted that in healthy subjects, sudden muscle fatigue could increase visual dependence, but this does not increase with global fatigue measured with the FSS.
This study has several limitations. Firstly, and although the sample size can be considered sufficient, a larger sample would allow more specific questions to be addressed through analyses of patient subgroups. On the other hand, the very specific geographical location of the patients included does not allow us to draw conclusions that can be extrapolated to other populations. In addition, in our case, the MAE was measured during the SVV test and RFT, with no data on other tests and other measures different from those obtained in this study that could enrich the results and conclusions in future studies.
5. Conclusions
In view of the results of this study, it can be concluded that around 70% of patients with FMS presented an alteration in the perception of VV: 11.1% presented an exclusive alteration in the SVV test, 35.2% an exclusive alteration in the RFT, and 24.1% in the two tests. Therefore, in approximately 60% of these patients, the disorder was detected using the RFT, which suggests that this percentage of patients present visual system dependence to the perception of verticality. Additionally, in approximately 35% of these patients, the disorder was detected using the SVV test, which means the possible alteration in the vestibular contribution to the perception of verticality.
The MAE of degrees deviation measured with the SVV test showed a moderate correlation with vertigo disability measured with the DHI, with confidence in balance measured with the ABC-16, with catastrophizing measured with the PCS and with the impact of the disease measured with the FIQ. For its part, the MAE of degrees deviation measured during the RFT presented a low correlation with the mean of the anterior–posterior position of the center of pressure with eyes open of the static posturography parameters and a moderate correlation with the dynamic balance measured with the JAEN scale, specifically with the subscales of Instability Support Reduced and Instability Gait Eyes Open.
These findings show the presence of a poor perception of verticality during the SVV test and RFT in patients with FMS. This could contribute to a better understanding of the pathophysiological mechanisms that are present in the alteration in the balance of these patients.
Author Contributions
Conceptualization, R.L.-V. and M.C.O.-P.; methodology, R.L.-V. and M.C.O.-P.; software, C.J.O.-A. and R.J.S.-S.; validation, A.J.R.-R., C.J.O.-A. and R.J.S.-S.; formal analysis, R.L.-V.; investigation, A.B.P.-R. and A.J.R.-R.; resources, C.J.O.-A., R.L.-V. and M.C.O.-P.; data curation, A.B.P.-R. and M.C.O.-P.; writing—original draft preparation, R.L.-V. and M.C.O.-P.; writing—review and editing, R.J.S.-S.; visualization, C.J.O.-A. and A.J.R.-R.; supervision, A.B.P.-R. and R.L.-V.; project administration, R.L.-V. and M.C.O.-P.; funding acquisition, R.L.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Project FEDER reference 1263880. The funding source had no involvement in the study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Ethics Committee for Biomedical Research of Andalusia PEIBA (protocol code: EPSF1).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors would like to thank the AFIXA (Asociación de Fibromialgia de Jaén) association and especially all of the participants in this study for their insightful contributions.
Conflicts of Interest
The authors Carlos Ogáyar-Anguita, Rafael Jesús Segura-Sánchez, Antonio Jesús Rueda-Ruiz, and Rafael Lomas-Vega own 14% each of the intellectual property rights of the software used to measure verticality. Said software is not commercialized yet.
References
- Wolfe, F.; Walitt, B. Culture, science and the changing nature of fibromyalgia. Nat. Rev. Rheumatol. 2013, 9, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, etiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.D.S.; Gamundí, A.; Miranda, J.G.V.; França, L.G.S.; De Santana, C.N.; Montoya, P. Altered Functional Performance in Patients with Fibromyalgia. Front. Hum. Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Cabo-Meseguer, A.; Cerdá-Olmedo, G.; Trillo-Mata, J.L. Fibromyalgia: Prevalence, epidemiologic profiles and economic costs. Med. Clin. 2017, 149, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Afshari, M.; Moosazadeh, M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol. Int. 2017, 37, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Gyorfi, M.; Rupp, A.; Abd-Elsayed, A. Fibromyalgia Pathophysiology. Biomedicines 2022, 10, 3070. [Google Scholar] [CrossRef]
- Boer, C.D.; Dries, L.; Terluin, B.; Van Der Wouden, J.C.; Blankenstein, A.H.; Van Wilgen, C.P.; Lucassen, P.J.; Van Der Horst, H.E. Central Sensitization in Chronic Pain and Medically Unexplained Symptom Research: A Systematic Review of Definitions, Operationalizations and Measurement Instruments. J. Psychosom. Res. 2019, 117, 32–40. [Google Scholar] [CrossRef]
- Hashimoto, K.; Takeuchi, T.; Ueno, T.; Suka, S.; Hiiragi, M.; Yamada, M.; Koyama, A.; Nakamura, Y.; Miyakoda, J.; Hashizume, M. Effect of Central Sensitization on Dizziness-Related Symptoms of Persistent Postural-Perceptual Dizziness. Biopsychosoc. Med. 2022, 16, 7. [Google Scholar] [CrossRef]
- Mucci, V.; Demori, I.; Rapallo, F.; Molinari, E.; Losacco, S.; Marinelli, L.; Browne, C.J.; Burlando, B. Vestibular Disability/Handicap in Fibromyalgia: A Questionnaire Study. J. Clin. Med. 2022, 11, 4017. [Google Scholar] [CrossRef]
- Klein, A.; Schankin, C.J. Visual snow syndrome, the spectrum of perceptual disorders, and migraine as a common risk factor: A narrative review. Headache 2021, 61, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Funabashi, M.; Santos-Pontelli, T.; Colafemina, J.; Pavan, T.; Carneiro, A.; Takayanagui, O. A new method to analyze the subjective visual vertical in patients with bilateral vestibular dysfunction. Clinics 2012, 67, 1127–1131. [Google Scholar] [CrossRef]
- De Winkel, K.N.; Edel, E.; Happee, R.; Bülthoff, H.H. Multisensory Interactions in Head and Body Centered Perception of Verticality. Front. Neurosci. 2021, 14, 599226. [Google Scholar] [CrossRef] [PubMed]
- Piscicelli, C.; Nadeau, S.; Barra, J.; Pérennou, D. Assessing the Visual Vertical: How Many Trials Are Required? BMC Neurol. 2015, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Isableu, B.; Gueguen, M.; Fourré, B.; Giraudet, G.; Amorim, M.-A. Assessment of visual field dependence: Comparison between the mechanical 3D rod-and-frame test developed by Oltman in 1968 with a 2D computer-based version. J. Vestib. Res. 2009, 18, 239–247. [Google Scholar] [CrossRef]
- Lomas-Vega, R.; Rodríguez-Almagro, D.; Peinado-Rubia, A.B.; Zagalaz-Anula, N.; Molina, F.; Obrero-Gaitán, E.; Ibáñez-Vera, A.J.; Osuna-Pérez, M.C. Joint Assessment of Equilibrium and Neuromotor Function: A Validation Study in Patients with Fibromyalgia. Diagnostics 2020, 10, 1057. [Google Scholar] [CrossRef]
- Manera, V.; Rovini, E.; Wais, P.E. Editorial: Early Detection of Neurodegenerative Disorders Using Behavioral Markers and New Technologies: New Methods and Perspectives. Front. Aging Neurosci. 2023, 15, 1149886. [Google Scholar] [CrossRef]
- Daikhes, N.A. The Interdisciplinary Approach and New Technologies in the Scientific and Clinical Development of Otorhinolaryngology. Her. Russ. Acad. Sci. 2021, 91, 438–444. [Google Scholar] [CrossRef]
- Realdon, O.; Adorni, R.; Ginelli, D.; Micucci, D.; Blasi, V.; Bellavia, D.; Schettini, F.; Carradore, R.; Polsinelli, P.; D’Addario, M.; et al. Embedding the Patient-Citizen Perspective into an Operational Framework for the Development and the Introduction of New Technologies in Rehabilitation Care: The Smart&Touch-ID Model. Healthcare 2023, 11, 1604. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Stasolla, F.; Seinfeld, S.; Caffò, A.O.; Banakou, D.; Bottiroli, S. Editorial: Neuropsychological and Cognitive-Behavioral Assessment of Neurodegenerative Disease and Rehabilitation Using New Technologies and Virtual Reality. Front. Psychol. 2021, 12, 691909. [Google Scholar] [CrossRef]
- EvaluateMedTech. World Preview 2018, Outlook to 2024. Available online: https://info.evaluategroup.com› (accessed on 16 August 2023).
- Zaleski-King, A.; Pinto, R.; Lee, G.; Brungart, D.S. Use of Commercial Virtual Reality Technology to Assess Verticality Perception in Static and Dynamic Visual Backgrounds. Ear Hear. 2019, 41, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Negrillo-Cárdenas, J.; Rueda-Ruiz, A.J.; Ogayar-Anguita, C.J.; Lomas-Vega, R.; Segura-Sánchez, R.J. A System for the Measurement of the Subjective Visual Vertical using a Virtual Reality Device. J. Med. Syst. 2018, 42, 124. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Almagro, D.; Obrero-Gaitán, E.; Lomas-Vega, R.; Zagalaz-Anula, N.; Osuna-Pérez, M.C.; Achalandabaso-Ochoa, A. New Mobile Device to Measure Verticality Perception: Results in Young Subjects with Headaches. Diagnostics 2020, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Piscicelli, C.; Pérennou, D. Visual verticality perception after stroke: A systematic review of methodological approaches and suggestions for standardization. Ann. Phys. Rehabil. Med. 2017, 60, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Bagust, J.; Docherty, S.; Haynes, W.; Telford, R.; Isableu, B. Changes in Rod and Frame Test Scores Recorded in Schoolchildren during Development–A Longitudinal Study. PLoS ONE 2013, 8, e65321. [Google Scholar] [CrossRef] [PubMed]
- Meireles, S.A.; Antero, D.C.; Kulczycki, M.M.; Skare, T.L. Prevalence of Falls in Fibromyalgia Patients. Acta Ortop. Bras. 2014, 22, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Gonzalez, T. The Fibromyalgia Impact Questionnaire: A Validated Spanish Version to Assess the Health Status in Women with Fibromyalgia. Clin. Exp. Rheumatol. 2004, 22, 554–560. [Google Scholar]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of Four Pain Intensity Rating Scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.I.; Neblett, R.; Gatchel, R.J.; Roldán-Jiménez, C. Cross-Cultural Adaptation and Validity of the Spanish Fear-Avoidance Components Scale and Clinical Implications in Primary Care. BMC Fam. Pract. 2020, 21, 44. [Google Scholar] [CrossRef]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validación de La Versión Española de La Escala de La Catastrofización Ante El Dolor (Pain Catastrophizing Scale) En La Fibromialgia. Med. Clín. 2008, 131, 487–492. [Google Scholar] [CrossRef]
- Gómez-Pérez, L.; López-Martínez, A.E.; Ruiz-Párraga, G.T. Psychometric Properties of the Spanish Version of the Tampa Scale for Kinesiophobia (TSK). J. Pain. 2011, 12, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Pérez, N.; Garmendia, I.; Martín, E.L.; García-Tapia, R. Cultural Adaptation of 2 Questionnaires for Health Measurement in Patients with Vertigo. Acta Otorrinolaringol. Esp. 2000, 51, 572–580. [Google Scholar] [PubMed]
- Montilla-Ibáñez, A.; Martínez-Amat, A.; Lomas-Vega, R.; Cruz-Díaz, D.; la Torre-Cruz, M.J.D.; Casuso-Pérez, R.; Hita-Contreras, F. The Activities-Specific Balance Confidence Scale: Reliability and Validity in Spanish Patients with Vestibular Disorders. Disabil. Rehabil. 2016, 39, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Lomas-Vega, R.; Hita-Contreras, F.; Mendoza, N.; Martínez-Amat, A. Cross-Cultural Adaptation and Validation of the Falls Efficacy Scale International in Spanish Postmenopausal Women. Menopause 2012, 19, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Téllez, N.; Río, J.; Tintoré, M.; Nos, C.; Galán, I.; Montalban, X. Does the Modified Fatigue Impact Scale Offer a More Comprehensive Assessment of Fatigue in MS? Mult. Scler. 2005, 11, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Romero-Franco, N.; Martínez-López, E.J.; Lomas-Vega, R.; Hita-Contreras, F.; Osuna-Pérez, M.C.; Martínez-Amat, A. Short-Term Effects of Proprioceptive Training with Unstable Platform on Athletes’ Stabilometry. J. Strength. Cond. Res. 2013, 27, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Embrechts, E.; Van Der Waal, C.; Anseeuw, D.; Van Buijnderen, J.; Leroij, A.; Lafosse, C.; Nijboer, T.C.W.; Truijen, S.; Saeys, W. Association between Spatial Neglect and Impaired Verticality Perception after Stroke: A Systematic Review. Ann. Phys. Rehabil. Med. 2023, 66, 101700. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.M.; De Carvalho Lopes, K.; De Abreu E Silva Grigol, T.A.; Ganança, M.M.; Caovilla, H.H. Subjective Visual Vertical and Vestibular Evoked Myogenic Potential in Meniere’s Disease. Braz. J. Otorhinolaryngol. 2023, 89, 485–493. [Google Scholar] [CrossRef]
- Al-Sharif, D.S.; Roehm, P.; Lindemann, T.L.; Dumenci, L.; Keshner, E.A. Visual-Vestibular Mismatch Correlates with Headache. J. Vestib. Res. 2021, 31, 173–180. [Google Scholar] [CrossRef]
- Lord, S.R.; Webster, I. Visual Field Dependence in Elderly Fallers and Non-Fallers. Int. J. Aging Hum. Dev. 1990, 31, 267–277. [Google Scholar] [CrossRef]
- Hafström, A.; Modig, F.; Karlberg, M.; Fransson, P.-A. Increased Visual Dependence and Otolith Dysfunction with Alcohol Intoxication. Neuroreport 2007, 18, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Willey, C.R.; Liu, Z. Re-Assessing the Role of Culture on the Visual Orientation Perception of the Rod and Frame Test. PLoS ONE 2022, 17, e0276393. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Razzak, R.; Bagust, J. Perceptual Lateralization on the Rod-And-Frame Test in Young and Older Adults. Appl. Neuropsychol. Adult 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, M.; Federici, A.; Serpino, C.; Vecchio, E.; Franco, G.; Sardaro, M.; Delussi, M.; Livrea, P. Clinical Features of Headache Patients with Fibromyalgia Comorbidity. J. Headache Pain. 2011, 12, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H.; Oh, S.-Y.; Kim, H.; Koo, J.-W.; Kim, J.S. Vestibular Dysfunction in Migraine: Effects of Associated Vertigo and Motion Sickness. J. Neurol. 2009, 257, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.A.; Mulavara, A.P.; Bloomberg, J.J.; Sangi-Haghpeykar, H.; Cohen, H.S. Visual Dependence and Spatial Orientation in Benign Paroxysmal Positional Vertigo. J. Vestib. Res. 2018, 27, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Benito-Orejas, J.I.; Ramírez-Salas, J.E. Rehabilitación vestibular en la dependencia visual y somatosensorial. Rev. ORL 2019, 11, 79. [Google Scholar] [CrossRef]
- Toprak Celenay, S.; Mete, O.; Coban, O.; Oskay, D.; Erten, S. Trunk Position Sense, Postural Stability, and Spine Posture in Fibromyalgia. Rheumatol. Int. 2019, 39, 2087–2094. [Google Scholar] [CrossRef]
- Reddy, R.S.; Meziat-Filho, N.; De Sá Ferreira, A.; Tedla, J.S.; Kandakurti, P.K.; Kakaraparthi, V.N. Comparison of Neck Extensor Muscle Endurance and Cervical Proprioception between Asymptomatic Individuals and Patients with Chronic Neck Pain. J. Bodyw. Mov. Ther. 2021, 26, 180–186. [Google Scholar] [CrossRef]
- Yu, Y.; Lauer, R.T.; Tucker, C.A.; Thompson, E.D.; Keshner, E.A. Visual Dependence Affects Postural Sway Responses to Continuous Visual Field Motion in Individuals with Cerebral Palsy. Dev. Neurorehabil 2018, 21, 531–541. [Google Scholar] [CrossRef]
- Gosselin, G.; Fagan, M.J. Effects of Cervical Muscle Fatigue on the Perception of the Subjective Vertical and Horizontal. SpringerPlus 2014, 3, 78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).