Histogram-Based Analysis of Low- and High-Grade Glioma and Its Surrounding Edema Using Arterial Spin Labeling Magnetic Resonance Imaging
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Pathological Diagnosis
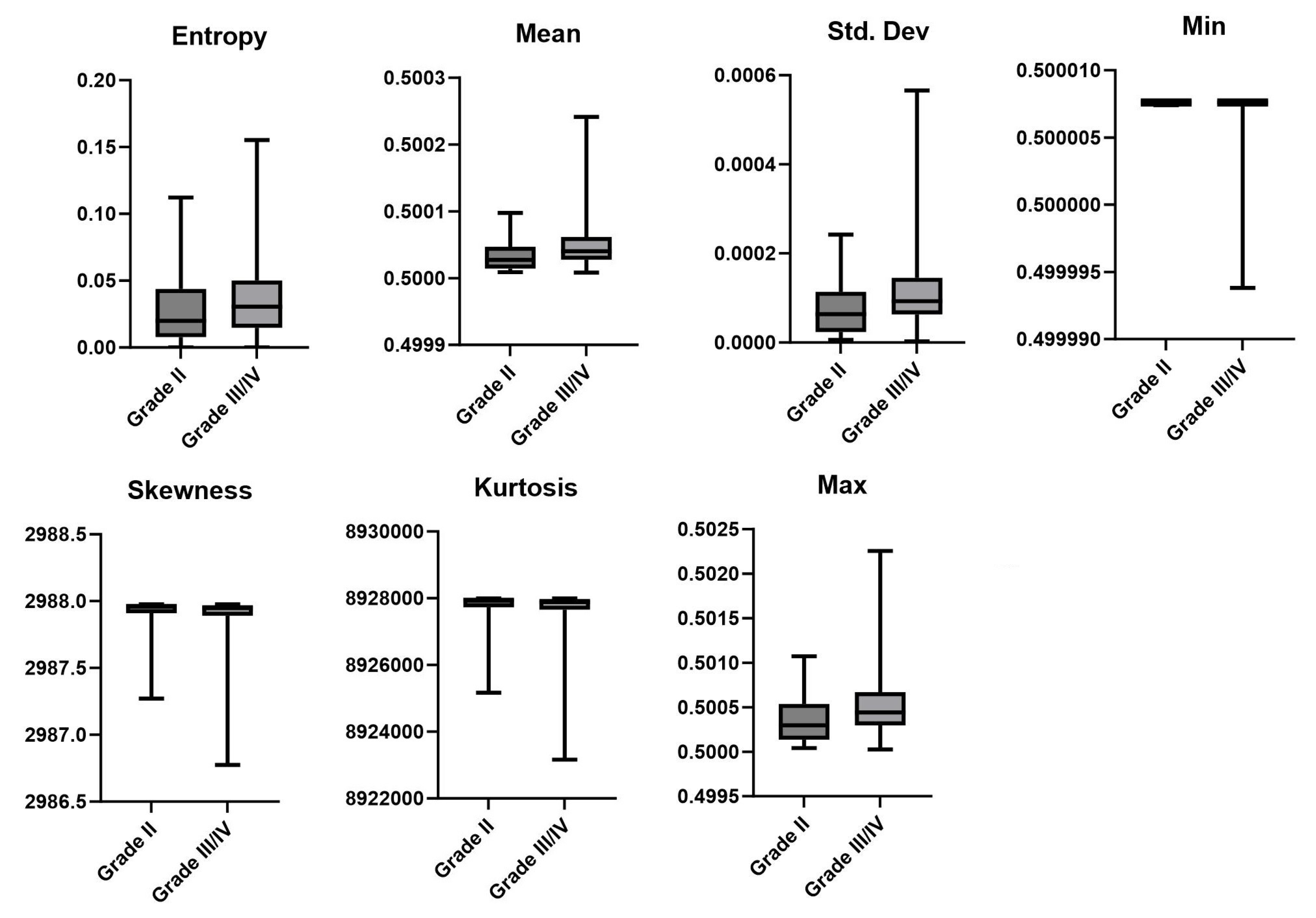
3.2. LGG vs. HGG
3.3. MGMT Status
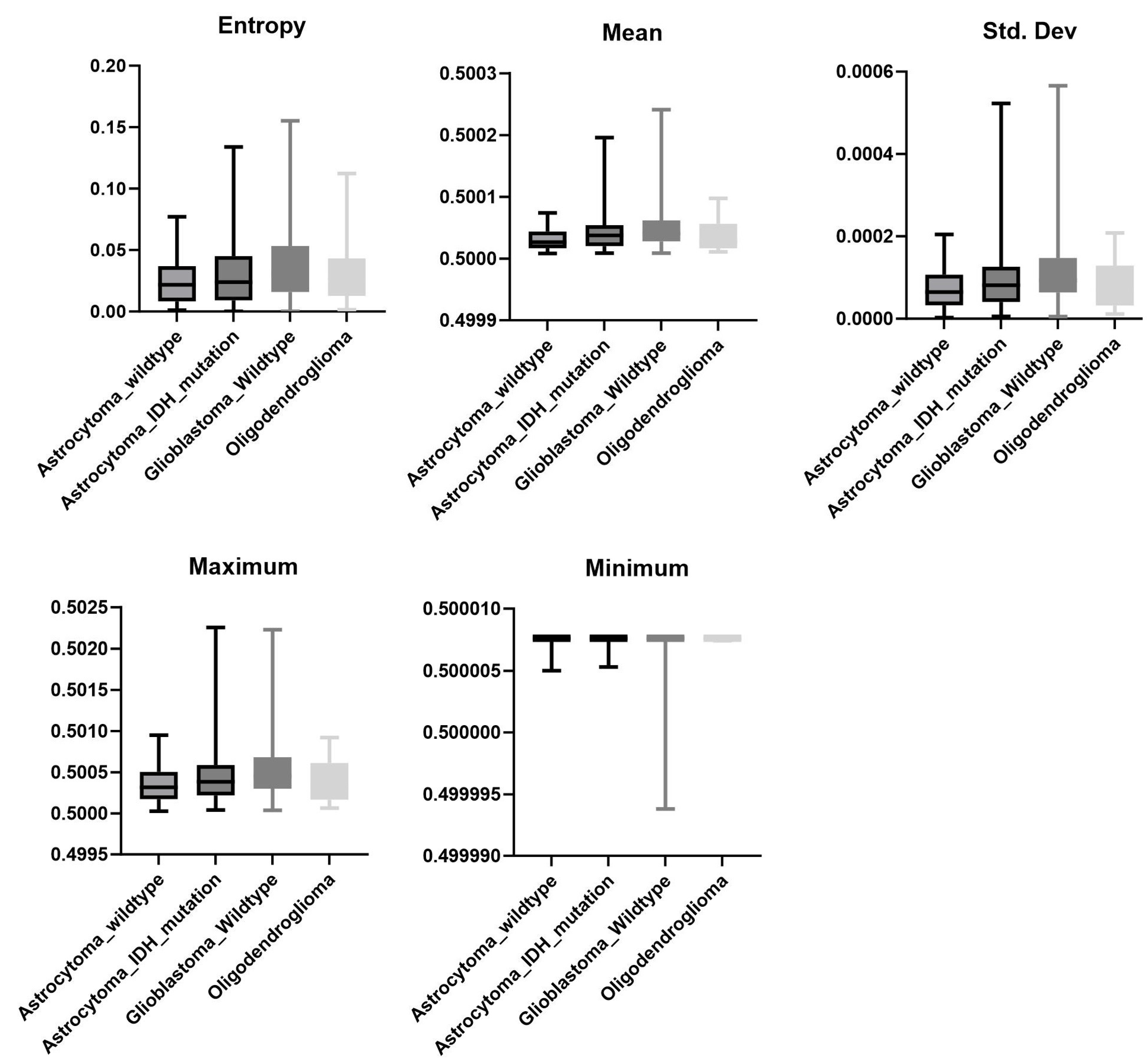
3.4. Mutation
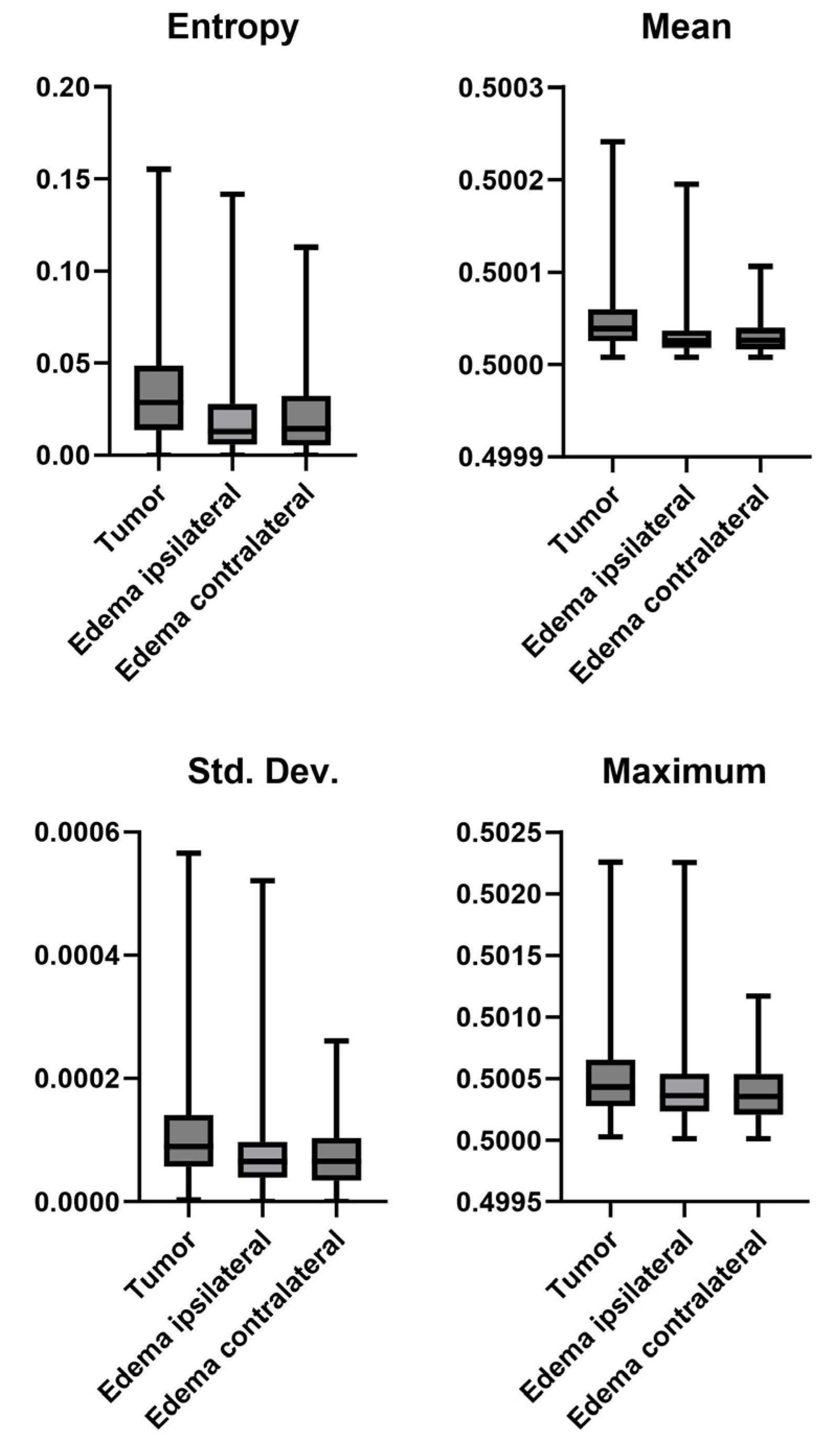
3.5. Tumor vs. Edema
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncolgy 2019, 21 (Suppl. S5), v1–v100. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Nehring, S.M.; Tadi, P.; Tenny, S. Cerebral Edema. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537272/ (accessed on 24 August 2023).
- Steen, R.G.; Petrella, J.R.; Provenzale, J.M. Edema and tumor perfusion: Characterization by quantitative 1H MR imaging. AJR Am. J. Roentgenol. 1992, 158, 259–264. [Google Scholar] [CrossRef][Green Version]
- Nihashi, T.; Dahabreh, I.J.; Terasawa, T. Diagnostic Accuracy of PET for Recurrent Glioma Diagnosis: A Meta-Analysis. AJNR Am. J. Neuroradiol. 2013, 34, 944–950. [Google Scholar] [CrossRef]
- Cui, M.; Zorrilla-Veloz, R.I.; Hu, J.; Guan, B.; Ma, X. Diagnostic Accuracy of PET for Differentiating True Glioma Progression From Post Treatment-Related Changes: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 671867. [Google Scholar] [CrossRef]
- de Zwart, P.L.; van Dijken, B.R.; Holtman, G.A.; Stormezand, G.N.; Dierckx, R.A.J.O.; van Laar, P.J.; van der Hoorn, A. Diagnostic Accuracy of PET Tracers for the Differentiation of Tumor Progression from Treatment-Related Changes in High-Grade Glioma: A Systematic Review and Metaanalysis. J. Nucl. Med. 2020, 61, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Haydar, N.; Alyousef, K.; Alanan, U.; Issa, R.; Baddour, F.; Al-Shehabi, Z.; Al-Janabi, M.H. Role of Magnetic Resonance Imaging (MRI) in grading gliomas comparable with pathology: A cross-sectional study from Syria. Ann. Med. Surg. 2022, 82, 104679. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, M.Z.; Li, T.; Yang, X. MRI combined with PET-CT of different tracers to improve the accuracy of glioma diagnosis: A systematic review and meta-analysis. Neurosurg. Rev. 2019, 42, 185–195. [Google Scholar] [CrossRef]
- Wei, R.-L.; Wei, X.-T. Advanced Diagnosis of Glioma by Using Emerging Magnetic Resonance Sequences. Front. Oncol. 2021, 11, 694498. [Google Scholar] [CrossRef]
- Alsop, D.C.; Detre, J.A.; Golay, X.; Günther, M.; Hendrikse, J.; Hernandez-Garcia, L.; Lu, H.; MacIntosh, B.; Parkes, L.M.; Smits, M.; et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015, 73, 102–116. [Google Scholar] [CrossRef]
- Lindner, T.; Bolar, D.S.; Achten, E.; Barkhof, F.; Bastos-Leite, A.J.; Detre, J.A.; Golay, X.; Günther, M.; Wang, D.J.J.; Haller, S.; et al. Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn. Reson. Med. 2023, 89, 2024–2047. [Google Scholar] [CrossRef] [PubMed]
- Falk Delgado, A.; De Luca, F.; van Westen, D.; Falk Delgado, A. Arterial spin labeling MR imaging for differentiation between high- and low-grade glioma—A meta-analysis. Neuro-Oncology 2018, 20, 1450–1461. [Google Scholar] [CrossRef]
- Alsaedi, A.; Doniselli, F.; Jäger, H.R.; Panovska-Griffiths, J.; Rojas-Garcia, A.; Golay, X.; Bisdas, S. The value of arterial spin labelling in adults glioma grading: Systematic review and meta-analysis. Oncotarget 2019, 10, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Flies, C.M.; Snijders, T.J.; Van Seeters, T.; Smits, M.; De Vos, F.Y.F.; Hendrikse, J.; Dankbaar, J.W. Perfusion imaging with arterial spin labeling (ASL)–MRI predicts malignant progression in low-grade (WHO grade II) gliomas. Neuroradiology 2021, 63, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, G.; Tang, H.; Hong, L.; Peng, W.; Zhang, X. Systematic review and meta-analysis of arterial spin-labeling imaging to distinguish between glioma recurrence and post-treatment radiation effect. Ann. Palliat. Med. 2021, 10, 12488–12497. [Google Scholar] [CrossRef]
- Luan, J.; Wu, M.; Wang, X.; Qiao, L.; Guo, G.; Zhang, C. The diagnostic value of quantitative analysis of ASL, DSC-MRI and DKI in the grading of cerebral gliomas: A meta-analysis. Radiat. Oncol. 2020, 15, 204. [Google Scholar] [CrossRef]
- Kang, X.-W.; Xi, Y.-B.; Liu, T.-T.; Wang, N.; Zhu, Y.-Q.; Wang, X.-R.; Guo, F. Grading of Glioma: Combined diagnostic value of amide proton transfer weighted, arterial spin labeling and diffusion weighted magnetic resonance imaging. BMC Med. Imaging 2020, 20, 50. [Google Scholar] [CrossRef]
- Kong, L.; Chen, H.; Yang, Y.; Chen, L. A meta-analysis of arterial spin labelling perfusion values for the prediction of glioma grade. Clin. Radiol. 2017, 72, 255–261. [Google Scholar] [CrossRef]
- Fu, M.; Han, F.; Feng, C.; Chen, T.; Feng, X. Based on arterial spin labeling helps to differentiate high-grade gliomas from brain solitary metastasis: A systematic review and meta-analysis. Medicine 2019, 98, e15580. [Google Scholar] [CrossRef]
- Calabrese, E.; Villanueva-Meyer, J.E.; Rudie, J.D.; Rauschecker, A.M.; Baid, U.; Bakas, S.; Cha, S.; Mongan, J.T.; Hess, C.P. The University of California San Francisco Preoperative Diffuse Glioma MRI Dataset. Radiol. Artif. Intell. 2022, 4, e220058. [Google Scholar] [CrossRef]
- Baid, U.; Ghodasara, S.; Mohan, S.; Bilello, M.; Calabrese, E.; Colak, E.; Farahani, K.; Kalpathy-Cramer, J.; Kitamura, F.C.; Pati, S.; et al. The rsna-asnr-miccai brats 2021 benchmark on brain tumor segmentation and radiogenomic classification. arXiv 2021, arXiv:2107.02314. [Google Scholar]
- Wang, N.; Xie, S.-Y.; Liu, H.-M.; Chen, G.-Q.; Zhang, W.-D. Arterial Spin Labeling for Glioma Grade Discrimination: Correlations with IDH1 Genotype and 1p/19q Status. Transl. Oncol. 2019, 12, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Lavrova, A.; Teunissen, W.H.T.; Warnert, E.A.H.; van den Bent, M.; Smits, M. Diagnostic Accuracy of Arterial Spin Labeling in Comparison With Dynamic Susceptibility Contrast-Enhanced Perfusion for Brain Tumor Surveillance at 3T MRI. Front. Oncol. 2022, 12, 849657. [Google Scholar] [CrossRef]
- Gihr, G.; Horvath-Rizea, D.; Kohlhof-Meinecke, P.; Ganslandt, O.; Henkes, H.; Härtig, W.; Donitza, A.; Skalej, M.; Schob, S. Diffusion Weighted Imaging in Gliomas: A Histogram-Based Approach for Tumor Characterization. Cancers 2022, 14, 3393. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Kamdar, M.R. MRI to MGMT: Predicting methylation status in glioblastoma patients using convolutional recurrent neural networks. In Proceedings of the 2018 Pacific Symposium on Biocomputing, Fairmont Orchid, HI, USA, 3–7 January 2018; Volume 23, pp. 331–342. [Google Scholar] [CrossRef]
- Sakai, Y.; Yang, C.; Kihira, S.; Tsankova, N.; Khan, F.; Hormigo, A.; Lai, A.; Cloughesy, T.; Nael, K. MRI Radiomic Features to Predict IDH1 Mutation Status in Gliomas: A Machine Learning Approach using Gradient Tree Boosting. Int. J. Mol. Sci. 2020, 21, 8004. [Google Scholar] [CrossRef] [PubMed]
- Halshtok Neiman, O.; Sadetzki, S.; Chetrit, A.; Raskin, S.; Yaniv, G.; Hoffmann, C. Perfusion-weighted imaging of peritumoral edema can aid in the differential diagnosis of glioblastoma mulltiforme versus brain metastasis. Isr. Med. Assoc. J. 2013, 15, 103–105. [Google Scholar]
- Carrete, L.R.; Young, J.S.; Cha, S. Advanced Imaging Techniques for Newly Diagnosed and Recurrent Gliomas. Front. Neurosci. 2022, 16, 787755. [Google Scholar] [CrossRef]
- van Santwijk, L.; Kouwenberg, V.; Meijer, F.; Smits, M.; Henssen, D. A systematic review and meta-analysis on the differentiation of glioma grade and mutational status by use of perfusion-based magnetic resonance imaging. Insights Imaging 2022, 13, 102. [Google Scholar] [CrossRef]
- Willats, L.; Calamante, F. The 39 steps: Evading error and deciphering the secrets for accurate dynamic susceptibility contrast MRI. NMR Biomed. 2013, 26, 913–931. [Google Scholar] [CrossRef]
- Pogosbekian, E.L.; Pronin, I.N.; Zakharova, N.E.; Batalov, A.I.; Turkin, A.M.; Konakova, T.A.; Maximov, I.I. Feasibility of generalised diffusion kurtosis imaging approach for brain glioma grading. Neuroradiology 2021, 63, 1241–1251. [Google Scholar] [CrossRef]

| Pathological Diagnosis | Mutation | ||
| Diagnosis | No. of Patients | Mutation | No. of Patients |
| Astrocytoma wildtype | 24 | IDH-1 | 70 |
| Astrocytoma IDH mutation | 90 | IDH-2 * | 2 |
| Wildtype glioblastoma | 374 | NOS | 31 |
| Oligodendroglioma | 13 | Wildtype | 398 |
| MGMT Status | LGG vs. HGG | ||
| MGMT | No. of Patients | Grade | No. of Patients |
| Positive | 302 | LGG | 99 |
| Negative | 114 | ||
| Indeterminate * | 6 | HGG | 402 |
| Not specified * | 80 | ||
| Pathological Diagnosis * | LGG vs. HGG † | MGMT Status † | |||
|---|---|---|---|---|---|
| Metric | p-Value | Metric | p-Value | Metric | p-Value |
| Entropy | 0.0364 | Entropy | 0.0072 | Entropy | 0.8595 |
| Mean | 0.0028 | Mean | 0.0001 | Mean | 0.9388 |
| Std. Dev. | 0.0045 | Std. Dev. | 0.0001 | Std. Dev. | 0.9271 |
| Skewness | 0.1204 | Skewness | 0.0144 | Skewness | 0.9278 |
| Kurtosis | 0.121 | Kurtosis | 0.0146 | Kurtosis | 0.9278 |
| Min | 0.0073 | Min | 0.0001 | Min | 0.0278 |
| Max | 0.0021 | Max | 0.0016 | Max | 0.7937 |
| Mutation * | Tumor vs. Edema * | Ipsi- vs. Contralateral Edema † | |||
| Metric | p-value | Metric | p-value | Metric | p-value |
| Entropy | 0.1409 | Entropy | 0.0001 | Entropy | 0.4866 |
| Mean | 0.1396 | Mean | 0.0001 | Mean | 0.9823 |
| Std. Dev. | 0.0985 | Std. Dev. | 0.0001 | Std. Dev. | 0.7005 |
| Skewness | 0.3051 | Skewness | 0.0001 | Skewness | 0.279 |
| Kurtosis | 0.305 | Kurtosis | 0.0001 | Kurtosis | 0.2701 |
| Min | 0.0048 | Min | 0.0001 | Min | 0.0001 |
| Max | 0.0354 | Max | 0.0001 | Max | 0.0767 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindner, T.; Dührsen, L.; Kyselyova, A.A.; Entelmann, W.; Hau, L.; Fiehler, J. Histogram-Based Analysis of Low- and High-Grade Glioma and Its Surrounding Edema Using Arterial Spin Labeling Magnetic Resonance Imaging. Appl. Sci. 2023, 13, 10581. https://doi.org/10.3390/app131910581
Lindner T, Dührsen L, Kyselyova AA, Entelmann W, Hau L, Fiehler J. Histogram-Based Analysis of Low- and High-Grade Glioma and Its Surrounding Edema Using Arterial Spin Labeling Magnetic Resonance Imaging. Applied Sciences. 2023; 13(19):10581. https://doi.org/10.3390/app131910581
Chicago/Turabian StyleLindner, Thomas, Lasse Dührsen, Anna Andriana Kyselyova, Wiebke Entelmann, Luis Hau, and Jens Fiehler. 2023. "Histogram-Based Analysis of Low- and High-Grade Glioma and Its Surrounding Edema Using Arterial Spin Labeling Magnetic Resonance Imaging" Applied Sciences 13, no. 19: 10581. https://doi.org/10.3390/app131910581
APA StyleLindner, T., Dührsen, L., Kyselyova, A. A., Entelmann, W., Hau, L., & Fiehler, J. (2023). Histogram-Based Analysis of Low- and High-Grade Glioma and Its Surrounding Edema Using Arterial Spin Labeling Magnetic Resonance Imaging. Applied Sciences, 13(19), 10581. https://doi.org/10.3390/app131910581







