A Palladium-Deposited Molybdenum Disulfide-Based Hydrogen Sensor at Room Temperature
Abstract
:1. Introduction
2. Nanocomposite Materials
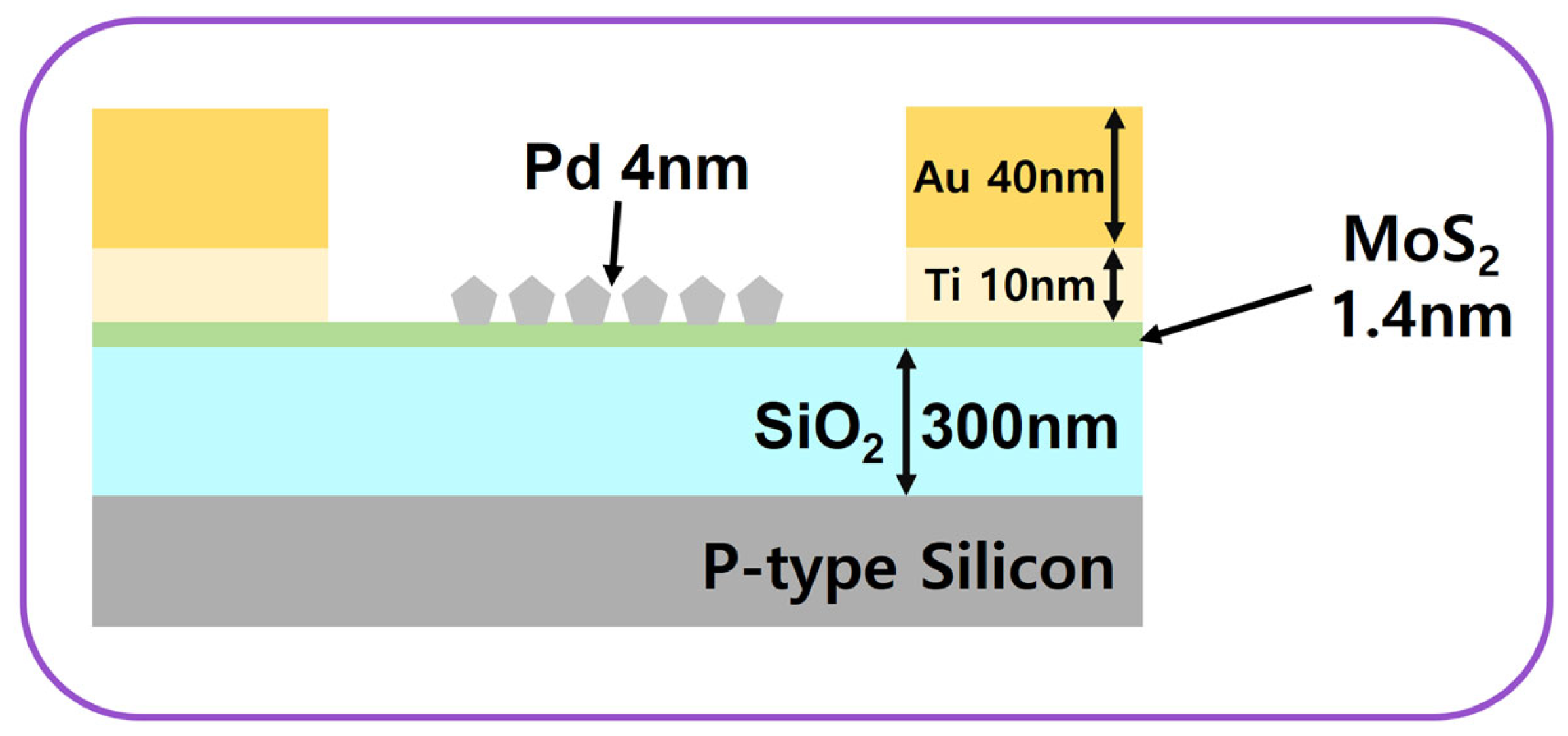
3. Fabrication Process
4. Results
4.1. Gas Measurement Method
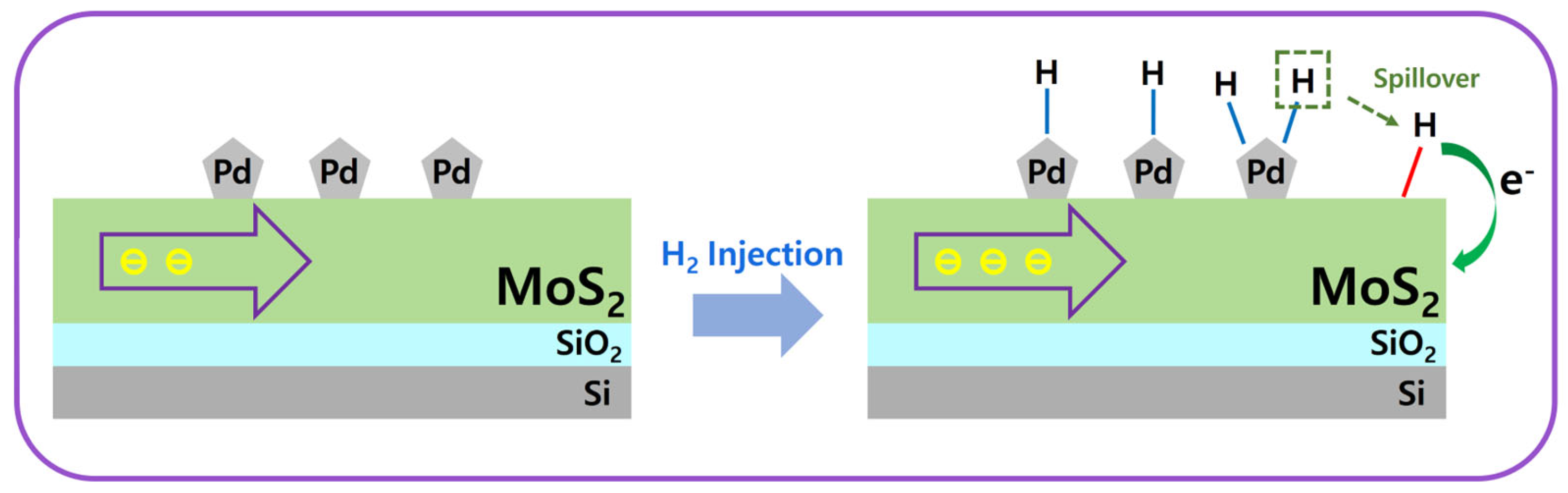
4.2. Gas Sensor Mechanism
4.3. Measurement Result
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrogen Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Kuru, C.; Choi, C.; Kargar, A.; Choi, D.; Kim, Y.J.; Liu, C.H.; Yavuz, S.; Jin, S. MoS2 Nanosheet–Pd Nanoparticle Composite for Highly Sensitive Room Temperature Detection of Hydrogen. Adv. Sci. 2015, 2, 1500004. [Google Scholar] [CrossRef] [PubMed]
- Rezende, N.P.; Cadore, A.R.; Gadelha, A.C.; Pereira, C.L.; Ornelas, V.; Watanabe, K.; Taniguchi, T.; Ferlauto, A.S.; Malachias, A.; Campos, L.C.; et al. Probing the Electronic Properties of Monolayer MoS2 via Interaction with Molecular Hydrogen. Adv. Electron. Mater. 2019, 5, 1800591. [Google Scholar] [CrossRef]
- Koo, W.-T.; Cho, H.-J.; Kim, D.-H.; Kim, Y.H.; Shin, H.; Penner, R.M.; Kim, I.-D. Chemiresistive Hydrogen Sensors: Fundamentals, Recent Advances, and Challenges. ACS Nano 2020, 14, 14284–14322. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Y.; Liu, J.; Li, H.Y.; Hu, Z.; Luo, X.; Gao, N.; Zhang, B.; Jiang, J.; Zhong, A.; et al. Sensitive H2 gas sensors based on SnO2 nanowires. Sens. Actuators B Chem. 2021, 345, 130334. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, P. Synthesis and sensor applications of MoS2-based nanocomposites. Nanoscale 2015, 7, 18364–18378. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.-H.; Kim, J. MoS2 gas sensor functionalized by Pd for the detection of hydrogen. Sens. Actuators B Chem. 2017, 250, 686–691. [Google Scholar] [CrossRef]
- Jaiswal, J.; Tiwari, P.; Singh, P.; Chandra, R. Fabrication of highly responsive room temperature H2 sensor based on vertically aligned edge-oriented MoS2 nanostructured thin film functionalized by Pd nanoparticles. Sens. Actuators B Chem. 2020, 325, 128800. [Google Scholar] [CrossRef]
- Lee, S.; Kang, Y.; Lee, J.; Kim, J.; Shin, J.W.; Sim, S.; Go, D.; Jo, E.; Kye, S.; Kim, J.; et al. Atomic layer deposited Pt nanoparticles on functionalized MoS2 as highly sensitive H2 sensor. Appl. Surf. Sci. 2022, 571, 151256. [Google Scholar] [CrossRef]
- Sarkar, D.; Xie, X.; Kang, J.; Zhang, H.; Liu, W.; Navarrete, J.; Moskovits, M.; Banerjee, K. Functionalization of Transition Metal Dichalcogenides with Metallic Nanoparticles: Implications for Doping and Gas-Sensing. Nano Lett. 2015, 15, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Int. J. Hydrogen Energy 2018, 42, 20386–20397. [Google Scholar] [CrossRef]
- Hao, L.; Liu, Y.; Du, Y.; Chen, Z.; Han, Z.; Xu, Z.; Zhu, J. Highly Enhanced H2 Sensing Performance of Few-Layer MoS2/SiO2/Si Heterojunctions by Surface Decoration of Pd Nanoparticles. Nanoscale Res. Lett. 2017, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Liu, H.; Wang, S.; Chen, S.; Li, W.; Yang, X.; Cai, M.; Yang, K. Probing the Optical Properties of MoS2 on SiO2/Si and Sapphire Substrates. Nanomaterials 2019, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, C. Pt-activated TiO2-MoS2 nanocomposites for H2 detection at low temperature. J. Alloys Compd. 2018, 747, 550–557. [Google Scholar] [CrossRef]
- Vivekanandan, A.K.; Huang, B.-R.; Kathiravan, D.; Saravanan, A.; Prasannan, A.; Tsai, H.-C.; Chen, S.-H. Effect of MoS2 solution on reducing the wall thickness of ZnO nanotubes to enhance their hydrogen gas sensing properties. J. Alloys Compd. 2021, 854, 157102. [Google Scholar] [CrossRef]



| Materials | Definition of Sensitivity | Concentration (ppm) | Temperature (°C) | Sensitivity | Response Time (s) | Reference |
|---|---|---|---|---|---|---|
| Pd/MoS2 | (Rg−Ra)/Ra × 100% | 50,000 | RT | 1000 | 40 | [2] |
| Pt/Tio2/MoS2 | (Rg-Ra)/Ra | 2000 | 100 | 0.749 | 150 | [14] |
| MoS2/ZnO | Rg/Ra × 100% | 500 | RT | 51.5 | 14 | [15] |
| Pd/MoS2 | (Rg−Ra)/Ra × 100% | 500 | RT | 33.7 | 16 | [8] |
| Pd/MoS2 | (Rg−Ra)/Ra × 100% | 10,000 | RT | 35.3 | 786 | [7] |
| Pd/MoS2 | Ig/I0 | 40,000 | RT | 2.77 | 4~5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, U.J.; Jang, D.; Jeon, Y.; Kim, T.; Jo, B.; Kim, R.; Kim, Y.; Kwon, M.-W. A Palladium-Deposited Molybdenum Disulfide-Based Hydrogen Sensor at Room Temperature. Appl. Sci. 2023, 13, 10594. https://doi.org/10.3390/app131910594
Cho UJ, Jang D, Jeon Y, Kim T, Jo B, Kim R, Kim Y, Kwon M-W. A Palladium-Deposited Molybdenum Disulfide-Based Hydrogen Sensor at Room Temperature. Applied Sciences. 2023; 13(19):10594. https://doi.org/10.3390/app131910594
Chicago/Turabian StyleCho, U Jin, Dongjun Jang, Youhyeong Jeon, Taeha Kim, Beomsu Jo, Ryangha Kim, Younglae Kim, and Min-Woo Kwon. 2023. "A Palladium-Deposited Molybdenum Disulfide-Based Hydrogen Sensor at Room Temperature" Applied Sciences 13, no. 19: 10594. https://doi.org/10.3390/app131910594





