Evaluation of the Reproductive Performance of Females of Anguilla anguilla Characterized by Different Levels of Silvering
Abstract
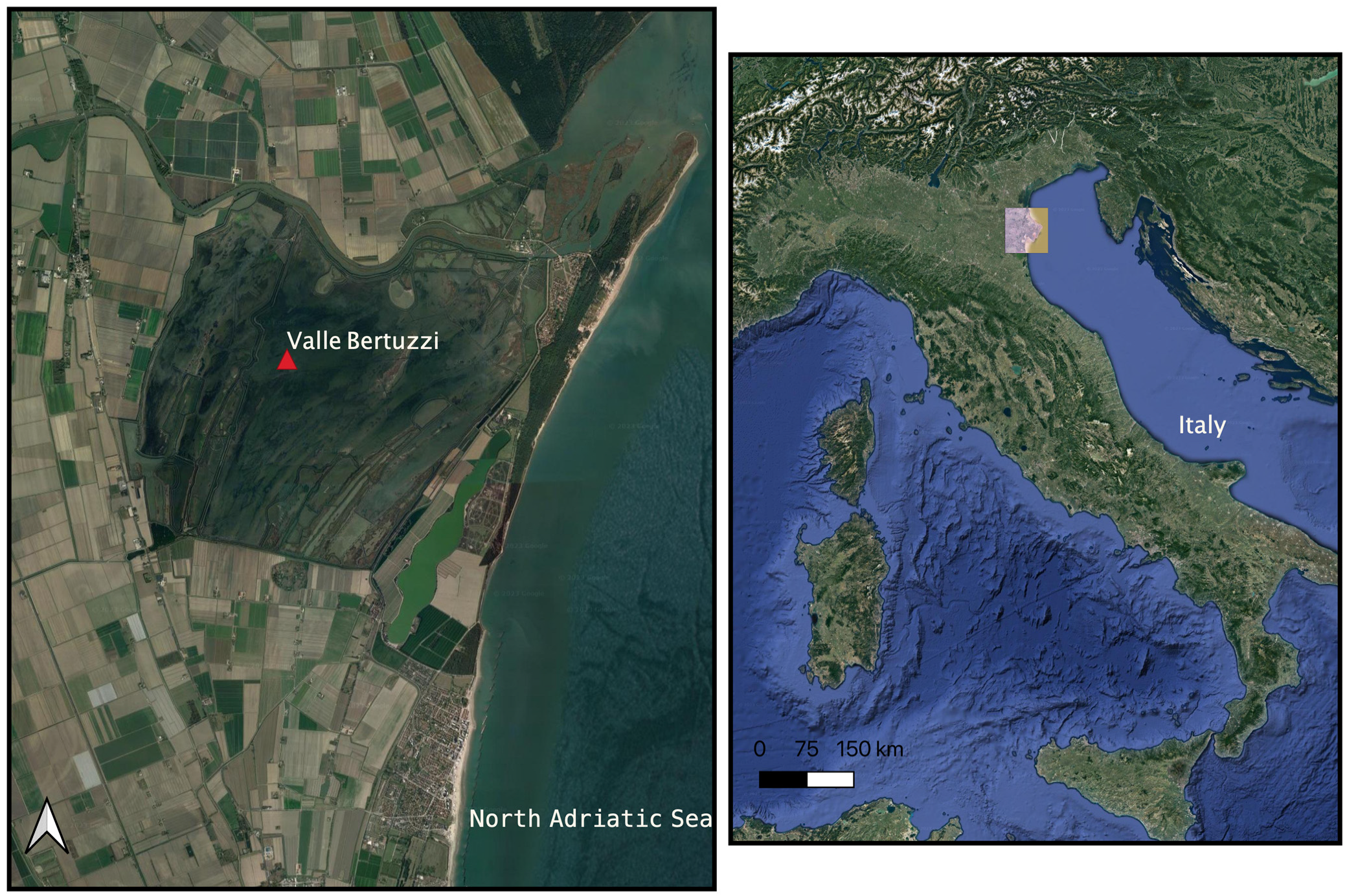
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Age and Gonadosomatic Index
2.3. Induction of Maturation
2.4. Reproductive Performance Comparison
2.5. Statistical Analysis
3. Results
3.1. Measurements
3.2. Age and Gonadosomatic Index
3.3. Reproductive Performance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- OECD. OECD-FAO Agricultural Outlook 2022–2031; OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Drouineau, H.; Durif, C.; Castonguay, M.; Mateo, M.; Rochard, E.; Verreault, G.; Yokouchi, K.; Lambert, P. Freshwater Eels: A Symbol of the Effects of Global Change. Fish Fish. 2018, 19, 903–930. [Google Scholar] [CrossRef]
- Pike, C.; Crook, V.; Gollock, M. Anguilla Anguilla. The IUCN Red List of Threatened Species; International Union for Conservation of Nature: Gland, Switzerland, 2020. [Google Scholar]
- Pujolar, J.M.; Jacobsen, M.W.; Bekkevold, D.; Lobón-Cervià, J.; Jónsson, B.; Bernatchez, L.; Hansen, M.M. Signatures of Natural Selection between Life Cycle Stages Separated by Metamorphosis in European Eel. BMC Genom. 2015, 16, 600–615. [Google Scholar] [CrossRef]
- Rousseau, K.; Aroua, S.; Schmitz, M.; Elie, P.; Dufour, S. Silvering: Metamorphosis or Puberty? In Spawning Migration of the European Eel; Van Den Thillart, G., Dufour, S., Rankin, J.C., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 30, pp. 39–63. [Google Scholar]
- Aroua, S.; Schmitz, M.; Baloche, S.; Vidal, B.; Rousseau, K.; Dufour, S. Endocrine Evidence That Silvering, a Secondary Metamorphosis in the Eel, Is a Pubertal Rather than a Metamorphic Event. Neuroendocrinology 2006, 82, 221–232. [Google Scholar] [CrossRef]
- Pankhurst, N.W. Relation of Visual Changes to the Onset of Sexual Maturation in the European Eel Anguilla anguilla (L.). J. Fish Biol. 1982, 21, 127–140. [Google Scholar] [CrossRef]
- Fontaine, Y.A.; Pisam, M.; Le Moal, C.; Rambourg, A. Silvering and Gill “Mitochondria-Rich” Cells in the Eel, Anguilla anguilla. Cell Tissue Res. 1995, 281, 465–471. [Google Scholar] [CrossRef]
- Ijiri, S.; Kayaba, T.; Takeda, N.; Tachiki, H.; Adachi, S.; Yamauchi, K. Pretreatment Reproductive Stage and Oocyte Development Induced by Salmon Pituitary Homogenate in the Japanese Eel Anguilla Japonica. Fish. Sci. 1998, 64, 531–537. [Google Scholar] [CrossRef]
- Oliveira, K.; Hable, W.E. Artificial Maturation, Fertilization, and Early Development of the American Eel (Anguilla Rostrata). Can. J. Zool. 2010, 88, 1121–1128. [Google Scholar] [CrossRef]
- Ijiri, S.; Tsukamoto, K.; Chow, S.; Kurogi, H.; Adachi, S.; Tanaka, H. Controlled Reproduction in the Japanese Eel (Anguilla Japonica), Past and Present. Aquac. Eur. 2011, 36, 13–17. [Google Scholar]
- Parmeggiani, A.; Govoni, N.; Zannoni, A.; Di Biase, A.; Sirri, R.; Forni, M.; Mandelli, M.; Mordenti, O. Effect of Photoperiod on Endocrine Profiles and Vitellogenin Expression in European Eels Anguilla anguilla during Artificially Induced Ovarian Development. Theriogenology 2015, 83, 478–484. [Google Scholar] [CrossRef]
- Mordenti, O.; Biase, A.D.; Bastone, G.; Sirri, R.; Zaccaroni, A.; Parmeggiani, A. Controlled Reproduction in the Wild European Eel (Anguilla anguilla): Two Populations Compared. Aquac. Int. 2013, 21, 1045–1063. [Google Scholar] [CrossRef]
- Di Biase, A.; Lokman, P.M.; Govoni, N.; Casalini, A.; Emmanuele, P.; Parmeggiani, A.; Mordenti, O. Co-Treatment with Androgens during Artificial Induction of Maturation in Female Eel, Anguilla anguilla: Effects on Egg Production and Early Development. Aquaculture 2017, 479, 508–515. [Google Scholar] [CrossRef]
- Mordenti, O.; Emmanuele, P.; Casalini, A.; Lokman, P.M.; Zaccaroni, A.; Di Biase, A.; Parmeggiani, A. Effect of Aromatable Androgen (17-Methyltestosterone) on Induced Maturation of Silver European Eels (Anguilla anguilla): Oocyte Performance and Synchronization. Aquac. Res. 2018, 49, 442–448. [Google Scholar] [CrossRef]
- Jéhannet, P.; Palstra, A.P.; Giménez Nebot, I.; Schipper, H.; Swinkels, W.; Heinsbroek, L.T.N.; Komen, H. Recombinant Gonadotropins to Induce Oocyte Development In Vitro and In Vivo in the European Eel Anguilla anguilla. Fishes 2023, 8, 123. [Google Scholar] [CrossRef]
- Palstra, A.P.; van de Ven, I.; Jéhannet, P.; Kruijt, L.; Schipper, H.; Swinkels, W.; Heinsbroek, L.T.N. Human Chorionic Gonadotropin Enhancement of Early Maturation and Consequences for Reproductive Success of Feminized European Eel (Anguilla anguilla). Fishes 2023, 8, 281. [Google Scholar] [CrossRef]
- Palstra, A.P.; Bouwman, L.J.; Jéhannet, P.; Kruijt, L.; Schipper, H.; Blokland, M.H.; Swinkels, W.; Heinsbroek, L.T.N.; Lokman, P.M. Steroid Implants for the Induction of Vitellogenesis in Feminized European Silver Eels (Anguilla anguilla L.). Front. Genet. 2022, 13, 969202. [Google Scholar] [CrossRef]
- Kjørsvik, E.; Jensen, A.M.; Holmefjord, T. Egg quality in fishes. Adv. Mar. Biol. 1990, 26, 71. [Google Scholar]
- Bromage, N.R.; Jones, J.; Randall, C.; Thrush, M.; Davies, B.; Springate, J.; Duston, J.; Barker, G. Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhiynchus mykiss). Aquaculture 1992, 100, 141. [Google Scholar] [CrossRef]
- Gallego, V.; Mazzeo, I.; Vílchez, M.C.; Peñaranda, D.S.; Carneiro, P.C.F.; Pérez, L.; Asturiano, J.F. Study of the Effects of Thermal Regime and Alternative Hormonal Treatments on the Reproductive Performance of European Eel Males (Anguilla anguilla) during Induced Sexual Maturation. Aquaculture 2012, 354–355, 7–16. [Google Scholar] [CrossRef]
- Herranz-Jusdado, J.G.; Rozenfeld, C.; Morini, M.; Pérez, L.; Asturiano, J.F.; Gallego, V. Recombinant vs Purified Mammal Gonadotropins as Maturation Hormonal Treatments of European Eel Males. Aquaculture 2019, 501, 527–536. [Google Scholar] [CrossRef]
- Okamura, A.; Horie, N.; Mikawa, N.; Yamada, Y.; Tsukamoto, K. Recent Advances in Artificial Production of Glass Eels for Conservation of Anguillid Eel Populations. Ecol. Freshw. Fish 2014, 23, 95–110. [Google Scholar] [CrossRef]
- Mordenti, O.; Di Biase, A.; Sirri, R.; Modugno, S.; Tasselli, A. Induction of Sexual Maturation in Wild Female European Eels (Anguilla anguilla) in Darkness and Light. Isr. J. Aquac. 2012, 64, 1–8. [Google Scholar]
- Seoka, M.; Yamada, S.; Iwata, Y.; Yanagisawa, T.; Nakagawa, T.; Kumai, H. Differences in the Biochemical Content of Buoyant and Non-Buoyant Eggs of the Japanese Eel, Anguilla japonica. Aquaculture 2003, 216, 355–362. [Google Scholar] [CrossRef]
- Kottmann, J.S.; Tomkiewicz, J.; Butts, I.A.E.; Lund, I.; Jacobsen, C.; Støttrup, J.G.; Holst, L. Effects of Essential Fatty Acids and Feeding Regimes on Egg and Offspring Quality of European Eel: Comparing Reproductive Success of Farm-Raised and Wild-Caught Broodstock. Aquaculture 2020, 529, 735581. [Google Scholar] [CrossRef]
- McEvoy, L.A. Ovulatory rhythms and over-ripening of eggs in cultivated turbot, Scphthalmus maximum. L. J. Fish Biol. 1984, 24, 48. [Google Scholar]
- Carrillo, M.; Bromage, N.; Zanuy, S.; Serrano, R.; Prat, F. The effect of modifications in photoperiod on spawning time, ovarian development and egg quality in the sea bass (Dicentrarchus labrax). Neth. J. Zool. 1989, 45, 204. [Google Scholar] [CrossRef]
- Pérez, L.; Peñaranda, D.S.; Dufour, S.; Baloche, S.; Palstra, A.P.; Van Den Thillart, G.E.E.J.M.; Asturiano, J.F. Influence of temperature regime on endocrine parameters and vitellogenesis during experimental maturation of European eel (Anguilla anguilla) females. Gen. Comp. Endocrinol. 2011, 174, 51–59. [Google Scholar] [CrossRef]
- Durif, C.M.F.; Dufour, S.; Elie, P. Impact of Silvering Stage, Age, Body Size and Condition on Reproductive Potential of the European Eel. Mar. Ecol. Prog. Ser. 2006, 327, 171–181. [Google Scholar] [CrossRef]
- Durif, C.; Dufour, S.; Elie, P. The Silvering Process of Anguilla anguilla: A New Classification from the Yellow Resident to the Silver Migrating Stage. J. Fish Biol. 2005, 66, 1025–1043. [Google Scholar] [CrossRef]
- Okamura, A.; Yamada, Y.; Horie, N.; Utoh, T.; Mikawa, N.; Tanaka, S.; Tsukamoto, K. Effects of Silvering State on Induced Maturation and Spawning in Wild Female Japanese Eel Anguilla japonica. Fish. Sci. 2008, 74, 642–648. [Google Scholar] [CrossRef]
- Daemen, E.; Cross, T.; Ollevier, F.; Volckaert, F. Analysis of the Genetic Structure of European Eel (Anguilla anguilla) Using Microsatellite DNA and MtDNA Markers. Mar. Biol. 2001, 139, 755–764. [Google Scholar] [CrossRef]
- Wirth, T.; Bernatchez, L. Genetic Evidence against Panmixia in the European Eel. Nature 2001, 409, 1037–1040. [Google Scholar] [CrossRef]
- Maes, G.E.; Volckaert, F.A.M. Clinal Genetic Variation and Isolation by Distance in the European Eel Anguilla anguilla (L.). Biol. J. Linn. Soc. 2002, 77, 509–521. [Google Scholar] [CrossRef]
- Dannewitz, J.; Maes, G.E.; Johansson, L.; Wickström, H.; Volckaert, F.A.M.; Järvi, T. Panmixia in the European Eel: A Matter of Time. Proc. R. Soc. B: Biol. Sci. 2005, 272, 1129–1137. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.; Maes, G.E. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: A literature review. Rev. Fish Biol. Fish. 2005, 15, 367–398. [Google Scholar] [CrossRef]
- Melià, P.; Bevacqua, D.; Crivelli, A.J.; Panfili, J.; De Leo, G.A.; Gatto, M. Sex differentiation of the European eel in brackish and freshwater environments: A comparative analysis. J. Fish Biol. 2006, 69, 1228–1235. [Google Scholar] [CrossRef]
- Durif, C.M.F.; van Ginneken, V.; Dufour, S.; Müller, T.; Elie, P. Seasonal Evolution and Individual Differences in Silvering Eels from Different Locations. In Spawning Migration of the European Eel: Reproduction Index, a Useful Tool for Conservation Management; van den Thillart, G., Dufour, S., Rankin, J.C., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 30, pp. 13–38. [Google Scholar]
- Panfili, J.; de Pontual, H.; Troadec, H.; Wright, P.J. Manual of Fish Sclerochronology; Ifremer-IRD Coedition: Brest, France, 2002; p. 464. [Google Scholar]
- Ohta, H.; Kagawa, H.; Tanaka, H.; Okuzawa, K.; Iinuma, N.; Hirose, K. Artificial Induction of Maturation and Fertilization in the Japanese Eel, Anguilla japonica. Fish Physiol. Biochem. 1997, 17, 163–169. [Google Scholar] [CrossRef]
- van Ginneken, V.; Vianen, G.; Muusze, B.; Palstra, A.; Verschoor, L.; Lugten, O.; Onderwater, M.; van Schie, S.; Niemantsverdriet, P.; van Heeswijk, R.; et al. Gonad Development and Spawning Behaviour of Artificially-Matured European Eel (Anguilla anguilla L.). Anim. Biol. 2005, 55, 203–218. [Google Scholar] [CrossRef]
- Mordenti, O.; Casalini, A.; Mandelli, M.; Di Biase, A. A Closed Recirculating Aquaculture System for Artificial Seed Production of the European Eel (Anguilla anguilla): Technology Development for Spontaneous Spawning and Eggs Incubation. Aquac. Eng. 2014, 58, 88–94. [Google Scholar] [CrossRef]
- Dou, S.-Z.; Yamada, Y.; Okamura, A.; Shinoda, A.; Tanaka, S.; Tsukamoto, K. Temperature Influence on the Spawning Performance of Artificially-Matured Japanese Eel, Anguilla japonica, in Captivity. Environ. Biol. Fish. 2008, 82, 151–164. [Google Scholar] [CrossRef]
- Palstra, A.P.; Jéhannet, P.; Heinsbroek, L.T.N.; Swinkels, W. Five Years of Optimizing the Assisted Reproduction Protocol for European Eel: What Worked and What Didn’t? In Proceedings of 12th World Congress on Genetics Applied to Livestock Production (WCGALP); Veerkamp, R.F., de Haas, Y., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2022; pp. 2028–2030. [Google Scholar]
- Guarniero, I.; Cariani, A.; Ferrari, A.; Sulliotti, V.; Emmanuele, P.; Casalini, A.; Mordenti, O. Sexual behaviour and reproductive performance of the endangered European eel Anguilla anguilla (Linnaeus, 1758) based on direct observations and paternity assignment in semi-natural conditions. Aquac. Rep. 2020, 16, 100258. [Google Scholar] [CrossRef]
- Emmanuele, P.; Casalini, A.; Pisati, D.; Andreini, R.; Guercilena, N.; Parmeggiani, A.; Zaccaroni, A.; Mordenti, O. Artificial Reproduction of Anguilla anguilla: Evaluation of Biometrics Characteristics of a Population from Valle Campo Lagoon, Comacchio (Italy). Aquacult. Int. 2020, 28, 777–790. [Google Scholar] [CrossRef]
- Tanaka, H.; Kagawa, H.; Ohta, H. Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture 2001, 201, 51–60. [Google Scholar] [CrossRef]
- Tanaka, H. Progression in artificial seedling production of Japanese eel Anguilla japonica. Fish Sci. 2015, 81, 11–19. [Google Scholar] [CrossRef]
- Butts, I.A.E.; Sørensen, S.R.; Politis, S.N.; Tomkiewicz, J. First-feeding by European eel larvae: A step towards closing the life cycle in captivity. Aquaculture 2016, 464, 451–458. [Google Scholar] [CrossRef]
- Parmeggiani, A.; Zannoni, A.; Tubon, I.; Casalini, A.; Emmanuele, P.; Forni, M.; Mordenti, O. Initial ontogeny of digestive enzymes in the early life stages of captive-bred European eels during fasting: A partial characterization. Res. Vet. Sci. 2020, 132, 54–56. [Google Scholar] [CrossRef]
- Palstra, A.P.; Cohen, E.G.H.; Niemantsverdriet, P.R.W.; Van Ginneken, V.J.T.; Van den Thillart, G.E.E.J.M. Artificial maturation and reproduction of European silver eel: Development of oocytes during final maturation. Aquaculture 2005, 249, 533–547. [Google Scholar] [CrossRef]
- Gentile, L.; Casalini, A.; Emmanuele, P.; Brusa, R.; Zaccaroni, A.; Mordenti, O. Gonadal Development in European Eel Populations of North Adriatic Lagoons at Different Silvering Stages. Appl. Sci. 2022, 12, 2820. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Fernández-Palacios, H.; Tacon, A.G.J. Effect of Broodstock Nutrition on Reproductive Performance of Fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- De Leo, G.A.; Gatto, M. Trends in Vital Rates of the European Eel: Evidence for Density Dependence? Ecol. Appl. 1996, 6, 1281–1294. [Google Scholar] [CrossRef]
- Politis, S.N.; Mazurais, D.; Servili, A.; Zambonino-Infante, J.L.; Miest, J.J.; Sørensen, S.R.; Butts, I.A. Temperature effects on gene expression and morphological development of European eel, Anguilla anguilla larvae. PLoS ONE 2017, 12, e0182726. [Google Scholar] [CrossRef]

| Silver Index | II | III | IV | V | |||
|---|---|---|---|---|---|---|---|
| Eels (n. 250) | n. | 14 | 184 | - | 52 | ||
| % | 5.6 | 73.6 | - | 20.8 | |||
| Body Weight (BW) | g | 431.16 ± 120.31 b | 552.34 ± 67.05 a | - | 555.44 ± 110.73 a | ||
| Body Lenght (BL) | mm | 622.44 ± 58.36 b | 674.48 ± 56.82 a | - | 671.74 ± 45.25 a | ||
| Pectoral Fin Length(PFL) | mm | 28.79 ± 3.4 b | 32.81 ± 2.60 a | - | 35.47 ± 2.12 a | ||
| Fulton’s Condition Factor | 1.73 ± 0.21 b | 1.81 ± 0.17 a | - | 1.83 ± 0.28 a | |||
| Eye Index (EI) | 3.82 ± 0.81 c | 6.26 ± 1.02 b | - | 9.28 ± 1.86 a | |||
| Pectoral Fin Lenght Index(PFI) | 4.64 ± 0.24 c | 4.87 ± 0.33 b | - | 5.29 ± 0.39 a | |||
| Gonadosomaic Index (GSI) | 0.64 ± 0.31 c | 1.65 ± 0.21 b | - | 1.98 ± 0.14 a | |||
| Age | <6 | - | % | - | - | - | - |
| 6–6+ | 5.3% | 20 | 5.7 | - | |||
| 7–7+ | 62.1% | 80 | 64.1 | 50 | |||
| 8–8+ | 20.8% | - | 18.9 | 33.3 | |||
| 9–9+ | 10.4% | - | 9.4 | 16.7 | |||
| 10–10+ | 1.4% | - | 1.9 | - | |||
| >10+ | - | - | - | - | |||
| Initial Weight (g) | Final Weight (g) | Weight Gain (%) | Ovulation n°/Total | Week (n°) | |
|---|---|---|---|---|---|
| PRE-MIGRANT III | 618.62 ± 62.05 | 762.72 ± 79.65 | 123.39 ± 6.92 | 10/10 | 20.2 ± 2 |
| MIGRANT V | 559.94 ± 112.4 | 704.72 ± 95.05 | 126.51 ± 7.65 | 8/10 | 20.4 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mordenti, O.; Gentile, L.; Emmanuele, P.; Hausz, B.L.; Brusa, R.; Casalini, A. Evaluation of the Reproductive Performance of Females of Anguilla anguilla Characterized by Different Levels of Silvering. Appl. Sci. 2023, 13, 10718. https://doi.org/10.3390/app131910718
Mordenti O, Gentile L, Emmanuele P, Hausz BL, Brusa R, Casalini A. Evaluation of the Reproductive Performance of Females of Anguilla anguilla Characterized by Different Levels of Silvering. Applied Sciences. 2023; 13(19):10718. https://doi.org/10.3390/app131910718
Chicago/Turabian StyleMordenti, Oliviero, Laura Gentile, Pietro Emmanuele, Bálint Lóránt Hausz, Riccardo Brusa, and Antonio Casalini. 2023. "Evaluation of the Reproductive Performance of Females of Anguilla anguilla Characterized by Different Levels of Silvering" Applied Sciences 13, no. 19: 10718. https://doi.org/10.3390/app131910718
APA StyleMordenti, O., Gentile, L., Emmanuele, P., Hausz, B. L., Brusa, R., & Casalini, A. (2023). Evaluation of the Reproductive Performance of Females of Anguilla anguilla Characterized by Different Levels of Silvering. Applied Sciences, 13(19), 10718. https://doi.org/10.3390/app131910718








