The Use of Chlorophyll Fluorescence as an Indicator of Predicting Potato Yield, Its Dry Matter and Starch in the Conditions of Using Microbiological Preparations
Abstract
:1. Introduction
- −
- To measure the fluorescence of chlorophyll, a spectrofluorometer can be used, which allows one to analyze the fluorescence of chlorophyll in plant leaves. Analyzing changes in the chlorophyll fluorescence intensity enables the evaluation of photosynthesis status and overall plant efficiency [8,21,22];
- −
- Fluorometric measurements at the cellular level: There are also techniques such as single cell fluorescence imaging that allow for a more accurate analysis of chlorophyll fluorescence at the level of individual plant cells. These methods can provide more detailed information on the efficiency of photosynthesis and starch accumulation in different areas of the plant [23,24];
- −
- The assessment of fluorescence during plant development: Regular measurements of chlorophyll fluorescence at various stages of plant development can provide information on the rate of growth and accumulation of dry matter or starch [20];
- −
- Chlorophyll fluorescence measurements yield valuable insights into how stress affects the functionality of a plant’s photosynthetic apparatus. Recent research has resulted in the intensive development of chlorophyll photoluminescence measurement methods and the miniaturization of measuring devices. Fluorimeters are integrated with gas exchange measurement devices, allowing for a comprehensive, non-invasive assessment of the photosynthesis intensity. The use of chlorophyll fluorescence measurement techniques is becoming more and more common, starting from the study of single cells of plant tissues, and ending with the imaging of the photoluminescence of agricultural fields using satellite images [24].
- −
- Treating seed potatoes with potential probiotic preparations can improve the quality of the raw material for both direct consumption and food processing;
- −
- The EM Farma Plus microbiological preparation enhances the biological and agrochemical properties of the soil. Consequently, it influences the potato cultivation technology and genetic characteristics of potato cultivars, impacting the accumulation of biologically active compounds;
- −
- The utilization of chlorophyll fluorescence as an indicator of crop yield and its components is a promising tool in agriculture. It facilitates the monitoring and optimization of plant production, to the null hypothesis that the application of the EM Farma Plus preparation will not affect the quantity and quality of potatoes, and that chlorophyll fluorescence will not provide valuable insights into the optimization of plant production.
2. Methodology
2.1. Characteristics of Microbial Preparation EM
2.2. Characteristics of the Cultivars
2.3. Field Studies
2.4. Assessment of Physiological Indicators
2.5. Determination of Selected Elements of the Chemical Composition of Tubers
2.6. Soil Conditions
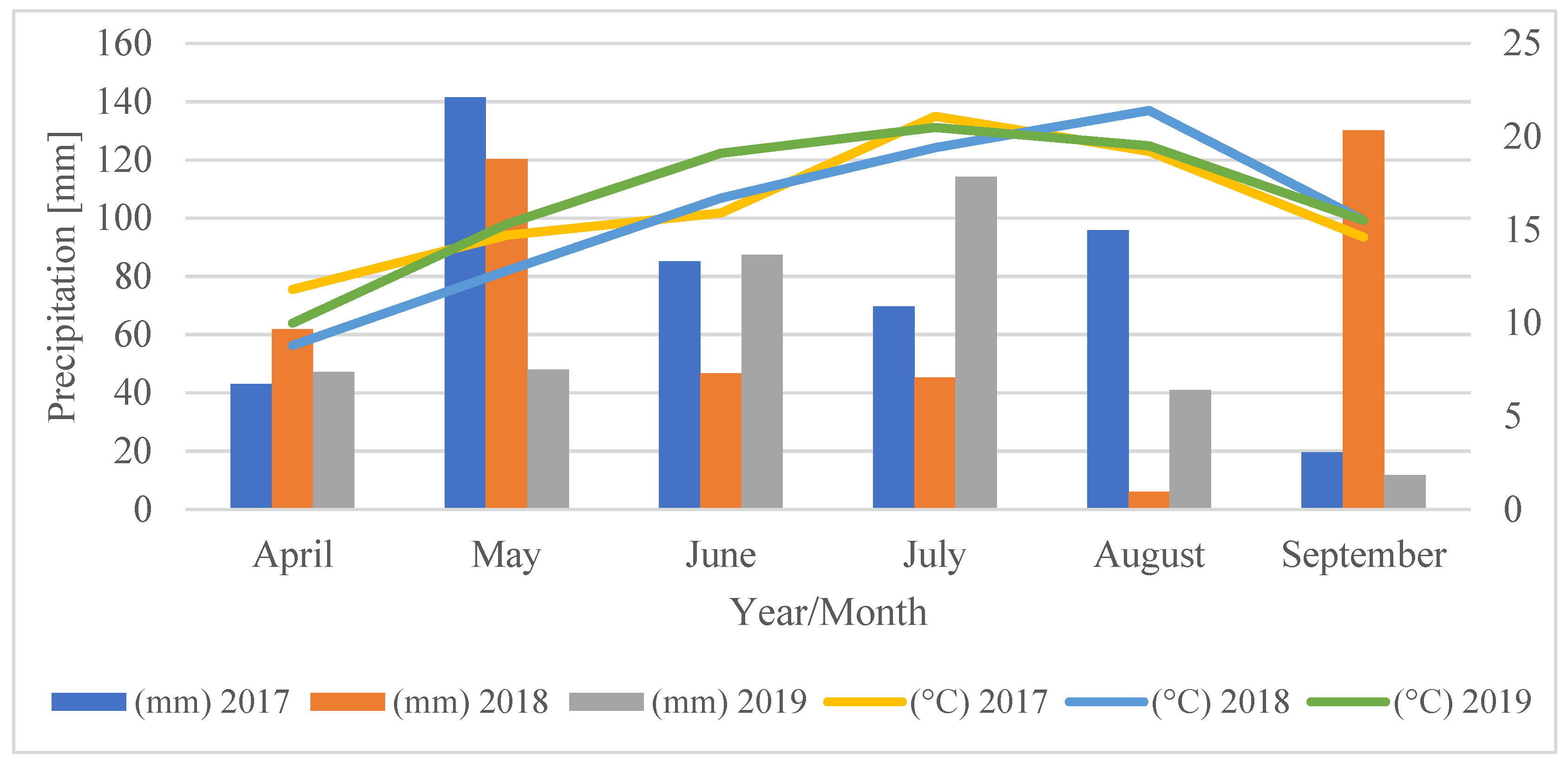
2.7. Meteorological Conditions
2.8. Statistical Calculations
3. Results
3.1. Physiological Indicators of Yield
3.2. Analyzing Dry Matter and Starch in Potato Tubers
3.3. Total Potato Yield and Yield of Dry Matter and Starch
3.4. Descriptive Analysis of the Yield, Its Most Important Economic Features, and the Physiological Indicators of Potato
3.5. Influence of Physiological Indicators of Chlorophyll Fluorescence on Tuber Yield, Dry Matter, and Starch Content
4. Discussion
4.1. Influence of Microbiological Organisms on Yield and Its Components as Well as Physiological Indicators of Chlorophyll Fluorescence
- −
- Fo (initial fluorescence): EM increased Fo after 10 min but not after 15 min compared to the control group.
- −
- Fm (maximum fluorescence): EM significantly increased Fm in both exposure periods.
- −
- Fo/Fm (quiescent to maximum fluorescence ratio): EM enhanced Fo/Fm, indicating improved photosynthetic performance, especially in the ‘Satina’, ‘Oberon’, and ‘Jelly’ cultivars.
- −
- Fo′ (initial fluorescence under light): EM influenced Fo′, with the shortest exposure (10 min) resulting in higher values.
- −
- Fm′ (maximum fluorescence on light): EM treatment increased Fm′, particularly with a 10-min exposure.
- −
- Qp (quantum efficiency of Photosystem II): EM significantly improved Qp in both exposure periods, with the most notable enhancement after 10 min.
- −
- Qn (fluorescence from other components): higher Qn was observed with the 10-min EM application (Table 3).
4.2. Genetic Variability Vs. Physiological and Yield Characteristics
4.3. The Impact of Abiotic Conditions on the Physiological Indicators of Potato
4.4. Interdependence of Feature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EM | Effective microorganisms |
| EMAS | Microbiologically activated solution |
| ETR | Electron transport rate |
| ETR(II) | Absolute rate of PS II turnover, electrons |
| Fm | Dark-acclimated maximal fluorescence yield |
| Fm′ | Maximal fluorescence yield during illumination |
| Fo | Dark-acclimated minimal fluorescence yield |
| Fo′ | Initial (minimal) fluorescence on the light |
| Fv | Variable fluorescence yield |
| Fv/Fm | Maximum photochemical efficiency of PSII |
| HSD | Honestly significant difference |
| PS II | Photosystem II |
| qN | Non-photochemical fluorescence quenching coefficient |
| qP | Photochemical fluorescence quenching coefficient |
| Y | Actual photochemical efficiency of PSII |
References
- Kosicka, D.; Wolna-Maruwka, A.; Trzeciak, M. Influence of microbiological preparations on soil and plant growth and development. Kosmos 2015, 64, 332. (In Polish) [Google Scholar]
- Olle, M.; Williams, I. The influence of effective microorganisms on the growth and nitrate content of vegetable transplants. J. Adv. Agric. Technol. 2015, 2, 25–28. [Google Scholar] [CrossRef]
- Sajid, M.; Butt, S.J.; Haq, Z.U.; Naseem, I.; Iqbal, A.; Khan, Q.A.; Ali, H. Effects of organic substrates and effective microorganisms (EM) on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse condition. Pure Appl. Biol. 2023, 2, 116–127. [Google Scholar] [CrossRef]
- Zamana, S.P.; Papaskiri, T.V.; Kondratieva, T.D.; Shapovalov, D.A.; Fedorovsky, T.D. The Influence of Beneficial Microorganisms on the Quality Indicators and Antioxidant Properties of Potatoes. In Proceedings of the 2nd International Scientific and Practical Conference: Improving Energy Efficiency, Environmental Safety and Sustainable Development in Agriculture 2022, Dushanbe, Tajikistan, 24–27 October 2022; Volume 1154. [Google Scholar] [CrossRef]
- Burgos, G.; Zum Felde, T.; Andre, C.; Kubow, S. The Potato and Its Contribution to the Human Diet and Health. In The Potato Crop; Campos, H., Ortiz, O., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Yap, C.K.; Al-Mutairi, K.A. Effective Microorganisms as Halal-Based Sources for Biofertilizer Production and Some Socio-Economic Insights: A Review. Foods 2023, 12, 1702. [Google Scholar] [CrossRef]
- Piotrowska, A.; Boruszko, D. Analysis of the potential of effective microorganisms in plant production. Econom. Environ. 2023, 83, 180–195. (In Polish) [Google Scholar] [CrossRef]
- Brock, C.; Geier, U.; Greiner, R.; Olbrich-Majer, M.; Fritz, J. Research in biodynamic food and farming—A review. Open Agric. 2019, 4, 743–757. [Google Scholar] [CrossRef]
- Bhandari, B.K.; Babasaheb, B.; Pravin, N.; Shubham, S.; Nikhil, S. Review on domestic waste water treatment by using effective microorganisms’ technology. IJSART 2021, 6, 792–796. [Google Scholar]
- Santoni, M.; Ferretti, L.; Migliorini, P.; Vazzana, C.; Pacini, G.C. A review of scientific research on biodynamic agriculture. Org. Agric. 2022, 12, 373–396. [Google Scholar] [CrossRef]
- Ramírez, E.; Prin, J.L.; de Astudillo, L.R. Evaluación del Efecto de los Microorganismos eficientes en el suelo usando Microscopía Electrónica de Barrido Analítica. Acta Microsc. 2022, 31, 63–71. [Google Scholar]
- Zhang, D.; Zhang, Q.S.; Yang, X.Q. Seasonal dynamics of photosynthetic activity in the representative brown macroalgae Sagrassum thunbergii (Sargassaceae phaeophyta). Plant Physiol. Biochem. 2017, 120, 88–94. [Google Scholar] [CrossRef]
- Higa, T. Effective Microorganisms—Technology of the 21st Century. In Proceedings of the Conference “Effective Microorganisms in the World”, London, UK, 23 July 2005; pp. 20–24. (In Polish). [Google Scholar]
- Zsom-Muha, V.; Le Phuong Lien, N.; Baranyai, L.; Hitka, G.; Horváth-Mezőfi, G.; Szabó, G.; Zsom, T. An attempt to the nondestructive investigation of photo-induced potato postharvest quality degradation—Preliminary results. Prog. Agric. Eng. Sci. 2021, 17, 99–109. [Google Scholar] [CrossRef]
- Xu, S.; Atherton, J.; Riikonen, A.; Zhang, C.; Oivukkamäki, J.; MacArthur, A.; Honkavaara, E.; Hakala, T.; Koivumäki, N.; Liu, Z.; et al. Structural and photosynthetic dynamics mediate the response of SIF to water stress in a potato crop. Remote Sens. Environ. 2021, 263, 112555. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Schreiber, U.; Klughammer, C.; Kolbowski, J. Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynth. Res. 2012, 113, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Zarzyńska, K.; Pietraszko, M. Possibility to predict the yield of potatoes grown under two crop production systems on the basis of selected morphological and physiological plant indicators. Plant Soil Environ. 2017, 63, 165–170. [Google Scholar] [CrossRef]
- Kwambai, T.K.; Griffin, D.; Nyongesa, M.; Byrne, S.; Gorman, M.; Struik, P.C. Dormancy and Physiological Age of Seed Tubers from a Diverse Set of Potato Cultivars Grown at Different Altitudes and in Different Seasons in Kenya. Potato Res. 2023, 66, 1–25. [Google Scholar] [CrossRef]
- Loayza, H.; Moya, I.; Quiroz, R.; Ounis, A.; Goulas, Y. Active and passive chlorophyll fluorescence measurements at canopy level on potato crops. Evidence of similitude of diurnal cycles of apparent fluorescence yields. Photosynth. Res. 2023, 155, 271–288. [Google Scholar] [CrossRef]
- Sulkiewicz, M.; Ciereszko, I. Fluorescence of chlorophyl a—History of discovery and application in plant research. Cosmos. Probl. Biol. Sci. 2016, 65, 103–115. (In Polish) [Google Scholar]
- WRB. World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. In World Soil Resources Reports 106; Food and Agriculture Organization of The United Nations: Rome, Italy, 2014. [Google Scholar]
- Iriti, M.; Scarafoni, A.; Pierce, S.; Castorina, G.; Vitalini, S. Soil application of effective microorganisms (EM) Maintains leaf photosynthetic efficiency, increases seed yield and quality traits of bean (Phaseolus vulgaris L.) plants grown so different substrates. Int. J. Mol. Sci. 2019, 20, 2327. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, A.; Kocoń, A. Evaluation of the effectiveness of preparations with beneficial microorganisms on the enzymatic activity of soil. Stud. I Rap. 2015, 45, 143–154. [Google Scholar]
- Lenartowicz, T. Descriptive List of Agricultural Cultivars. Potato; COBORU Publishing House: Słupia Wielka, Poland, 2022; p. 38. (In Polish) [Google Scholar]
- Duer, I.; Fotyma, M.; Madej, A. Code of Good Agricultural Practice, 2nd revised ed.; Foundation of Assistance Programs for Agriculture: Warsaw, Poland, 2004. (In Polish) [Google Scholar]
- Roztropowicz, S.; Czerko, Z.; Głuska, A.; Goliszewski, W.; Gruczek, T.; Lis, B.; Lutomirska, B.; Nowacki, W.; Rykaczewska, K.; Sowa-Niedziałkowska, G.; et al. Methodic of Observation, Measurements and Sample Take in Agricultural Experiments with Potato; Plant Breeding Acclimatization Institute: Jadwisin, Poland, 1999; p. 50. (In Polish) [Google Scholar]
- Krełowska-Kułas, M. Badanie Jakości Produktów Spożywczych; Państ. Wydaw. Ekonomiczne: Warszawa, Poland, 1993; (Krak.: DNar.). 554 ss. Identyfikator; Available online: http://polona.pl/item/1555918 (accessed on 25 September 2023). (In Polish)
- Tajner-Czopek, A. Methodology for determining the technological value and consumer quality of potato. Zeszyty Problemowe Postępów Nauk Rolniczych. Adv. Agric. Sci. 2006, 511, 95–103. (In Polish) [Google Scholar]
- Scope of Accreditation for Testing, PCA Scope of Accreditation No. AB 128. Available online: https://www.bosmal.com.pl/userfiles/plik/1525079239.pdf (accessed on 25 September 2023).
- ISO 10390:2005; Soil Quality—Determination of pH. ISO: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/40879.html (accessed on 9 June 2023).
- Polish Standard PN-R-04023:1996; Chemical and Agricultural Analysis of Soil. Determination of Available Phosphorus Content in Mineral Soils. Polish Committee for Standardization: Warsaw, Poland, 1996. (In Polish)
- Polish Standard PN-R-04022: 1996 + AZ1: 2002; Chemical and Agricultural Analysis of Soil. Determination of Available Potassium Content in Mineral Soils. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Polish Standard PN-R-04020 1994 + AZ1: 2004; Chemical and Agricultural Analysis of Soil. Polish Committee for Standardization: Warsaw, Poland, 2004. (In Polish)
- Mocek, A. Soil Science; State Scientific Publisher: Warsow, Poland, 2015; p. 571. [Google Scholar]
- Nawrocki, S. Fertilizer Recommendations. Limit Numbers for Valuation of Macro- and Micronutrient Content in Soils; IUNG Publishing House: Pulawy, Poland, 1985; p. 38. (In Polish) [Google Scholar]
- Skowera, B. Changes of hydrothermal conditions in the Polish area (1971–2010). Fragm. Agron. 2014, 31, 74–87. [Google Scholar]
- SAS Institute Inc. SAS/STAT®9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Koronacki, J.; Mielniczuk, J. Statistics for Students of Technical and Natural Sciences; Scientific and Technical Publishing House: Warsaw, Poland, 2009; p. 491. (In Polish) [Google Scholar]
- IBM. SPSS Statistics 28 User’s Guide—IBM Core System. Available online: https://www.ibm.com/docs/en/SSLVMB_28.0.0/pdf/IBM_SPSS_Statistics_Core_System_User_Guide.pdf (accessed on 25 September 2023).
- Starck, Z. The role of aquaporins in water transport in plants. Zesz. Probl. Postępów Nauk Rol. Adv. Agric. Sci. 2010, 1, 9–26. (In Polish) [Google Scholar]
- Figueroa, F.L.; Conde-Álvarez, R.; Gómez, I. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth. Res. 2003, 75, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Strihz, I.G.; Lysenko, G.G.; Neverov, K.V. Photoreduction of molecular oxygen in preparation of photosystem II under photoinhibitors conditions. Rus. J. Plant Physiol. 2005, 52, 717–723. [Google Scholar]
- Eyvazov, E.G. The Evaluation of the Potato Collection Samples According to Some Physiological Indicators and the Choosing of the Primary Donors for the Selection. Adv. Stud. Biol. 2023, 15, 137–147. [Google Scholar] [CrossRef]
- Kaczmarek, Z.; Jakubus, M.; Grzelak, M.; Mrugalska, L. Impact of the addition of various doses of Effective Microorganisms to arable-humus horizons of mineral soils on their physical and water properties. J. Res. Appl. Agric. Eng. 2008, 53, 118–121. [Google Scholar]
- Janas, R. Possibilities of using Effective Microorganisms in ecological crop production systems. Probl. Inżynierii Rol. 2009, 3, 111–119. (In Polish) [Google Scholar]
- Brestic, M.; Zivcak, M. PSII Fluorescence techniques for measurement of drought and Hight temperature stress signal in crop plants: Protocols and applications. In Molecular Stress Physiology of Plants; Springer: Dordrecht, Switzerland, 2013; pp. 81–131. [Google Scholar]
- Li, X.; Guo, Q.; Wang, Y.; Xu, J.; Wei, Q.; Chen, L.; Liao, L. Enhancing Nitrogen and Phosphorus Removal by Applying Effective Microorganisms to Constructed Wetlands. Water 2020, 12, 2443. [Google Scholar] [CrossRef]
- Okumoto, S.; Shintani, M.; Higa, T. The use of effective microorganisms and biochar inhibits the transfer of radioactive cesium from soil to plant during continuous Komatsu cultivation. In Proceedings of the International Scientific Conference “Radiobiology: Present”, Gomel, Belarus, 26–27 September 2019. [Google Scholar]
- Van Vliet, P.C.J.; Bloem, J.; de Goede, R.G.M. Microbial diversity. nitrogen loss and grass production after addition of Effective Micro-organisms (EM) to slurry manure. Appl. Soil Ecol. 2006, 32, 188–198. [Google Scholar] [CrossRef]
- Martyniuk, S.; Księżak, J. Evaluation of the impact of pseudo-microbial biopreparations used in plant cultivation. Pol. J. Argonomy 2011, 6, 27–33. (In Polish) [Google Scholar]
- Małuszyńska, E.; Szydłowska, A.; Martyniak, D.; Dziamba, S.; Dziamba, J. Effect of preparations containing effective microorganisms on the germination capacity of seeds from organic farming. Bull. IHAR 2012, 263, 33–42. (In Polish) [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A. The Influence of Effective Microorganisms on the Content of Selected Nutrients in Vegetables. In Current Trends in Quality Science–Design, Quality and Safety of Products; Institute of Quality Science, Poznań University of Economics & Business: Poznań, Poland, 2021; pp. 104–110. [Google Scholar]
- Polivanova, O.B.; Tiurin, K.N.; Sivolapova, A.B.; Goryunova, S.V.; Zhevora, S.V. Influence of Increased Radiation Background on Antioxidative Responses of Helianthus tuberosus L. Antioxidants 2023, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Vaitkevičienė, N.; Kulaitiene, J.; Jariene, E.; Levickiene, D.; Danilcenko, H.; Średnicka-Tober, D.; Rembiałkowska, E.; Hallmann, E. Characterization of Bioactive Compounds in Colored Potato (Solanum tuberosum L.) Cultivars Grown with Conventional, Organic, and Biodynamic Methods. Sustainability 2020, 12, 2701. [Google Scholar] [CrossRef]
- Dighton, J.; Tugay, T.; Zhdanova, N. Fungi and Ionizing Radiation from Radionuclides: Fungi and Ionizing Radiation. FEMS Microbiol. Lett. 2008, 281, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kalita, M.C.; Thakur, D. Broad Spectrum Antimicrobial Activity of Forest-Derived Soil Actinomycete, Nocardia sp. PB-52. Front. Microbiol. 2016, 18, 347. [Google Scholar] [CrossRef]
- Chęciński, G.; Czernaś, K. Beneficial microorganisms “ProBio-Emy” and their practical application. Gas Water Sanit. Technol. 2012, 2, 58–61. (In Polish) [Google Scholar]
- FAO. FAOO Chart—The State of Food Security and Nutrition in the World 2023. In Urbanization, Agrifood Systems Transformation and Healthy Diets across the Rural–Urban Continuum; FAO: Rome, Italy, 2023; Available online: https://www.fao.org (accessed on 7 August 2023).
- Kondhare, K.R.; Kumar, A.; Patil, N.S.; Malankar, N.N.; Saha, K.; Banerjee, A.K. Development of aerial and belowground tubers in potato are governed by photoperiod and epigenetic mechanism. Plant Physiol. 2021, 187, 1071–1086. [Google Scholar] [CrossRef]
- Sawicka, B.; Hulail Noaema, A.; Hameed, S.T.; Skiba, D. Genotype and environmental variability of chemical elements in potato tubers. Acta Sci. Pol. Agric. 2016, 15, 79–91. [Google Scholar]
- Seid, E.; Tessema, L.; Abebe, T.; Solomon, A.; Chindi, A.; Hirut, B.; Negash, K.; Shunka, E.; Mogse, Z.; Burgos, G.; et al. Genetic Variability for Micronutrient Content and Tuber Yield Traits among Biofortified Potato (Solanum tuberosum L.) Clones in Ethiopia. Plants 2023, 12, 2625. [Google Scholar] [CrossRef]
- Ahmad, D.; Zhang, Z.; Rasheed, H.; Xu, X.; Bao, J. Recent Advances in Molecular Improvement for Potato Tuber Traits. Int. J. Mol. Sci. 2022, 1, 9982. [Google Scholar] [CrossRef]
- Yan, W.; Qin, J.; Jian, Y.; Liu, J.; Bian, C.; Jin, L.; Li, G. Analysis of Potato Physiological and Molecular Adaptation in Response to Different Water and Nitrogen Combined Regimes. Plants 2023, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.V.; Hoekenga, O.A.; Gordin, M.; Broeckling, C.; De Jong, W.S. Applying network and genetic analysis to the potato metabolome. Front. Plant Sci. 2023, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska-Mańkowska, D.; Ruszczak, B.; Zarzyńska, K. Classification of Potato Varieties Drought Stress Tolerance Using Supervised Learning. Appl. Sci. 2022, 12, 1939. [Google Scholar] [CrossRef]
| Cultivars | Maturity Group | Color of Skin | Color of the Flesh | Shape of the Tubers | Type Consumption | Taste 9° Scale * |
|---|---|---|---|---|---|---|
| ‘Denar’ | Very early | Yellow | Light Yellow | Round Oval | AB | 7 |
| ‘Bellarosa’ | Early | Red | Yellow | Round Oval | B | 7 |
| ‘Gwiazda’ | Early | Yellow | Yellow | Round Oval | B | 7 |
| ‘Ignacy’ | Early | Yellow | Light Yellow | Round Oval | B | 6.5 |
| ‘Owacja’ | Early | Yellow | Light Yellow | Round Oval | B-BC | 7 |
| ‘Vineta’ | Early | Yellow | Yellow | Round | AB | 7 |
| ‘Finezja’ | Medium early | Yellow | Light Yellow | Round Oval | BC | 6.5 |
| ‘Oberon’ | Medium early | Red | Light Yellow | Oval | AB | 7 |
| ‘Satina’ | Medium early | Yellow | Yellow | Round Oval | B | 7.5 |
| ‘Tajfun’ | Medium early | Yellow | Yellow | Oval | B-BC | 7 |
| ‘Jelly’ | Medium late | Yellow | Yellow | Oval | B | 7.5 |
| ‘Mondeo’ | Medium late | Yellow | Creamy | Round Oval | B-BC | 6.7 |
| ‘Syrena’ | Medium late | Yellow | Yellow | Oval | B | 7 |
| ‘Kuras’ | late | Yellow | Creamy | Round | B | 6 |
| Years of Study | Assimilable Macronutrient Content [mg kg−1 of soil] | Humus Content [g kg−1 of soil] | pH in KCl | ||
|---|---|---|---|---|---|
| P2O5 | K2O | Mg | |||
| 2017 | 172 | 138 | 49 | 1.11 | 6.10 |
| 2018 | 184 | 126 | 54 | 1.14 | 6.20 |
| 2019 | 176 | 141 | 52 | 1.12 | 6.30 |
| Average | 177 | 135 | 52 | 1.12 | 6.20 |
| Experimental Factors | Physiological Indicators of Plant Growth | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fo | Fm | Fv/FM | Fo′ | Fm′ | Y | Qp | Qn | ||
| Pre-plantingtreatments | Control object | 0.218 a | 0.814 a | 0.580 a | 0.188 b | 0.324 a | 0.363 a | 0.499 a | 0.130 a |
| Exposition I * | 0.230 b | 0.861 b | 0.626 b | 0.211 c | 0.381 c | 0.402 b | 0.581 c | 0.145 b | |
| Exposition II ** | 0.227 a | 0.866 b | 0.616 b | 0.178 a | 0.350 b | 0.361 a | 0.529 b | 0.125 a | |
| HSD *** p≤0.05 | 0.011 | 0.043 | 0.031 | 0.010 | 0.018 | 0.017 | 0.028 | 0.007 | |
| Cultivars | ‘Denar’ | 0.205 a | 0.836 a | 0.586 a | 0.155 a | 0.329 a | 0.341 a | 0.489 a | 0.120 a |
| ‘Bellarosa’ | 0.217 a | 0.777 a | 0.542 a | 0.165 a | 0.311 a | 0.331 a | 0.460 a | 0.128 a | |
| ‘Gwiazda’ | 0.204 a | 0.749 a | 0.548 a | 0.160 a | 0.318 a | 0.337 a | 0.470 a | 0.140 b | |
| ‘Ignacy’ | 0.210 a | 0.786 a | 0.583 a | 0.172 a | 0.347 a | 0.376 a | 0.509 a | 0.139 ab | |
| ‘Owacja’ | 0.208 a | 0.756 a | 0.559 a | 0.161 a | 0.323 a | 0.335 a | 0.484 a | 0.128 a | |
| ‘Vineta’ | 0.225 a | 0.856 a | 0.592 a | 0.181 a | 0.349 a | 0.380 a | 0.590 b | 0.138 ab | |
| ‘Finezja’ | 0.226 a | 0.835 a | 0.610 a | 0.204 b | 0.343 a | 0.401 ab | 0.556 a | 0.134 ab | |
| ‘Oberon’ | 0.254 a | 0.975 b | 0.672 b | 0.210 b | 0.397 b | 0.372 a | 0.604 b | 0.134 ab | |
| ‘Satina’ | 0.252 a | 0.985 b | 0.687 b | 0.225 b | 0.375 a | 0.408 b | 0.641 b | 0.152 b | |
| ‘Tajfun’ | 0.228 a | 0.857 a | 0.615 a | 0.214 b | 0.357 a | 0.404 b | 0.548 a | 0.135 a | |
| ‘Jelly’ | 0.242 a | 0.899 a | 0.670 b | 0.213 b | 0.390 ab | 0.405 b | 0.591 b | 0.137 a | |
| ‘Mondeo’ | 0.240 a | 0.887 a | 0.629 a | 0.217 b | 0.371 a | 0.420 b | 0.570 b | 0.141 b | |
| ‘Syrena’ | 0.230 a | 0.861 a | 0.641 a | 0.221 b | 0.372 a | 0.421 b | 0.551 a | 0.131 a | |
| ‘Kuras’ | 0.203 a | 0.800 a | 0.575 a | 0.188 a | 0.338 a | 0.327 a | 0.445 a | 0.110 a | |
| HSD p≤0.05 | ns **** | 0.202 | 0.125 | 0.046 | 0.084 | 0.077 | 0.125 | 0.030 | |
| Years | 2017 | 0.228 b | 0.823 a | 0.627 a | 0.194 a | 0.356 b | 0.345 a | 0.490 a | 0.138 b |
| 2018 | 0.244 c | 0.842 a | 0.592 a | 0.194 a | 0.324 a | 0.403 c | 0.541 b | 0.115 a | |
| 2019 | 0.202 a | 0.876 b | 0.605 a | 0.187 a | 0.375 c | 0.379 b | 0.578 c | 0.147 c | |
| HSD p≤0.05 | 0.013 | 0.050 | ns **** | ns **** | 0.018 | 0.022 | 0.032 | 0.008 | |
| Mean | 0.225 | 0.847 | 0.608 | 0.192 | 0.351 | 0.376 | 0.536 | 0.133 | |
| Experimental Factors | Dry Matter (%) | Starch (%) | |
|---|---|---|---|
| Pre-planting treatments | Control object | 20.2 a | 15.2 a |
| Exposition I * | 20.4 a | 15.4 a | |
| Exposition II ** | 20.6 a | 15.4 a | |
| HSD *** p≤0.05 | ns **** | ns **** | |
| Cultivars | ‘Denar’ | 16.3 a | 12.4 a |
| ‘Bellarosa’ | 18.0 a | 13.8 a | |
| ‘Gwiazda’ | 16.5 a | 13.3 a | |
| ‘Ignacy’ | 18.0 a | 13.6 a | |
| ‘Owacja’ | 17.7 a | 13.8 a | |
| ‘Vineta’ | 19.3 a | 14.5 a | |
| ‘Finezja’ | 23.9 c | 18.0 bc | |
| ‘Oberon’ | 20.8 b | 15.6 ab | |
| ‘Satina’ | 21.5 b | 15.7 ab | |
| ‘Tajfun’ | 23.0 bc | 17.4 bc | |
| ‘Jelly’ | 20.2 b | 14.8 b | |
| ‘Mondeo’ | 22.1 b | 16.2 b | |
| ‘Syrena’ | 21.0 b | 15.5 b | |
| ‘Kuras’ | 28.0 d | 20.3 c | |
| HSD p≤0.05 | 3.8 | 2.9 | |
| Years | 2017 | 20.1 a | 15.1 a |
| 2018 | 20.7 a | 15.7 b | |
| 2019 | 20.3 a | 15.2 a | |
| HSD p≤0.05 | ns **** | 0.6 | |
| Mean | 20.4 | 15.4 | |
| Experimental Factors * | Yield (t ha−1) | |||
|---|---|---|---|---|
| Yield of Fresh Mass | Dry Mass Yield | Starch Yield | ||
| Pre-planting treatments | Control object | 37.78 a | 7.57 a | 5.73 a |
| Exposition I | 46.63 b | 9.53 b | 7.15 b | |
| Exposition II | 45.09 b | 9.28 b | 6.96 b | |
| HSD *** p≤0.05 | 2.37 | 0.42 | 0.33 | |
| Cultivars | ‘Denar’ | 48.57 c | 7.98 b | 6.05 b |
| ‘Bellarosa’ | 38.09 a | 6.88 a | 5.23 a | |
| ‘Gwiazda’ | 43.56 b | 7.35 a | 5.71 b | |
| ‘Ignacy’ | 48.58 c | 8.79 b | 6.63 b | |
| ‘Owacja’ | 36.46 a | 6.56 a | 5.02 a | |
| ‘Vineta’ | 40.86 b | 7.86 b | 5.92 b | |
| ‘Finezja’ | 40.18 b | 9.56 bc | 7.20 c | |
| ‘Oberon’ | 39.88 b | 8.26 b | 6.21 b | |
| ‘Satina’ | 48.48 c | 10.33 c | 7.66 c | |
| ‘Tajfun’ | 42.97 b | 9.88 c | 7.46 c | |
| ‘Jelly’ | 41.42 b | 8.31 b | 6.17 b | |
| ‘Mondeo’ | 48.14 c | 10.49 c | 7.82 c | |
| ‘Syrena’ | 45.63 c | 9.42 bc | 7.05 c | |
| ‘Kuras’ | 41.56 b | 11.41c | 8.48 c | |
| HSD p≤0.05 | 4.54 | 1.97 | 1.54 | |
| Years | 2017 | 45.47 b | 9.10 b | 6.84 b |
| 2018 | 35.66 a | 7.35 a | 5.57 a | |
| 2019 | 48.22 c | 9.89 c | 7.43 c | |
| HSD p≤0.05 | 2.37 | 0.42 | 0.33 | |
| Mean | 43.17 | 8.79 | 6.61 | |
| Specification | y1 | y2 | y3 | y4 | y5 | x1 | x2 | x3 | x4 | x5 | x6 | x7 | x8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 43.17 | 20.38 | 8.79 | 15.36 | 6.61 | 0.22 | 0.85 | 0.61 | 0.19 | 0.35 | 0.37 | 0.54 | 0.13 |
| Median | 43.51 | 20.13 | 8.74 | 15.10 | 6.57 | 0.22 | 0.85 | 0.60 | 0.19 | 0.35 | 0.37 | 0.52 | 0.14 |
| Standard error | 0.45 | 0.15 | 0.13 | 0.11 | 0.10 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| Standard deviation | 8.52 | 3.00 | 2.54 | 2.18 | 1.86 | 0.04 | 0.10 | 0.08 | 0.04 | 0.06 | 0.06 | 0.10 | 0.02 |
| Kurtosis | −0.69 | 0.48 | −0.46 | 0.44 | −0.50 | −0.66 | 1.96 | 0.29 | 2.32 | 7.27 | −0.73 | 0.00 | 0.00 |
| Skewness | −0.09 | 0.68 | 0.26 | 0.69 | 0.25 | 0.41 | 0.77 | 0.15 | 1.03 | 1.61 | 0.39 | 0.30 | 0.11 |
| Range | 49.38 | 14.53 | 11.55 | 10.90 | 8.60 | 0.15 | 0.65 | 0.44 | 0.25 | 0.50 | 0.27 | 0.55 | 0.14 |
| Minimum | 19.52 | 14.53 | 3.78 | 10.90 | 2.90 | 0.16 | 0.60 | 0.37 | 0.10 | 0.23 | 0.26 | 0.32 | 0.07 |
| Maximum | 68.90 | 29.07 | 15.33 | 21.80 | 11.50 | 0.31 | 1.25 | 0.80 | 0.35 | 0.73 | 0.53 | 0.87 | 0.21 |
| V (%) | 24.99 | 14.70 | 28.85 | 14.22 | 28.12 | 15.74 | 12.39 | 12.99 | 20.40 | 15.72 | 16.63 | 19.38 | 18.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pszczółkowski, P.; Sawicka, B.; Skiba, D.; Barbaś, P.; Noaema, A.H. The Use of Chlorophyll Fluorescence as an Indicator of Predicting Potato Yield, Its Dry Matter and Starch in the Conditions of Using Microbiological Preparations. Appl. Sci. 2023, 13, 10764. https://doi.org/10.3390/app131910764
Pszczółkowski P, Sawicka B, Skiba D, Barbaś P, Noaema AH. The Use of Chlorophyll Fluorescence as an Indicator of Predicting Potato Yield, Its Dry Matter and Starch in the Conditions of Using Microbiological Preparations. Applied Sciences. 2023; 13(19):10764. https://doi.org/10.3390/app131910764
Chicago/Turabian StylePszczółkowski, Piotr, Barbara Sawicka, Dominika Skiba, Piotr Barbaś, and Ali Hulail Noaema. 2023. "The Use of Chlorophyll Fluorescence as an Indicator of Predicting Potato Yield, Its Dry Matter and Starch in the Conditions of Using Microbiological Preparations" Applied Sciences 13, no. 19: 10764. https://doi.org/10.3390/app131910764






