Preclinical Studies of the Antimicrobial and Wound-Healing Effects of the High-Intensity Optical Irradiation “Zarnitsa-A” Apparatus
Abstract
:1. Introduction
- UV-C therapy could be much more cost-effective than commonly used antibiotics. This is because, in addition to inactivating microorganisms, adequate exposure to short-wave UV radiation promotes accelerated wound healing and restores skin homeostasis. The effects of UV-C radiation on wound healing include hyperplasia and enhanced re-epithelialization, granulation tissue formation, and the shedding of necrotic tissue [3,21,28].
2. Materials and Methods
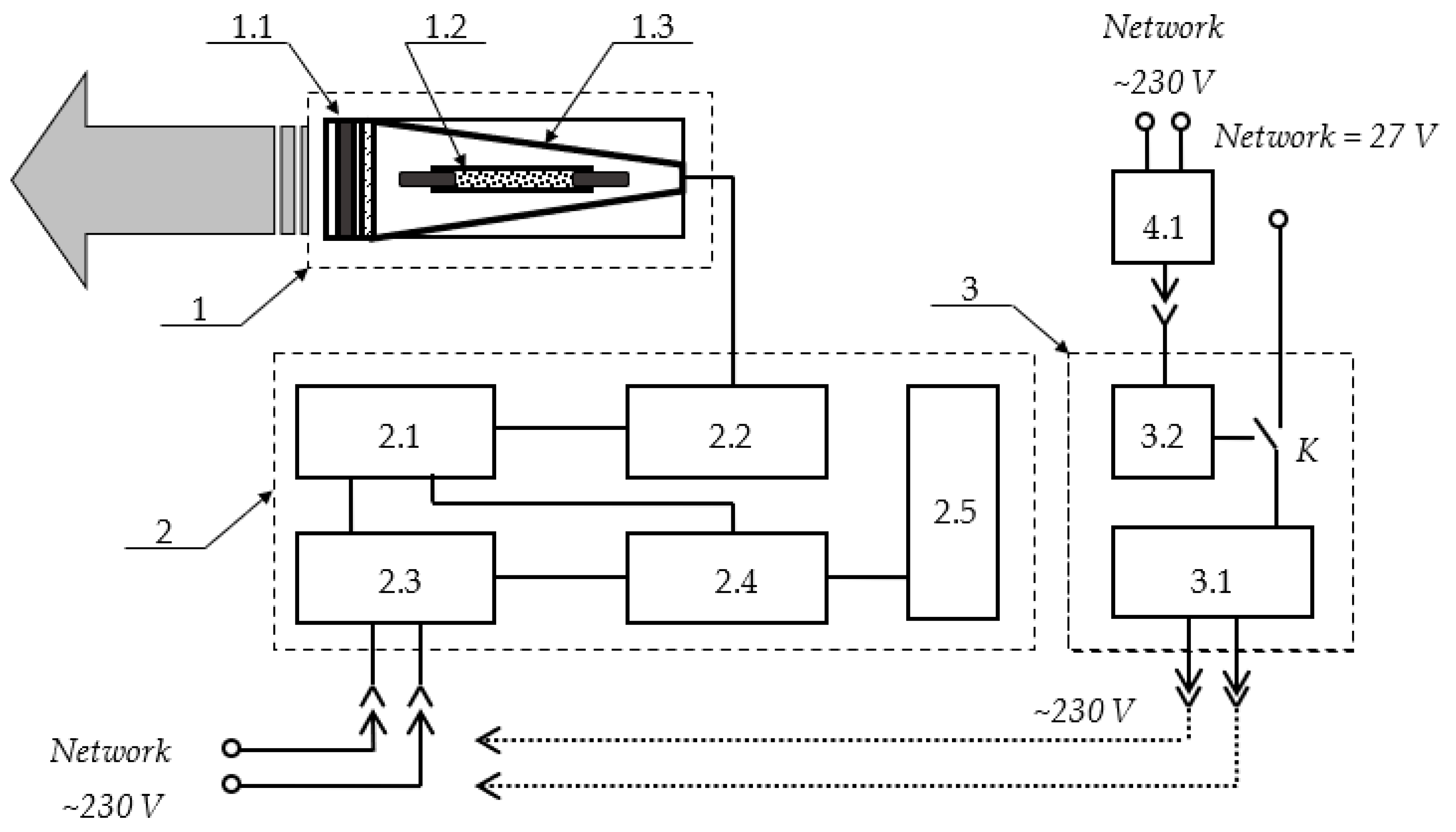
2.1. Description of the “Zarnitsa-A” Prototype
- A key for supplying power to the device: when the key is turned clockwise to the end, operating voltages are applied to the power and control circuits;
- A “Start” button: when it is pressed, the apparatus starts working; the irradiator emits light flashes that follow at a frequency of 5 Hz for 20 s (100 flashes in total) for one irradiation cycle;
- A “Stop” button: when it is pressed, the irradiator stops emitting light flashes, but power continues to be supplied to the circuits of the apparatus. When one presses the “Start” button again, the operation of the device is resumed.
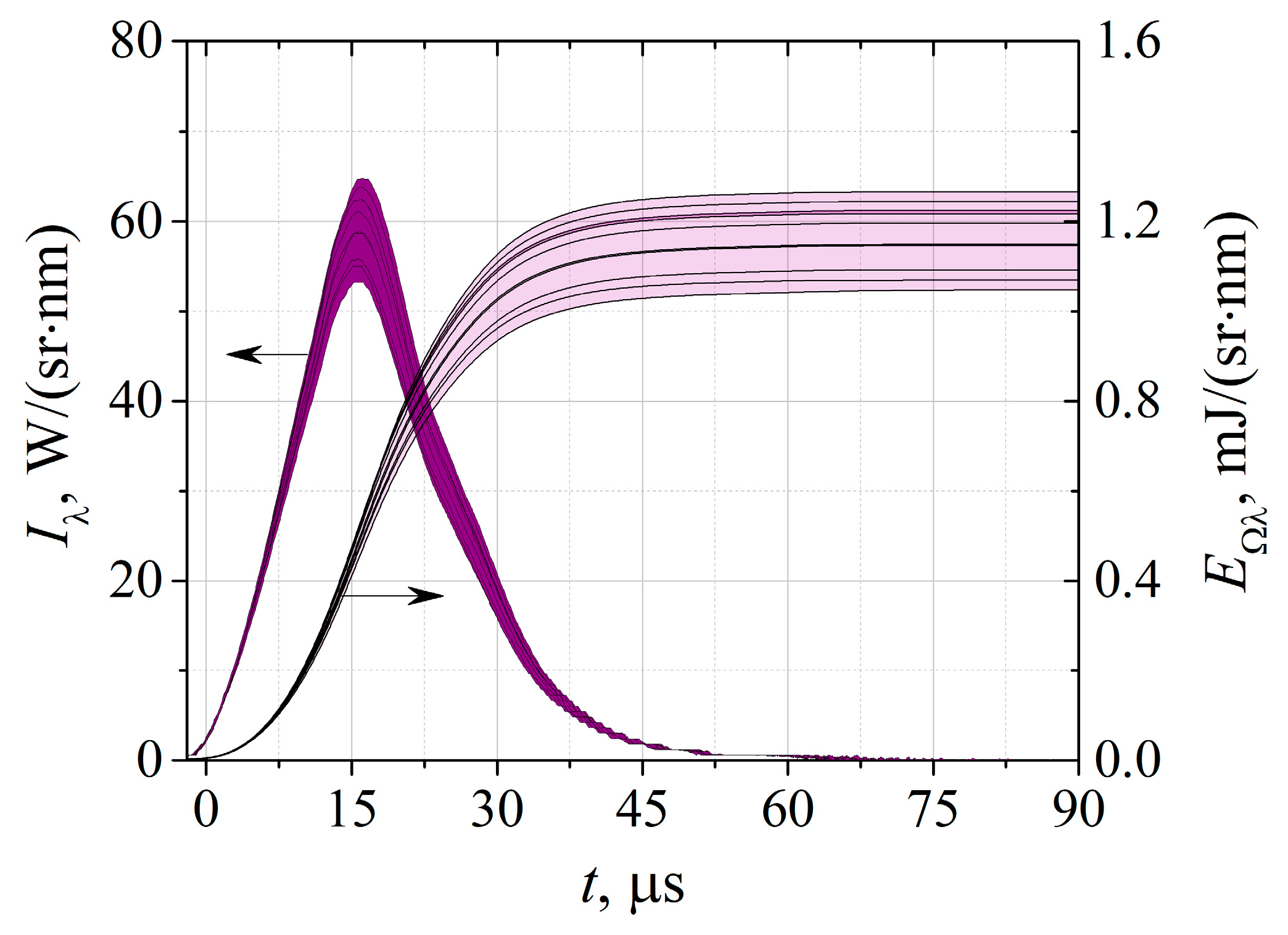
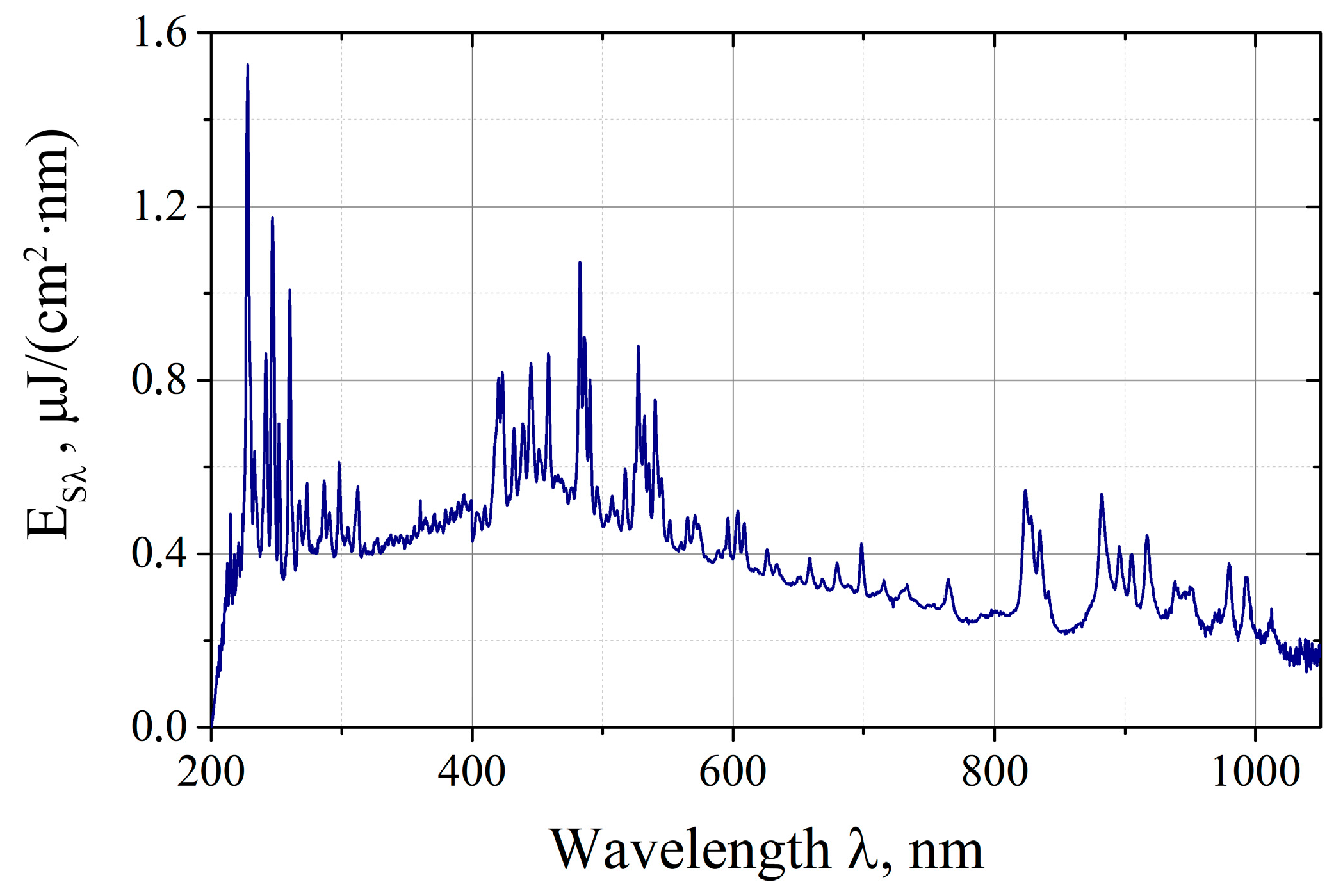
2.2. Spectral Energy Measurements
2.3. Studies In Vitro
- Gram-negative bacteria: Escherichia coli (M-17 (O2:H6)) and Pseudomonas aeruginosa (ATCC 9027/NCTC 12924);
- Gram-positive bacteria: Staphylococcus aureus (ATCC 6538-P/FDA 209-P) and Streptococcus pyogenes (ATCC 12344/NCTC 8198).
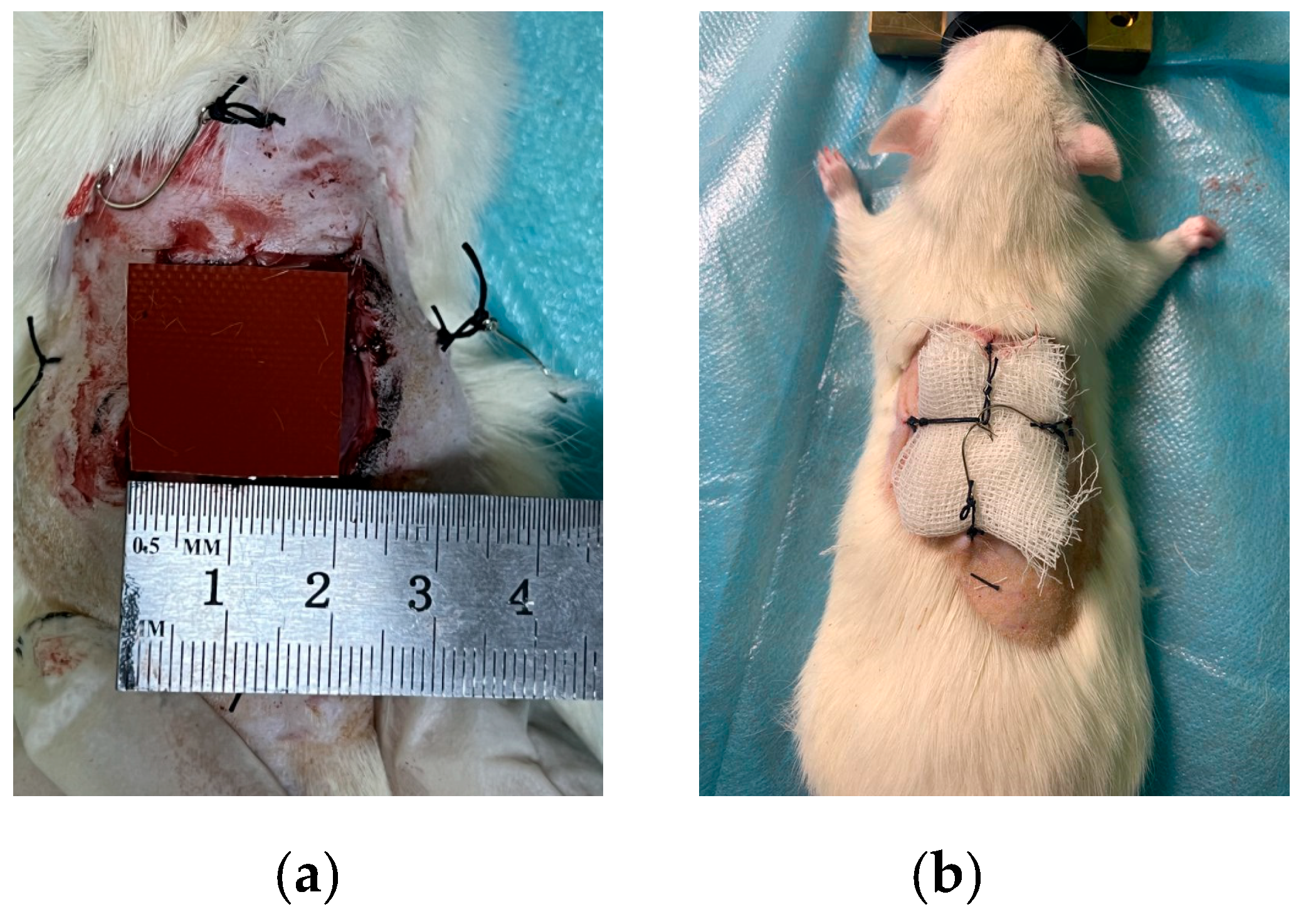
2.4. Preclinical Studies In Vivo
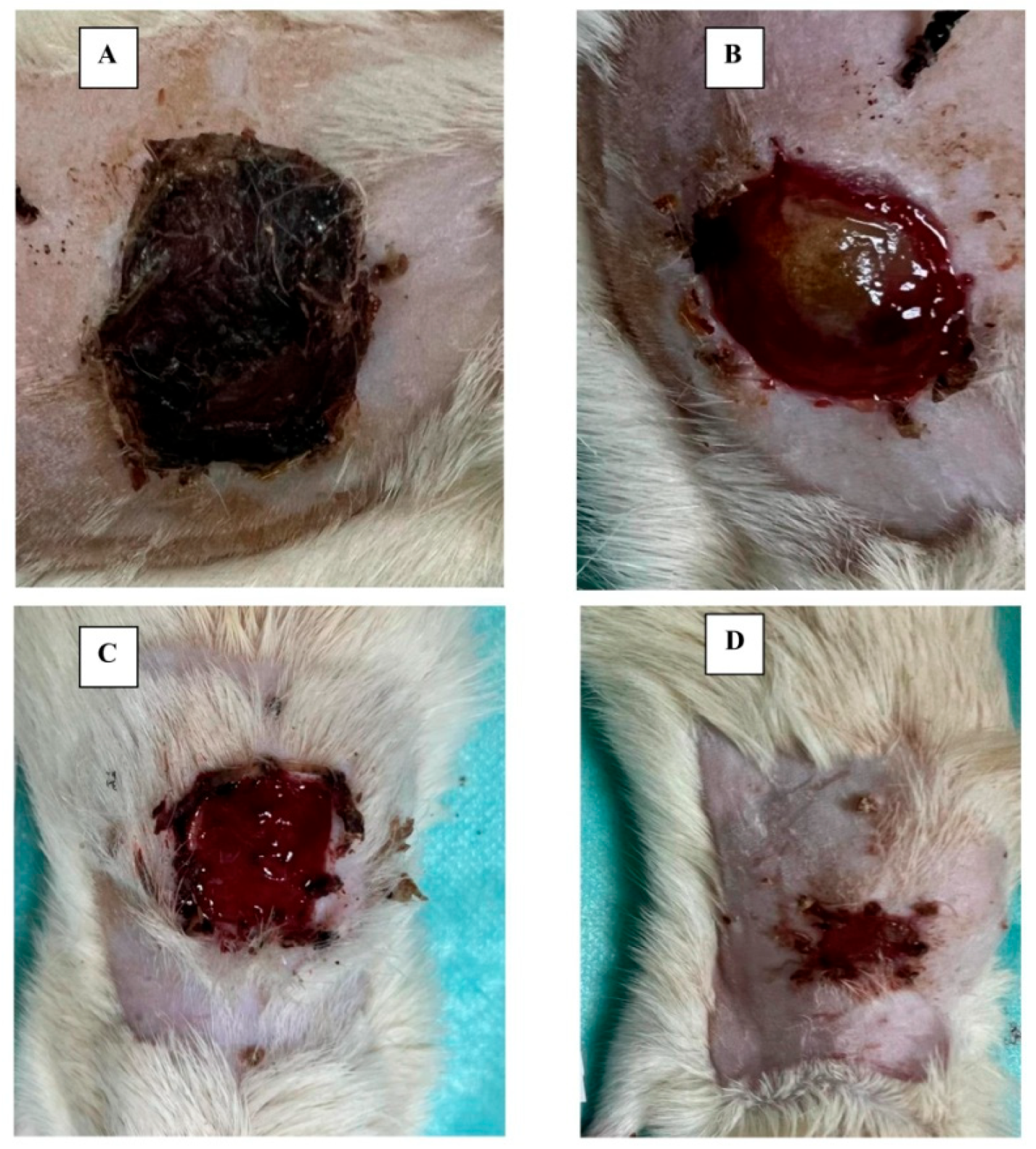
Pathology (Wound) Modeling
2.5. Statistical Processing
3. Results and Discussion
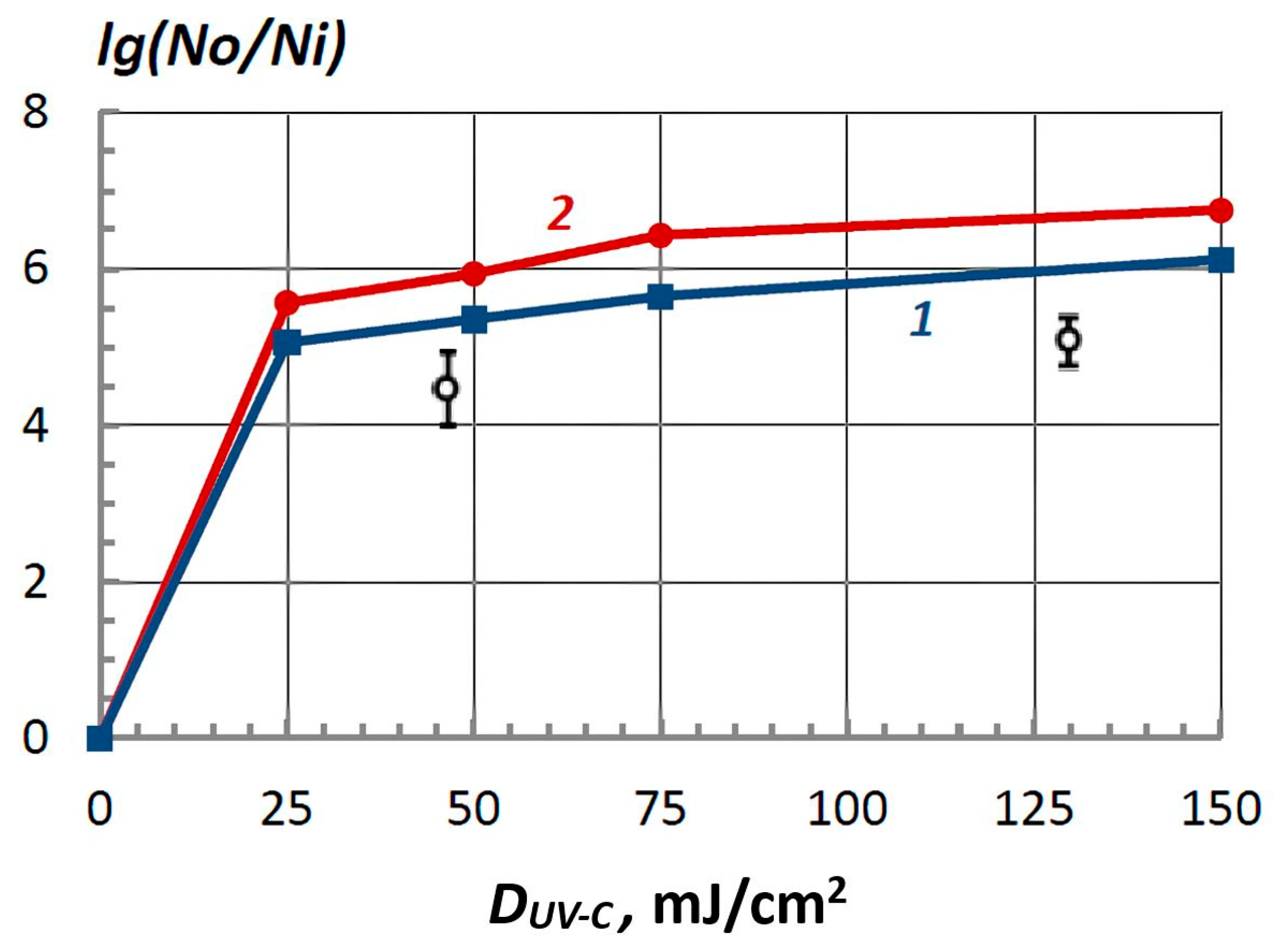
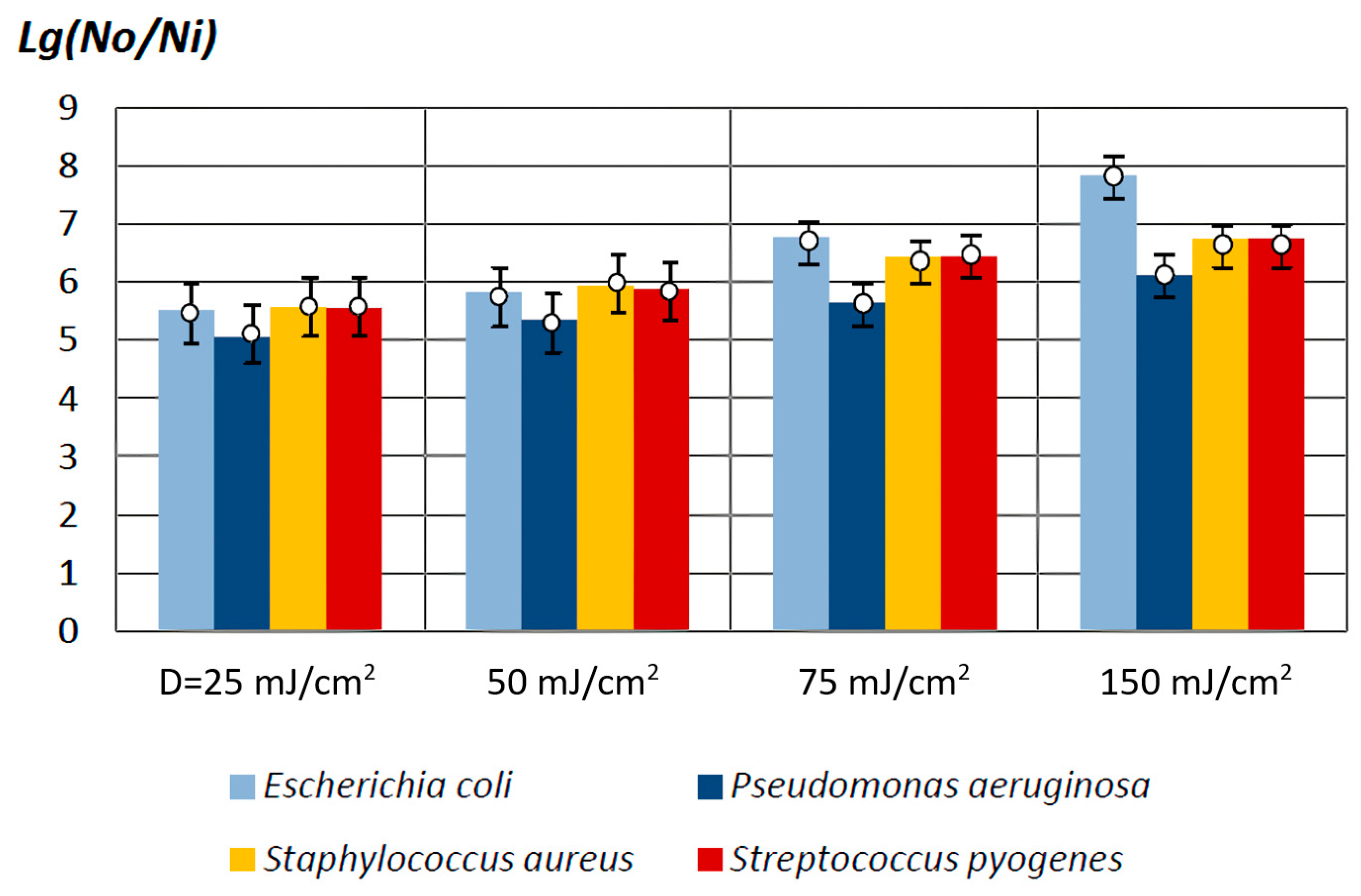
3.1. Main Results of the In Vitro Studies
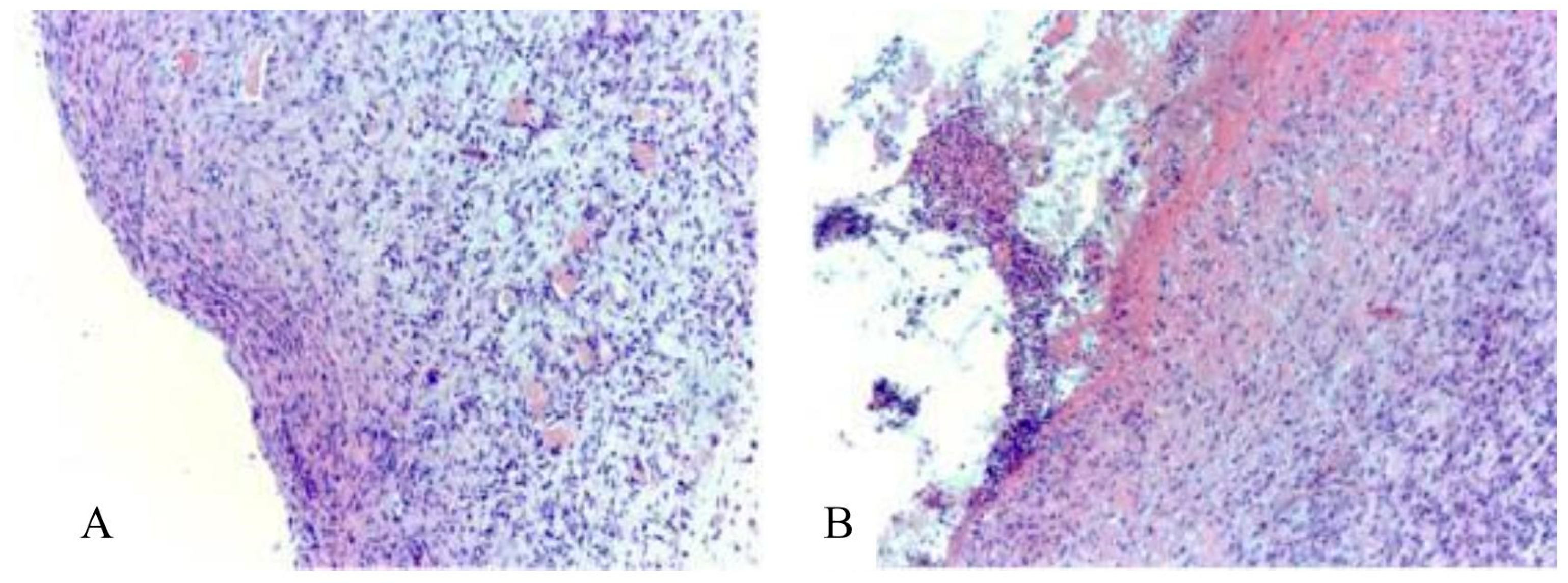
3.2. Main Results of Preclinical In Vivo Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Control Time | Experimental Groups | ||
|---|---|---|---|
| Control № 1 | Control № 2 | Main № 3 | |
| Background | 37.6 (37.6–37.8) | 37.5 (37.5–37.6) | 37.6 (37.5–37.6) |
| 3 days | 37.0 (36.9–37.5) | 36.9 (36.5–37.0) | 37.2 (37.0–37.4) |
| 7 days | 37.2 (37.1–37.6) | 37.1 (36.9–37.4) | 37.3 (37.0–37.5) |
| 10 days | 37.2 (36.9–37.8) | 36.8 (36.4–37.0) | 37.2 (36.9–37.3) |
| 14 days | 36.7 (36.4–37.3) | 36.8 (36.5–36.9) | 37.2 (36.9–37.2) |
Appendix B
| Control Time | Experimental Groups | ||
|---|---|---|---|
| Control № 1 | Control № 2 | Main № 3 | |
| Background | 220.0 (218.5–226.5) | 220.0 (218.5–229.0) | 220.5 (217.5–235.5) |
| 3 days | 217.0 (214.5–220.0) | 211.0 (207.5–220.0) | 216.0 (210.0–225.5) |
| 7 days | 226.0 (219.0–232.5) | 221.0 (213.0–224.5) | 224.0 (217.0–231.0) |
| 10 days | 225.0 (223.5–230.5) | 212.0 (209.0–226.5) | 215.5 (209.5–225.0) |
| 14 days | 221.0 (211.5–231.0) | 214.0 (203.0–230.5) | 228.5 (217.5–236.0) |
Appendix C
| Investigated Indicators | Experimental Groups | ||
|---|---|---|---|
| Control № 1 | Control № 2 | Main № 3 | |
| 1 Day | |||
| Number of white blood cells (WBC), 109/L | 14.2 ± 5.0 | 12.9 ± 4.7 | 13.4 ± 2.9 |
| Lymphocytes (LYM), 109/L | 2.3 ± 0.7 | 2.1 ± 0.8 | 2.4 ± 0.7 |
| Monocytes and eosinophils (MID), 109/L | 4.8 ± 1.3 | 4.1 ± 1.6 | 4.8 ± 1.0 |
| Granulocytes (GRAN), 109/L | 7.1 ± 3.9 | 6.7 ± 3.0 | 6.2 ± 1.9 |
| Erythrocytes (RBC), 1012/L | 8.9 ± 1.6 | 8.7 ± 1.5 | 9.2 ± 1.1 |
| Hemoglobin concentration (HGB), g/L | 156.5 ± 27.4 | 152.0 ± 25.8 | 159.5 ± 16.2 |
| Hematocrit (HCT), % | 35.6 ± 6.3 | 34.9 ± 5.9 | 37.0 ± 4.5 |
| Average red blood cell volume (MCV), fl | 40.2 ± 0.6 | 40.0 ± 0.8 | 40.2 ± 0.6 |
| Average hemoglobin content in erythrocyte (MCH), pg | 17.6 ± 0.8 | 17.4 ± 0.4 | 17.3 ± 0.6 |
| Average concentration of hemoglobin in erythrocyte (MCHC), g/dL | 439.1 ± 23.8 | 434.9 ± 8.6 | 432. ± 15.7 |
| Platelet count (PLT), 109/L | 311.8 ± 86.0 | 351 ± 99.7 | 347.8 ± 81.1 |
| Average platelet volume (MPV), fl | 9.5 ± 0.8 | 9.5 ± 0.9 | 9.3 ± 0.6 |
| Platelet distribution latitude (PDW), fl | 8.1 ± 0.6 | 8.5 ± 0.8 | 8.7 ± 1.1 |
| Plateletcrit, (PCT), % | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Investigated Indicators | Experimental Groups | ||
|---|---|---|---|
| Control № 1 | Control № 2 | Main № 3 | |
| 3 Days | |||
| Number of white blood cells (WBC), 109/L | 19.5 ± 7.7 | 19.1 ± 13.5 | 19.5 ± 7.7 |
| Lymphocytes (LYM), 109/L | 3.4 ± 1.4 # | 3.9 ± 2.6 | 3.4 ± 1.4 # |
| Monocytes and eosinophils (MID), 109/L | 5.1 ± 1.6 | 5.6 ± 2.8 | 5.1 ± 1.7 |
| Granulocytes (GRAN), 109/L | 11.0 ± 5.1 | 9.6 ± 8.5 | 11.0 ± 5.1 |
| Erythrocytes (RBC), 1012/L | 6.4 ± 1.9 | 6.4 ± 2.7 # | 6.4 ± 1.9 #* |
| Hemoglobin concentration (HGB), g/L | 122.1 ± 31.3 | 121.9 ± 48.5 | 122.1 ± 31.3 # |
| Hematocrit (HCT), % | 27.6 ± 6.8 | 28.3 ± 10.9 | 27.6 ± 6.8 # |
| Average red blood cell volume (MCV), fl | 43.9 ± 3.2 # | 44.8 ± 2.7 *# | 43.9 ± 3.2 # |
| Average hemoglobin content in erythrocyte (MCH), pg | 19.3 ± 1.6 # | 19.1 ± 1.0 # | 19.3 ± 1.6 # |
| Average concentration of hemoglobin in erythrocyte (MCHC), g/dL | 440.9 ± 19.0 | 402.3 ± 104.6 | 440.9 ± 19.0 |
| Platelet count (PLT), 109/L | 309.6 ± 86.8 | 350.7 ± 121.2 | 309.6 ± 86.8 |
| Average platelet volume (MPV), fl | 9.6 ± 0.5 | 9.4 ± 0.5 | 9.6 ± 0.5 |
| Platelet distribution latitude (PDW), fl | 7.8 ± 1.3 | 6.7 ± 2.3 | 7.8 ± 1.3 |
| Plateletcrit, (PCT), % | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Investigated Indicators | Experimental Groups | ||
|---|---|---|---|
| Control № 1 | Control № 2 | Main № 3 | |
| 7 Days | |||
| Number of white blood cells (WBC), 109/L | 19.3 ± 4.5 # | 18.7 ± 14.1 | 14.7 ± 3.4 * |
| Lymphocytes (LYM), 109/L | 3.0 ± 1.0 | 3.4 ± 2.4 | 2.5 ± 0.8 |
| Monocytes and eosinophils (MID), 109/L | 6.0 ± 1.5 | 5.3 ± 2.8 | 4.7 ± 1.1 * |
| Granulocytes (GRAN), 109/L | 10.4 ± 3.3 # | 9.3 ± 9.7 | 7.5 ± 2.0 * |
| Erythrocytes (RBC), 1012/L | 8.4 ± 2.6 | 7.3 ± 1.3 # | 6.4 ± 1.7 # |
| Hemoglobin concentration (HGB), g/L | 163.8 ± 43.1 | 143.5 ± 23.1 | 127.9 ± 28.6 #* |
| Hematocrit (HCT), % | 37.6 ± 9.7 | 33.0 ± 4.3 | 29.4 ± 6.7 #* |
| Average red blood cell volume (MCV), fl | 45.3 ± 2.8 # | 45.4 ± 2.2 # | 40.8 ± 15.3 |
| Average hemoglobin content in erythrocyte (MCH), pg | 19.6 ± 1.2 # | 19.6 ± 1.0 # | 19.5 ± 3.2 |
| Average concentration of hemoglobin in erythrocyte (MCHC), g/dL | 434.7 ± 15.9 | 432.6 ± 21.8 | 434.9 ± 7.4 |
| Platelet count (PLT), 109/L | 331.2 ± 70.2 | 405.4 ± 60.7 | 406.6 ± 90.4 #* |
| Average platelet volume (MPV), fl | 9.6 ± 0.7 | 8.9 ± 9.2 | 9.2 ± 0.3 |
| Platelet distribution latitude (PDW), fl | 8.1 ± 1.1 | 8.9 ± 7.5 | 7.6 ± 1.3 |
| Plateletcrit, (PCT), % | 0.3 ± 0.1 | 0.2 ± 0.3 | 0.4 ± 0.1 # |
| Investigated Indicators | Experimental Groups | ||
|---|---|---|---|
| Control № 1 | Control № 2 | Main № 3 | |
| 10 Days | |||
| Number of white blood cells (WBC), 109/L | 17.2 ± 4.9 # | 20.0 ± 7.6 # | 15.7 ± 3.5 |
| Lymphocytes (LYM), 109/L | 3.9 ± 1.5 # | 4.4 ± 1.7 # | 3.2 ± 1.7 |
| Monocytes and eosinophils (MID), 109/L | 7.1 ± 1.7 # | 7.4 ± 2.6 # | 6.1 ± 1.1 # |
| Granulocytes (GRAN), 109/L | 6.2 ± 2.7 | 8.2 ± 4.3 | 5.8 ± 2.9 |
| Erythrocytes (RBC), 1012/L | 7.7 ± 1.9 # | 6.9 ± 2.3 # | 7.6 ± 1.9 |
| Hemoglobin concentration (HGB), g/L | 141.5 ± 33.0 | 128.7 ± 43.8 | 143.6 ± 34.7 |
| Hematocrit (HCT), % | 33.4 ± 7.2 | 30.0 ± 9.3 | 31.1 ± 11.3 |
| Average red blood cell volume (MCV), fl | 44.1 ± 3.1 # | 43.8 ± 2.4 # | 44.1 ± 1.8 # |
| Average hemoglobin content in erythrocyte (MCH), pg | 18.6 ± 1.2 # | 18.7 ± 1.1 # | 18.8 ± 0.8 # |
| Average concentration of hemoglobin in erythrocyte (MCHC), g/dL | 400.7 ± 90.0 | 426.6 ± 18.9 | 428.9 ± 18.4 |
| Platelet count (PLT), 109/L | 326.9 ± 124.0 | 328.9 ± 108.2 | 327.1 ± 120.4 |
| Average platelet volume (MPV), fl | 9.3 ± 0.3 | 8.9 ± 9.2 | 9.2 ± 0.3 |
| Platelet distribution latitude (PDW), fl | 7.8 ± 2.3 | 8.9 ± 7.5 | 8.3 ± 1.0 |
| Plateletcrit, (PCT), % | 0.3 ± 0.1 | 0.2 ± 0.3 | 0.3 ± 0.1 |
| Investigated Indicators | Experimental Groups | ||
|---|---|---|---|
| Control № 1 | Control № 2 | Main № 3 | |
| 14 Days | |||
| Number of white blood cells (WBC), 109/L | 13.2 ± 4.4 | 12.2 ± 3.2 | 10.8 ± 2.1 |
| Lymphocytes (LYM), 109/L | 3.0 ± 0.9 | 3.3 ± 1.4 # | 2.1 ± 0.6 * |
| Monocytes and eosinophils (MID), 109/L | 4.8 ± 1.3 | 4.7 ± 1.3 | 4.0 ± 1.0 |
| Granulocytes (GRAN), 109/L | 5.4 ± 3.3 | 4.3 ± 1.0 # | 4.7 ± 1.4 |
| Erythrocytes (RBC), 1012/L | 7.7 ± 1.2 # | 6.9 ± 2.4 # | 7.9 ± 1.2 |
| Hemoglobin concentration (HGB), g/L | 140.2 ± 22.7 # | 127.5 ± 36.4 | 141.6 ± 22.1 |
| Hematocrit (HCT), % | 33.9 ± 5.6 | 31.4 ± 5.8 | 33.7 ± 4.9 |
| Average red blood cell volume (MCV), fl | 44.9 ± 8.7 | 42.4 ± 2.7 # | 42.9 ± 1.9 |
| Average hemoglobin content in erythrocyte (MCH), pg | 18.3 ± 1.6 # | 17.9 ± 1.2 | 18.0 ± 1.1 |
| Average concentration of hemoglobin in erythrocyte (MCHC), g/dL | 415.5 ± 43.2 | 424.5 ± 16.1 | 419.4 ± 17.3 # |
| Platelet count (PLT), 109/L | 552.6 ± 154.8 # | 532.8 ± 117.1 # | 421.0 ± 107.7 #* |
| Average platelet volume (MPV), fl | 9.3 ± 0.8 | 8.9 ± 9.2 | 9.1 ± 0.4 |
| Platelet distribution latitude (PDW), fl | 8.2 ± 0.9 | 8.9 ± 7.5 | 8.2 ± 1.0 |
| Plateletcrit, (PCT), % | 0.5 ± 0.2 # | 0.2 ± 0.3 # | 0.4 ± 0.1 #* |
Appendix D
| Control Time | Experimental Groups | ||
|---|---|---|---|
| Control № 1 | Control № 2 | Main № 3 | |
| 3 days | 325.0 (290.0–370.0) | 370.0 (327.5–385.0) | 340.0 (300.0–385.0) |
| 7 days | 420.0 (367.5–452.5) | 397.5 * (361.5–479.0) | 340.0 * (322.5.0–370.0) |
| 10 days | 385.0 (350.0–437.5) | 342.5 (311.0–405.0) | 320.0 * (262.5–342.5) |
| 14 days | 290.0 (227.5–357.5) | 292.5 (167.5–317.5) | 245.0 (210.0–290.0) |
References
- Tretyakov, A.; Petrov, S.; Neverov, A.; Shchetinin, A. Treatment of Purulent Wounds. Nov. Khirurgii 2015, 23, 680–687. [Google Scholar] [CrossRef]
- Lee, C.-R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to Minimize Antibiotic Resistance. Int. J. Environ. Res. Public Health 2013, 10, 4274–4305. [Google Scholar] [CrossRef]
- Gupta, A.; Avci, P.; Dai, T.; Huang, Y.-Y.; Hamblin, M.R.; Yan, J.-X.; Liao, X.; Li, S.-H.; Liu, H.-W.; Chang, H.-Y.; et al. Ultraviolet Radiation in Wound Care: Sterilization and Stimulation. Adv. Wound Care 2013, 2, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Ushakov, A.A. Modern Physiotherapy in Clinical Practice; ANMI: Moscow, Russia, 2002. [Google Scholar]
- Motorina, I.G.; Kulikov, L.K.; Meleshko, T.I. Modern idea about physiotherapeutic ways of treatments of chronic injuries. Sib. Sci. Med. J. 2012, 114, 8–11. [Google Scholar]
- Zaslavskii, A.Y.; Gelis, Y.S.; Markarov, G.S.; Gudkov, A.G.; Leushin, V.Y.; Agasieva, S.V. A Pulsed Low-Frequency Physiotherapy Device. Biomed. Eng. 2022, 56, 84–87. [Google Scholar] [CrossRef]
- Butenko, A.V.; Shekhter, A.B.; Pekshev, A.V.; Vagapov, A.B.; Fayzullin, A.L.; Serejnikova, N.B.; Sharapov, N.A.; Zaborova, V.A.; Vasilets, V.N. Review of clinical applications of nitric oxide-containing air-plasma gas flow generated by Plason device. Clin. Plasma Med. 2020, 19, 100112. [Google Scholar] [CrossRef]
- Ponomarenko, G.N.; Enin, L.D. The action of low-intensity infrared laser radiation on skin afferent. Vopr. Kurortol. Fizioter. Lech. Finishes Kul’tury 1995, 5, 10–13. [Google Scholar]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Dolganova, I.N.; Shikunova, I.A.; Zotov, A.K.; Shchedrina, M.A.; Reshetov, I.V.; Zaytsev, K.I.; Tuchin, V.V.; Kurlov, V.N. Microfocusing sapphire capillary needle for laser surgery and therapy: Fabrication and characterization. J. Biophotonics 2020, 13, e202000164. [Google Scholar] [CrossRef]
- Ponomarenko, G.N. Use of polychromatic polarized incoherent radiation of “Bioptron” set in the complex treatment of patients having wounds, trophic ulcers, burns and decubituses. Physiotherapist 2010, 7, 48–59. [Google Scholar]
- Nussbaum, E.L.; Mazzulli, T.; Pritzker, K.P.; Heras, F.L.; Jing, F.; Lilge, L. Effects of low intensity laser irradiation during healing of skin lesions in the rat. Lasers Surg. Med. 2009, 41, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Enwemeka, C.S.; Williams, D.; Enwemeka, S.K.; Hollosi, S.; Yens, D. Blue 470-nm Light Kills Methicillin-Resistant Staphylococcus aureus (MRSA) in Vitro. Photomed. Laser Surg. 2009, 27, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ataliev, A.E.; Mavlyanov, A.R.; Rakhimov, B.K.; Pardaev, Y. Results of the use of narrow-spectrum far infrared radiation in patients with diffuse and diffuse peritonitis. J. Theor. Clin. Med. 2001, 4, 135–139. [Google Scholar]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Konev, S.V.; Volotovsky, I.D. Introduction to Molecular Photobiology; Science and Technique Institute: Minsk, Belarus, 1971. [Google Scholar]
- Meyer, A.E.; Zeitz, E.O. Ultraviolet Radiation; Foreign Literature: London, UK, 1952. [Google Scholar]
- Karmazinov, F.V.; Kostyuchenko, S.V.; Kudryavtsev, N.N.; Khramenkov, S.V. Ultraviolet Technologies in the Modern World; ID Intellect: Dolgoprudny, Russia, 2012. [Google Scholar]
- Kowalski, W. Ultraviolet Germicidal Irradiation Handbook, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Bogolyubov, V.M.; Ponomarenko, G.N. General Physiotherapy; Medicine: Moscow, Russia, 1999. [Google Scholar]
- Dai, T.; Vrahas, M.S.; Murray, C.K.; Hamblin, M.R. Ultraviolet C irradiation: An alternative antimicrobial approach to localized infections? Expert Rev. Anti Infect. Ther. 2012, 10, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Gugg-Helminger, A. Health hazard assessment of ultraviolet radiation. Light Eng. 1999, 1, 12–15. [Google Scholar]
- Klyachkin, L.M.; Vinogradova, M.N. Physiotherapy; Medicine: Moscow, Russia, 1988. [Google Scholar]
- Ponomarenko, G.N. Electromagnetic Therapy and Phototherapy; Peace and Family-95: Saint-Petersburg, Russia, 1995. [Google Scholar]
- Ushakov, A.A. Practical Physiotherapy; LLC Medical Information Agency: Church, VA, USA, 2009. [Google Scholar]
- Conner-Kerr, T.A.; Sullivan, P.K.; Gaillard, J.; Franklin, M.E.; Jones, R.M. The effects of ultraviolet radiation on antibi-otic-resistant bacteria in vitro. Ostomy Wound Manag. 1998, 44, 50–56. [Google Scholar]
- Thai, T.P.; Houghton, P.E.; Campbell, K.E.E.; Woodbury, M.G. Ultraviolet light C in the treatment of chronic wounds with MRSA: A case study. Ostomy Wound Manag. 2002, 48, 52–60. [Google Scholar]
- Rennekampff, H.O.; Busche, M.N.; Knobloch, K.; Tenenhaus, M. Is UV radiation beneficial in postburn wound healing? Med. Hypotheses 2010, 75, 436–438. [Google Scholar] [CrossRef]
- Dai, T.; Garcia, B.; Murray, C.K.; Vrahas, M.S.; Hamblin, M.R. UVC Light Prophylaxis for Cutaneous Wound Infections in Mice. Antimicrob. Agents Chemother. 2012, 56, 3841–3848. [Google Scholar] [CrossRef]
- Dai, T.; Kharkwal, G.B.; Zhao, J.; St Denis, T.G.; Wu, Q.; Xia, Y.; Huang, L.; Sharma, S.K.; d’Enfert, C.; Hamblin, M.R. Ultra-violet-C light for treatment of Candida albicans burn infection in mice. Photochem. Photobiol. 2011, 87, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Marshak, I.S. (Ed.) Pulsed Light Sources; Energy: Moscow, Russia, 1978. [Google Scholar]
- Liltved, H.; Landfald, B. Effects of high intensity light on ultraviolet-irradiated and non-irradiated fish pathogenic bacteria. Water Res. 2000, 34, 481–486. [Google Scholar] [CrossRef]
- Takeshita, K.; Shibato, J.; Sameshima, T.; Fukunaga, S.; Isobe, S.; Arihara, K.; Itoh, M. Damage of yeast cells induced by pulsed light irradiation. Int. J. Food Microbiol. 2003, 85, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wekhof, A. Disinfection with flash lamps. PDA J. Pharm. Sci. Technol. 2000, 54, 264–267. [Google Scholar] [PubMed]
- Wekhof, A.; Trompeter, F.J.; Franken, O. Pulsed UV-disintegration (PUVD): A new sterilization mechanism for packaging and broad medical-hospital applications. In Proceedings of the First International Conference on Ultraviolet Technologies, Washington, DC, USA, 14–16 June 2001; pp. 1–15. [Google Scholar]
- Dunn, J.; Ott, T.; Clark, W. Pulsed-light treatment of food and packaging. Food Technol. 1995, 49, 95–98. [Google Scholar]
- Gómez-López, V.M.; Ragaert, P.; Debevere, J.; Devlieghere, F. Pulsed light for food decontamination: A review. Trends Food Sci. Technol. 2007, 18, 464–473. [Google Scholar] [CrossRef]
- Elmnasser, N.; Guillou, S.; Leroi, F.; Orange, N.; Bakhrouf, A.; Federighi, M. Pulsed-light system as a novel food decontami-nation technology: A review. Can. J. Microbiol. 2007, 53, 813–821. [Google Scholar] [CrossRef]
- Mandal, R.; Mohammadi, X.; Wiktor, A.; Singh, A.; Singh, A.P. Applications of Pulsed Light Decontamination Technology in Food Processing: An Overview. Appl. Sci. 2020, 10, 3606. [Google Scholar] [CrossRef]
- McDonald, K.; Curry, R.; Clevenger, T.; Brazos, B.; Unklesbay, K.; Eisestark, A.; Baker, S.; Golden, J.; Morgan, R. A comparison of pulsed vs. continuous ultraviolet light sources for de-contamination of surfaces. In Proceedings of the Pulsed Power Conference, Digest of Technical Papers—12th IEEE International, Arlington, VA, USA, 27 June 1999; Volume 2, pp. 1484–1488. [Google Scholar] [CrossRef]
- Arkhipov, V.P.; Kamrukov, A.S.; Kozlov, N.P.; Korop, E.D.; Shashkovsky, S.G.; Yalovik, M.S. New ultraviolet technology for deep purification and disinfection of water. J. Convers. 1996, 6, 46–50. [Google Scholar]
- Kamrukov, A.S.; Kozlov, N.P.; Shashkovsky, S.G.; Yalovik, M.S. New biocidal ultraviolet technologies and devices for sani-tation, microbiology and medicine. Life Saf. 2003, 1, 32–40. [Google Scholar]
- Kamrukov, A.S.; Kozlov, N.P.; Shashkovsky, S.G.; Yalovik, M.S. High-intensity plasma-optical technologies for solving urgent environmental and biomedical problems. Technosphere Saf. 2009, 3, 31–38. [Google Scholar]
- Goldstein, Y.A.; Golubtsov, A.A.; Shashkovsky, S.G. Disinfection of air and surfaces of premises of medical organizations and bureaus of forensic medical examination with pulsed ultraviolet radiation. Bull. Forensic Med. 2016, 5, 50–55. [Google Scholar]
- Shestopalov, N.V.; Akimkin, V.G.; Fedorova, L.S.; Skopin, A.Y.; Goldstein, Y.A.; Golubtsov, A.A.; Kireev, S.G.; Polikarpov, N.A.; Shashkovsky, S.G. Research of germicidal efficiency of air and open surfaces disinfection by pulsed ultraviolet light of continuous spectrum. Med. Alph. 2017, 19, 6–9. [Google Scholar]
- Arkhipov, V.P.; Bagrov, V.V.; Byalovsky, Y.Y.; Kamrukov, A.S.; Kuspanalieva, D.S.; Maslova, M.V.; Odegov, A.K.; Davydov, V.V.; Voronin, R.M. The organization of pre-clinical studies of bactericidal and wound healing effects of the impulse photoherapy device “Zarya”. Probl. Soc. Hyg. Public Health Hist. Med. 2021, 29, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Zverev, A.Y.; Borisevich, S.V.; Chepurenkov, N.Y.; Masyakin, D.N.; Kovalchuk, E.A.; Bykov, V.A.; Trufanova, V.V.; Tutelyan, A.V.; Tivanova, E.V.; Kvasova, O.A.; et al. Virucidal activity of pulsed ultraviolet radiation of continuous spectrum against SARS-CoV-2 coronavirus. Med. Alph. 2020, 18, 55–58. [Google Scholar] [CrossRef]
- Kitagawa, H.; Mori, M.; Kashiyama, S.; Sasabe, Y.; Ukon, K.; Shimokawa, N.; Shime, N.; Ohge, H. Effect of pulsed xenon ultraviolet disinfection on methicillin-resistant Staphylococcus aureus contamination of high-touch surfaces in a Japanese hospital. Am. J. Infect. Control 2020, 48, 139–142. [Google Scholar] [CrossRef]
- Arkhipov, V.P.; Zhelaev, I.A.; Ivashkin, A.B.; Kamrukov, A.S.; Semenov, K.A. Multispectral photoelectric converters for measuring the radiative characteristics of pulsed sources of broadband optical radiation. Prikl. Fiz. Appl. Phys. 2017, 3, 107–114. [Google Scholar]


| Experimental Groups | Control Time | ||||
|---|---|---|---|---|---|
| 4 Hours | 3 Days | 7 Days | 10 Days | 14 Days | |
| Control № 1 * | 44.0 | 300.0 | 300.0 | 300.0 | 300.0 |
| (20.5–300.0) | (300.0–300.0) | (250.0–300.0) | (300.0–300.0) | (300.0–300.0) | |
| Control № 2 | 77.0 * | 1.0 * | 13 * | 20 * | 7.0 * |
| (5.5–294.0) | (0.0–3.0) | (2.0–58.0) | (9.5–197.5) | (0.5–125.5) | |
| Main № 3 | 3.5 * | 0.0 * | 0.0 *# | 0.0 *# | 0.5 *# |
| (2.0–13.0) | (0.0–0.0) | (0.0–1.0) | (0.0–2.5) | (0.0–2.5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagrov, V.V.; Bukhtiyarov, I.V.; Volodin, L.Y.; Zibarev, E.V.; Kamrukov, A.S.; Kondratiev, A.V.; Krylov, V.I.; Nikonova, S.M.; Novikov, D.O.; Semenov, K.A. Preclinical Studies of the Antimicrobial and Wound-Healing Effects of the High-Intensity Optical Irradiation “Zarnitsa-A” Apparatus. Appl. Sci. 2023, 13, 10794. https://doi.org/10.3390/app131910794
Bagrov VV, Bukhtiyarov IV, Volodin LY, Zibarev EV, Kamrukov AS, Kondratiev AV, Krylov VI, Nikonova SM, Novikov DO, Semenov KA. Preclinical Studies of the Antimicrobial and Wound-Healing Effects of the High-Intensity Optical Irradiation “Zarnitsa-A” Apparatus. Applied Sciences. 2023; 13(19):10794. https://doi.org/10.3390/app131910794
Chicago/Turabian StyleBagrov, Valery V., Igor V. Bukhtiyarov, Lev Y. Volodin, Evgeny V. Zibarev, Alexander S. Kamrukov, Andrey V. Kondratiev, Vladimir I. Krylov, Sofya M. Nikonova, Dmitry O. Novikov, and Kirill A. Semenov. 2023. "Preclinical Studies of the Antimicrobial and Wound-Healing Effects of the High-Intensity Optical Irradiation “Zarnitsa-A” Apparatus" Applied Sciences 13, no. 19: 10794. https://doi.org/10.3390/app131910794
APA StyleBagrov, V. V., Bukhtiyarov, I. V., Volodin, L. Y., Zibarev, E. V., Kamrukov, A. S., Kondratiev, A. V., Krylov, V. I., Nikonova, S. M., Novikov, D. O., & Semenov, K. A. (2023). Preclinical Studies of the Antimicrobial and Wound-Healing Effects of the High-Intensity Optical Irradiation “Zarnitsa-A” Apparatus. Applied Sciences, 13(19), 10794. https://doi.org/10.3390/app131910794






