Enhanced Effect of Phytoextraction on Arsenic-Contaminated Soil by Microbial Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Isolation of Iron-Sulfur-Reducing Bacteria
2.3. Preparation of Plant Organic Matter (POM)
2.4. Soil-Microbial Batch Tests
2.5. Plant Potting Experiment
2.6. Analysis of Arsenic Speciation in Pteris vittata
2.7. Microbial Community Structure Analysis
2.8. Data Analysis
3. Results and Discussion
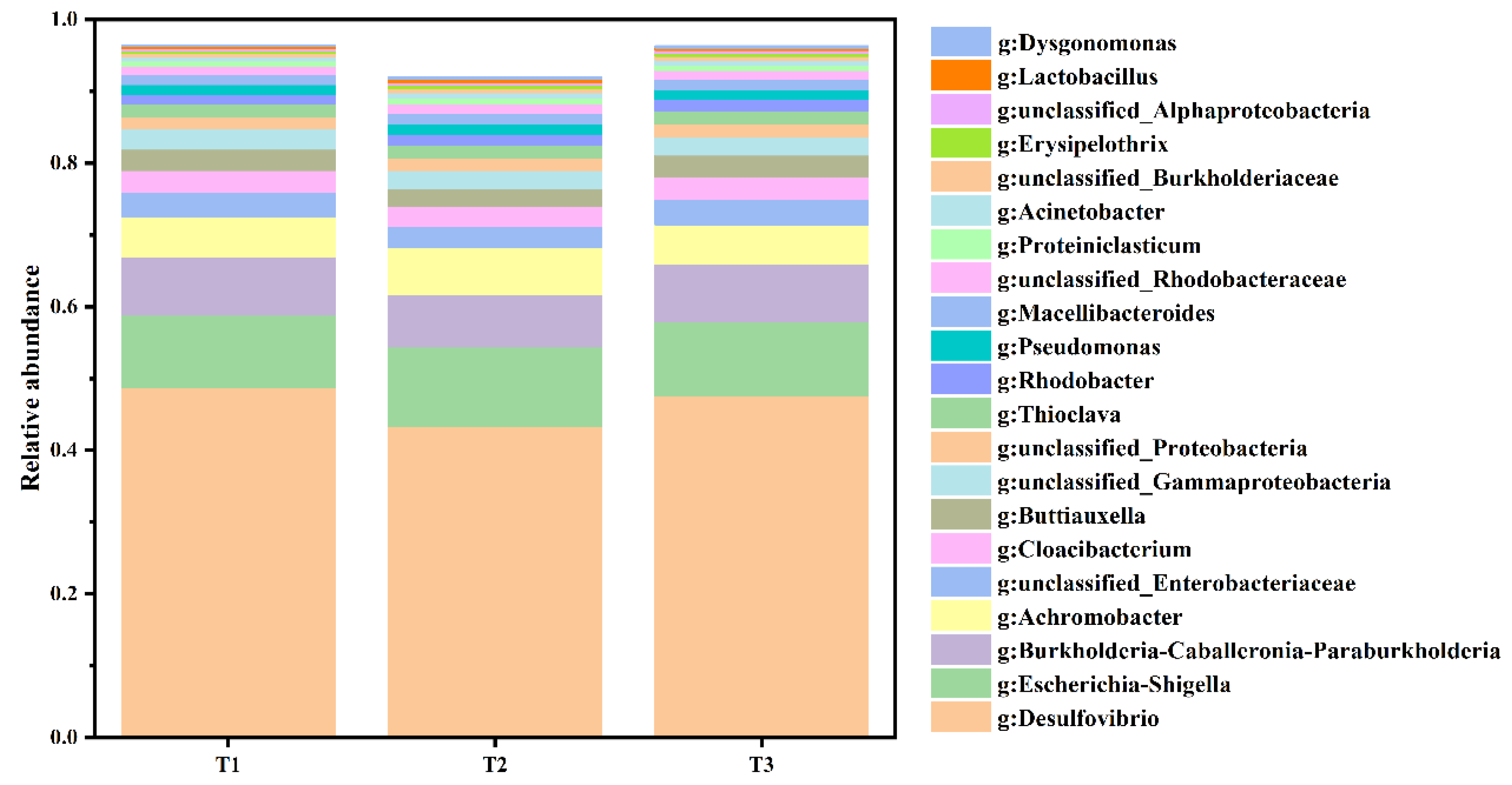
3.1. Composition of Iron-Sulfur-Reducing Flora
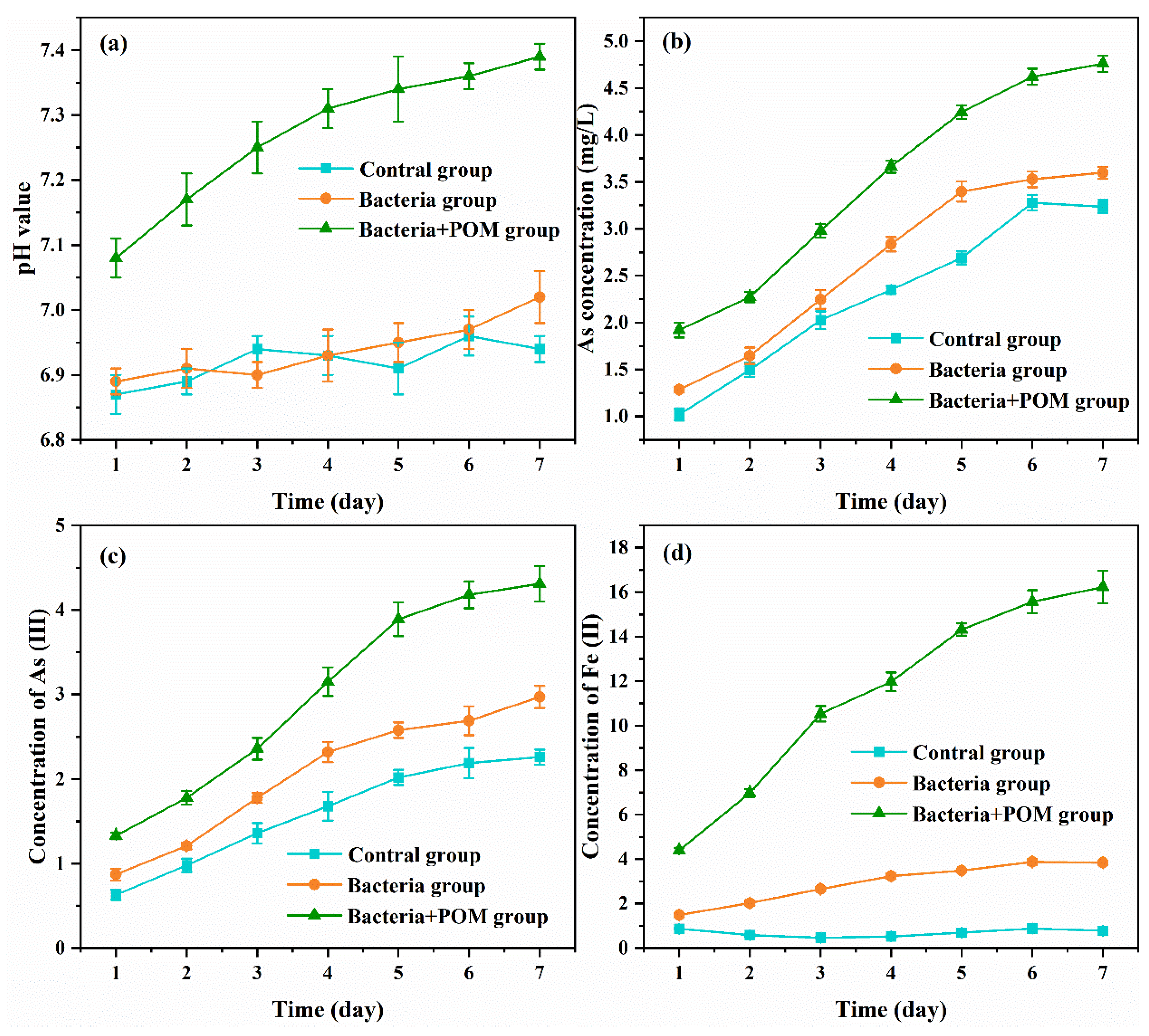
3.2. Function of Iron–Sulfur-Reducing Microbial
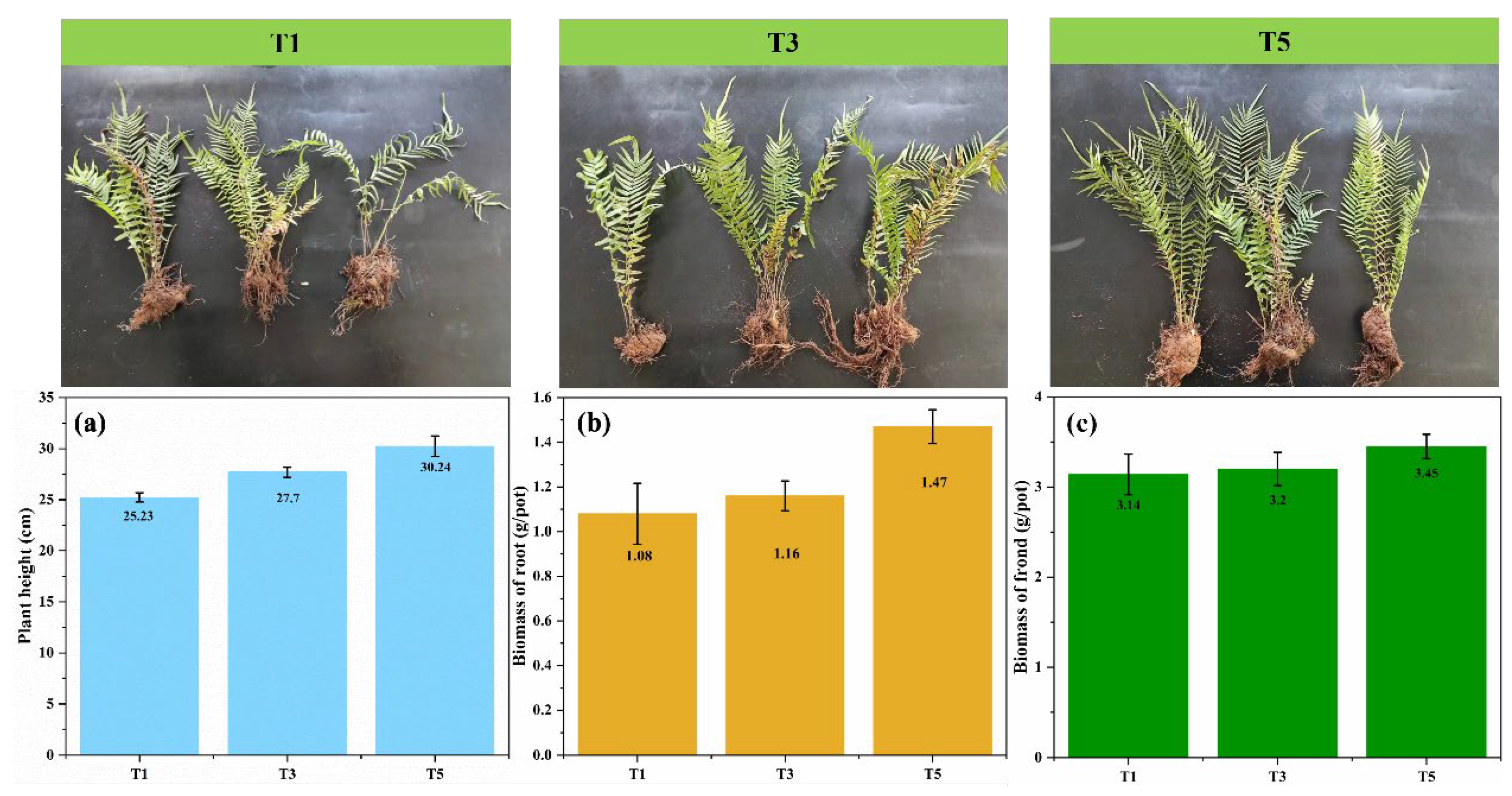
3.3. Growth and Biomass of Pteris vittata after Bio-Reductive Enhancement
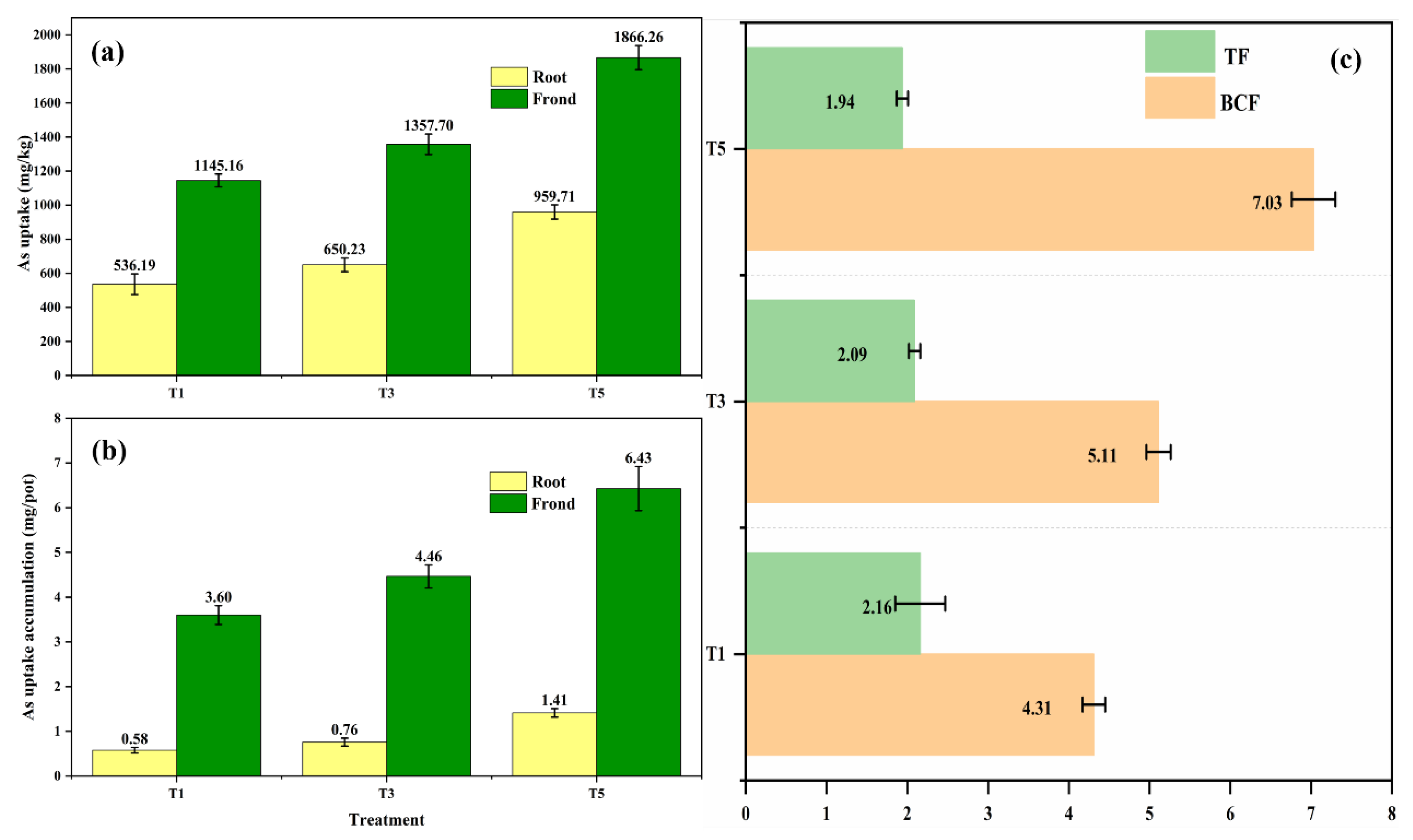
3.4. Extraction and Transport of Arsenic by the Pteris vittata
3.5. Speciation of Arsenic Uptake by Pteris vittata
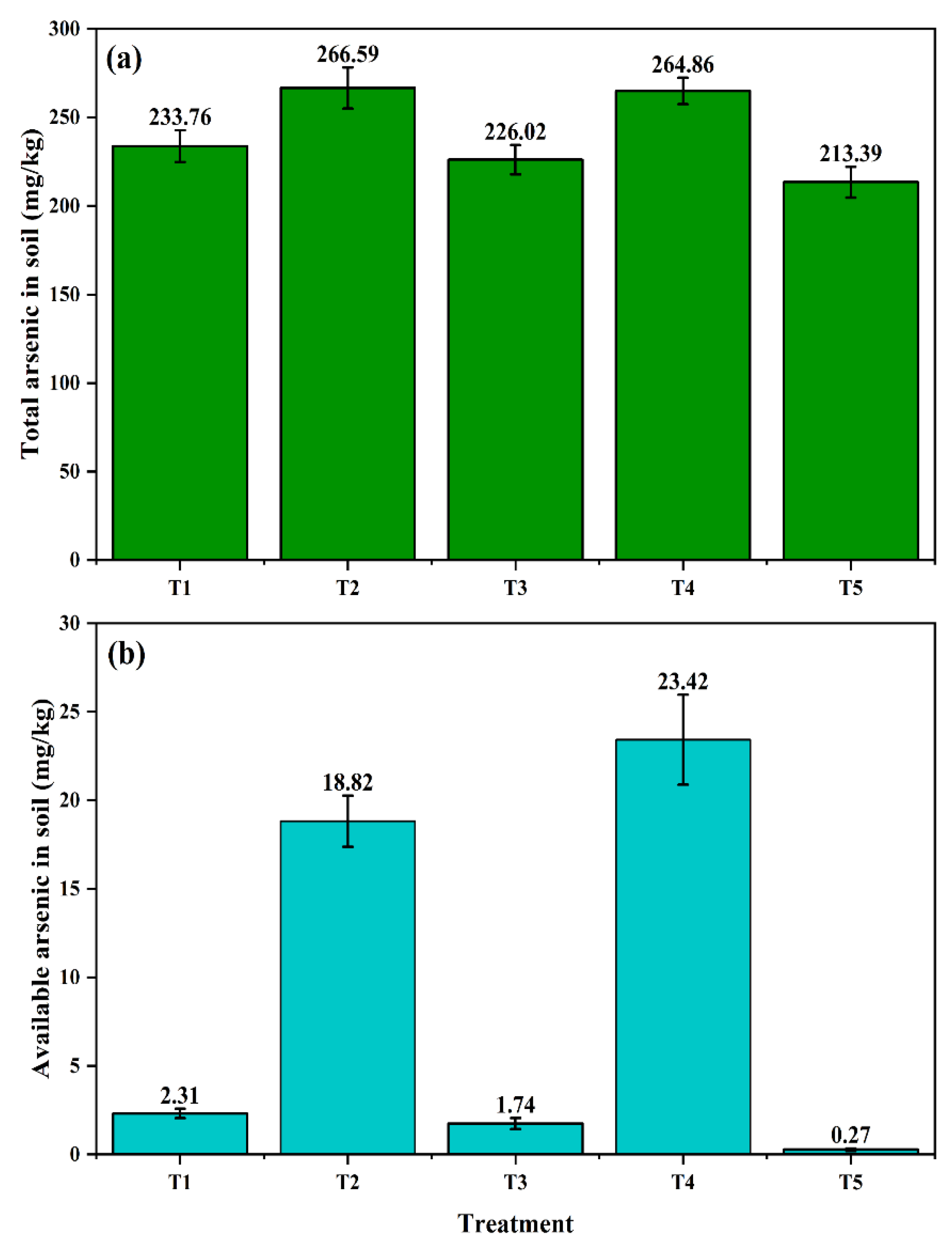
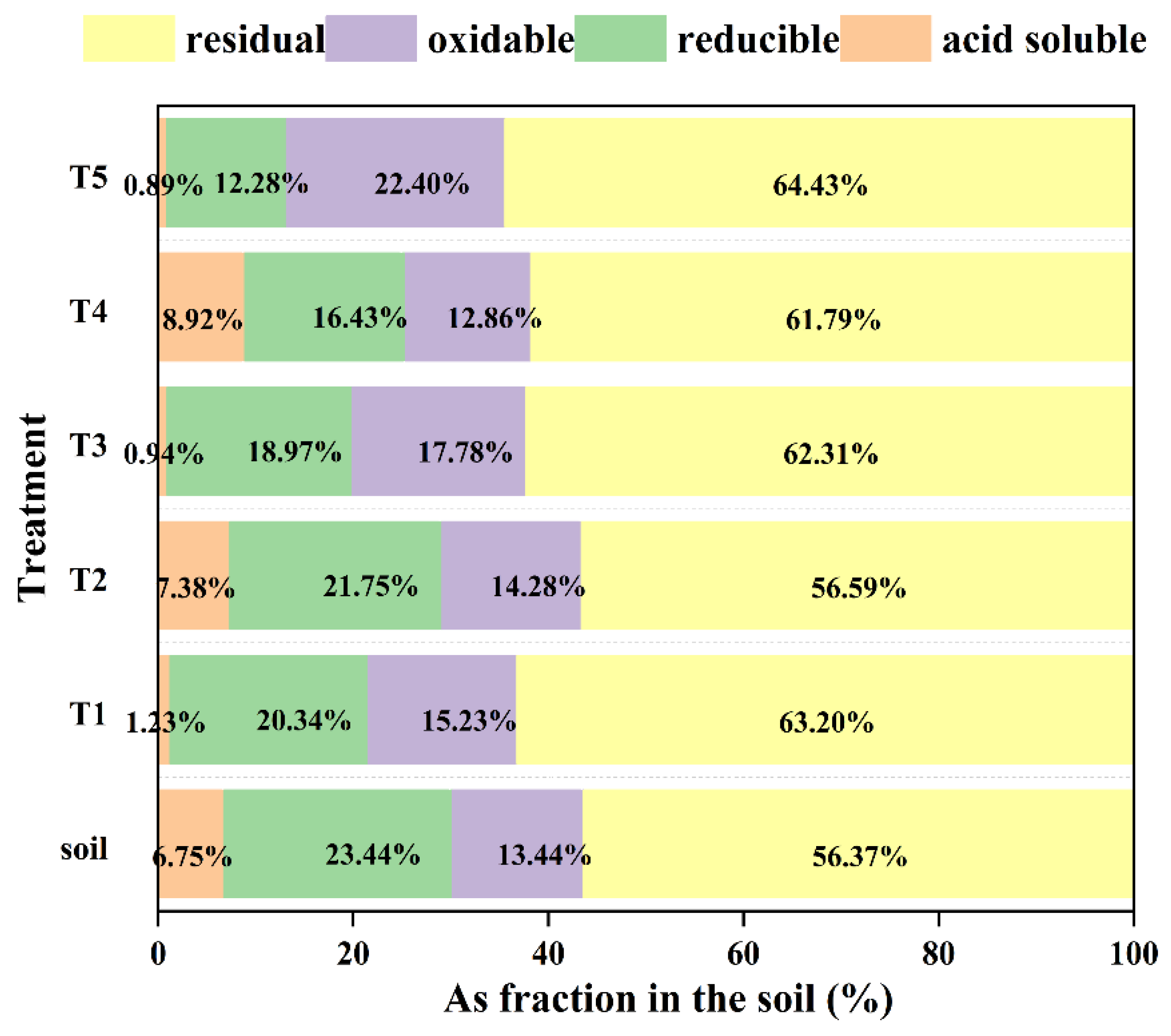
3.6. Changes in Soil Arsenic Content and Fractionation
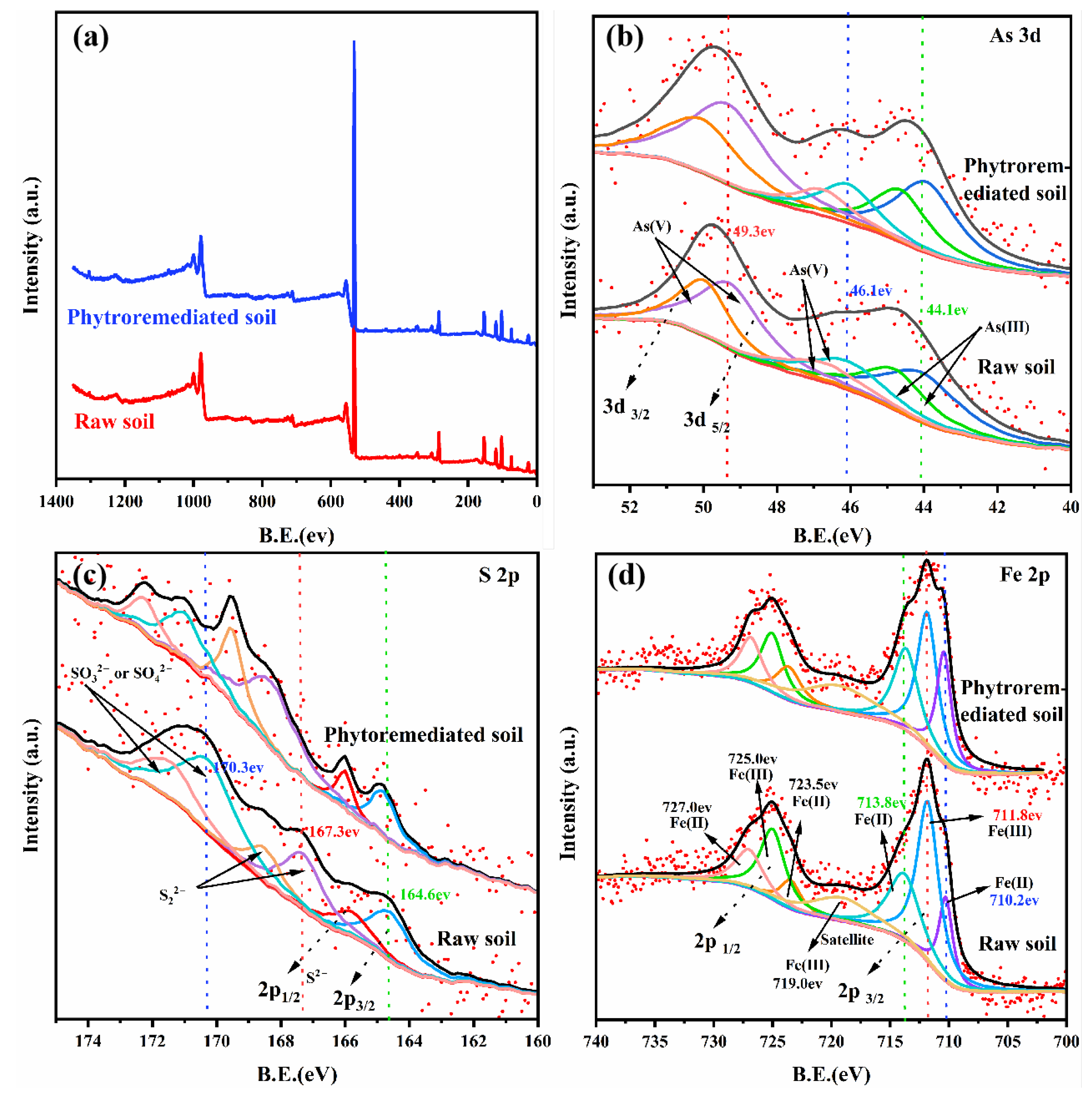
3.7. Transformation of Soil Element Valance
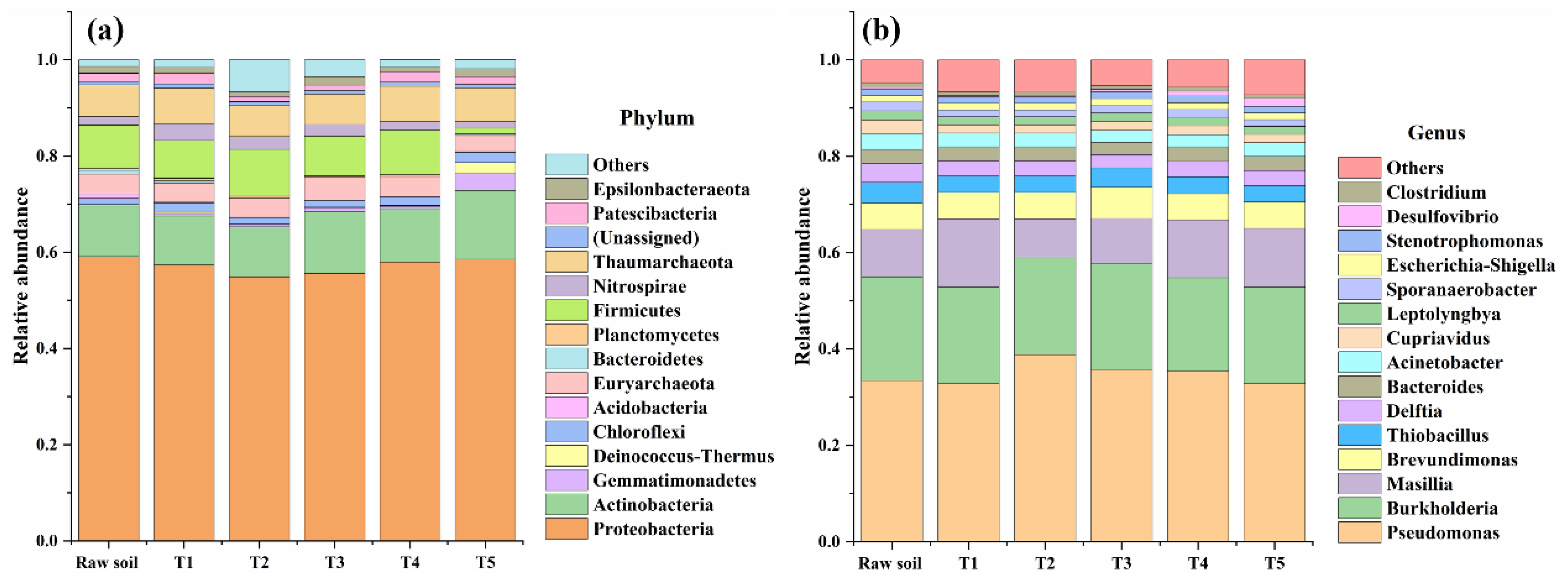
3.8. Effects of Iron-Sulfur-Reducing Flora on Soil Microbial Community
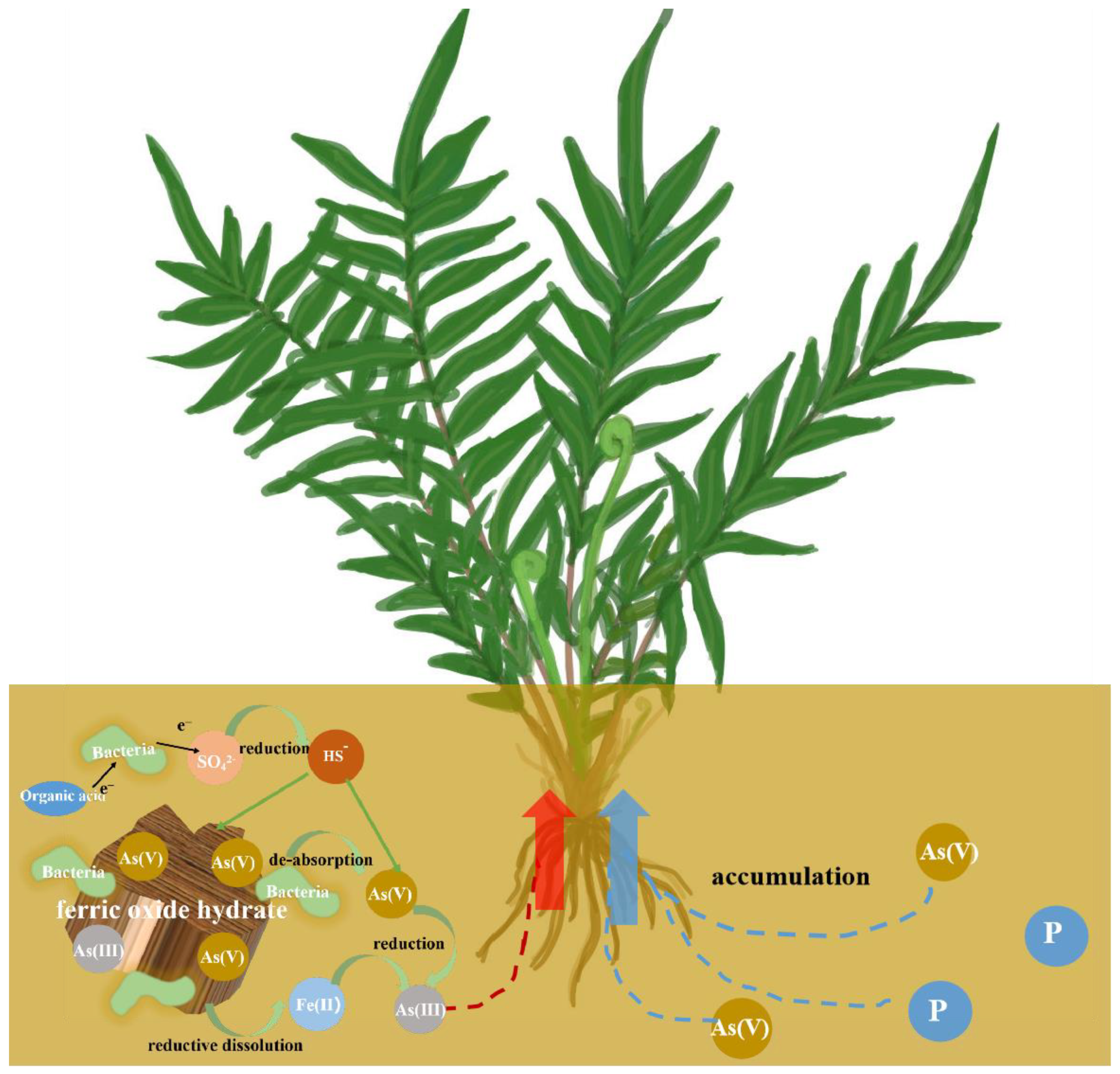
3.9. Mechanism of Bio-Reductive Enhanced Phytoremediation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, R.; Ma, G.; Liu, C.; Wang, C.; Kang, X.; Wu, M.; Zhang, B. Effects of different heavy metal pollution levels on microbial community structure and risk assessment in Zn-Pb mining soils. Environ. Sci. Pollut. Res. 2023, 30, 52749–52761. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Yang, S.; Chen, S.; Zhao, S.; Ai, Y.; Huang, D.; Yang, K.; Cheng, H. Soil microbial responses to simultaneous contamination of antimony and arsenic in the surrounding area of an abandoned antimony smelter in Southwest China. Environ. Int. 2023, 174, 107897. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, M.; Watanabe, C.; Ahmad, S.A.; Ohtsuka, R. Arsenic contamination in drinking water and skin manifestations in lowland Nepal: The first community-based survey. Am. J. Trop. Med. Hyg. 2005, 73, 477. [Google Scholar] [CrossRef]
- Fontcuberta, M.; Calderon, J.; Villalbí, J.R.; Centrich, F.; Portaña, S.; Espelt, A.; Duran, J.; Nebot, M. Total and inorganic arsenic in marketed food and associated health risks for the Catalan (Spain) population. J. Agric. Food Chem. 2011, 59, 10013–10022. [Google Scholar] [CrossRef]
- Wu, H.; Yang, F.; Li, H.; Li, Q.; Zhang, F.; Ba, Y.; Cui, L.; Sun, L.; Lv, T.; Wang, N.; et al. Heavy metal pollution and health risk assessment of agricultural soil near a smelter in an industrial city in China. Int. J. Environ. Health Res. 2020, 30, 174–186. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Yun, Z.; Shi, J.; Jiang, G. Research progress of heavy metal pollution in China: Sources, analytical methods, status, and toxicity. Chin. Sci. Bull. 2013, 58, 134–140. [Google Scholar] [CrossRef]
- Moniuszko, H.; Malonga, W.A.M.; Koczoń, P.; Thijs, S.; Popek, R.; Przybysz, A. Accumulation of Plastics and Trace Elements in the Mangrove Forests of Bima City Bay, Indonesia. Plants 2023, 12, 462. [Google Scholar] [CrossRef]
- Popek, R.; Fornal-Pieniak, B.; Dąbrowski, P.; Chyliński, F. The Role of Spontaneous Flora in the Mitigation of Particulate Matter from Traffic Roads in an Urbanised Area. Sustainability 2023, 15, 7568. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; He, X.; Li, B.; Du, S. Plant growth-promoting rhizobacteria: A good companion for heavy metal phytoremediation. Chemosphere 2023, 338, 139475. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Souri, Z.; Sharifan, H.; de Oliveira, L.M.; Ngatia, L. Arsenic Removal by Phytoremediation Techniques. In Arsenic in Plants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 267–286. [Google Scholar] [CrossRef]
- Vithanage, M.; Dabrowska, B.B.; Mukherjee, A.B.; Sandhi, A.; Bhattacharya, P. Arsenic uptake by plants and possible phytoremediation applications: A brief overview. Environ. Chem. Lett. 2012, 10, 217–224. [Google Scholar] [CrossRef]
- Gumaelius, L.; Lahner, B.; Salt, D.E.; Banks, J.A. Arsenic Hyperaccumulation in Gametophytes of Pteris vittata. A New Model System for Analysis of Arsenic Hyperaccumulation. Plant Physiol. 2004, 136, 3198–3208. [Google Scholar] [CrossRef]
- Kertulis, G.M.; Ma, L.Q.; MacDonald, G.E.; Chen, R.; Winefordner, J.D.; Cai, Y. Arsenic speciation and transport in Pteris vittata L. and the effects on phosphorus in the xylem sap. Environ. Exp. Bot. 2005, 54, 239–247. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Q.; Wei, Z.; Zhu, Y.; Pan, X. Simultaneous removal of As(III) and Cu(II) from real bottom ash leachates by manganese-oxidizing aerobic granular sludge: Performance and mechanisms. Sci. Total Environ. 2020, 700, 134510. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Han, N.; Inoue, C.; Yang, Y.L.; Nojiri, H.; Ho, Y.N.; Chien, M.F. Rhizospheric plant-microbe synergistic interactions achieve efficient arsenic phytoextraction by Pteris vittata. J. Hazard. Mater. 2022, 434, 128870. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Lu, Y.; Zhen, D.; Guo, Z.; Wang, G.; Shi, K.; Liao, S. Enterobacter sp. E1 increased arsenic uptake in Pteris vittata by promoting plant growth and dissolving Fe-bound arsenic. Chemosphere 2023, 329, 138663. [Google Scholar] [CrossRef]
- Ganghui, Z.; Liu, W.; Yi, W.; Liao, X.; Sun, L. Potential of arsenate-reducing bacterial inoculants to enhance field-scale remediation of arsenic contaminated soils by Pteris vittata L. Ecol. Eng. 2021, 169, 106312. [Google Scholar]
- Xu, Y.; Wan, L.; Wang, K.; Liu, C.; Zhang, J. Enhanced mobilization of arsenic from tailing soil by four types of low molecular weight organic acids with different functional groups. J. Soils Sediments 2021, 21, 3834–3844. [Google Scholar] [CrossRef]
- Li, N.; Jiang, L.; Li, X.; Su, Y. Enhancing phytoremediation of arsenic-contaminated soil by agronomic practices (drip irrigation and intercropping): Influence of soil organic matter. Sci. Total Environ. 2023, 891, 164463. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Sun, S.; Wang, Y. Arsenic transformation and redistribution in groundwater induced by the complex geochemical cycling of iron and sulfur. Sci. Total Environ. 2023, 894, 164941. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Hongguang, C.; Liu, Q.; Wang, S.; Wang, X. Arsenic release from arsenopyrite weathering in acid mine drainage: Kinetics, transformation, and effect of biochar. Environ. Int. 2022, 170, 107558. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.; Johnston, S.; Bush, R. Microbial sulfidogenesis in ferrihydrite-rich environments: Effects on iron mineralogy and arsenic mobility. Geochim. Cosmochim. Acta 2011, 75, 3072–3087. [Google Scholar] [CrossRef]
- Heidelberg, J.F.; Seshadri, R.; Haveman, S.A.; Hemme, C.L.; Paulsen, I.T.; Kolonay, J.F.; Eisen, J.A.; Ward, N.; Methe, B.; Brinkac, L.M.; et al. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat. Biotechnol. 2004, 22, 554–559. [Google Scholar] [CrossRef]
- Muyzer, G.; Stams, A.J. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.; Kjellerup, B.V. Biogeochemical cycling of metals impacting by microbial mobilization and immobilization. J. Environ. Sci. 2018, 66, 146–154. [Google Scholar] [CrossRef]
- Kumar, N.; Couture, R.M.; Millot, R.; Battaglia-Brunet, F.; Rose, J. Microbial Sulfate Reduction Enhances Arsenic Mobility Downstream of Zerovalent-Iron-Based Permeable Reactive Barrier. Environ. Sci. Technol. 2016, 50, 7610–7617. [Google Scholar] [CrossRef]
- Nghiem, A.A.; Prommer, H.; Mozumder, M.R.H.; Siade, A.; Jamieson, J.; Ahmed, K.M.; van Geen, A.; Bostick, B.C. Sulfate reduction accelerates groundwater arsenic contamination even in aquifers with abundant iron oxides. Nat. Water 2023, 1, 151–165. [Google Scholar] [CrossRef]
- Xu, X.; Wang, P.; Zhang, J.; Chen, C.; Wang, Z.; Kopittke, P.M.; Kretzschmar, R.; Zhao, F.J. Microbial sulfate reduction decreases arsenic mobilization in flooded paddy soils with high potential for microbial Fe reduction. Environ. Pollut. 2019, 251, 952–960. [Google Scholar] [CrossRef]
- Chen, C.; Li, L.; Huang, K.; Zhang, J.; Xie, W.-Y.; Lu, Y.; Dong, X.; Zhao, F.-J. Sulfate-reducing bacteria and methanogens are involved in arsenic methylation and demethylation in paddy soils. ISME J. 2019, 13, 2523–2535. [Google Scholar] [CrossRef]
- Sun, J.; Quicksall, A.N.; Chillrud, S.N.; Mailloux, B.J.; Bostick, B.C. Arsenic mobilization from sediments in microcosms under sulfate reduction. Chemosphere 2016, 153, 254–261. [Google Scholar] [CrossRef]
- Mackay, J.E.; Macdonald, L.M.; Smernik, R.J.; Cavagnaro, T.R. Organic amendments as phosphorus fertilisers: Chemical analyses, biological processes and plant P uptake. Soil Biol. Biochem. 2017, 107, 50–59. [Google Scholar] [CrossRef]
- Liu, X.; Yang, G.-M.; Guan, D.-X.; Ghosh, P.; Ma, L.Q. Catecholate-siderophore produced by As-resistant bacterium effectively dissolved FeAsO4 and promoted Pteris vittata growth. Environ. Pollut. 2015, 206, 376–381. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Q.; Sun, Z.; Zhang, W.; Chen, J.; Chen, Z.; Na, S.; Sun, H. Chemical oxidation of arsenic in the environment and its application in remediation: A mini review. Pedosphere 2023, 33, 185–193. [Google Scholar] [CrossRef]
- Mishra, R.K.; Tiwari, S.; Patel, A.; Prasad, S.M. Arsenic contamination, speciation, toxicity and defense strategies in plants. Braz. J. Bot. 2021, 44, 1–10. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef]
- Mathews, S.; Ma, L.Q.; Rathinasabapathi, B.; Natarajan, S.; Saha, U.K. Arsenic transformation in the growth media and biomass of hyperaccumulator Pteris vittata L. Bioresour. Technol. 2010, 101, 8024–8030. [Google Scholar] [CrossRef]
- Cesaro, P.; Cattaneo, C.; Bona, E.; Berta, G.; Cavaletto, M. The arsenic hyperaccumulating Pteris vittata expresses two arsenate reductases. Sci. Rep. 2015, 5, 14525. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Isayenkov, S.V.; Zhao, F.J.; Maathuis, F.J. Arsenite transport in plants. Cell. Mol. Life Sci. 2009, 66, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yan, H.; Chen, Y.; Shen, H.; Xu, W.; Zhang, H.; Shi, L.; Zhu, Y.G.; Ma, M. An aquaporin PvTIP4;1 from Pteris vittata may mediate arsenite uptake. New Phytol. 2016, 209, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; He, X.; Jiang, X.; Pan, W.; Li, W.; Xia, L.; Wu, C. Arsenic biotransformation genes and As transportation in soil-rice system affected by iron-oxidizing strain (Ochrobactrum sp.). Environ. Pollut. 2022, 314, 120311. [Google Scholar] [CrossRef]
- Hou, J.; Tan, X.; Xiang, Y.; Zheng, Q.; Chen, C.; Sha, Z.; Ren, L.; Wang, M.; Tan, W. Insights into the underlying effect of Fe vacancy defects on the adsorption affinity of goethite for arsenic immobilization. Environ. Pollut. 2022, 314, 120268. [Google Scholar] [CrossRef]
- Ren, X.; Yan, N.; Chen, S.; Yao, J.; Liu, J. Remediation of arsenic-containing ferrihydrite in soil using iron- and sulfate-reducing bacteria: Implications for microbially-assisted clean technology. J. Environ. Chem. Eng. 2022, 10, 108876. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Shi, Y.; Zhao, Z.; Zhang, Z.; Zhao, P.; Gao, Q.; Zhang, X.; Chen, B.; Li, Y.; et al. Polyethylene and poly (butyleneadipate-co-terephthalate)-based biodegradable microplastics modulate the bioavailability and speciation of Cd and As in soil: Insights into transformation mechanisms. J. Hazard. Mater. 2023, 445, 130638. [Google Scholar] [CrossRef]
- Qin, L.; Wang, L.; Zhao, S.; Sun, X.; Yu, L.; Wang, M.; Chen, S. A new insight into Cd reduction by flooding in paddy soil: The different dominant roles of Fe and S on Cd immobilization under fluctuant pe + pH conditions. Sci. Total Environ. 2022, 847, 157604. [Google Scholar] [CrossRef]
- Qin, L.; He, L.; Yang, W.; Lin, A. Preparation of a novel iron-based biochar composite for removal of hexavalent chromium in water. Environ. Sci. Pollut. Res. 2020, 27, 9214–9226. [Google Scholar] [CrossRef]
- Kumkrong, P.; Dy, E.; Tyo, D.D.; Jiang, C.; Gedara Pihilligawa, I.; Kingston, D.; Mercier, P.H.J. Investigation of metal mobility in gold and silver mine tailings by single-step and sequential extractions. Environ. Monit. Assess. 2022, 194, 423. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Castor, J.M.; Portugal, L.; Ferrer, L.; Guzmán-Mar, J.L.; Hernández-Ramírez, A.; Cerdà, V.; Hinojosa-Reyes, L. Arsenic fractionation in agricultural soil using an automated three-step sequential extraction method coupled to hydride generation-atomic fluorescence spectrometry. Anal. Chim. Acta 2015, 874, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zeng, W.; Lei, M.; Chen, T. The influence of diverse fertilizer regimes on the phytoremediation potential of Pteris vittata in an abandoned nonferrous metallic mining site. Sci. Total Environ. 2023, 880, 163246. [Google Scholar] [CrossRef] [PubMed]

| Properties | Value |
|---|---|
| pH | 6.98 ± 0.04 |
| cation exchange capacity (cmol/kg) | 14.54 ± 0.53 |
| Organic carbon (g/kg) | 20.43 ± 2.16 |
| Total nitrogen (g/kg) | 1.56 ± 0.13 |
| Available nitrogen (mg/kg) | 193.22 ± 17.86 |
| Total phosphorous (g/kg) | 2.18 ± 0.21 |
| Available phosphorous (mg/kg) | 85.35 ± 13.64 |
| As(mg/kg) | 265.48 ± 23.36 |
| Available As(mg/kg) | 16.43 ± 2.38 |
| KH2PO4 | K2HPO4 | MgCl2 | CaCl2·2H2O | Na2SO4 | Fe2(SO4)3 | Yeast Extract | C3H5NaO3 |
|---|---|---|---|---|---|---|---|
| 0.5 g/L | 1 g/L | 0.25 g/L | 0.1 g/L | 1.2 g/L | 0.5 g/L | 1 g/L | 0.5 g/L |
| pH | Lactic Acid | Acetic Acid | Fiber | Protein |
|---|---|---|---|---|
| 4.52 | 32.48 g/kg | 8.26 g/kg | 38.43% | 4.28% |
| Group | As Contaminated Soil | Pteris vittata | Microbial Flora | POM |
|---|---|---|---|---|
| T1 | 1 kg | 1 plant | / | / |
| T2 | 1 kg | / | 50 mL | / |
| T3 | 1 kg | 1 plant | 50 mL | / |
| T4 | 1 kg | / | 50 mL | 50 g |
| T5 | 1 kg | 1 plant | 50 mL | 50 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Cao, J.; Chen, P. Enhanced Effect of Phytoextraction on Arsenic-Contaminated Soil by Microbial Reduction. Appl. Sci. 2023, 13, 10921. https://doi.org/10.3390/app131910921
Zhao Y, Cao J, Chen P. Enhanced Effect of Phytoextraction on Arsenic-Contaminated Soil by Microbial Reduction. Applied Sciences. 2023; 13(19):10921. https://doi.org/10.3390/app131910921
Chicago/Turabian StyleZhao, Yuxin, Jian Cao, and Pan Chen. 2023. "Enhanced Effect of Phytoextraction on Arsenic-Contaminated Soil by Microbial Reduction" Applied Sciences 13, no. 19: 10921. https://doi.org/10.3390/app131910921






