Abstract
Polysaccharide biopolymers have been shown to be alternatives to established dust suppressants. This study investigates the potential of 14 polysaccharides and proteins from diverse botanical (corn, pea, wheat, cellulose, potato, and fava bean) and animal (pig, chicken, and cow) sources as dust suppressants on two mine soils (medium-grained sand and fine-grained silica sand). Results of laboratory tests demonstrate that the type of biopolymer and its concentration have a significant effect on penetration resistance, moisture retention, and crust thickness. Depending on biopolymer type, concentration, and soil type, moisture retention range from 1.0 to 19.5 wt% (control, Cmedium-gr. = 2.5 and Cfine-gr. = 6.9 wt%), penetration resistance from 1.7 to 37.9 N (Cmedium-gr. = 1.5 and Cfine-gr. = 1.7 N), and crust thickness from 0.3 to 18.1 mm (Cmedium-gr. and Cfine-gr. = 0 mm). Proteins form crusts with penetration resistances similar to polysaccharides but mainly require higher concentrations than polysaccharides. Based on the test results, xanthan gum, carboxymethyl cellulose, corn starch, fava bean protein concentrate, and plasma protein exhibit the highest potential to act as dust suppressants. This research contributes to evaluating biopolymers as environmentally friendly soil amendments that may be used to control fugitive dust emissions from large, barren surfaces.
1. Introduction
Fugitive dust emissions from industries such as mining pose a significant threat to the environment and the health of workers and surrounding communities [1,2,3,4]. These emissions can cause respiratory diseases such as pneumoconiosis, asthma, or chronic obstructive pulmonary disease (COPD) [5], increase vehicle maintenance, and reduce occupational safety by limiting visibility [6,7]. As climate change increases the frequency and severity of extreme weather events [8,9], fugitive dust emissions are expected to rise in the coming decades. This is because prolonged heat waves lead to a faster depletion of soil moisture, increasing the susceptibility of soil particles to be suspended and become fugitive dust. Industries, such as mining, quarrying, construction, and agriculture, constitute the main sources of anthropogenic fugitive industrial (non-combustion) dust [10]. By their very nature, these domains encompass large, barren surfaces with a scarce vegetative cover exposed to frequent windblow and mechanical disturbances. Hence, these industries are challenged to reduce fugitive dust emissions at their sites.
Measures such as (vegetative) barriers or encapsulations reduce fugitive dust emissions but are unsuitable for protecting large, exposed areas or mitigating emissions caused by mechanical disturbance. In mining, the spray-on application of water is the oldest yet-established means for decreasing dust emissions, but its effect rapidly diminishes upon evaporation. Dust suppressants constitute an alternative solution for controlling dust emissions. They act by either agglomerating small particles, making them less prone to be suspended in the air, or are hygroscopic, absorbing moisture from the air to increase soil moisture [11]. However, many traditional dust suppressants, such as chloride salts or petroleum-based products, can adversely affect the environment [12] or are costly, resulting in the need for environmentally friendly and cost-effective alternatives.
Recently, the potential of biopolymers as environmentally friendly alternatives to traditional soil amendments has been increasingly investigated in the fields of soil stabilisation and dust control (e.g., [13,14,15,16,17]). Biopolymers are produced by living organisms, such as plants, microbes, and animals, are biodegradable, and can be classified into polysaccharides, proteins, and polynucleotides (e.g., deoxyribonucleic acid (DNA) and ribonucleic acid (RNA)) [18]. Soil stabilisation refers to the process of mixing soil amendments into the soil to enhance its engineering properties, while dust control refers to the spray-on application of dust suppressants [19]. Table A1 provides a comprehensive review of previous studies on different biopolymer types, and several reviews summarise the current research in the respective fields [20,21,22,23,24].
Based on previous studies (cf. Table A1) and reviews, the current knowledge on biopolymers tested in soil stabilisation and dust control can be summarised as follows. (a) Most research focuses on polysaccharides, with xanthan and guar gum being the most studied, whereas only a few studies investigated proteins. Polynucleotides have not yet been investigated, likely because of their extremely high cost. (b) Many previous studies examined biopolymers from botanical sources native to tropical, arid, and partially temperate climates (e.g., guar, Persian, acacia, and locust bean gum, or soybeans), hindering the ability of regional sourcing in regions with a continental climate, such as central and eastern Europe. (c) Some tested biopolymers are only dissolvable at high temperatures (>80 °C) or in acetic or alkaline solutions. While such properties make these biopolymers more effective or resistant to degradation [25], such dissolution behaviour prevents large-scale field testing and site applications. (d) Most studies have focused on extensive testing with a single or a few biopolymers, with only a few comparative studies analysing multiple biopolymers.
Based on the current knowledge, the following research needs can be identified. (a) Research on underrepresented polysaccharides and especially proteins should be expanded to identify further biopolymers with potential as dust suppressants. (b) Research on biopolymers that can be sourced regionally in areas with continental climates should be increased to identify alternative sources that may be procurable at a lower carbon footprint. (c) Emphasis should be on biopolymers that dissolve readily in water, enabling the conduct of large field tests under real site conditions.
This study aimed to investigate the dust suppression potential of selected polysaccharides and proteins (meeting the needs above) by performing laboratory experiments on local mine soils. Penetrometer tests were performed to measure the penetration resistance of the formed crusts, as it is an established and recommended indicator for evaluating potential dust suppressants [13,17,26,27]. Moisture retention tests were conducted to evaluate the ability of biopolymer-treated soil to retain moisture, which is a further relevant indicator for analysing potential dust suppressants [17,27,28]. Crust thickness measurements were performed to assess the ability of different biopolymer types and concentrations to agglomerate particles and form crusts. The results of this research contribute to the evaluation of polysaccharide and protein biopolymers as environmentally friendly dust suppressants on large, barren surfaces.
2. Materials and Methods
2.1. Materials
2.1.1. Soils
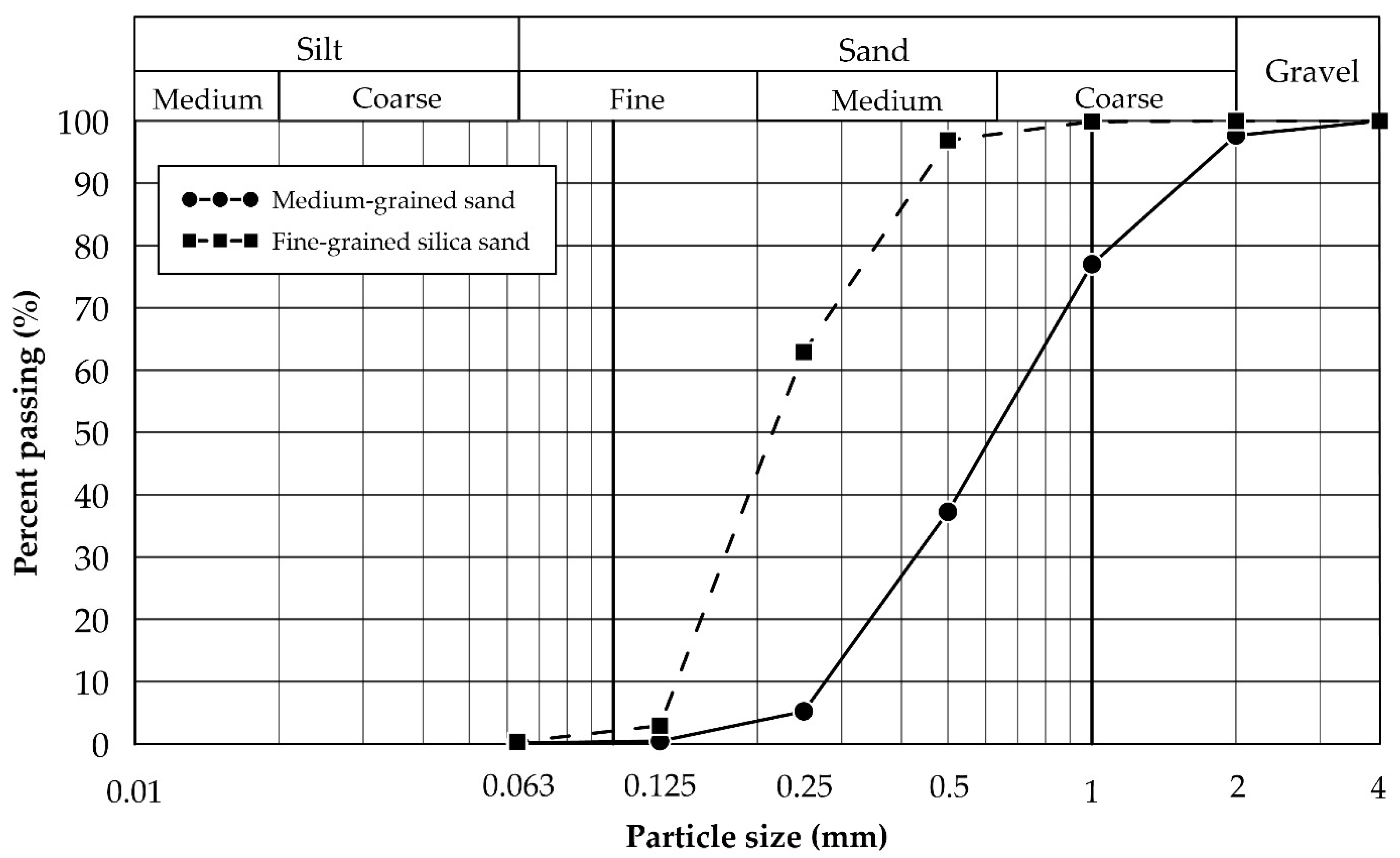
Medium-grained sand. A 2 t bulk sample of medium-grained sand was provided by the Rheinische Baustoffwerke GmbH, which represents a sand and gravel operation located 16 km northeast of Aachen, Germany. The bulk sample is representative of overburden material that is extracted during lignite open-pit mining in the region. The material was air-dried at room temperature and homogenised by coning and quartering. Relevant soil properties (i.e., pH, specific gravity, and soil colour) and grain size distributions were determined at RWTH Aachen University. The particle-size distribution was determined according to DIN EN ISO 17892-4 [29] (Table 1 and Figure 1) and based on the unified soil classification system (USCS); the material can be classified as medium-grained, poorly graded sand (SP). The sample’s geochemistry was determined at ALS Geochemistry (Loughrea, Ireland), which performed whole-rock analysis by X-ray fluorescence spectroscopy (XRF) and inductively coupled plasma mass spectrometry (ICP-MS) with four-acid digestion. Its mineralogy was established by semi-quantitative X-ray diffraction (XRD) using an X’Pert Pro (PANalytical) instrument with a data collector and an X’Pert High Score system equipped with a Co-LFF (Empyrian) tube and an automated divergence slit (Clausthal University of Technology, Germany). The sand primarily consists of quartz with plagioclase, ankerite, and rutile as accessories.

Table 1.
Physical and chemical properties of mine soils used by this study.
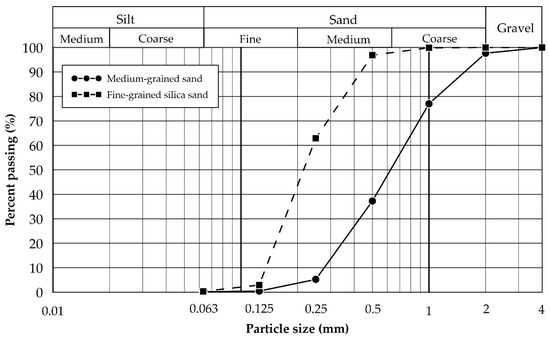
Figure 1.
Particle-size distribution of medium-grained sand and fine-grained silica sand, determined according to DIN EN ISO 17892-4 [29].
Fine-grained silica sand. A 1.5 t bulk sample of fine-grained silica sand was obtained from the Quarzwerke Frechen open-pit mine, which represents a silica sand operation located 8 km west of Cologne, Germany. The sand can be classified as medium- to fine-grained, poorly graded sand (SP) and its properties, as well as its geochemistry, are listed in Table 1. Semi-quantitative XRD showed that the sand primarily consists of quartz with ankerite and clinochlore as accessories.
2.1.2. Biopolymers
Selection methodology. Fourteen different biopolymers (seven polysaccharides and seven proteins) were selected for this study (Table 1). The polysaccharides, xanthan gum (XG), and sodium lignosulfonate (NLS) were preselected because they have already been studied in detail by previous works [13,17,33], and hence experimental results can be compared. Three qualitative criteria were used to select biopolymers relevant to this study:
- The biopolymer should be able to be sourced regionally within central European countries where continental climate prevails.
- The biopolymer should be commercially available at a relatively low cost compared to biopolymers investigated in previous studies, such as agar gum (avg. 18 USD/kg), alginates (avg. 12 USD/kg), carrageenan (avg. 10.5 USD/kg), chitosan (avg. 35 USD/kg), or pectin (avg. 15 USD/kg) [34,35,36].
- The biopolymer should be readily soluble in water to enable large-scale field testing and potential industrial implementation. Such a requirement precludes biopolymers, whose dissolution would rely on either high temperature (e.g., agar or gellan gum) or acetic/alkaline solutions (e.g., chitosan or casein).
Selected biopolymers. Selected proteins and polysaccharides and relevant product information were obtained from the manufacturers (Table 2). Due to commercial sensitivity, the manufacturers did not allow the disclosure of their products’ bulk prices. Thus, indicative bulk prices obtained from other articles are provided below. The polysaccharides used include carboxymethyl cellulose (CMC, ~1.4 USD/kg), NLS (0.2–0.5 USD/kg), XG (~2.0–3.0 USD/kg), and four different modified starches (corn, pea, potato, and wheat, typically <1.0 USD/kg in bulk) [20,37,38,39,40]. The chosen proteins comprise the plant-based fava bean protein concentrate (FBPC, ~1.4–2.5 USD/kg) and wheat protein (WP, ~1.4–2.5 USD/kg) and the animal-based proteins hen egg albumen (HEA, ~6.0–8.0 €/kg), porcine haemoglobin protein (HG, ~0.7–1.0 USD/kg), porcine plasma protein (PP, ~3.5–4.5 USD/kg), technical gelatine (TG, ~4–6 USD/kg), and whey protein concentrate (WPC, ~5.5 USD/kg) [41,42]. While technical gelatine does not strictly meet criterion (c), it was still selected because the temperature required to dissolve it (40 °C) is still modest.

Table 2.
Biopolymers investigated by this study and their product data.
2.2. Laboratory Experiments
All experiments were conducted on two substrates (medium-grained sand and fine-grained silica sand), using replicates (3x) and control (distilled water) samples and 14 different biopolymers at a fixed application rate and two different concentrations. The fixed application rate was set at 1.6 L/m2 (20.3 mL per sample), and the biopolymer concentrations were chosen as 1.0 and 2.0 wt%. These values were chosen as they are within the range recommended by the literature and have been used in previous studies [12,13,16,17,46]. XG was applied at only 0.25 and 0.50 wt% because higher concentrations yield too viscous solutions for spraying. Each sample was subject to (a) moisture retention tests, (b) penetrometer testing, and (c) crust weight and thickness measurements.
2.2.1. Sample Preparation
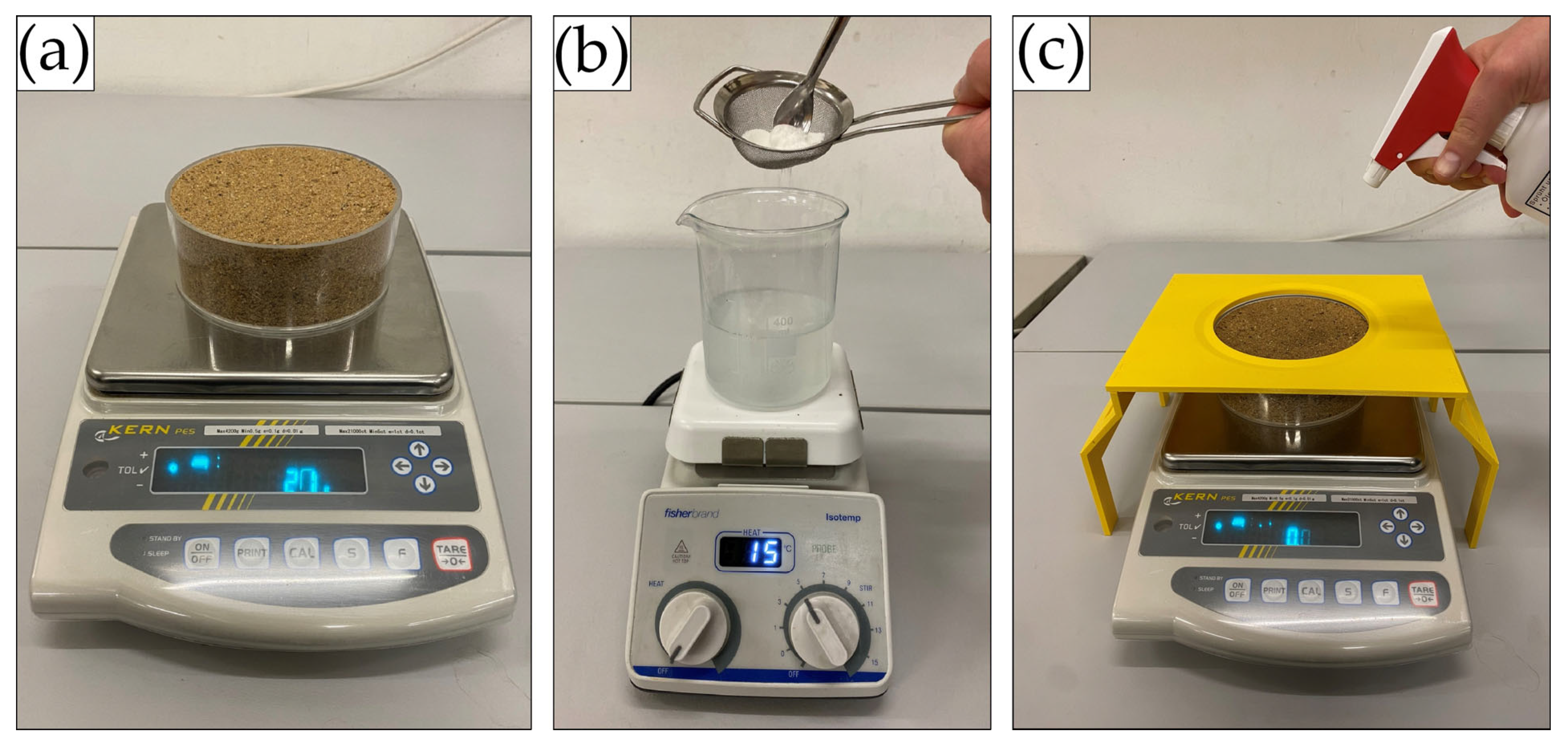
Overall, 174 substrate samples were prepared and tested. Air-dried soil was placed into acryl glass cylinder moulds (127 mm diameter, 50 mm height, 126.7 cm2 sample surface area). Samples were gently shaken to ensure slight and uniform compaction. Subsequently, sample surfaces were levelled with a ruler so that they were flush with the edge of the acryl glass cups. The resulting medium-grained sand samples weighed, on average, 1027 g (SD = 40.6 g) and the fine-grained silica sand samples 1109 g (SD = 38.6 g) (Figure 2a).
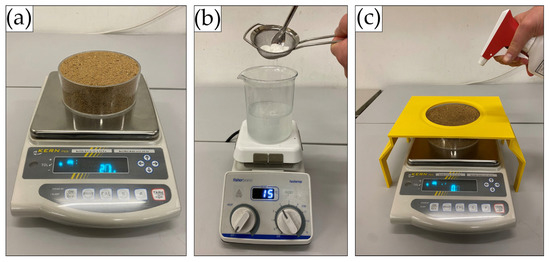
Figure 2.
(a) Weighing of dry sample, (b) preparation of biopolymer solution, (c) gravimetric spray-on application of biopolymer solution with splash guard.
The calculation of the biopolymer mass required for the preparation of the individual solutions also accounted for the biopolymers’ respective dry mass (Table 2). The biopolymer powders were dissolved at room temperature in distilled water at the specified concentrations for 10 min using a magnetic stirrer until fully dissolved (Figure 2b). To avoid clumping of the biopolymers, powders were slowly added to the distilled water through a sieve. The TG solution required preparation in 40 °C warm water.
The biopolymer solutions were sprayed onto the samples using a trigger sprayer with a nozzle suitable for viscous solutions (Ballistol hand atomizer, standard nozzle, Figure 2c). Uniform and accurate spray-on application was achieved by placing the untreated samples on a precision scale (Kern PES 4200-2M, 0.001 g resolution) and spraying the solution until the required application rate was achieved. Changes in solution density caused by the addition of biopolymer were considered negligible, so a fixed solution density of 1.0 g/mL was assumed for converting the required volumetric application rate (20.3 mL) to the gravimetric application rate (20.3 g). A 3D-printed splash guard was used to prevent the biopolymer solution from inadvertently touching the scale’s weighing plate and thus distorting the scale readings (Figure 2c). After achieving the required application rate, the edges of the cylinder moulds were wiped dry. In the sample preparation process, the following weight measurements were recorded: weight of the empty sample moulds, dry sample weight, and total sample weight after biopolymer application.
2.2.2. Moisture Retention Tests
The ability of a biopolymer to enhance the soil moisture retention capacity is one determinant of its potential as a dust suppressant. Soil with increased moisture retention capacity can bind water over longer periods and slow the evaporation effect [47]. Moisture makes soil particles heavier and enhances the interparticle binding force [17], causing it to be less susceptible to being suspended in the atmosphere by erosive forces.
For the moisture retention tests performed in this study, the weights of the samples were recorded before and immediately after the biopolymer application. The treated samples were then cured in the laboratory (RWTH Aachen, spring) for four days (96 h) at ambient temperature (21 ± 1 °C) and humidity (45 ± 2.5%), their weights were recorded every 24 h (t = 0, 24, 48, 72, and 96 h post application). The moisture retention, ω (wt%), was calculated as the proportion of the initial moisture applied at t = 0 h that was retained in the sample at t = 96 h and was calculated according to Equation (1):
where m1 denotes the sample weight after 96 h of curing (g), m0 is the sample weight after application of the biopolymer solution (g), and mdry represents the dry sample weight prior to the application of the biopolymer solution (g).
2.2.3. Penetrometer Tests
Penetrometer tests allow measuring the maximum penetration resistance of soil crusts under controlled conditions. This analytical approach has already been applied by numerous studies investigating dust control agents [13,14,16,17,28,33,48,49]. A penetrometer is a stationary loading machine mounted with a pin to penetrate the soil crust at a set penetration angle and rate, continuously recording the penetration force and depth. Ding et al. [13] concluded that penetration resistance is a good indicator for predicting the dust control performance of a biopolymer and is even better suited than the UCS. In addition, Toufigh and Ghassemi [17] reported a strong correlation between results from the penetrometer and wind tunnel tests. Thus, penetrometer tests are a recommended and established method for evaluating potential dust suppressants.
The penetrometer tests were performed with a 6.4 mm (1/4 inch) diameter flat-ended cylindrical penetrometer pin that was mounted to a Wille UL 60/100 loading machine equipped with a 0.001 N resolution calibrated load cell (Institute of Geomechanics and Underground Technology, RWTH Aachen University, Germany). One penetration test was performed in the centre of each sample, pursuing a 4 mm penetration depth at an advance rate of 2 mm/min, a data logging interval of 5 Hz, and a fixed penetration angle of 90° (Figure 3). Since the penetrometer records penetration resistance alongside the penetration depth, the crusts’ modulus of elasticity (Me, kN m−1) could be calculated by dividing the maximum penetration resistance (N) by the penetration depth reached at the moment of rupture.

Figure 3.
Penetrometer testing of fine-grained silica sand sample.
2.2.4. Crust Thickness Measurements
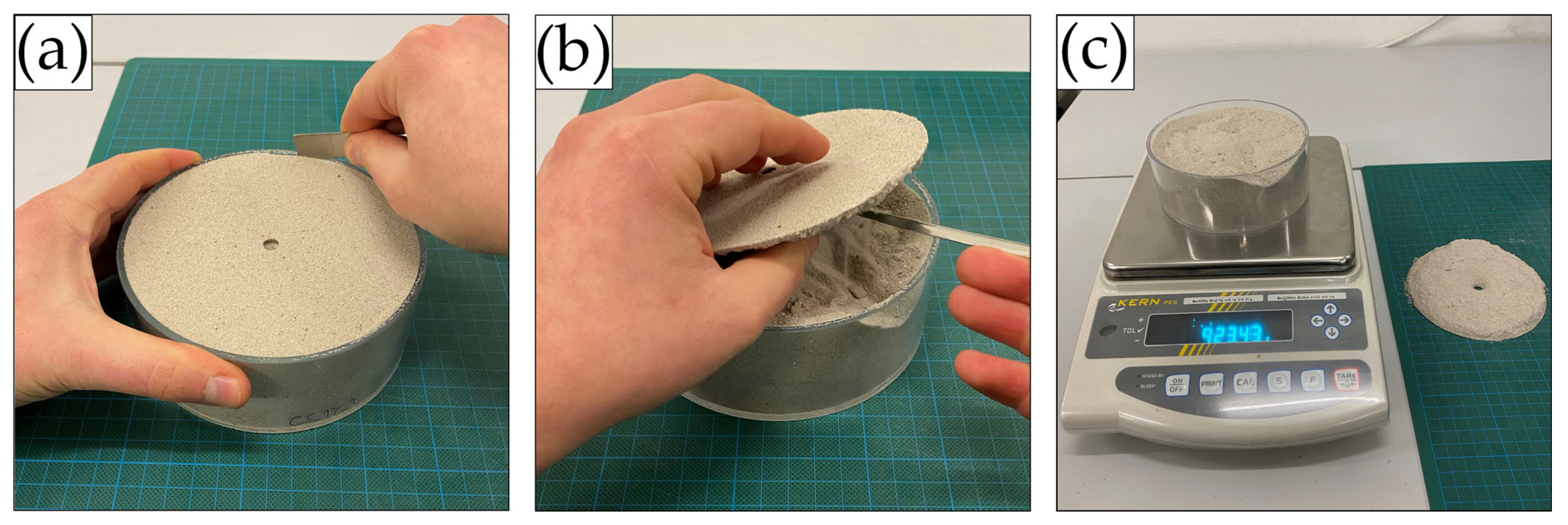
After penetration testing, the weight and thickness of every crust were measured. Therefore, crusts were carefully removed from the sample mould using a small spatula (Figure 4a,b). Depending on crust strength and brittleness, crusts could either be recovered in one piece, broken down into multiple recoverable pieces, or were very thin, weak, and brittle and thus barely recoverable. The samples were weighed again after the removal of the crusts, which allowed the calculation of the crust weight (Figure 4c). Together with the weight recordings and known dimensions of every sample mould, each crust’s average density and thickness were established.
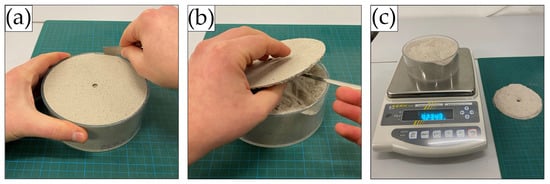
Figure 4.
Process of removing the crust from the sample. (a) Loosening of the crust with a spatula from the mould rim, (b) removal of the crust with a spatula, (c) weighing of the sample without the crust.
2.2.5. Statistical Analysis
Two-way analysis of variance (ANOVA) with α = .05 was performed to analyse whether biopolymer type and concentration had a significant effect on the measured parameters. Two-way ANOVA is a statistical method used to analyse whether two individual independent variables (i.e., biopolymer type and concentration), as well as their interaction (biopolymer type * concentration), have a significant effect on one dependent variable (i.e., moisture retention, penetration resistance, and crust thickness) or not. If the resulting p-value is < .05, it can be concluded that the corresponding independent variable or their interaction has a significant effect on the analysed dependent variable. Separate statistical analyses were performed for the polysaccharides and proteins, medium-grained sand, and fine-grained silica sand. As recommended by the literature, any percentage data (moisture retention tests) were subject to square-root data transformation before performing the two-way ANOVA [50].
3. Results
3.1. Moisture Retention Tests
3.1.1. Medium-Grained Sand
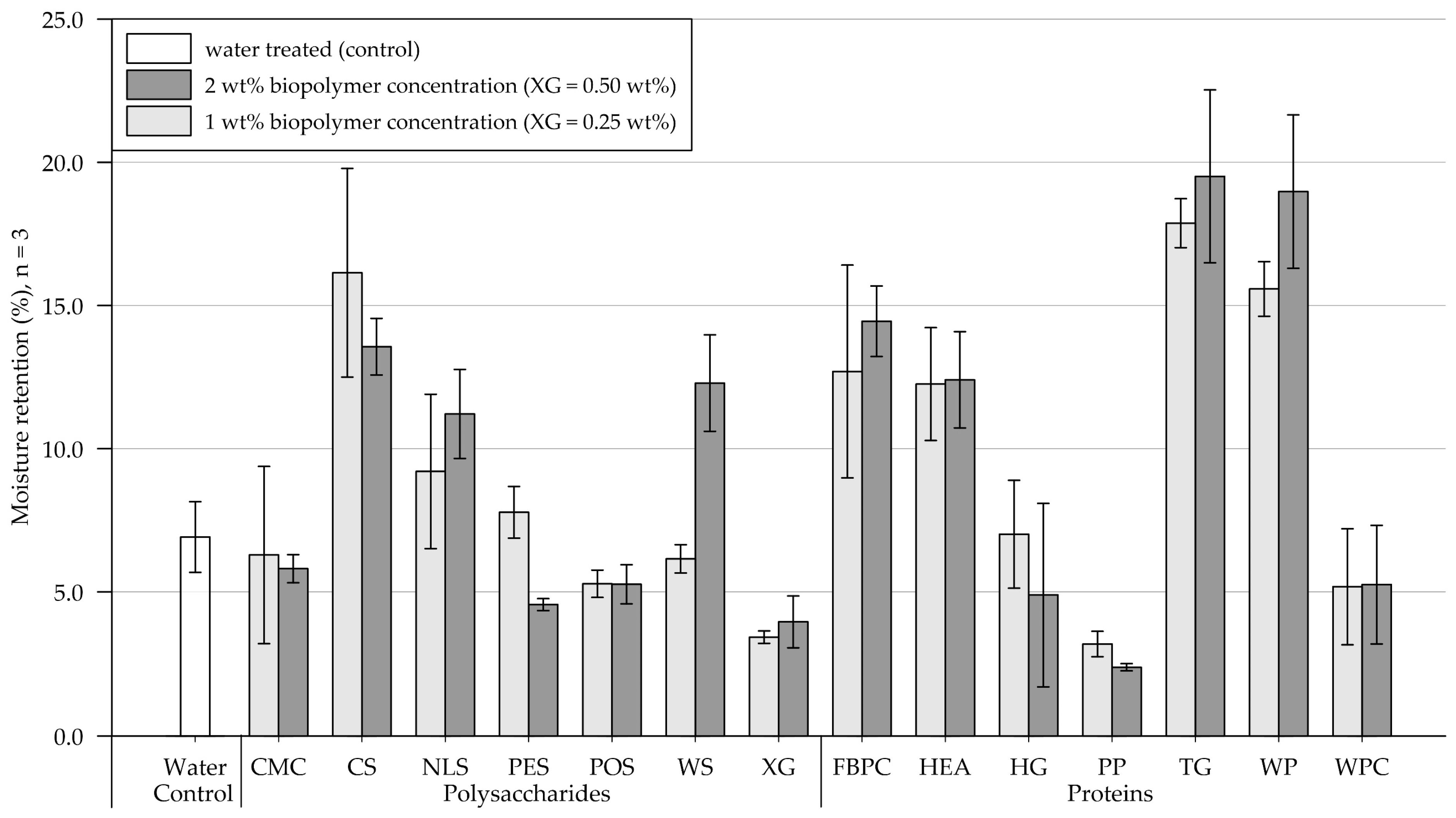
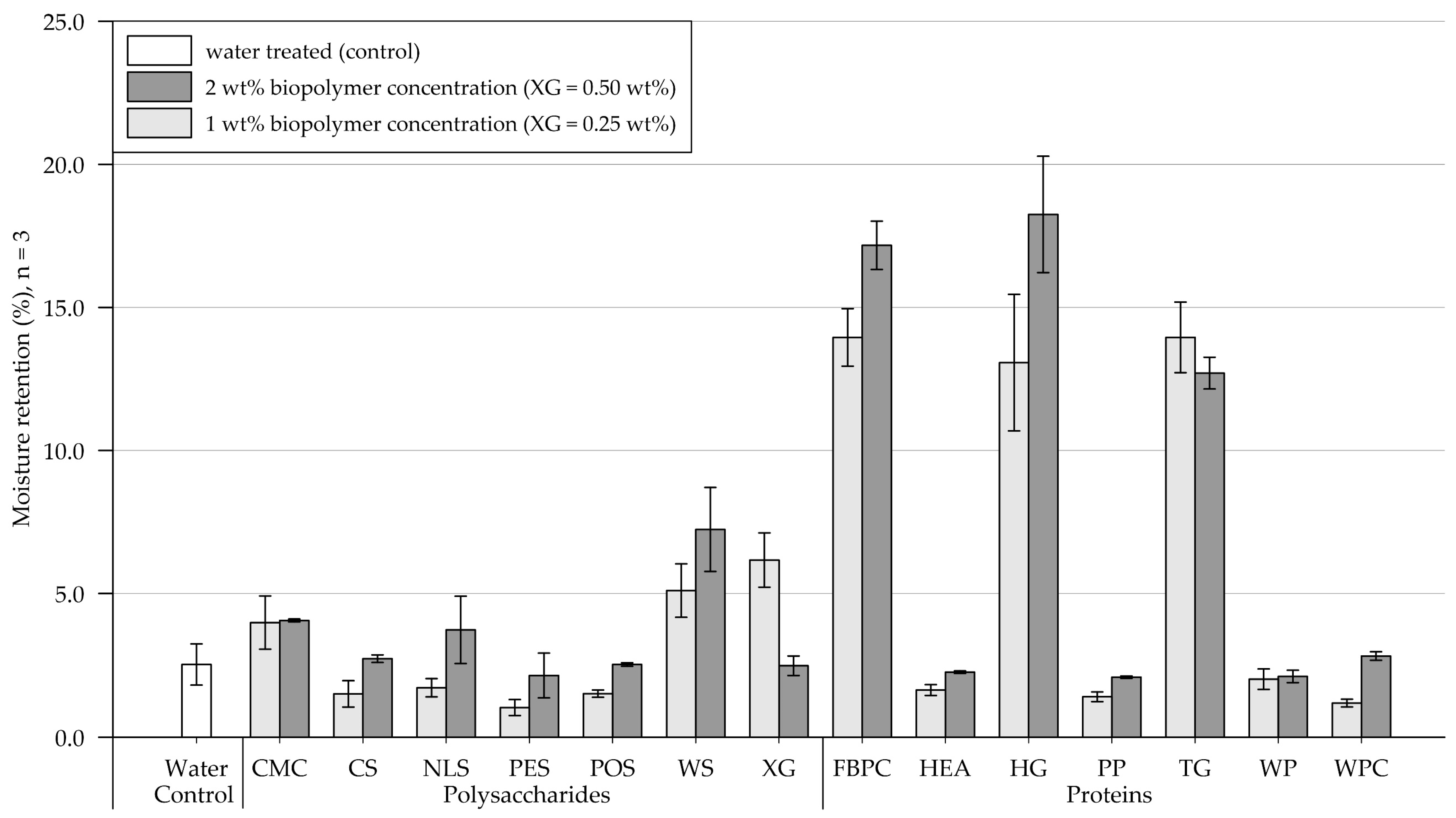
Results of the moisture retention tests are shown in Figure 5 (exact values are appended in Table A2). Four days (96 h) after treatment, the control sample, which was treated with water, contained 6.9% (SD = 1.2) of the initially applied 20.3 g water. In the following, results of the polysaccharide and protein applications on the medium-grained sand are presented and compared.
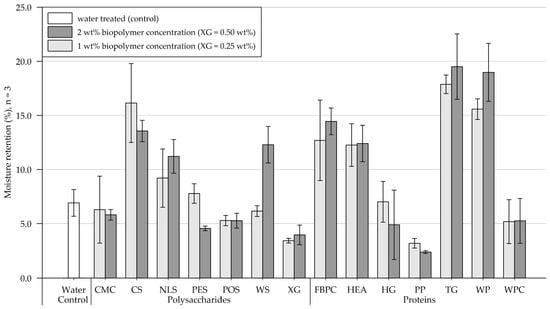
Figure 5.
Mean moisture retention of medium-grained sand samples 4 days (96 h) after treatment at 1.6 L/m2 and biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Biopolymers are grouped into polysaccharides and proteins with water as control. Tests were performed in triplicates (n = 3), and error bars indicate the standard deviation (SD). Note. The exact values of experimental results are appended in Table A2.
Polysaccharides. Compared to the control, the 1 wt% (XG = 0.25 wt%) treatments either increased or decreased the samples’ moisture retention. A slight reduction in moisture retention was observed for samples treated with CMC, POS, WS, and XG, whereas it increased for samples treated with CS, NLS, and PES. Relative to the 1 wt% treatment, the application of 2 wt% resulted in the samples’ moisture retention either decreasing, increasing, or changing negligibly. It decreased slightly for CMC and CS and significantly for PES, while no change was observed for POS. By contrast, it moderately increased for NLS and XG and even doubled for WS-amended samples.
Proteins. Protein applications at 1 wt% concentration reduced the moisture retention for samples amended with PP and WPC relative to the control group, while a negligible effect was observed for HG. Doubling the concentration decreased moisture retention for HG- and PP-treated samples, increased it for FBPC-, TG-, and WP-amended samples and had a negligible effect on applications with HEA and WP.
Comparison of polysaccharide and protein treatments. At the 1 wt% concentration, the polysaccharide-treated samples had, on average, higher moisture retention than the protein-amended samples. Doubling the concentration resulted in a slight increase in the average moisture retention of polysaccharide and protein-treated substrate. A direct comparison of the polysaccharide and protein amendments that resulted in the highest moisture retention shows that the material treated with proteins achieved higher moisture retention regardless of the concentration tested.
Statistical analysis. Results of the two-way ANOVA (Table 3) show that the types of polysaccharide (p < .001) and protein (p < .001) have a significant effect on moisture retention, whereby no general trend could be identified: whether the treatments increase or decrease moisture retention. Doubling the concentration has no significant effect on moisture retention among polysaccharide- (p = .596) and protein-amended samples (p = .470), and there is no general trend, whether doubling the concentration results in the moisture retention to increase or decrease.

Table 3.
Results of two-way ANOVA (α = .05) of moisture retention. Percentage values were transformed via square root transformation prior to performing two-way ANOVA.
Conclusions. On medium-grained sand, the type of biopolymers has a significant effect on the samples’ moisture retention, whereby some biopolymers significantly increased moisture retention. In contrast, others have a negligible effect or even decrease moisture retention relative to the control sample treated with water. The biopolymer’s concentration does not have a significant effect on the moisture retention of the treated samples.
3.1.2. Fine-Grained Silica Sand
Results of moisture retention tests are depicted in Figure 6 (exact values are appended in Table A2). Four days (96 h) after treatment, the control sample, which was treated with water, contained 2.5% (SD = 0.7) of the initially applied 20.3 g of water. In the following, results of the polysaccharide and protein applications to the fine-grained silica sand are presented and compared.
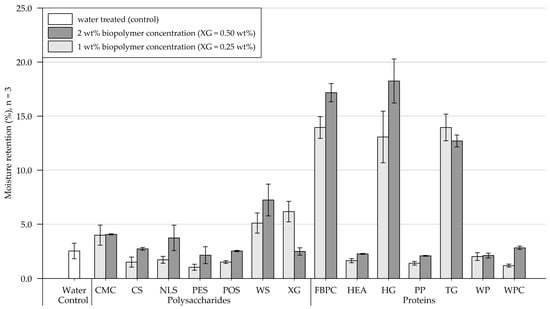
Figure 6.
Mean moisture retention of fine-grained silica sand samples 4 days (96 h) after treatment at 1.6 L/m2 and biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Biopolymers are grouped into polysaccharides and proteins with water as control. Tests were performed in triplicates (n = 3), and error bars indicate the standard deviation (SD). Note. The exact values of experimental results are appended in Table A2.
Polysaccharides. In relation to the control sample, a 1 wt% (XG = 0.25 wt%) biopolymer treatment decreased the moisture retention of substrates treated with CS, NLS, PES, and POS, whereas an increase was observed for samples amended with CMC, WS, and XG. Increasing the concentration to 2 wt% (XG = 0.50 wt%) significantly decreased the moisture retention of XG-treated samples, and CMC had a negligible effect. By contrast, significant increases in moisture retention were observed for samples subject to applications with CS, NLS, PES, POS, and WS.
Proteins. Biopolymer applications at 1 wt% reduced the moisture retention for samples amended with HEA, PP, WP, and WPC compared to the control group. At the same time, significant increases were observed for FBPC, HG, and TG, respectively. When the concentration was doubled to 2 wt%, the moisture retention of the TG-treated silica sand decreased slightly, while the remaining proteins increased moisture retention.
Comparison of polysaccharide and protein treatments. At both tested biopolymer concentrations, the protein-treated samples, on average, showed significantly higher moisture retention than the polysaccharide-amended soils. A comparison of the polysaccharide- and protein-treated silica sand samples that achieved the highest moisture retention shows that the proteins performed better regardless of the tested concentration.
Statistical analysis. Results of the two-way ANOVA (Table 3) show that the types of polysaccharide (p < .001) and protein (p < .001) have a significant effect on moisture retention, with some biopolymers having a negligible effect, others decreasing moisture retention, and some resulting in the retention rate increasing significantly. Doubling the biopolymer concentration slightly or even considerably increases moisture retention for all biopolymers except XG and TG. A significant effect of concentration on moisture retention is only indicated for the tested proteins (p < .001) and not for the polysaccharides (p = .052).
Conclusions. On fine-grained silica sand, the biopolymer type has a significant effect on the samples’ moisture retention, with some biopolymers increasing moisture retention, whereas others only result in minor changes or even decrease it relative to the control sample treated with water. Moisture retention of the treated silica sand is significantly influenced by the protein concentration.
3.2. Penetrometer Tests
3.2.1. Medium-Grained Sand
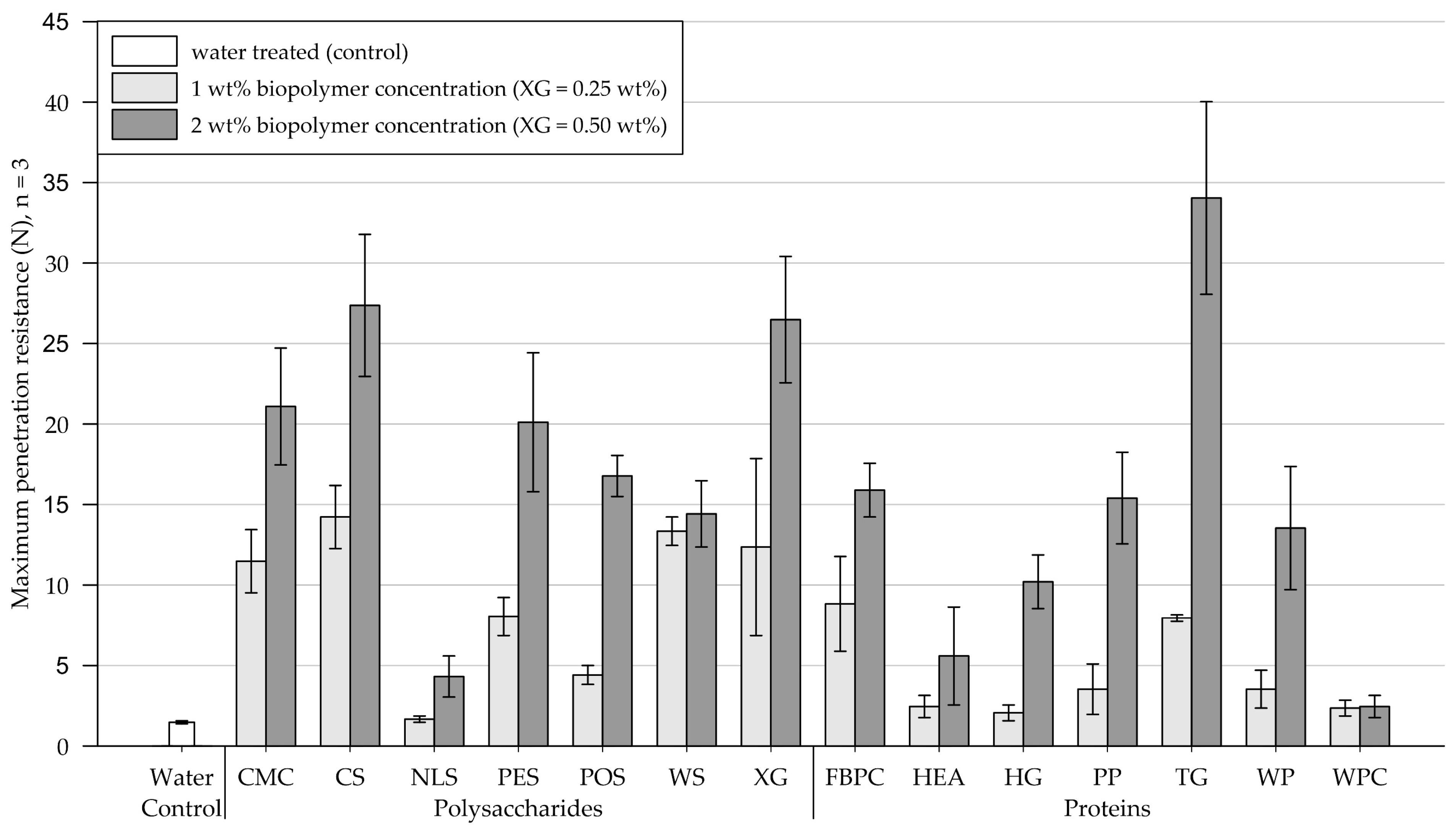
On medium-grained sand, all tested biopolymer applications formed crusts, and the results of the penetrometer tests are shown in Figure 7 (exact penetration resistance values and the results of the modulus of elasticity calculations are appended in Table A3 and Table A4, respectively). Four days after treatment, the control group, treated with water, endured a maximum penetration resistance of 1.5 N (SD = 0.1).
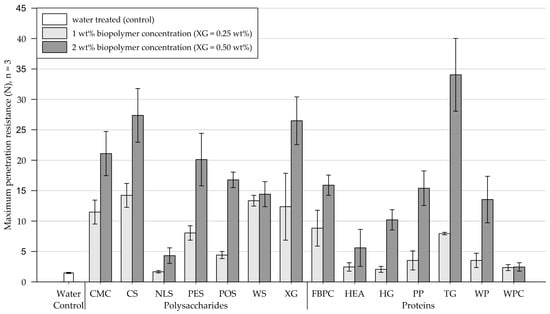
Figure 7.
Mean maximum penetration resistance of crusts from medium-grained sand samples measured by penetrometer tests. Biopolymers are grouped into polysaccharides and proteins with water as control. Tests were performed in triplicates (n = 3), with error bars indicating the standard deviation (SD). Note. The exact values of experimental results are appended in Table A3.
Polysaccharides. Compared to the control, 1 wt% (XG = 0.25 wt%) treatments with NLS had a negligible effect on the penetration resistance. By contrast, treatments with POS resulted in a slight increase in penetration resistance, whereas the remainder of the tested polysaccharide applications showed significant increases in penetration resistance. When the concentration was increased to 2 wt% (XG = 0.50 wt%), the penetration resistance of substrate amended with WS slightly increased, while all other tested polysaccharides showed significant increases, with CS and XG achieving the highest penetration resistances among all tested biopolymers.
Proteins. The application at a concentration of 1 wt% slightly increased the penetration resistance of sand treated with HEA, HG, PP, WP, and WPC compared to the control group treated with water. By contrast, substrates amended with FBPC and TG exhibited significantly higher penetration resistances. Relative to 1 wt%, the 2 wt% protein applications increased the maximum penetration resistance of all treated samples, except for WPC. Applications with HG, PP, and TG displayed the greatest increases in penetration resistance.
Comparison of polysaccharide and protein treatments. When biopolymers were applied at concentrations of 1 wt% (XG = 0.25 wt%), polysaccharide-induced crusts were, on average, more than twice as resistant to penetration than protein-induced crusts. Doubling the concentration to 2 wt% (XG = 0.50 wt%) resulted in a disproportionate increase in the penetration resistance of the protein-induced crusts compared to the polysaccharide-induced crusts, with the polysaccharide treatments still achieving higher absolute penetration resistances.
Statistical analysis. Results of the two-way ANOVA (Table 4) show that the types of polysaccharide and protein have a significant effect on the penetration resistance (p < .001), with some biopolymer treatments achieving significantly higher penetration resistances than others. Among the proteins and polysaccharides, the concentration has a significant effect (p < .001) on the penetration resistance, whereby higher concentrations mostly enhanced the penetration resistance significantly.

Table 4.
Results of two-way ANOVA (α = .05) of maximum penetration resistance measured by penetrometer.
Conclusions. On medium-grained sand, the type of biopolymer and concentration have a significant effect on the resulting penetration resistance of the cured crusts. Some biopolymers enhanced the penetration resistance only slightly or negligibly, while others had a considerable effect. For most biopolymers, doubling the concentration increased the achieved penetration resistance considerably.
3.2.2. Fine-Grained Silica Sand
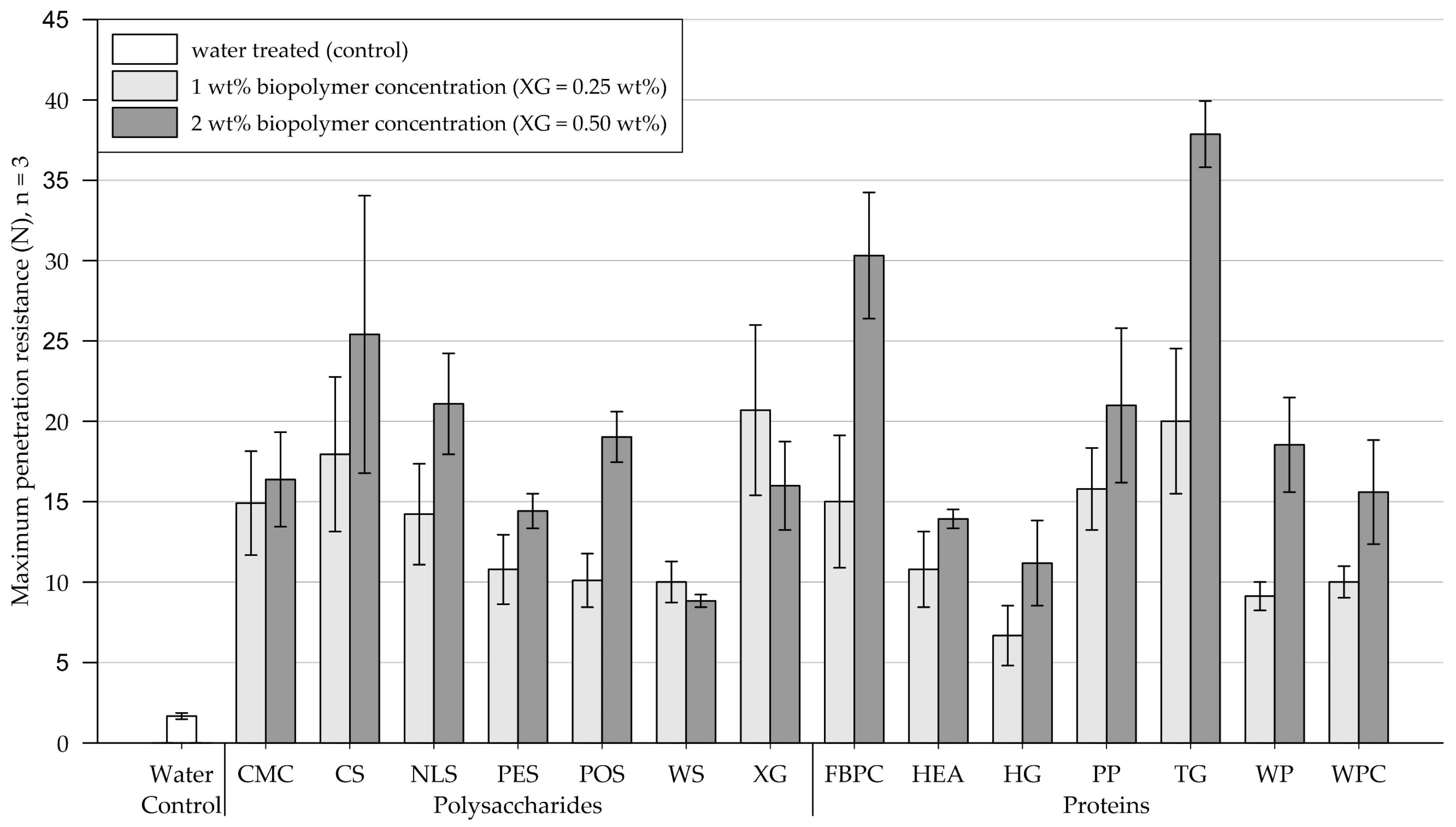
Results of the penetrometer tests performed on fine-grained silica sand samples are shown in Figure 8 (exact values are appended in Table A3). Four days after treatment, the water-treated control group had a maximum penetration resistance of 1.7 N (SD = 0.2).
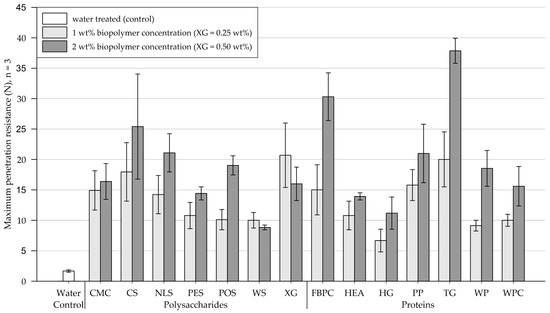
Figure 8.
Mean maximum penetration resistance of crusts from fine-grained silica sand samples measured by penetrometer tests. Biopolymers are grouped into polysaccharides and proteins with water as control. Tests were performed in triplicates (n = 3), with error bars indicating the standard deviation (SD). Note. The exact values of experimental results are appended in Table A3.
Polysaccharides. At biopolymer concentrations of 1 wt% (XG = 0.25 wt%), all tested polysaccharide types formed crusts with considerably enhanced penetration resistances compared to the control group treated with water. Increasing the concentration significantly enhanced the penetration resistance of all tested polysaccharides, except for WS and XG, whose penetration resistance decreased.
Proteins. Relative to the control group treated with water, all protein admixtures applied at 1 wt% concentration formed crusts with significantly increased penetration resistances. Compared to treatments at 1 wt% concentration, applications at 2 wt% resulted in the penetration resistance of all tested proteins increasing significantly, with FBPC, TG, and WP experiencing the most significant increases.
Comparison of polysaccharide and protein treatments. For amendments at 1 wt% concentration, polysaccharide-induced crusts, on average, endured slightly higher penetration resistances than protein-induced crusts. While doubling the concentration significantly increased the penetration resistance of almost all samples, the penetration resistance of protein-induced crusts displayed a disproportionate increase relative to polysaccharide crusts. At the higher biopolymer concentration, the protein-treated substrates, on average, showed higher penetration resistances than the polysaccharides.
Statistical analysis. Results of the two-way ANOVA (Table 4) show that the types of polysaccharide (p < .001) and protein (p < .001) have a significant effect on the penetration resistance, with some biopolymers forming significantly stronger crusts than others. The concentration also has a significant effect on the penetration resistance (polysaccharide: p = .026, proteins: p < .001), whereby doubling the concentration significantly increased the penetration resistance of most tested amendments.
Conclusions. On fine-grained silica sand, all biopolymers produced crusts with significantly increased penetration resistances relative to the control group, with the biopolymer type having a significant effect on the resulting penetration resistance. The effect of doubling the concentration strongly depends on the biopolymer type, whereby most biopolymer treatments exhibited significant increases.
3.3. Crust Thickness Measurements
3.3.1. Medium-Grained Sand
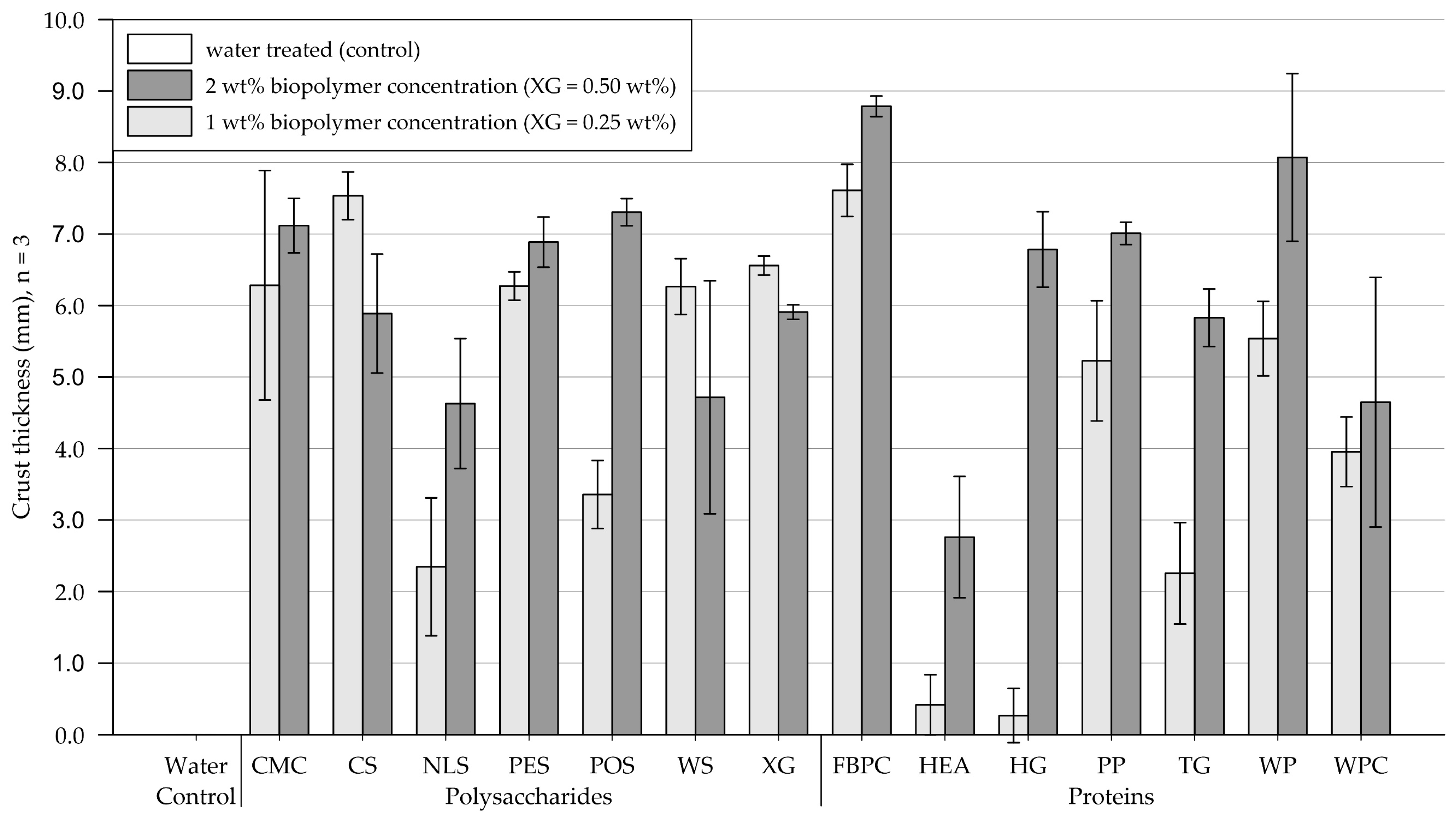
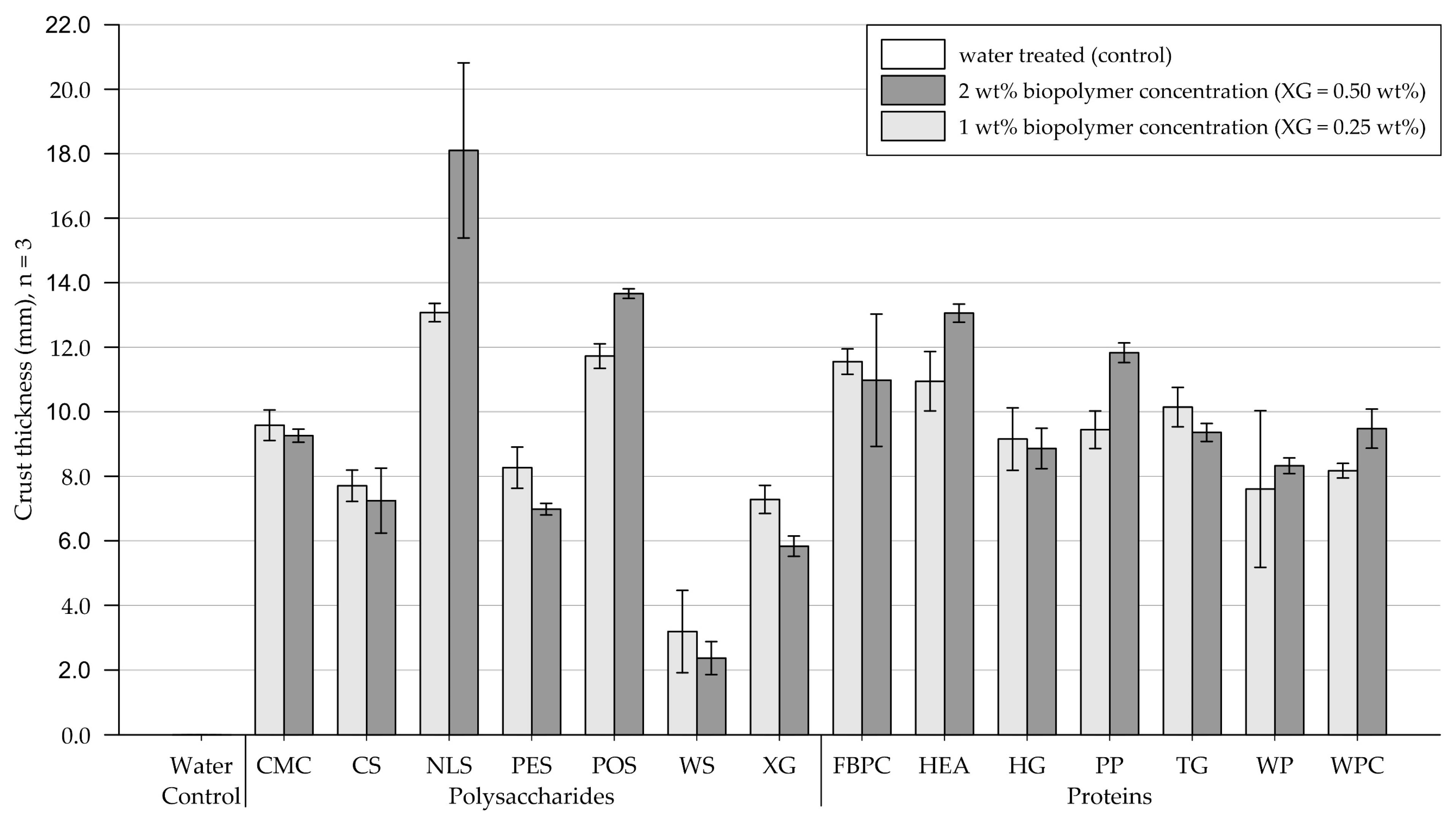
The results of the crust thickness measurements are shown in Figure 9 (exact values are appended in Table A5). Figure 10 and Figure 11 show exemplary pictures of upside-down-facing crusts formed by the tested biopolymer amendments (visual classification appended in Table A6). While the control group, treated with water, formed no recoverable crust, all tested biopolymers formed crusts of varying thicknesses.
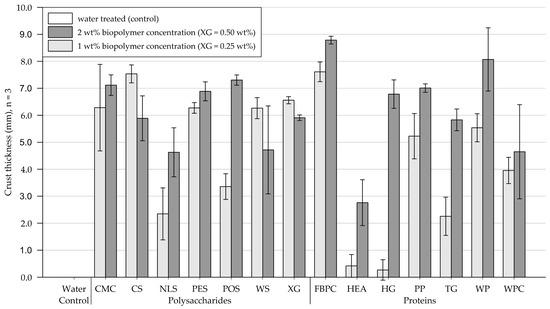
Figure 9.
Mean thickness of crust formed on medium-grained sand samples. Biopolymers are grouped into polysaccharides and proteins with water as control. Tests were performed with six replicas (n = 3), with error bars indicating the standard deviation (SD). Note. The exact values of experimental results are appended in Table A5.

Figure 10.
Examples of polysaccharide-induced crusts recovered from the samples (cf. Table A6 for classification).

Figure 11.
Examples of protein-induced crusts recovered from the samples (cf. Table A6 for classification).
Polysaccharides. Treatments at 1 wt% (XG = 0.25 wt%) produced crusts ranging from 2 to 7.2 mm in thickness. While amendments with NLS and POS formed relatively thin to medium-thick crusts, the remaining polysaccharide applications yielded thick crusts on medium-grained sand. Compared to the 1 wt% treatment, doubling the concentration decreased the crust thickness for substrate treated with XG, CS, and WS, while crusts resulting from amendments with CMC and PES displayed a slight, and NLS and POS a significant, increase in thickness, respectively.
Proteins. Protein applications at 1 wt% concentration produced crusts of varying thickness. While treatments with HEA and HG formed fragile, brittle, and barely recoverable crusts, TG already formed slightly thicker crusts. Thick crusts formed on medium-grained sand amended with PP, WP, WPC, and FBPC, with the latter resulting in the thickest crusts of all biopolymers tested at 1 wt%. At the higher tested concentration, the crust thicknesses of all treatments increased, whereby HEA, TG, and HG displayed the most significant increases.
Comparison of polysaccharide and protein treatments. At treatments with 1 wt% (XG = 0.25 wt%) biopolymer concentration, medium-grained sand amended with polysaccharides, on average, formed slightly thicker crusts than applications with proteins. Doubling the concentration to 2 wt% (XG = 0.50 wt%) resulted in the thickness of polysaccharide-induced crusts to increase slightly, while protein-induced crusts displayed significant increases.
Statistical analysis. Results of the two-way ANOVA (Table 5) show that the types of polysaccharide (p < .001) and protein (p < .001) have a significant effect on the crust thickness, with some biopolymers forming only very thin crusts and others thick crusts. Increasing the concentration only had a significant effect on the crust thickness of protein amendments (p < .001), whereby increasing the concentration caused the crust thickness of all protein-treated samples to increase.

Table 5.
Results of two-way ANOVA (α = .05) of average calculated crust thickness.
Conclusions. On medium-grained sand, all biopolymer treatments formed crusts, with the resulting crust thickness varying significantly among the biopolymers tested. For most polysaccharides, doubling the concentration only slightly increased the crust thickness, whereas most protein treatments exhibited significant increases in crust thickness.
3.3.2. Fine-Grained Silica Sand
The results of the crust thickness calculation are shown in Figure 12 (exact values are appended in Table A5). Exemplary depictions of the cured crusts are displayed in Figure 10 and Figure 11. The control group formed no recoverable crust, whereas all tested biopolymers formed crusts of varying thicknesses.
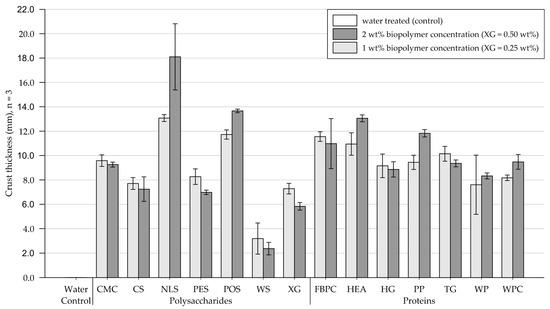
Figure 12.
Mean thickness of crust formed on fine-grained silica sand samples. Biopolymers are grouped into polysaccharides and proteins with water as control. Tests were performed with six replicas (n = 3), with error bars indicating the standard deviation (SD). Note. The exact values of experimental results are appended in Table A5.
Polysaccharides. Polysaccharide applications at 1 wt% (XG = 0.25 wt%) formed crusts of varying thickness. WS formed only very thin and rather ductile crusts, while the remainder of the tested polysaccharide applications yielded significantly thicker crusts. Doubling the concentration increased the thickness of silica sand crusts amended with NLS and POS, while the other tested polysaccharide applications displayed decreased crust thicknesses. Notably, crusts formed because of treatment with WS even curled up throughout the curing period (Figure 10).
Proteins. Treatments at 1 wt% produced crusts of similar thicknesses, whereby amendments with FBPC, HEA, and TG formed the thickest crusts among the proteins tested. Doubling the concentration slightly decreased the thickness of crusts formed after treatment with FBPC, TG, and HG, whereas the other protein applications displayed slight increases in crust thickness.
Comparison of polysaccharide and protein treatments. At a biopolymer concentration of 1 wt% (XG = 0.25 wt%), protein amendments, on average, formed slightly thicker crusts than polysaccharide treatments. Doubling the concentration had a similar effect for most biopolymer applications, only slightly reducing or increasing the resulting crust thickness.
Statistical analysis. Two-way ANOVA (Table 4) shows that the biopolymer type has a significant effect on crust thickness among polysaccharides (p < .001) and proteins (p < .001), whereby the crust thickness differs significantly depending on the biopolymer type. By contrast, doubling the concentration has no significant effect (polysaccharides: p = .288, proteins: p = .075) on the resulting crust thickness, as it only slightly increased or even decreased the crust thickness.
Conclusions. All biopolymers tested formed crusts on fine-grained silica sand. While the crust thicknesses were relatively uniform among the tested proteins, the tested polysaccharide amendments resulted in a greater variability. For all biopolymers, doubling the concentration had only a small effect on the resulting crust thickness.
4. Discussion
4.1. Moisture Retention Tests
Several studies have investigated the effect of different biopolymer types and concentrations on moisture retention of biopolymer-treated soils [17,28,46,47,49,51,52]. The studies found that biopolymer treatments enhance soil moisture retention, with the biopolymer type having a significant effect on the resulting moisture retention. Most studies concluded that moisture retention increases at higher biopolymer concentrations [17,28,49], although one study did not confirm this effect [46]. Soil type also appears to significantly influence the moisture retention that can be achieved by a biopolymer amendment [17]. In the following, the key trends of this study’s results are discussed in the context of the previous literature. A more detailed discussion is limited by the significant differences in soil, treatment, and experimental setup among the studies.
4.1.1. Effect of Biopolymer Type
Medium-grained sand. The water-treated control group exhibited a higher moisture retention than several biopolymer treatments, which is not consistent with the existing literature [17,46,49,53]. It is assumed that the relatively low viscosity of water allowed it to penetrate deeper into the medium-grained sand, making it less prone to evaporation. By contrast, the more viscous biopolymer solutions did not infiltrate as deeply into the sample, resulting in a higher evaporation rate.
Some biopolymer treatments significantly increased moisture retention (Figure 5), which is consistent with the findings of other studies examining the ability of biopolymers to improve soil moisture retention [17,25,28,46,49,54,55]. This increase is likely due to the high water-absorption capacity of the biopolymers, as well as the effects of the solution viscosity, bio-clogging, and crust formation. Low solution viscosity allows water molecules to infiltrate deeper into the soil, making them less susceptible to evaporation. Bio-clogging occurs when hydrated biopolymers clog the soil’s pore space, reducing capillary conductivity and, thus, the evaporation rate [52,56]. Additionally, biopolymer-induced surface crusts may act as barriers between moisture and air, slowing the evaporation rate [47,57]. Biopolymers such as XG, PP, POS, and CMC did not significantly affect moisture retention (Figure 5), which does not correspond with the published literature [17,28,49]. Conversely to the biopolymer treatments that increased the moisture retention of medium-grained sand, it is likely that a low water-absorption capacity (PP and POS) or a relatively high viscosity (XG and CMC) contributed to a faster evaporation rate and thus lower moisture retention.
Fine-grained silica sand. Similar trends in moisture retention were found on fine-grained silica sand as on medium-grained sand, with some biopolymers decreasing it, some having no effect, and some increasing it significantly compared to the water-treated control group (Figure 6). In addition, as found for medium-grained sand, the control did not have the lowest moisture retention. The same reasons as identified for medium-grained sand likely caused these results.
Comparison of polysaccharides and proteins. On average, the well-performing proteins, such as FBPC, HEA, WP, and HG, achieved a higher moisture retention capacity than the well-performing polysaccharides, such as CS, NLS, and WS (Figure 5 and Figure 6). It is likely that this trend has been caused by the lower viscosity exhibited by most protein solutions and potentially a higher water-absorption capacity.
4.1.2. Effect of Biopolymer Concentration
Medium-grained sand. Increasing the biopolymer concentration had no significant effect on moisture retention (Figure 5 and Table 3). The published literature also reported mixed findings, with most studies concluding that higher biopolymer concentrations increase moisture retention [17,28,49], while one study could not confirm this observation [46]. For biopolymer applications that increased moisture retention (Figure 5), it is assumed that doubling the concentration amplified the effects of water absorption capacity, bio-clogging [52,56], and crust formation [47,57] (as discussed in Section 4.1.1).
For some biopolymers, doubling the concentration had little effect on moisture retention or even caused it to decrease. Ding et al. [28] reported similar results when testing applications of lignosulfonates on red sand, with moisture retention only increasing noticeably up to a certain concentration. This suggests that each biopolymer has a specific threshold concentration, beyond which only minor changes or even a decrease in soil moisture retention occurs. This trend is likely related to an increase in biopolymer viscosity, which reduces infiltration depth and increases evaporation rate, resulting in the adverse effects of higher viscosity outweighing the positive effects of increased water absorption capacity, bio-clogging, and crust formation (see Section 4.1.1).
Fine-grained silica sand. On fine-grained silica sand, doubling the concentration resulted in a slight increase in moisture retention for most tested biopolymers (Figure 6 and Table 3), which agrees with previous studies [17,28,49]. It is believed that the same factors assumed for medium-grained sand caused these trends on fine-grained silica sand. Samples treated with XG and TG exhibited decreased moisture retention when the concentration was doubled, likely because of an increase in viscosity that limited the infiltration depth and, thus, increased the evaporation rate. CMC-treated samples showed no significant change, assumably because of the balancing of positive effects (e.g., increased water absorption, bio-clogging, and crust formation) and the negative effect of higher viscosity. Fine-grained silica sand samples, on average, exhibited a lower moisture-retention capacity than medium-grained sand samples (Figure 5) because of the higher hydraulic conductivity of medium-grained sand that enhanced the infiltration depth and decreased evaporation throughout the curing period.
4.2. Penetrometer Tests
Multiple studies performed penetrometer tests to investigate the effects of different biopolymers and concentrations on the penetration resistance of biopolymer-induced soil crusts [13,14,16,17,28,33,48,49]. A compilation of these studies’ experimental results has been appended in Table A7 and Table A8. The results suggest that the biopolymer type has a significant effect on the crusts’ penetration resistance, with some biopolymers forming firmer crusts than others. Increasing the concentration generally increases the penetration resistance [33,46,49], although one study found that this relationship plateaus at a specific concentration [16]. On sandy soil, biopolymers act by coating and binding sand particles together, forming a cross-linked 3D network that increases inter-particle cohesion [21]. In the following sections, the key trends of this study’s results are discussed in the context of the previous literature, as a more detailed discussion is limited by the significant differences in soil, treatment, and experimental setup among the studies.
4.2.1. Effect of Biopolymer Type
Medium-grained sand. Penetrometer test results showed that all tested biopolymers formed crusts with differing penetration resistances, with the biopolymer type significantly affecting the penetration resistance (Table 4 and Figure 7). The water-treated control group formed no crust and endured a very low penetration resistance. These trends are consistent with findings from previous studies (Table A7 and Table A8), which also showed that the water-treated control endured low penetration resistances relative to the biopolymer-treated samples.
Comparison with previous studies on XG, NLS, and CMC [17,33] (cf. Table A7 and Table A8) reveals that XG forms stronger crusts than NLS, which agrees with this study’s results (Figure 7). By contrast, Toufigh and Ghassemi [17] reported that CMC tends to form firmer crusts than XG, which contradicts the results of this study. This discrepancy may be due to the different XG types used in the two studies. The XG used in this study formed highly viscous, not-sprayable gels at concentrations >0.5 wt%, while Toufigh and Ghassemi tested XG up to 1.5 wt% without reporting issues regarding the solution viscosity and spray-ability. Hence, it is likely that the XG used in this study had a higher gel strength and viscosity than the one tested by Toufigh and Ghassemi.
The starches (CS, POS, PES, and WS) also formed crusts, some with high penetration resistances. While the penetration resistance of starch-treated soil has not yet been investigated, other studies have already demonstrated their ability to improve soil mechanical properties. A wind tunnel study found that starch-treated soil exhibited reduced wind erosion [58], while other studies reported that starches and starch–xanthan blends enhanced the soil’s geotechnical properties [59,60,61].
All tested proteins formed crusts, some of which had high penetration resistances (Figure 7). While previous studies have not investigated the crust strength of protein-treated soils, some studies demonstrated proteins’ ability to enhance geotechnical soil properties [47,62,63,64]. Analogous to PP, bovine plasma protein was found to increase the compressive strength of biopolymer-bound soil composites [62]; while corresponding to WPC, cottage cheese whey was found to improve aggregate stability and reduce furrow erosion [64]. Likewise, gelatine has been used as a constituent in the formulation of dust suppressants because of its film-forming properties [47,63]. The remaining proteins tested in this study have not yet been tested regarding their ability to enhance soil properties but are commonly used in the food industry for their thickening, gelling, or water-retention abilities [65,66].
Fine-grained silica sand. The penetrometer tests revealed that all biopolymer treatments formed crusts, with the biopolymer type significantly affecting the penetration resistance (Figure 8 and Table 4). This trend corresponds with previous research (Table A7 and Table A8). Compared to medium-grained sand, crusts on fine-grained silica sand, on average, had a higher penetration resistance. This can be attributed to the silica sands’ smaller particle size, which results in a larger surface area, tighter packing, and lower hydraulic conductivity. As a result, the biopolymers coat a larger surface area, must bridge shorter distances between particles, and do not infiltrate as deep into the soil, and thus form a stronger crust.
4.2.2. Effect of Biopolymer Concentration
Medium-grained sand. Penetrometer test results showed that doubling the concentration significantly increased the penetration resistance of most biopolymers tested (Figure 7 and Table 4). This trend corresponds with previous research (Table A7 and Table A8) [14,17,28,33]. As stated by Owji et al. [14], this is because higher biopolymer concentrations increase the thickness of the inter-particle bonds forming between the coated sand particles, which causes the crust strength to increase. Doubling the concentration of WS and WPC only slightly increased the penetration resistance (Figure 7). The minor effect of WPC suggests that it generally has limited potential to enhance soil properties. Regarding WS, it is likely that its high viscosity limited the infiltration depth, leading to the presence of more biopolymer molecules in the pores than required to form a stable 3D network. Thus, for WS doubling, the concentration likely exceeded a threshold concentration, beyond which only minor increases in crust strength occur. This corresponds with findings of Lemboye et al. [16], who observed that applications of sodium alginate and pectin only increased penetration resistance up to 2 or 3 wt%.
Fine-grained silica sand. Doubling the concentration increased the penetration resistance of most biopolymers tested (Figure 8), similar to the findings on medium-grained sand (Figure 7) and the published literature (Table A7 and Table A8). WS and XG exhibit a different trend, as doubling their concentration resulted in decreased penetration resistances. This can be attributed to the high viscosities of their respective solutions, which limited infiltration depth and crust thickness, causing the crust to endure less load. For WS, the low infiltration and high local biopolymer concentration even caused the crust to curl up because of tension forces that occurred as the swollen biopolymers dehydrated and shrank (Figure 10).
Comparison of polysaccharides and proteins. On average, protein-treated samples exhibited larger incremental increases in penetration resistance than polysaccharide-treated samples upon doubling the concentration (Figure 7 and Figure 8). Thereby, on medium-grained sand, some protein treatments only formed stable crusts at 2 wt%, while most tested polysaccharides formed stable crusts at 1 wt% on both soils (XG = 0.25 wt%). This suggests that for the tested polysaccharides, doubling the concentration likely surpassed a threshold beyond which only small increases in crust strength occur. As a result, proteins must be applied at higher concentrations to achieve comparable penetration resistances as polysaccharide-induced crusts on medium-grained sand.
4.3. Crust Thickness Measurements
Several studies have shown that the type and concentration of biopolymers significantly affect the thickness of biopolymer-induced soil crusts, and a compilation of their results has been appended for reference (Table A9) [13,14,16,28,46,67]. Thereby, viscosity and the ability to enhance inter-particle cohesion influence the resulting crust thickness and depend on the biopolymer type and concentration. In the following, the main trends of this study’s results are discussed in the context of previous studies. A more detailed discussion is limited by the significantly different soil types, treatments, and experimental setups used in previous studies.
4.3.1. Effect of Biopolymer Type
Medium-grained sand. The crust thickness measurements showed that all tested biopolymers produced crusts, with the biopolymer type significantly affecting the resulting crust thickness (Figure 9 and Table 5). These findings correspond with the published literature (Table A9). In contrast to the other biopolymers, NLS, HEA, HG, and TG partially formed very thin and brittle crusts (Figure 10 and Figure 11). Regarding NLS, this can be explained as previous studies found that lignosulfonates must be applied at concentrations >2 wt% to enhance soil mechanical properties effectively [19,33,68]. The ability of HEA and HG to enhance soil mechanical properties has not yet been studied, but their treatments at 1 wt% and 2 wt% (only HEA) did not increase inter-particle cohesion sufficiently to form a competent crust.
Fine-grained silica sand. The test results showed that the biopolymer type significantly affects the crust thickness (Figure 12 and Table 5), which agrees with the existing literature (Table A9). Compared to the other tested biopolymers, NLS, POS, HEA, and PP formed relatively thick crusts, likely because of a favourable combination of viscosity and bonding strength, allowing for a high infiltration depth and sufficient inter-particle cohesion to form a thick crust. By contrast, WS and XG only formed thin crusts because of their high viscosity. Hence, on fine-grained silica sand, biopolymer viscosity is a limiting factor regarding the achievable crust thickness. Treatments on fine-grained silica sand generally formed thicker crusts than on medium-grained sand, as the finer particle size and tighter packing of the silica sand favour particle agglomeration and crust formation. In this context, NLS, HG, and HEA exhibited significant differences, forming only thin and brittle crusts on medium-grained sand but achieving considerably higher crust thicknesses on fine-grained silica sand.
4.3.2. Effect of Biopolymer Concentration
Medium-grained sand. The results of crust thickness measurements revealed that doubling the biopolymer concentration significantly affected crust thickness (Figure 9 and Table 5). For most polysaccharides, crust thickness only increased slightly or even decreased upon doubling the concentration, which corresponds with the published literature (Table A9). This is likely because the increased viscosity limited the infiltration depth, resulting in a thinner crust. By contrast, NLS and POS treatment benefitted significantly from doubling the concentration, which increased the inter-particle cohesion sufficiently to form a thick crust.
In contrast to the polysaccharide treatments, doubling the concentration significantly increased the thickness of protein-induced crusts. This is because doubling the concentration increased inter-particle cohesion without limiting the infiltration depth. While most polysaccharides formed relatively thick crusts at 1 wt%, most proteins required concentrations of 2 wt% to achieve crusts of similar thickness. Thus, proteins must be applied at higher concentrations to achieve similar crust thicknesses as polysaccharide treatments.
Fine-grained silica sand. Results showed that doubling the concentration had no significant effect on the resulting crust thickness, leading only to slight increases or decreases (Figure 12 and Table 5). It appears that concentrations of 1 wt% are already sufficient to form a thick, stable crust, so doubling the concentration mainly resulted in adverse effects caused by the increase in viscosity.
4.4. Evaluation of Tested Biopolymers as Dust Suppressants
This study found that the tested biopolymers formed crusts with significantly increased penetration resistances relative to the water-treated control and, in part, enhanced moisture retention. As penetration resistance [13,17,26] and moisture retention [28] are commonly used indicators for assessing potential dust suppressants, it is concluded that most tested polysaccharides and proteins show potential as dust suppressants. XG was incorporated as a benchmark in the study, as it is the most widely studied biopolymer in soil stabilisation and dust control [22,69]. The results on both tested soil types showed that some polysaccharides (CMC, CS, and WS) and proteins (FBPC, PP, and WP) achieved similar crust penetration resistances as XG (Figure 7 and Figure 8). However, it must be considered that XG was only tested at a quarter of the concentration of the other biopolymers.
Commercially available dust control products, such as chloride salts, petroleum-based products, and synthetic polymers, are effective dust suppressants but remain costly and can have adverse environmental effects [12,70]. Thus, there is a need for affordable, environmentally friendly alternatives that are easy to apply. While approaches such as microbially induced carbonate precipitation show potential to mitigate wind erosion [71,72,73], their cultivation and application are challenging [23], rendering them not easy-to-apply off-the-shelf solutions. Alternatively, by-products and wastes from food processing have shown potential as dust suppressants [74,75], but their inconsistent composition and dry-matter content may limit their application potential. By contrast, biopolymers show potential to meet the needs mentioned above.
This study and previous research [13,14,15,16] have shown that biopolymers have potential as dust suppressants. Moreover, they are biodegradable [76], well-studied, and frequently applied in the food industry and medical applications [40,66,77]. The tested biopolymers have relatively low cost, are easily dissolvable in water, and can be applied using conventional water-spraying equipment. In addition, the tested biopolymers originate from botanical (corn, pea, wheat, cellulose, potato, and fava bean) and animal (pig, chicken, and cow) sources that are widely cultivated and bred. Consequently, biopolymers show potential as environmentally benign, readily available, low-cost, and easy-to-use alternatives to traditional dust suppressants. Further laboratory and field studies are needed to investigate their potential at different dosages and under realistic operating conditions to raise industry awareness.
5. Conclusions
This study examined the potential of 14 polysaccharides and proteins as dust suppressants by testing spray-on treatments at two different biopolymer concentrations on a medium-grained sand and a fine-grained silica sand. Moisture retention tests, penetrometer tests, and crust thickness measurements were performed, and the following conclusions were drawn:
- Penetrometer test results on biopolymer-treated medium-grained sand ranged from 1.7 to 34.0 N (control = 1.5 N) and on fine-grained silica sand from 6.7 to 37.9 N (control = 1.7 N), respectively. The results showed that all tested biopolymers formed crusts with significantly differing penetration resistances depending on the biopolymer type (p < .05). Increasing the biopolymer concentration significantly increased the penetration resistance on medium-grained sand (p < .001). In contrast, on fine-grained silica sand, it only increased the penetration resistance of protein treatments significantly (p < .001). Proteins achieved similar penetration resistances as polysaccharides but required higher concentrations.
- Moisture-retention test results on medium-grained sand ranged from 3.4 to 19.5 wt% (control = 6.9 wt%) and on fine-grained silica sand from 1.0 to 18.2 wt% (control = 2.5 wt%). On both tested soil types, the biopolymer type had a significant effect (p < .001) on the samples’ moisture retention, resulting in it decreasing or increasing relative to the water-treated control. Increasing the concentration increased moisture retention of protein-treated fine-grained silica sand samples significantly (p < .001).
- The thicknesses of crusts formed on biopolymer-amended medium-grained sand samples ranged from 0.3 to 8.8 mm (control = 0 mm) and on fine-grained silica sand from 3.2 to 18.1 mm (control = 0 mm). The results showed that the different biopolymers formed crusts of varying thicknesses, with the biopolymer type significantly affecting the crust thickness (p < .001). On medium-grained sand, doubling the concentration only had a significant effect for protein amendments (p < .001) and resulted in the crust thickness increasing. On fine-grained sand, increasing the concentration slightly reduced the crust thickness of most treatments because of a lower infiltration depth.
The results of this study demonstrate that the tested polysaccharides and proteins have the potential to be applied as dust suppressants in industries such as mining. Thereby, biopolymers can contribute to reducing the industry’s environmental and human health impacts.
Author Contributions
Conceptualisation, J.L.S. and J.F.; methodology, J.L.S. and J.F.; formal analysis, J.L.S.; investigation, J.L.S.; data curation, J.L.S.; writing—original draft preparation, J.L.S.; writing—review and editing, J.L.S., B.G.L. and J.F.; visualisation, J.L.S.; project administration, J.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data supporting the findings of this study will be made available from the corresponding author upon reasonable request.
Acknowledgments
We thank Rheinische Baustoffwerke GmbH and Quarzwerke Frechen AG for providing the mine soils used in this study. We thank Devrim Gürsel, Martin Knippertz, and Stephanie Lohmeier for assisting in characterising the soil samples and Johannes J. Emontsbotz for designing and printing the 3D-printed splash guard. Last, the authors would like to thank the anonymous reviewers for their helpful comments and suggestions, which improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Compilation of previous studies investigating biopolymer applications in the disciplines of soil stabilisation and dust control.
Table A1.
Compilation of previous studies investigating biopolymer applications in the disciplines of soil stabilisation and dust control.
| Biopolymer | Source | Solubility | Type of Application | References |
|---|---|---|---|---|
| Polysaccharides | ||||
| Arabic gum (Acacia gum) | Exudate from acacia trees | Cold-water soluble | Spray-on (dust control) | [16] |
| Mix-in (soil stabilisation) | [16,78] | |||
| Agar gum | Red algae (Gelidium and Gracilaria) | Hot-water soluble (>86 °C) | Mix-in (soil stabilisation) | [25,79,80] |
| β-glucan | Extracted from cells of yeast, fungi, certain bacteria and cereals | Cold-water soluble | Mix-in (soil stabilisation) | [25,81,82,83,84,85,86] |
| Carrageenan | Red algae (Chondracanthus) | Cold-water soluble | Spray-on (dust control) | [87,88] |
| Mix-in (soil stabilisation) | [58,88] | |||
| Chitosan | Chitin shells of crustaceans | Soluble in acetic solutions | Mix-in (soil stabilisation) | [55,89,90,91,92,93] |
| Spray-on (dust control) | [87,94,95] | |||
| Carboxymethyl cellulose | Cellulose derivative | Cold-water soluble | Mix-in (soil stabilisation) | [96,97,98,99] |
| Spray-on (dust control) | [14,17] | |||
| Dextran | Microbial | Mix-in (soil stabilisation) | [100] | |
| Spray-on (dust control) | [101] | |||
| Gellan gum | Bacteria | Poor solubility at low temperature (fully dissolvable > 80 °C) | Mix-in (soil stabilisation) | [37,96,102,103,104,105,106] |
| Guar gum | Guar beans | Cold-water soluble | Mix-in (soil stabilisation) | e.g., [13,33,82,83,96,107,108,109,110,111] |
| Spray-on (dust control) | [14,15,46] | |||
| Lignosulfonate | By-product of wood pulp production | Cold-water soluble | Spray-on (dust control) | [19,26,33,112] |
| Locust bean gum | Carob tree seeds | Mix-in (soil stabilisation) | [113] | |
| Pectin | Citrus fruit lamella and cell walls | Cold-water soluble | Spray-on (dust control) | [16] |
| Persian gum | Exudate from almond tree trunk and branches | Cold-water soluble (30% soluble, 70% insoluble) | Mix-in (soil stabilisation) | [114] |
| Sodium alginate | Brown algae | Cold-water soluble | Spray-on (dust control) | [15,16,67,115] |
| Mix-in (soil stabilisation) | [16,91,116,117,118,119,120] | |||
| Starch | ||||
| Corn | Corn | Cold-water soluble | Spray-on (dust control) | [121] |
| Mix-in (soil stabilisation) | [58,80] | |||
| Potato | Potato | Cold-water soluble | Spray-on (dust control) | [47] |
| Xanthan gum | Bacteria | Cold-water soluble | Spray-on (dust control) | [17,122] |
| Mix-in (soil stabilisation) | e.g., [82,107,123,124,125,126,127,128] | |||
| Proteins | ||||
| Casein | Milk | Soluble in alkaline solutions | Mix-in (soil stabilisation) | [129,130,131,132,133,134] |
| Bovine blood plasma | Bovine blood | [62] | ||
| Gelatin | Collagen from animal bones and tissues | Warm-water soluble (>40 °C) | Spray-on (dust control) | [47,63] |
| Soybean | Soybean | Cold-water soluble | Spray-on (dust control) | [135,136] |

Table A2.
Moisture retention of medium-grained sand and fine-grained silica sand samples treated at biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Tests were performed in triplicates (n = 3), with M = mean and SD = standard deviation. On medium-grained sand the water-treated control group achieved a mean moisture retention of 6.9 wt% (SD = 1.2) and on fine-grained silica sand 2.5 wt% (SD = 0.7).
Table A2.
Moisture retention of medium-grained sand and fine-grained silica sand samples treated at biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Tests were performed in triplicates (n = 3), with M = mean and SD = standard deviation. On medium-grained sand the water-treated control group achieved a mean moisture retention of 6.9 wt% (SD = 1.2) and on fine-grained silica sand 2.5 wt% (SD = 0.7).
| Moisture Retention (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Medium-Grained Sand | Fine-Grained Silica Sand | |||||||
| 1 wt% | 2 wt% | 1 wt% | 2 wt% | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Polysaccharides | ||||||||
| Carboxymethyl cellulose | 6.3 | 3.1 | 5.8 | 0.5 | 4.0 | 0.9 | 4.1 | 0.1 |
| Corn starch | 16.1 | 3.6 | 13.6 | 1.0 | 1.5 | 0.5 | 2.7 | 0.1 |
| Sodium lignosulfonate | 9.2 | 2.7 | 11.2 | 1.6 | 1.7 | 0.3 | 3.7 | 1.2 |
| Pea starch | 7.8 | 0.9 | 4.6 | 0.2 | 1.0 | 0.3 | 2.1 | 0.8 |
| Potato starch | 5.3 | 0.5 | 5.3 | 0.7 | 1.5 | 0.1 | 2.5 | 0.1 |
| Wheat starch | 6.2 | 0.5 | 12.3 | 1.7 | 5.1 | 0.9 | 7.2 | 1.5 |
| Xanthan gum | 3.4 | 0.2 | 4.0 | 0.9 | 6.2 | 0.9 | 2.5 | 0.3 |
| Average | 7.8 | 3.8 | 8.1 | 3.8 | 3.0 | 1.9 | 3.6 | 1.6 |
| Proteins | ||||||||
| Fava bean protein concentrate | 12.7 | 3.7 | 14.5 | 1.2 | 14.0 | 1.0 | 17.2 | 0.8 |
| Hen egg albumen | 12.3 | 2.0 | 12.4 | 1.7 | 1.6 | 0.2 | 2.3 | 0.0 |
| Haemoglobin protein | 7.0 | 1.9 | 4.9 | 3.2 | 13.1 | 2.4 | 18.2 | 2.0 |
| Plasma protein | 3.2 | 0.4 | 2.4 | 0.1 | 1.4 | 0.2 | 2.1 | 0.0 |
| Technical gelatin | 17.9 | 0.9 | 19.5 | 3.0 | 14.0 | 1.2 | 12.7 | 0.6 |
| Wheat protein | 15.6 | 1.0 | 19.0 | 2.7 | 2.0 | 0.4 | 2.1 | 0.2 |
| Whey protein concentrate | 5.2 | 2.0 | 5.3 | 2.1 | 1.2 | 0.1 | 2.8 | 0.1 |
| Average | 10.5 | 5.1 | 11.1 | 6.5 | 6.7 | 6.0 | 8.2 | 7.0 |

Table A3.
Penetration resistance of medium-grained and fine-grained silica sand samples treated at biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Tests were performed in triplicates (n = 3), with M = mean and SD = standard deviation. The water-treated control group achieved a penetration resistance of 1.5 N (SD = 0.1) on medium-grained sand and 1.7 N (SD = 0.2) on fine-grained silica sand.
Table A3.
Penetration resistance of medium-grained and fine-grained silica sand samples treated at biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Tests were performed in triplicates (n = 3), with M = mean and SD = standard deviation. The water-treated control group achieved a penetration resistance of 1.5 N (SD = 0.1) on medium-grained sand and 1.7 N (SD = 0.2) on fine-grained silica sand.
| Penetration Resistance (N) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Medium-Grained Sand | Fine-Grained Silica Sand | |||||||
| 1 wt% | 2 wt% | 1 wt% | 2 wt% | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Polysaccharides | ||||||||
| Carboxymethyl cellulose | 11.5 | 2.0 | 21.1 | 3.6 | 14.9 | 3.2 | 16.4 | 2.9 |
| Corn starch | 14.2 | 2.0 | 27.4 | 4.4 | 18.0 | 4.8 | 25.4 | 8.6 |
| Sodium lignosulfonate | 1.7 | 0.2 | 4.3 | 1.3 | 14.2 | 3.1 | 21.1 | 3.1 |
| Pea starch | 8.0 | 1.2 | 20.1 | 4.3 | 10.8 | 2.2 | 14.4 | 1.1 |
| Potato starch | 4.4 | 0.6 | 16.8 | 1.3 | 10.1 | 1.7 | 19.0 | 1.6 |
| Wheat starch | 13.3 | 0.9 | 14.4 | 2.1 | 10.0 | 1.3 | 8.8 | 0.4 |
| Xanthan gum | 12.4 | 5.5 | 26.5 | 3.9 | 20.7 | 5.3 | 16.0 | 2.8 |
| Average | 9.4 | 4.5 | 18.7 | 7.3 | 14.1 | 3.8 | 17.3 | 4.9 |
| Proteins | ||||||||
| Fava bean protein concentrate | 8.8 | 2.9 | 15.9 | 1.7 | 15.0 | 4.1 | 30.3 | 3.9 |
| Hen egg albumen | 2.5 | 0.7 | 5.6 | 3.0 | 10.8 | 2.4 | 13.9 | 0.6 |
| Haemoglobin protein | 2.1 | 0.5 | 10.2 | 1.7 | 6.7 | 1.9 | 11.2 | 2.7 |
| Plasma protein | 3.5 | 1.6 | 15.4 | 2.8 | 15.8 | 2.6 | 21.0 | 4.8 |
| Technical gelatin | 8.0 | 0.2 | 34.0 | 6.0 | 20.0 | 4.5 | 37.9 | 2.1 |
| Wheat protein | 3.5 | 1.2 | 13.5 | 3.8 | 9.1 | 0.9 | 18.5 | 2.9 |
| Whey protein concentrate | 2.4 | 0.5 | 2.5 | 0.7 | 10.0 | 1.0 | 15.6 | 3.2 |
| Average | 4.4 | 2.6 | 13.9 | 9.5 | 12.5 | 4.3 | 21.2 | 8.9 |

Table A4.
Mean modulus of elasticity (Me) of crusts formed on medium-grained and fine-grained silica sand samples treated at biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Tests were performed in triplicates (n = 3), with M = mean and SD = standard deviation. The water-treated control group formed no crust, so no Me could be derived.
Table A4.
Mean modulus of elasticity (Me) of crusts formed on medium-grained and fine-grained silica sand samples treated at biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Tests were performed in triplicates (n = 3), with M = mean and SD = standard deviation. The water-treated control group formed no crust, so no Me could be derived.
| Modulus of Elasticity (kN m−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Medium-Grained Sand | Fine-Grained Silica Sand | |||||||
| 1 wt% | 2 wt% | 1 wt% | 2 wt% | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Polysaccharides | ||||||||
| Carboxymethyl cellulose | 17.2 | 4.0 | 21.7 | 5.7 | 20.7 | 4.3 | 20.7 | 6.3 |
| Corn starch | 27.7 | 14.1 | 27.2 | 5.9 | 69.1 | 50.5 | 38.7 | 26.2 |
| Sodium lignosulfonate | 0.6 | 0.1 | 3.7 | 2.1 | 22.2 | 9.1 | 28.7 | 6.4 |
| Pea starch | 6.8 | 1.4 | 20.1 | 3.2 | 24.2 | 8.3 | 43.4 | 18.8 |
| Potato starch | 4.8 | 0.3 | 20.2 | 6.2 | 25.7 | 5.9 | 65.0 | 13.9 |
| Wheat starch | 15.9 | 1.5 | 13.2 | 3.0 | 8.6 | 2.4 | 7.4 | 0.7 |
| Xanthan gum | 23.9 | 9.3 | 36.0 | 11.4 | 41.2 | 12.1 | 20.7 | 3.5 |
| Average | 13.8 | 9.4 | 20.3 | 9.4 | 30.2 | 18.2 | 32.1 | 17.5 |
| Proteins | ||||||||
| Fava bean protein concentrate | 13.0 | 8.6 | 21.1 | 5.0 | 36.3 | 7.8 | 87.2 | 44.8 |
| Hen egg albumen | 1.3 | 0.3 | 7.4 | 3.3 | 19.7 | 9.3 | 26.3 | 3.7 |
| Haemoglobin protein | 1.2 | 0.2 | 13.1 | 5.6 | 14.1 | 7.1 | 27.3 | 12.8 |
| Plasma protein | 4.3 | 3.1 | 18.3 | 9.3 | 22.7 | 6.1 | 30.9 | 3.0 |
| Technical gelatin | 9.4 | 1.4 | 31.2 | 5.3 | 32.8 | 9.3 | 33.7 | 6.2 |
| Wheat protein | 3.7 | 2.8 | 13.8 | 3.8 | 14.1 | 3.7 | 47.0 | 16.6 |
| Whey protein concentrate | 1.8 | 1.6 | 1.5 | 0.5 | 19.9 | 2.6 | 22.4 | 6.2 |
| Average | 5.0 | 4.2 | 15.2 | 8.9 | 22.8 | 8.0 | 39.3 | 20.9 |
Medium-grained sand. For 1 wt% treatments, the Me of polysaccharides-induced crusts was on average 2.8 times greater than the Me of protein-induced crusts (Table A4). Amendments with CMC, CS, and XG resulted in the highest Me among the tested polysaccharides and FBPC and TG among the tested proteins, respectively. Doubling the concentration resulted in the Me of most biopolymer-induced crusts increasing, whereby protein-induced crusts exhibited a disproportionate increase relative to the polysaccharide-induced crusts. Fine-grained silica sand. On samples treated at 1 wt% biopolymer concentration, polysaccharide-induced crusts on average exhibited a larger Me than protein-induced crusts, with CS, XG, FBPC, and TG achieving the highest Me within their respective groups. Treatment at 2 wt% increased the Me of most biopolymer treatments tested, with protein-induced crusts increasing disproportionately in thickness relative to the polysaccharide-induced crusts.

Table A5.
Mean crust thickness of medium-grained sand and fine-grained silica sand samples treated at biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Tests were performed in triplicates (n = 3), with M = mean and SD = standard deviation. Water-treated control groups formed no crusts.
Table A5.
Mean crust thickness of medium-grained sand and fine-grained silica sand samples treated at biopolymer concentrations of 1 and 2 wt% (XG = 0.25 and 0.50 wt%). Tests were performed in triplicates (n = 3), with M = mean and SD = standard deviation. Water-treated control groups formed no crusts.
| Crust Thickness (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Medium-Grained Sand | Fine-Grained Silica Sand | |||||||
| 1 wt% | 2 wt% | 1 wt% | 2 wt% | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Polysaccharides | ||||||||
| Carboxymethyl cellulose | 6.3 | 1.6 | 7.1 | 0.4 | 9.6 | 0.5 | 9.3 | 0.2 |
| Corn starch | 7.5 | 0.3 | 5.9 | 0.8 | 7.7 | 0.5 | 7.3 | 1.0 |
| Sodium lignosulfonate | 2.4 | 1.0 | 4.6 | 0.9 | 13.1 | 0.3 | 18.1 | 2.7 |
| Pea starch | 6.3 | 0.2 | 6.9 | 0.4 | 8.3 | 0.6 | 7.0 | 0.2 |
| Potato starch | 3.4 | 0.5 | 7.3 | 0.2 | 11.7 | 0.4 | 13.7 | 0.2 |
| Wheat starch | 6.3 | 0.4 | 4.7 | 1.6 | 3.2 | 1.3 | 2.4 | 0.5 |
| Xanthan gum | 6.6 | 0.1 | 5.9 | 0.1 | 7.3 | 0.4 | 5.8 | 0.3 |
| Average | 5.5 | 1.8 | 6.1 | 1.0 | 8.7 | 3.0 | 9.1 | 4.9 |
| Proteins | ||||||||
| Fava bean protein concentrate | 7.6 | 0.4 | 8.8 | 0.1 | 11.6 | 0.4 | 11.0 | 2.1 |
| Hen egg albumen | 0.4 | 0.4 | 2.8 | 0.9 | 11.0 | 0.9 | 13.1 | 0.3 |
| Haemoglobin protein | 0.3 | 0.4 | 6.8 | 0.5 | 9.2 | 1.0 | 8.9 | 0.6 |
| Plasma protein | 5.2 | 0.8 | 7.0 | 0.2 | 9.4 | 0.6 | 11.8 | 0.3 |
| Technical gelatin | 2.3 | 0.7 | 5.8 | 0.4 | 10.1 | 0.6 | 9.4 | 0.3 |
| Wheat protein | 5.5 | 0.5 | 8.1 | 1.2 | 7.6 | 2.4 | 8.3 | 0.2 |
| Whey protein concentrate | 4.0 | 0.5 | 4.7 | 1.7 | 8.2 | 0.2 | 9.5 | 0.6 |
| Average | 3.6 | 2.6 | 6.3 | 1.9 | 9.6 | 1.3 | 10.3 | 1.6 |

Table A6.
Qualitative classification of biopolymers according to their crust’s visual appearance (cf. Figure 10 and Figure 11).
| Classification | Biopolymers | Description |
|---|---|---|
| Medium-grained sand | ||
| Solid crusts | Polysaccharides: CS, CMC, WS, XG Proteins: FBPC, TG | 1 and 2 wt% (XG = 0.25 and 0.50 wt%) concentrations. Crusts were recoverable in a single piece or up to four fully recoverable pieces. |
| Mediocre crusts | Polysaccharides: PES, POS Proteins: PP, WP, WPC | 1 wt% concentration. Crusts broke into multiple large pieces, whereby some pieces were only partially recoverable and crumbled into countless pieces. 2 wt% concentration. Crusts were almost fully recoverable in several pieces. |
| Weak crusts | Polysaccharides: NLS Proteins: HEA, HG | 1 wt% concentration. Crusts were very weak and brittle, crumbling into countless unrecoverable pieces. 2 wt% concentration. Crusts had increased stability but were still extremely fragile. |
| Fine-grained silica sand | ||
| Solid crusts | Polysaccharides: CS, CMC, PES, XG | 1 and 2 wt% (XG = 0.25 and 0.50 wt%) concentrations. Crusts were recoverable in a single piece or up to four fully recoverable pieces. |
| Mediocre crusts | Polysaccharides: NLS, POS Proteins: FBPC, HEA, HG, PP, TG, WP, WPC | 1 and 2 wt% concentrations. Crusts were thick and almost fully recoverable in a few pieces at both tested concentrations. (TG exhibited a unique characteristic, as the uppermost part of its crust peeled off from the lower part while recovering the crust.) |
| Ductile crusts | Polysaccharides: WP | 1 and 2 wt% concentrations. Crusts were very thin and ductile and even curled up during the curing period. |

Table A7.
Compilation of results from previous studies performing penetrometer testing on different soils to assess the penetration resistance of spray-on biopolymer applications.
Table A7.
Compilation of results from previous studies performing penetrometer testing on different soils to assess the penetration resistance of spray-on biopolymer applications.
| Biopolymer | Soil | D50 (mm) | Cu | Penetrometer | AR | Maximum Penetration Resistance (N) at Different Tested Concentrations (wt%) | Reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shape | d (mm) | (L/m2) | 0 | 0.3 | 0.5 | 0.6 | 0.7 | 0.8 | 1.0 | 1.5 | 1.6 | 2.0 | 3.0 | 5.0 | |||||
| Acacia gum | Poorly graded sand (SP) | 0.15 a | 2.1 a | flat | 6 | 1.3 | 1.1 a | 10.0 a | 15.0 a | 21.0 a | 30.0 a | 50.0 a | [16] | ||||||
| 0.15 a | 2.1 a | flat | 6 | 3.5 | 6.4 a | 18.0 a | 20.0 a | 50.0 a | 70.0 a | 145.0 a | [16] | ||||||||
| Sodium alginate | 0.15 a | 2.1 a | flat | 6 | 1.3 | 1.1 a | 9.0 a | 17.5 a | 18.0 a | 15.0 a | N/A | [16] | |||||||
| 0.15 a | 2.1 a | flat | 6 | 3.5 | 6.4 a | 7.5 a | 21.0 a | 25.0 a | N/A | N/A | [16] | ||||||||
| Pectin | 0.15 a | 2.1 a | flat | 6 | 1.3 | 1.1 a | 15.0 a | 20.0 a | 28.0 a | 22.0 a | 15.0 a | [16] | |||||||
| 0.15 a | 2.1 a | flat | 6 | 3.5 | 6.4 a | 20.0 a | 39.0 a | 33.0 a | 30.0 a | N/A | [16] | ||||||||
| Carboxymethyl cellulose | Poorly graded sand with silt (SP-SM) | 0.16 a | 2.2 a | flat | 6 | 1 | 0.0 b | 1.0 b | 2.2 b | 2.3 b | [14] | ||||||||
| 0.16 a | 2.2 a | flat | 6 | 2 | 0.0 b | 2.8 b | 3.6 b | 5.7 b | [14] | ||||||||||
| Guar gum | 0.16 a | 2.2 a | flat | 6 | 1 | 0.0 b | 0.7 b | 1.4 b | 2.4 b | [14] | |||||||||
| 0.16 a | 2.2 a | flat | 6 | 2 | 0.0 b | 2.3 b | 2.8 b | 3.5 b | [14] | ||||||||||
| Guar gum | Mine tailings | 0.15 a | 33.9 a | flat | 6 | 1.9 | 212.8 | 263.0 | 358.8 | 428.0 | [49] | ||||||||
| Xanthan gum | 0.15 a | 33.9 a | flat | 6 | 1.9 | 212.8 | 250.8 | 304.5 | 340.6 | [49] | |||||||||
| Xanthan gum | Mine tailings | 0.13 a | 56.3 a | flat | 6 | 1.9 | 213.8 | 278.4 | 312.5 | 331.8 | [137] | ||||||||
| Xanthan gum | Hooralazim lagoon sand | 0.22 a | 7.5 a | flat | 6 | 1.9 | 5.0 a | 22.0 a | 28.0 a | 32.0 a | [17] | ||||||||
| Urmia lake sand | 0.13 a | 2.1 a | flat | 6 | 1.9 | 5.0 a | 6.0 a | 6.0 a | 11.0 a | [17] | |||||||||
| Mine tailings | 0.28 a | 9.4 a | flat | 6 | 1.9 | 12.0 a | 14.0 a | 27.0 a | 30.0 a | [17] | |||||||||
| Carboxymethyl cellulose | Hooralazim lagoon sand | 0.22 a | 7.5 a | flat | 6 | 1.9 | 5.0 a | 15.0 a | 38.0 a | 58.0 a | [17] | ||||||||
| Urmia lake sand | 0.13 a | 2.1 a | flat | 6 | 1.9 | 5.0 a | 14.0 a | 16.0 a | 28.0 a | [17] | |||||||||
| Mine tailings | 0.28 a | 9.4 a | flat | 6 | 1.9 | 12.0 a | 18.0 a | 27.0 a | 30.0 a | [17] | |||||||||
| Guar gum | Hooralazim lagoon sand | 0.22 a | 7.5 a | flat | 6 | 1.9 | 5.0 a | 8.0 a | 28.0 a | 39.0 a | [17] | ||||||||
| Urmia lake sand | 0.13 a | 2.1 a | flat | 6 | 1.9 | 5.0 a | 10.0 a | 14.0 a | 20.0 a | [17] | |||||||||
| Mine tailings | 0.28 a | 9.4 a | flat | 6 | 1.9 | 12.0 a | 16.0 a | 25.0 a | 31.0 | [17] | |||||||||
| Sodium alginate + CaCl2 | Poorly graded sand (SP) | 0.24 a | 1.7 | flat | 6 | 2.2 | N/A | 2.8 c | 5.4 c | 5.4 c | [67] | ||||||||
Note. AR = application rate, N/A = not available, SP = poorly graded sand, SP-SM = poorly graded sand with silt, a = values extracted from diagram in source, b = original values given in kPA and converted into N by multiplying with pin area, c = results from pocket penetrometer tests. Original values given in kg/cm2 and converted into N using penetrometer pin area and g = 9.81 m/s2.

Table A8.
Compilation of results from previous studies performing penetrometer testing on different types of red sand (bauxite residue) to assess the penetration resistance of spray-on biopolymer applications.
Table A8.
Compilation of results from previous studies performing penetrometer testing on different types of red sand (bauxite residue) to assess the penetration resistance of spray-on biopolymer applications.
| Biopolymer | Soil | D50 (mm) | Cu | Penetrometer | AR | Maximum Penetration Resistance (N) at Different Tested Concentrations (wt%) | Reference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| shape | d (mm) | (L/m2) | 0 | 0.4 | 0.8 | 1.0 | 1.2 | 1.6 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 | 8.0 | 10.0 | |||||
| Polyacrylamide | Red loam sand (SP) | N/A | <5 | cone | 2 | 2 | 3.0 a | 5.5 a | 7.5 a | 9.5 a | 15.0 a | [46] | ||||||||
| Guar gum | N/A | <5 | cone | 2 | 2 | 3.0 a | 5.5 a | 7.0 a | 7.5 a | 8.5 a | [46] | |||||||||
| Xanthan gum | N/A | <5 | cone | 2 | 2 | 3.0 a | 5.5 a | 6.5 a | 7.0 a | 7.5 a | [46] | |||||||||
| Polyacrylamide | Red loam sand (SP) | N/A | <5 | cone | 2 | 2 | N/A | 6.0 a | 8.0 a | 9.8 a | [48] | |||||||||
| Guar gum | N/A | <5 | cone | 2 | 2 | N/A | 5.5 a | 7.0 a | 8.0 a | [48] | ||||||||||
| Xanthan gum | N/A | <5 | cone | 2 | 2 | N/A | 5.3 a | 6.5 a | 7.0 a | [48] | ||||||||||
| Sodium lignosulfonate | Red loam sand (SP) | N/A | <5 | cone | 2 | 2 | 3.0 a | 4.8 a | 5.0 a | 5.3 a | 6.5 a | 7.5 a | 7.8 a | 8.0 a | 9.5 a | [28] | ||||
| Calcium lignosulfonate | N/A | <5 | cone | 2 | 2 | 3.0 a | 3.5 a | 4.0 a | 4.5 a | 5.0 a | 5.3 a | 5.8 a | 6.3 a | 7.3 a | [28] | |||||
| Polyacrylamide | Red sand (d < 0.15) | N/A | N/A | cone | 2 | 2 | 3.2 | 6.2 | 7.8 | 9.6 | 12.3 | 15.3 | [13] | |||||||
| Red sand (0.15 < d < 0.3) | N/A | N/A | cone | 2 | 2 | 3.2 | 7.4 | 9.6 | 11.4 | 14.2 | 16.5 | [13] | ||||||||
| Red sand (0.3 < d < 0.45) | N/A | N/A | cone | 2 | 2 | 3.3 | 8.1 | 11.1 | 13.2 | 16.0 | 18.0 | [13] | ||||||||
| Guar gum | Red sand (d < 0.15) | N/A | N/A | cone | 2 | 2 | 3.2 | 5.7 | 7.0 | 8.3 | 9.3 | 10.2 | [13] | |||||||
| Red sand (0.15 < d < 0.3) | N/A | N/A | cone | 2 | 2 | 3.2 | 6.7 | 9.0 | 10.2 | 11.7 | 14.2 | [13] | ||||||||
| Red sand (0.3 < d < 0.45) | N/A | N/A | cone | 2 | 2 | 3.3 | 7.1 | 9.9 | 11.0 | 12.8 | 14.4 | [13] | ||||||||
| Xanthan gum | Red sand (d < 0.15) | N/A | N/A | cone | 2 | 2 | 3.2 | 5.4 | 6.4 | 7.2 | 7.7 | 8.1 | [13] | |||||||
| Red sand (0.15 < d < 0.3) | N/A | N/A | cone | 2 | 2 | 3.2 | 5.7 | 6.9 | 8.6 | 10.8 | 13.5 | [13] | ||||||||
| Red sand (0.3 < d < 0.45) | N/A | N/A | cone | 2 | 2 | 3.3 | 6.5 | 8.0 | 10.0 | 12.3 | 13.4 | [13] | ||||||||
| Sodium lignosulfonate | Red sand (d < 0.15) | N/A | N/A | cone | 2 | 2 | 3.0 a | 5.0 a | 7.0 a | 7.5 a | 8.0 a | 9.0 a | [33] | |||||||
| Red sand (0.15 < d < 0.3) | N/A | N/A | cone | 2 | 2 | 3.0 a | 6.5 a | 8.0 a | 9.0 a | 10.5 a | 13.0 a | [33] | ||||||||
| Red sand (d > 0.3) | N/A | N/A | cone | 2 | 2 | 3.0 a | 6.0 a | 8.5 a | 10.0 a | 12.5 a | 14.0 a | [33] | ||||||||
| Calcium lignosulfonate | Red sand (d < 0.15) | N/A | N/A | cone | 2 | 2 | 3.0 a | 3.5 a | 4.0 a | 5.5 a | 6.5 a | 7.5 a | [33] | |||||||
| Red sand (0.15 < d < 0.3) | N/A | N/A | cone | 2 | 2 | 3.0 a | 4.0 a | 5.5 a | 7.0 a | 8.5 a | 11.5 a | [33] | ||||||||
| Red sand (d > 0.3) | N/A | N/A | cone | 2 | 2 | 3.0 a | 5.0 a | 6.0 a | 8.0 a | 10.0 a | 12.5 a | [33] | ||||||||
Note. AR = application rate, N/A = not available, SP = poorly graded sand, SP-SM = poorly graded sand with silt, a = values extracted from diagram in source. Original values given in kg/cm2 and converted into N using penetrometer pin area and g = 9.81 m/s2.

Table A9.
Compilation of results from previous studies analysing the crust thickness of biopolymer-treated soils.
Table A9.
Compilation of results from previous studies analysing the crust thickness of biopolymer-treated soils.
| Biopolymer | Soil Type | AR | Crust Thickness (mm) at Different Tested Concentrations (%) | Reference | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (L/m2) | 0 | 0.3 | 0.4 | 0.5 | 0.7 | 0.8 | 1.0 | 1.2 | 1.6 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 | 7.0 | 8.0 | 9.0 | 10.0 | |||
| Acacia gum | Poorly graded sand (SP) | 1.3 | 7.4–11.9 | 5.1–11.4 | 5.2–11.5 | 3.8–10.9 | 2.8–10.9 | [16] | |||||||||||||
| Sodium alginate | 1.3 | 8.5–15.3 | 7.6–15.2 | 2.2–10.3 | 3.0–6.7 | [16] | |||||||||||||||
| Pectin | 1.3 | 3.8–8.4 | 3.6–7.3 | 4.6–6.1 | 2.4–3.0 | 0.9–1.2 | [16] | ||||||||||||||
| Acacia gum | 3.5 | 15.1–29.8 | 12.6–29.3 | 11.5–22.1 | 11.5–22.0 | 9.7–21.6 | [16] | ||||||||||||||
| Sodium alginate | 3.5 | 9.0–12.1 | 2.6–4.7 | 1.6–3.2 | 1.2–2.1 | [16] | |||||||||||||||
| Pectin | 3.5 | 8.5–15.3 | 7.6–15.2 | 2.2–10.3 | 3.0–6.7 | 0.7–1.9 | [16] | ||||||||||||||
| Sodium alginate + CaCl2 | Poorly graded sand (SP) | 2.2 | 5.2–7.8 | 4.9–8.0 | 5.9–7.7 | [67] | |||||||||||||||
| Carboxymethyl cellulose | Poorly graded sand with silt (SP-SM) | 1.0 | 4.0 | 3.8 | 3.4 | [14] | |||||||||||||||
| Guar gum | 2.0 | 3.9 | 4.5 | 4.2 | [14] | ||||||||||||||||
| Carboxymethylcellulose | 1.0 | 7.9 | 6.3 | 6.0 | [14] | ||||||||||||||||
| Guar gum | 2.0 | 6.6 | 7.8 | 10.3 | [14] | ||||||||||||||||
| Sodium lignosulfonate | Red sand (SP) | 2.0 | 11.0 a | 10.5 a | 11.0 a | 8.5 a | 7.5 a | 7.5 a | 7.0 a | 6.5 a | 6.5 a | 6.0 a | [28] | ||||||||
| Calcium lignosulfonate | 2.0 | 11.0 a | 10.5 a | 10.5 a | 10.0 a | 10.0 a | 9.5 a | 9.0 a | 8.5 a | 8.0 a | 7.5 a | [28] | |||||||||
| Polyacrylamide | Red sand (SP) | 2.0 | 30.0 a | 26.0 a | 15.0 a | 14.5 a | 12.0 a | [46] | |||||||||||||
| Guar gum | 2.0 | 30.0 a | 19.0 a | 23.0 a | 27.0 a | 22.5 a | [46] | ||||||||||||||
| Xanthan gum | 2.0 | 30.0 a | 13.0 a | 12.0 a | 10.0 a | 8.0 a | [46] | ||||||||||||||
| Polyacrylamide | Red sand (d < 0.15) | 2.0 | 30.0 a | 27.5 a | 22.5 a | 19.0 a | 15.0 a | 15.0 a | [33] | ||||||||||||
| Guar gum | 2.0 | 30.0 a | 26.0 a | 20.0 a | 14.0 a | 14.5 a | 14.0 a | [33] | |||||||||||||
| Xanthan gum | 2.0 | 30.0 a | 13.5 a | 11.5 a | 12.0 a | 12.5 a | 10.5 a | [33] | |||||||||||||
Note. AR = application rate, SP = poorly graded sand, SP-SM = poorly graded sand with silt, a = values extracted from diagram in source.
References
- Entwistle, J.A.; Hursthouse, A.S.; Marinho Reis, P.A.; Stewart, A.G. Metalliferous mine dust: Human health impacts and the potential determinants of disease in mining communities. Curr. Pollut. Rep. 2019, 5, 67–83. [Google Scholar] [CrossRef]
- Zota, A.R.; Riederer, A.M.; Ettinger, A.S.; Schaider, L.A.; Shine, J.P.; Amarasiriwardena, C.J.; Wright, R.O.; Spengler, J.D. Associations between metals in residential environmental media and exposure biomarkers over time in infants living near a mining-impacted site. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Liang, T.; Li, K. Fine road dust contamination in a mining area presents a likely air pollution hotspot and threat to human health. Environ. Int. 2019, 128, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zanetta-Colombo, N.C.; Fleming, Z.L.; Gayo, E.M.; Manzano, C.A.; Panagi, M.; Valdés, J.; Siegmund, A. Impact of mining on the metal content of dust in indigenous villages of northern Chile. Environ. Int. 2022, 169, 107490. [Google Scholar] [CrossRef]
- Khan, R.K.; Strand, M.A. Road dust and its effect on human health: A literature review. Epidemiol. Health 2018, 40, e2018013. [Google Scholar] [CrossRef]
- du Plessis, J.J.; Janse van Rensburg, L. Effectiveness of applying dust suppression palliatives on haul roads. J. Mine Vent. Soc. S. Afr. 2015, 69, 15–19. [Google Scholar]
- Thompson, R.J.; Visser, A.T. Selection, performance and economic evaluation of dust palliatives on surface mine haul roads. J. S. Afr. Inst. Min. Metall. 2007, 107, 435–450. [Google Scholar]
- Clarke, B.; Otto, F.; Stuart-Smith, R.; Harrington, L. Extreme weather impacts of climate change: An attribution perspective. Environ. Res. Clim. 2022, 1, 12001. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaption and Vulnerability: Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- Philip, S.; Martin, R.V.; Snider, G.; Weagle, C.L.; van Donkelaar, A.; Brauer, M.; Henze, D.K.; Klimont, Z.; Venkataraman, C.; Guttikunda, S.K.; et al. Anthropogenic fugitive, combustion and industrial dust is a significant, underrepresented fine particulate matter source in global atmospheric models. Environ. Res. Lett. 2017, 12, 44018. [Google Scholar] [CrossRef]
- Parvej, S.; Naik, D.L.; Sajid, H.U.; Kiran, R.; Huang, Y.; Thanki, N. Fugitive dust suppression in unpaved roads: State of the art research review. Sustainability 2021, 13, 2399. [Google Scholar] [CrossRef]
- Piechota, T.C.; van Ee, J.; Stave, K.; James, D. Potential Environmental Impacts of Dust Suppressants: “Avoiding Another Times Beach”; U.S. Environmental Protection Agency: Las Vegas, NV, USA, 2002.
- Ding, X.; Luo, Z.; Xu, G.; Chang, P. Characterization of red sand dust pollution control performance via static and dynamic laboratorial experiments when applying polymer stabilizers. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Owji, R.; Habibagahi, G.; Nikooee, E.; Afzali, S.F. Wind erosion control using carboxymethyl cellulose: From sand bombardment performance to microfabric analysis. Aeolian Res. 2021, 50, 100696. [Google Scholar] [CrossRef]
- Wade, E.; Zowada, R.; Foudazi, R. Alginate and guar gum spray application for improving soil aggregation and soil crust integrity. Carbohydr. Polym. 2021, 2, 100114. [Google Scholar] [CrossRef]
- Lemboye, K.; Almajed, A.; Alnuaim, A.; Arab, M.; Alshibli, K. Improving sand wind erosion resistance using renewable agriculturally derived biopolymers. Aeolian Res. 2021, 49, 100663. [Google Scholar] [CrossRef]
- Toufigh, V.; Ghassemi, P. Control and stabilization of fugitive dust: Using eco-friendly and sustainable materials. Int. J. Geomech. 2020, 20, 4020140. [Google Scholar] [CrossRef]
- Runnels, C.M.; Lanier, K.A.; Williams, J.K.; Bowman, J.C.; Petrov, A.S.; Hud, N.V.; Williams, L.D. Folding, assembly, and persistence: The essential nature and origins of biopolymers. J. Mol. Evol. 2018, 86, 598–610. [Google Scholar] [CrossRef]
- Katra, I. Comparison of diverse dust control products in wind-induced dust emission from unpaved roads. Appl. Sci. 2019, 9, 5204. [Google Scholar] [CrossRef]
- Jang, J. A review of the application of biopolymers on geotechnical engineering and the strengthening mechanisms between typical biopolymers and soils. Adv. Mater. Sci. Eng. 2020, 2020, 1465709. [Google Scholar] [CrossRef]
- Fatehi, H.; Ong, D.E.L.; Yu, J.; Chang, I. Biopolymers as green binders for soil improvement in geotechnical applications: A review. Geosci. J. 2021, 11, 291. [Google Scholar] [CrossRef]
- Mendonça, A.; Morais, P.V.; Pires, A.C.; Chung, A.P.; Oliveira, P.V. A Review on the importance of microbial biopolymers such as xanthan gum to improve soil properties. Appl. Sci. 2021, 11, 170. [Google Scholar] [CrossRef]
- Choi, S.-G.; Chang, I.; Lee, M.; Lee, J.-H.; Han, J.-T.; Kwon, T.-H. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Cho, G.-C. Introduction of microbial biopolymers in soil treatment for future environmentally-friendly and sustainable geotechnical engineering. Sustainability 2016, 8, 251. [Google Scholar] [CrossRef]
- Chang, I.; Prasidhi, A.K.; Im, J.; Shin, H.-D.; Cho, G.-C. Soil treatment using microbial biopolymers for anti-desertification purposes. Geoderma 2015, 253–254, 39–47. [Google Scholar] [CrossRef]
- Xu, G.; Ding, X.; Kuruppu, M.; Zhou, W.; Biswas, W. Research and application of non-traditional chemical stabilizers on bauxite residue (red sand) dust control, a review. Sci. Total Environ. 2018, 616–617, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, T.; Jin, L.; Li, G.; Wang, S.; Wei, Y.; Ou, S.; Wang, Y.; Xu, J.; Lin, M.; et al. Suppression Characteristics and Mechanism of Molasses Solution on Coal Dust: A Low-Cost and Environment-Friendly Suppression Method in Coal Mines. Int. J. Environ. Res. Public Health 2022, 19, 16472. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, G.; Kizil, M.; Zhou, W.; Guo, X. Lignosulfonate treating bauxite residue dust pollution: Enhancement of mechanical properties and wind erosion behavior. Water Air Soil Pollut. 2018, 229, 1084. [Google Scholar] [CrossRef]
- German Institute for Standardization. DIN EN ISO 17892-4; Geotechnical Investigation and Testing-Laboratory Testing of Soil-Part 4: Determination of Particle Size Distribution. German Institute for Standardization: Berlin, Germany, 2017.
- AST D2487-17; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2018.
- German Institute for Standardization. DIN EN ISO 11508-2017; Soil Quality-Determination of Particle Density. German Institute for Standardization: Berlin, Germany, 2017.
- DIN EN 15933:2012-11; Sludge, Treated Biowaste and Soil—Determination of pH. German Institute for Standardization: Berlin, Germany, 2012.
- Ding, X.; Xu, G.; Zhang, Y.; Luo, Z.; Deng, J. Reduction of airborne bauxite residue dust pollution by enhancing the structural stability via the application of non-traditional stabilizers. Water Air Soil Pollut. 2021, 232, 100. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Zuorro, A.; Moreno-Sader, K.A.; González-Delgado, Á.D. Economic Evaluation and Techno-Economic Sensitivity Analysis of a Mass Integrated Shrimp Biorefinery in North Colombia. Polymers 2020, 12, 2397. [Google Scholar] [CrossRef]
- Criminna, R.; Fidalgo, A.; Delisi, R.; Ilharco, L.M.; Pagliaro, M. Pectin production and global market. Agro Food Ind. Hi-Tech 2016, 27, 17–20. [Google Scholar]
- Chang, I.; Im, J.; Cho, G.-C. Geotechnical engineering behaviors of gellan gum biopolymer treated sand. Can. Geotech. J. 2016, 53, 1658–1670. [Google Scholar] [CrossRef]
- Gosselink, R.J.A. Lignin as a Renewable Aromatic Resource for the Chemical Industry. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2011. [Google Scholar]
- Hailu, A. Production and Optimisation of Carboxymethylcellulose from Waste Cartons. Master Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2016. [Google Scholar]
- Phillips, G.O.; Edwards, C.A.; Garcia, A.L.; Williams, P.A.; Dickinson, E.; Armisén, R.; Taggart, P.; Mitchell, J.R.; Haug, I.J.; Draget, K.I.; et al. Handbook of Hydrocolloids; CRC: Boca Raton, FL, USA; Woodhead: Cambridge, UK, 2009; ISBN 978-1-84569-414-2. [Google Scholar]
- Mulder, W.; van der Peet-Schwering, C.; Hua, N.-P.; van Ree, R. Proteins for Food, Feed and Biobased Applications: Biorefining of Protein Containing Biomass. 2016. Available online: https://www.ieabioenergy.com/wp-content/uploads/2016/10/Proteins-for-Food_Feed_and_Biobased_Applications_-IEA-Bioenergy-Task42_September-2016.pdf (accessed on 6 January 2023).
- Heusala, H.; Sinkko, T.; Sözer, N.; Hytönen, E.; Mogensen, L.; Knudsen, M.T. Carbon footprint and land use of oat and faba bean protein concentrates using a life cycle assessment approach. J. Clean. Prod. 2020, 242, 118376. [Google Scholar] [CrossRef]
- German Institute for Standardization. EN ISO 1666-1997; Starch-Determination of Moisture Content-Oven-Drying Method. German Institute for Standardization: Berlin, Germany, 1998.
- International Organization for Standardization. ISO 6496:1999; Animal Feeding Stuffs-Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Genève, Switzerland, 1999.
- Association of Official Analytical Chemists. Official Methods of Analysis-Agricultural Chemicals; Contaminants Drugs; Association of Official Analytical chemists: Arlington, TX, USA, 1990. [Google Scholar]
- Ding, X.; Xu, G.; Zhou, W.; Kuruppu, M. Effect of synthetic and natural polymers on reducing bauxite residue dust pollution. Environ. Technol. 2018, 41, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shi, L.; Shan, Z.; Dai, R.; Chen, H. Efficient removal of atmospheric dust by a suppressant made of potato starch, polyacrylic acid and gelatin. Environ. Chem. Lett. 2020, 18, 1701–1711. [Google Scholar] [CrossRef]
- Ding, X.; Xu, G.; Liu, W.V.; Yang, L.; Albijanic, B. Effect of polymer stabilizers’ viscosity on red sand structure strength and dust pollution resistance. J. Powder Technol. 2019, 352, 117–125. [Google Scholar] [CrossRef]
- Chen, R.; Lee, I.; Zhang, L. Biopolymer stabilization of mine tailings for dust control. J. Geotech. Geoenviron. Eng. 2014, 141, 4014100. [Google Scholar] [CrossRef]
- Maidapwad, S.L.; Sananse, S.L. On analysis of two-way ANOVA using data transformation techniques. Int. J. Sci. Res. 2014, 3, 480–483. [Google Scholar]
- Tran, T.P.A.; Cho, G.-C.; Ilhan, C. Water retention characteristics of biopolymer hydrogel containing sandy soils. Hue Univ. J. Sci. Earth Sci. Environ. 2020, 129, 5–17. [Google Scholar] [CrossRef]
- Tran, T.P.; Cho, G.C.; Lee, S.J.; Chang, I. Effect of xanthan gum biopolymer on the water retention characteristics of unsaturated sand. In Proceedings of the UNSAT2018 the 7th International Conference on Unsaturated Soils, Hong Kong, China, 3–6 August 2018. [Google Scholar]
- Huang, J.; Ho, C.-H.; Gao, Y.; Wu, Z.; Zhang, Y. Evaluation of polymer based dust suppressant mixed with clayey soil in unpaved road: Lab experiment. In Transportation and Geotechniques: Materials, Sustainability and Climate: Proceedings of the 5th GeoChina International Conference 2018-Civil Infrastructures Confronting Severe Weathers and Climate Changes: From Failure to Sustainability, Held on July 23 to 25, 2018 in HangZhou, China, 1st ed.; Barman, M., Zaman, M., Chang, J.-R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–9. ISBN 978-3-319-95768-5. [Google Scholar]
- Parameswaran, K.; Ekholm, J.; Zhang, L. Evaluation of mine tailings dust control. In Proceedings of the Geoenvironmental Engineering, Selected Papers from the 2014 Geoshanghai International Congress, Shanghai, China, 26–28 May 2014; Reddy, K.R., Ed.; Curran: New York, NY, USA, 2014; pp. 80–89, ISBN 978-0-784-41343-2. [Google Scholar]
- Hataf, N.; Ghadir, P.; Ranjbar, N. Investigation of soil stabilization using chitosan biopolymer. J. Clean. Prod. 2018, 170, 1493–1500. [Google Scholar] [CrossRef]
- Tran, T.P.A.; Im, J.; Cho, G.-C.; Chang, I. Soil-water characteristics of xanthan gum biopolymer containing soils. In Proceedings of the UNSAT2018 the 7th International Conference on Unsaturated Soils, Hong Kong, China, 3–6 August 2018. [Google Scholar]
- Liu, J.; Shi, B.; Lu, Y.; Jiang, H.; Huang, H.; Wang, G.; Kamai, T. Effectiveness of a new organic polymer sand-fixing agent on sand fixation. Environ. Earth Sci. 2012, 65, 589–595. [Google Scholar] [CrossRef]
- Ayeldeen, M.; Negm, A.; El Sawwaf, M.; Gädda, T. Laboratory study of using biopolymer to reduce wind erosion. Int. J. Geo-Eng. 2017, 12, 228–240. [Google Scholar] [CrossRef]
- Seo, S.; Lee, M.; Im, J.; Kwon, Y.-M.; Chung, M.-K.; Cho, G.-C.; Chang, I. Site application of biopolymer-based soil treatment (BPST) for slope surface protection: In-situ wet-spraying method and strengthening effect verification. Constr. Build. Mater. 2021, 307, 124983. [Google Scholar] [CrossRef]
- Kwon, Y.-M.; Ham, S.-M.; Kwon, T.-H.; Cho, G.-C.; Chang, I. Surface-erosion behaviour of biopolymer-treated soils assessed by EFA. Géotechnique Lett. 2020, 10, 106–112. [Google Scholar] [CrossRef]
- Vishweshwaran, M.; Padmashree, S.; Kalambari, R.; Sathya Bhaarathi, C.R.; Ramani Sujatha, E. Pre-gelatinized starch-a sustainable soil stabilizer. J. Adv. Res. Dyn. Control Syst. 2018, 10, 543–546. [Google Scholar]
- Rosa, I.; Roedel, H.I.; Allende, M.D.; Lepech, M.J.; Loftus, D. On Designing Biopolymer-Bound Soil Composites (BSC) for Peak Compressive Strength. J. Renew. Mater. 2020, 8, 845–861. [Google Scholar] [CrossRef]
- Dang, X.; Shan, Z.; Chen, H. Usability of oxidized corn starch-gelatin blends for suppression and prevention of dust. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Brown, M.J.; Robbins, C.W.; Freeborn, L.L. Combining cottage cheese whey and straw reduces erosion while increasing infiltration in furrow irrigation. J. Soil Water Conserv. 1998, 53, 152–156. [Google Scholar]
- Sharan, S.; Zanghelini, G.; Zotzel, J.; Bonerz, D.; Aschoff, J.; Saint-Eve, A.; Maillard, M.-N. Fava bean (Vicia faba L.) for food applications: From seed to ingredient processing and its effect on functional properties, antinutritional factors, flavor, and color. Compr. Rev. Food Sci. Food Saf. 2021, 20, 401–428. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.O.; Williams, P.A.; O’Kennedy, B.T.; Boland, M.; Tarté, R.; Mayer, O.; Haug, I.J.; Draget, K.I.; Tahergorabi, R.; Hosseini, S.V.; et al. Handbook of Food Proteins; Woodhead Publishing: Oxford, UK, 2011; ISBN 978-1-84569-758-7. [Google Scholar]
- Almajed, A.; Lemboye, K.; Arab, M.G.; Alnuaim, A. Mitigating wind erosion of sand using biopolymer-assisted EICP technique. Soils Found. 2020, 60, 356–371. [Google Scholar] [CrossRef]
- Blanck, G.; Cuisinier, O.; Masrouri, F. Soil treatment with organic non-traditional additives for the improvement of earthworks. Acta Geotech. 2014, 9, 1111–1122. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Vydehi, K.V. State-of-the-art review on efficacy of xanthan gum and guar gum inclusion on the engineering behavior of soils. Innov. Infrastruct. Solut. 2021, 6, 108. [Google Scholar] [CrossRef]
- Kunz, B.K.; Little, E.E.; Barandino, V.L. Aquatic toxicity of chemical road dust suppressants to freshwater organisms. Arch. Environ. Contam. Toxicol. 2021, 82, 294–305. [Google Scholar] [CrossRef]
- Katebi, H.; Fahmi, A.; Ouria, A.; Babaeian Amini, A.; Kafil, H.S. Microbial surface treatment of sand with sporosarcina pasteurii to improve the wind erosion resistance in Urmia Lake. Appl. Environ. Soil Sci. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Li, X. Geotechnical engineering properties of soils solidified by microbially induced CaCO3 precipitation (MICP). Adv. Civ. Eng. 2021, 2021, 6683930. [Google Scholar] [CrossRef]
- Meng, H.; Gao, Y.; He, J.; Qi, Y.; Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 2021, 383, 114723. [Google Scholar] [CrossRef]
- Freer, J.; Lübeck, M.; Sieger, J.L.; Lottermoser, B.G.; Braun, M. Effectiveness of food processing by-products as dust suppressants for exposed mine soils: Results from laboratory experiments and field trials. Appl. Sci. 2022, 12, 11551. [Google Scholar] [CrossRef]
- Freer, J.; Bucher, P.G.; Braun, M.; Lottermoser, B.G. Food processing by-products and wastes as potential dust suppressants at mine sites: Results from unconfined compressive strength testing. J. Air Waste Manag. Assoc. 2022, 72, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Niaounakis, M. Definitions of terms and types of biopolymers. In Biopolymers: Applications and Trends; Niaounakis, M., Ed.; Elsevier: Oxford, UK, 2015; pp. 1–90. ISBN 9780323353991. [Google Scholar]
- Thomas, S.; Durand, D.; Chassenieux, C.; Jyotishkumar, P. Handbook of Biopolymer-Based Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 9783527652457. [Google Scholar]
- Rimbarngaye, A.; Mwero, J.N.; Ronoh, E.K. Effect of gum arabic as partial replacement of cement on the durability properties of compressed laterite blocks. Open J. Civ. Eng. 2021, 11, 398–410. [Google Scholar] [CrossRef]
- Smitha, S.; Sachan, A. Use of agar biopolymer to improve the shear strength behavior of sabarmati sand. Int. J. Geo. Eng. 2016, 10, 387–400. [Google Scholar] [CrossRef]
- Khatami, H.R.; O’Kelly, B.C. Improving mechanical properties of sand using biopolymers. J. Geotech. Geoenviron. Eng. 2013, 139, 1402–1406. [Google Scholar] [CrossRef]
- Vishweshwaran, M.; Sujatha, E.R. β-glucan as a sustainable alternative to stabilize pavement subgrade. Polymers 2022, 14, 2850. [Google Scholar] [CrossRef]
- Soldo, A.; Aguilar, V.; Miletić, M. Macroscopic stress-strain response and strain-localization behavior of biopolymer-treated soil. Polymers 2022, 14, 997. [Google Scholar] [CrossRef] [PubMed]
- Anandha Kumar, S.; Sujatha, E.R. An appraisal of the hydro-mechanical behaviour of polysaccharides, xanthan gum, guar gum and β-glucan amended soil. Carbohydr. Polym. 2021, 265, 118083. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Im, J.; Cho, G.-C. An environmentally-friendly geotechnical approach for soil erosion reduction using microbial biopolymers. In Proceedings of the Geo-Chicago 2016, Sustainability and Resiliency in Geotechnical Engineering, Chicago, IL, USA, 14–18 August 2016; Zekkos, D., Farid, A., De, A., Reddy, K.R., Yesiller, N., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2016; pp. 17–24, ISBN 9780784480120. [Google Scholar]
- Chang, I.; Cho, G.-C. Geotechnical behavior of a beta-1,3/1,6-glucan biopolymer-treated residual soil. Geomech. Eng. 2014, 7, 633–647. [Google Scholar] [CrossRef]
- Chang, I.; Cho, G.-C. Strengthening of Korean residual soil with β-1, 3/1, 6-glucan biopolymer. Constr. Build. Mater. 2012, 30, 30–35. [Google Scholar] [CrossRef]
- Donayre, A.; Sanchez, L.F.; Kim, S.; Aguilar, R.; Nakamatsu, J. Eco-friendly improvement of water erosion resistance of unstable soils with biodegradable polymers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 12044. [Google Scholar] [CrossRef]
- Nakamatsu, J.; Kim, S.; Ayarza, J.; Ramírez, E.; Elgegren, M.; Aguilar, R. Eco-friendly modification of earthen construction with carrageenan: Water durability and mechanical assessment. Constr. Build. Mater. 2017, 139, 193–202. [Google Scholar] [CrossRef]
- Bocheńska, M.; Bujko, M.; Dyka, I.; Srokosz, P.; Ossowski, R. Effect of chitosan solution on low-cohesive soil’s shear modulus G determined through resonant column and torsional shearing tests. Appl. Sci. 2022, 12, 5332. [Google Scholar] [CrossRef]
- Shariatmadari, N.; Reza, M.; Tasuji, A.; Ghadir, P.; Javadi, A.A. Experimental study on the effect of chitosan biopolymer on sandy soil stabilization. E3S Web Conf. 2020, 195, 6007. [Google Scholar] [CrossRef]
- Soldo, A.; Miletić, M.; Auad, M.L. Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci. Rep. 2020, 10, 267. [Google Scholar] [CrossRef]
- Aguilar, R.; Nakamatsu, J.; Ramírez, E.; Elgegren, M.; Ayarza, J.; Kim, S.; Pando, M.A.; Ortega-San-Martin, L. The potential use of chitosan as a biopolymer additive for enhanced mechanical properties and water resistance of earthen construction. Constr. Build. Mater. 2016, 114, 625–637. [Google Scholar] [CrossRef]
- Taytak, B.; Pulat, H.F.; Yukselen-Aksoy, Y. Improvement of engineering properties of soils by biopolymer additives. In Proceedings of the 3. ICNDSMGE-ZM 2012 3rd International Conference on New Developments, Nicosia, Cyprus, 28–30 June 2012. [Google Scholar]
- Alsand, A. Novel Biopolymer Treatment for Wind Induced Soil Erosion. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2011. [Google Scholar]
- Kavazanjian, E.; Iglesias, E.; Karatas, I. Biopolymer soil stabilization for wind erosion control. In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering; Hamza, M., Shahien, M., El-Mossallamy, Y., Eds.; IOS Press: Amsterdam, The Netherlands, 2009. ISBN 978-1-60750-031-5. [Google Scholar]
- Armistead, S.J.; Rawlings, A.E.; Smith, C.C.; Staniland, S.S. Biopolymer stabilization/solidification of soils: A rapid, micro-macro, cross-disciplinary approach. Environ. Sci. Technol. 2020, 54, 13963–13972. [Google Scholar] [CrossRef] [PubMed]
- Ojuri, O.O.; Ramdas, V.; Aderibigbe, E.A.; Williams, C.G.; Ramchuran, S.; Al-Nageim, H. Improving strength and hydraulic characteristics of regional clayey soils using biopolymers. Case Stud. Constr. Mater. 2022, 17, e01319. [Google Scholar] [CrossRef]
- Yang, Q.; Pei, X.; Huang, R. Impact of polymer mixtures on the stabilization and erosion control of silty sand slope. J. Mt. Sci. 2019, 16, 470–485. [Google Scholar] [CrossRef]
- Inyang, H.I.; Bae, S.; Pando, M.A. Contaminant dust suppression materials: A cost-effectiveness estimation methodology. Measurement 2016, 93, 563–571. [Google Scholar] [CrossRef]
- Ham, S.-M.; Chang, I.; Noh, D.-H.; Kwon, T.-H.; Muhunthan, B. Improvement of surface erosion resistance of sand by microbial biopolymer formation. J. Geotech. Geoenviron. Eng. 2018, 144, 6018004. [Google Scholar] [CrossRef]
- Ramachandran, A.L.; Mukherjee, A.; Dhami, N.K. Nanoscale to macroscale characterization of in-situ bacterial biopolymers for applications in soil stabilization. Front. Mater. 2022, 8, 546. [Google Scholar] [CrossRef]
- Bitar, L. Optimum Mixing Design of Xanthan and Gellan Treated Soils for Slope Stabilization for Weathered Shales and Glacial Tills in Nebraska. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, 2020. [Google Scholar]
- Chang, I.; Cho, G.-C. Shear strength behavior and parameters of microbial gellan gum-treated soils: From sand to clay. Acta Geotech. 2019, 14, 361–375. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Lee, S.-W.; Cho, G.-C. Strength durability of gellan gum biopolymer-treated Korean sand with cyclic wetting and drying. Constr. Build. Mater. 2017, 143, 210–221. [Google Scholar] [CrossRef]
- Im, J.; Tran, A.T.; Chang, I.; Cho, G.-C. Dynamic properties of gel-type biopolymer-treated sands evaluated by Resonant Column (RC) Tests. Geomech. Eng. 2017, 12, 815–830. [Google Scholar] [CrossRef]
- Ferruzzi, G.; Pan, N.; Casey, W.H. Mechanical properties of gellan and polyacrylamide gels with implications for soil stabilization. Soil Sci. 2000, 165, 778–792. [Google Scholar] [CrossRef]
- Bonal, N.S.; Prasad, A.; Verma, A.K. Effect of microbial biopolymers on mechanical properties of bauxite residue. KSCE J. Civ. Eng. 2021, 25, 2437–2450. [Google Scholar] [CrossRef]
- Bozyigit, I.; Javadi, A.; Altun, S. Strength properties of xanthan gum and guar gum treated kaolin at different water contents. J. Rock Mech. Geotech. Eng. 2021, 13, 1160–1172. [Google Scholar] [CrossRef]
- Muguda, S.; Hughes, P.N.; Augarde, C.E.; Perlot, C.; Walter Bruno, A.; Gallipoli, D. Cross-linking of biopolymers for stabilizing earthen construction materials. Build. Res. Inf. 2021, 50, 502–514. [Google Scholar] [CrossRef]
- Bonal, N.S.; Prasad, A.; Verma, A.K. Use of biopolymers to enhance the geotechnical properties of coal mine overburden waste. Géotech. Lett. 2020, 10, 179–185. [Google Scholar] [CrossRef]
- Muguda, S.; Lucas, G.; Hughes, P.N.; Augarde, C.E.; Perlot, C.; Bruno, A.W.; Gallipoli, D. Durability and hygroscopic behaviour of biopolymer stabilised earthen construction materials. Constr. Build. Mater. 2020, 259, 119725. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, X.; Han, F.; Song, Z.; Wang, D.; Fan, J.; Jia, Z.; Jiang, G. A research on dust suppression mechanism and application technology in mining and loading process of burnt rock open pit coal mines. J. Air Waste Manag. Assoc. 2021, 71, 1568–1584. [Google Scholar] [CrossRef] [PubMed]
- Armistead, S.J.; Smith, C.C.; Staniland, S.S. Sustainable biopolymer soil stabilization in saline rich, arid conditions: A ‘micro to macro’ approach. Sci. Rep. 2022, 12, 2880. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Modiri, F. Application of novel Persian gum hydrocolloid in soil stabilization. Carbohydr. Polym. 2020, 246, 116639. [Google Scholar] [CrossRef]
- Yan, J.; Nie, W.; Xiu, Z.; Yuan, M.; Zhou, W.; Bao, Q.; Peng, H.; Niu, W.; Yu, F. Development and characterization of a dust suppression spray agent based on an adhesive NaAlg−gln−poly/polysaccharide polymer. Sci. Total Environ. 2021, 785, 147192. [Google Scholar] [CrossRef]
- Soltani, A.; Raeesi, R.; Taheri, A.; Deng, A.; Mirzababaei, M. Improved shear strength performance of compacted rubberized clays treated with sodium alginate biopolymer. Polymers 2021, 13, 764. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.G.; Mousa, R.A.; Gabr, A.R.; Azam, A.M.; El-Badawy, S.M.; Hassan, A.F. Resilient behavior of sodium alginate–treated cohesive soils for pavement applications. J. Mater. Civ. Eng. 2019, 31, 4018361. [Google Scholar] [CrossRef]
- Fatehi, H.; Bahmani, M.; Noorzad, A. Strengthening of dune sand with sodium alginate biopolymer. In Proceedings of the Geo-Congress 2019 8th International Conference on Case Histories in Geotechnical Engineering, Philadelphia, PA, USA, 24–27 March 2019; Meehan, C.L., Kumar, S., Pando, M.A., Coe, J.T., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2019; pp. 157–166, ISBN 9780784482117. [Google Scholar]
- Zhao, Y.; Zhuang, J.; Wang, Y.; Jia, Y.; Niu, P.; Jia, K. Improvement of loess characteristics using sodium alginate. Bull. Eng. Geol. Environ. 2019, 79, 1879–1891. [Google Scholar] [CrossRef]
- Bouazza, A.; Gates, W.P.; Ranjith, P.G. Hydraulic conductivity of biopolymer-treated silty sand. Géotechnique 2009, 59, 71–72. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, L.; Tong, L.; Liu, Y.; Yang, X.; Zhang, B.; Liao, Z.; Chen, A.; Hu, Y. Preparation of high-temperature resistant and environment friendly dust suppressant. J. Phys. Conf. Ser. 2021, 2076, 12054. [Google Scholar] [CrossRef]
- Chen, R.; Ding, X.; Lai, H.; Zhang, L. Improving dust resistance of mine tailings using green biopolymer. Environ. Geotech. 2019, 8, 382–391. [Google Scholar] [CrossRef]
- Chen, C.; Peng, Z.; Gu, J.; Peng, Y.; Huang, X.; Wu, L. Exploring environmentally friendly biopolymer material effect on soil tensile and compressive behavior. Int. J. Environ. Res. Public Health 2020, 17, 9032. [Google Scholar] [CrossRef]
- Ni, J.; Hao, G.-L.; Chen, J.-Q.; Ma, L.; Geng, X.-Y. The optimisation analysis of sand-clay mixtures stabilised with xanthan gum biopolymers. Sustainability 2021, 13, 3732. [Google Scholar] [CrossRef]
- Ramachandran, A.L.; Dubey, A.A.; Dhami, N.K.; Mukherjee, A. Multiscale study of soil stabilization using bacterial biopolymers. J. Geotech. Geoenviron. Eng. 2021, 147, 4021074. [Google Scholar] [CrossRef]
- Sujatha, E.R.; Atchaya, S.; Sivasaran, A.; Keerdthe, R.S. Enhancing the geotechnical properties of soil using xanthan gum—An eco-friendly alternative to traditional stabilizers. Bull. Eng. Geol. Environ. 2021, 80, 1157–1167. [Google Scholar] [CrossRef]
- Joga, J.R.; Varaprasad, B.J.S. Effect of xanthan gum biopolymer on dispersive properties of soils. World J. Eng. 2020, 17, 563–571. [Google Scholar] [CrossRef]
- Singh, S.P.; Das, R. Geo-engineering properties of expansive soil treated with xanthan gum biopolymer. Geomech. Eng. 2020, 15, 107–122. [Google Scholar] [CrossRef]
- Theyab, A.F.; Muhauwiss, F.M.; Alabdraba, W.M. Enhancing gypseous soil behavior using casein from milk wastes. J. Mech. Behav. Mater. 2022, 31, 306–313. [Google Scholar] [CrossRef]
- Park, S.-S.; Woo, S.-W.; Jeong, S.-W.; Lee, D.-E. Durability and strength characteristics of casein-cemented sand with slag. Materials 2020, 13, 3182. [Google Scholar] [CrossRef] [PubMed]
- Gopika, A.S.; Mohandas, T.V. Soil strengthening using caseinate: A protein based biopolymer. Int. J. Res. Eng. Sci. Manag. 2019, 2, 538–540. [Google Scholar]
- Chang, I.; Im, J.; Chung, M.-K.; Cho, G.-C. Bovine casein as a new soil strengthening binder from diary wastes. Constr. Build. Mater. 2018, 160, 1–9. [Google Scholar] [CrossRef]
- Fatehi, H.; Abtahi, S.M.; Hashemolhosseini, H.; Hejazi, S.M. A novel study on using protein based biopolymers in soil strengthening. Constr. Build. Mater. 2018, 167, 813–821. [Google Scholar] [CrossRef]
- Im, J.; Cho, G.-C.; Chang, I. A new soil treatment method using casein from bovine milk. In Proceedings of the Geo-Chicago 2016, Sustainability and Resiliency in Geotechnical Engineering, Chicago, IL, USA, 14–18 August 2016; Zekkos, D., Farid, A., De, A., Reddy, K.R., Yesiller, N., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2016; pp. 1–6, ISBN 9780784480120. [Google Scholar]
- Jin, H.; Nie, W.; Zhang, Y.; Wang, H.; Zhang, H.; Bao, Q.; Yan, J. Development of environmental friendly dust suppressant based on the modification of soybean protein isolate. Processes 2019, 7, 165. [Google Scholar] [CrossRef]
- Cruse, R.M.; Berghoefer, B.E.; Mize, C.W.; Ghaffarzadeh, M. Water drop impact angle and soybean protein amendment effects on soil detachment. Soil Sci. Soc. Am. J. 2000, 64, 1474–1478. [Google Scholar] [CrossRef]
- Chen, R.; Ding, X.; Ramey, D.; Lee, I.; Zhang, L. Experimental and numerical investigation into surface strength of mine tailings after biopolymer stabilization. Acta Geotech. 2016, 11, 1075–1085. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).