1. Introduction
Trans-septal punctures are performed to gain access to the left atrium for several minimally invasive cardiac interventions such as mitral valve repair, left atrial appendage closure, and several left-sided cardiac ablations [
1]. Moreover, measurement of left atrial pressure is an assessment of heart diseases. Although trans-septal access to the left side of the heart provides a very promising method for cardiologists, the maneuverability of the catheter remains challenging. Thus, making it difficult to access the desired location of the left atrium.
Patent foramen ovale (PFO) is a common condition with probe patency in about 15–35% of the population [
2]. It is involved in the etiology of several different pathologies including cryptogenic stroke and decompression sickness in divers. To analyze the concept of atrial septal defect (ASD) and PFO, the anatomical functional characteristic of the interatrial septum is of paramount importance. It not only assists device selection but also helps in the evaluation of the outcome of the trans-septal procedure [
2]. The puncture is performed at the thinnest region of the interatrial septum known as the fossa ovalis (FO). A morphological study conducted in apparently 50 normal human hearts confirmed the probe patency and an oval shape of fossa ovalis. The measured transverse and vertical diameters of FO were 14.53 mm and 12.60 mm, respectively [
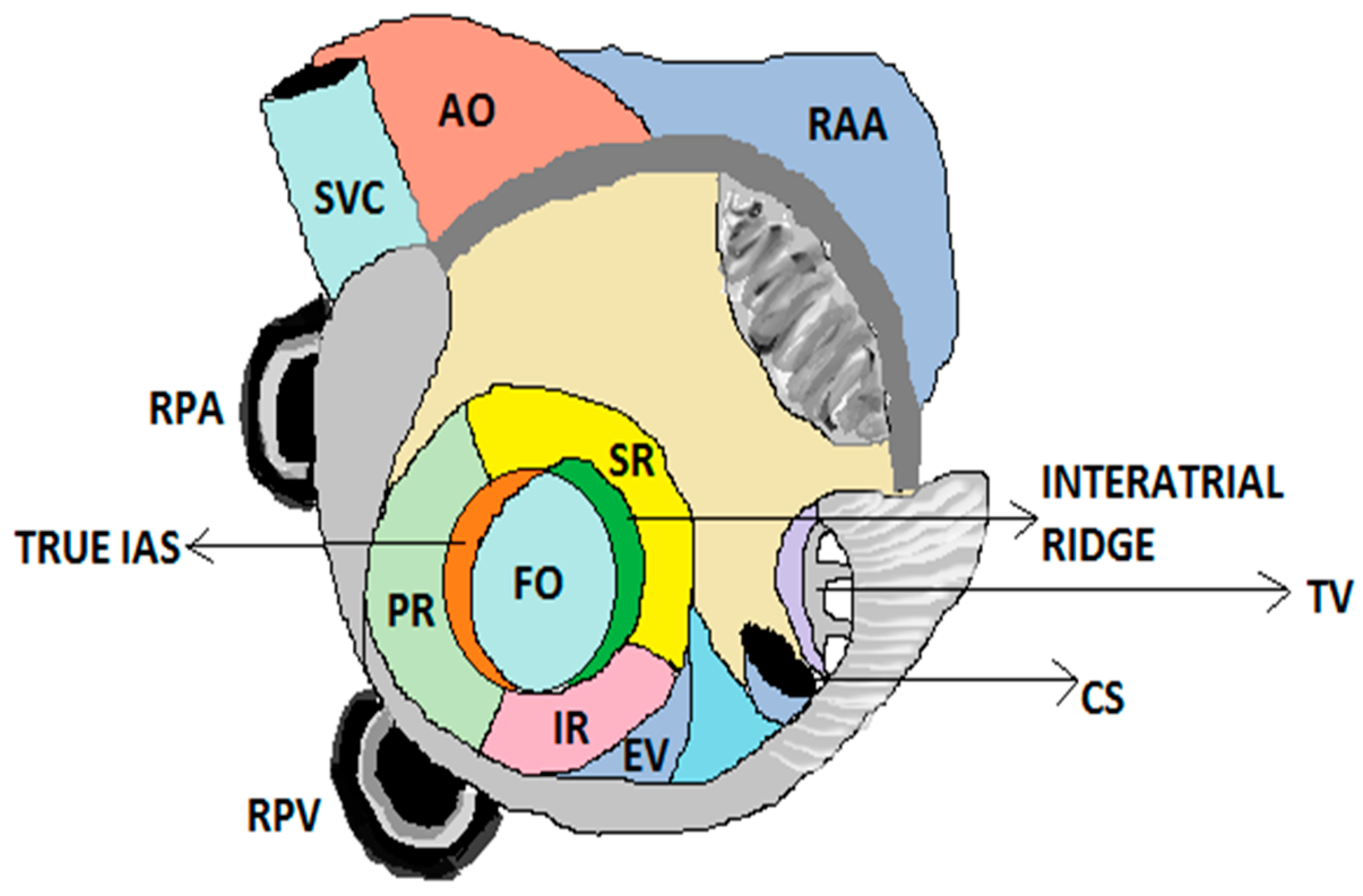
2]. Fossa ovalis is the route for trans-septal access to the left atrium. A detailed study of the anatomy of fossa ovalis and surrounding landmarks plays a vital role in achieving a successful trans-septal procedure. FO is an oval or circular depression of the atrial septum located towards the right atrium above and to the left of the orifice of the inferior vena cava (IVC) [
3]. Analysis of the clinical anatomy is essential for atrial septal defect, device selection, and overall success of the trans-septal procedure [
4]. A schematic representation of FO and its surrounding landmarks is shown in
Figure 1.
A recent study on trans-septal procedures and the clinical relevance of FO presented similar data such as anteroposterior diameter of FO (14.5 mm), craniocaudal diameter (12.6 mm), interatrial septum width (29.3 mm), PFO channel length (10.5 mm), and shape of FO: oval (86%) v circular (14%) [
5]. Apart from morphology, this study also focused on imaging modalities such as fluoroscopy and intracardiac echocardiography, which play a vital role in the case of septal repair, PFO portal crossing, or when the anatomy of FO remains ambiguous [
5]. A major complication in the trans-septal procedure is the iatrogenic atrial septal defect (iASD), which comes into effect due to the large size of sheaths that cross the septum. Other factors that contribute to the formation of iASD include long procedural times, high pulmonary pressures, and catheter manipulation [
6].
A study conducted by S. P. Lake et al. aimed to evaluate the initial collagen fiber orientation of soft tissues subjected to indentation testing. This was achieved using a collagen-based tissue-equivalent (TEs) model that helped to explore the structural and functional relationship [
7]. Samples with both the isotropic and anisotropic fiber alignment were fabricated using the mold-grip system, where the samples can be secured uniaxially (rectangular mold) and biaxially (cruciform mold). This study subjected the Tes with either isotropic or highly anisotropic fiber alignment to stress–relaxation indentation tests with four-step increments. The collagen reorientation and mechanical properties were compared at each indentation step between different groups, and significant differences were observed in the relaxation behavior and fiber orientation. Significant differences were observed in the collagen fiber alignment due to differences in mold geometries. The relative strength of alignment for the isotropic group was very low, whereas the relative strength for the anisotropic group was significantly higher, demonstrating a more strongly aligned network. Results demonstrated that the slower the relaxation rate, the more highly aligned the samples, the smaller the changes in collagen fiber orientation, and the larger the strain magnitudes compared to isotropic samples. He analyzed the mechanics and kinematics of soft tissues under indentation. The purpose of performing indentation tests on soft biological tissues varies in several studies including evaluation of biomechanical properties such as the Young’s modulus, puncture force, and displacement, as well as variations in the collagen fiber orientation upon indentation, and mechanics of dynamic needle insertions into soft biological tissues [
7]. In general, understanding the mechanical properties of soft tissue will help scientists to predict and analyze the behavior of the tissue subjected to various biological forces, or the forces that they need to bear or respond to during the surgical procedure; this insight leads to a better device design and improvement [
8,
9].
Needle insertion procedures involve several transitions between tissue layers that give rise to rupture events. These events involve large forces and tissue deformations that lead to crack extensions. M. Mahvash et al. presented the theory behind the mechanics of rupture events and determined the impact of insertion velocity on needle force and tissue deformation [
10]. Needle insertion experiments were performed on porcine heart tissues and the non-linear viscoelastic properties of the tissues were determined through implementation of the Kelvin–Voight model. This helped in analyzing the relationship between tissue deformation and rupture force at different velocities [
10].
Another study conducted by J. Y. Bourges et al. was aimed at establishing a relationship between the fiber orientation and tensile strength of an artificial collagen model made of porcine skin. The model was subjected to uniaxial tensile tests and the collagen fiber orientation (parallel or perpendicular) was determined prior to testing using small-angle light scattering (SALS). This helps in characterizing the microstructure without damaging the material [
11]. Results illustrated the stress–elongation response, where the collagen fibers in the parallel direction were observed to be less extensible compared to fibers aligned in the perpendicular direction. It was also observed that the stiffness significantly varies with respect to the direction of orientation [
11].
Collagen is the most important protein responsible for the mechanical framework of the myocardium [
12]. There are two types of collagens dominantly in myocardium: collagen I and collagen III; where collagen I is stiffer compared to collagen III and responsible for regulating the mechanical properties of the myocardium under large deformation. Collagen IV, V, and VI play significant roles in fiber formation and cell–ECM interactions. However, they do not directly contribute to the mechanical properties of tissues. Collagen fibers consist of bundles of proteins that are cross-linked together. Collagen fibers exhibit a coiled structure. When stretched, they change from crimpled state to an uncrumpled state (straight) creating a toe region. At the end of this region, the fibers respond linearly to the load. When stretched to a high level, the fibers completely straighten [
12,
13].
Lamb hearts were chosen because of their biological and physiological similarities with human hearts. Lambs and sheep are common animals for translational research in cardiovascular surgery [
14] due to their similar cardiac output and heart mass [
15]. Lamb hearts were relatively easier and more ethical to obtain than human cadaveric hearts. Hence, mechanical tests were carried out in lamb hearts which then correlate to a human heart. The main objective of this study is to provide an accurate location of fossa ovalis through lamb heart dissection and to evaluate the mechanical properties of fossa ovalis through indentation and tensile testing.
2. Methods and Materials
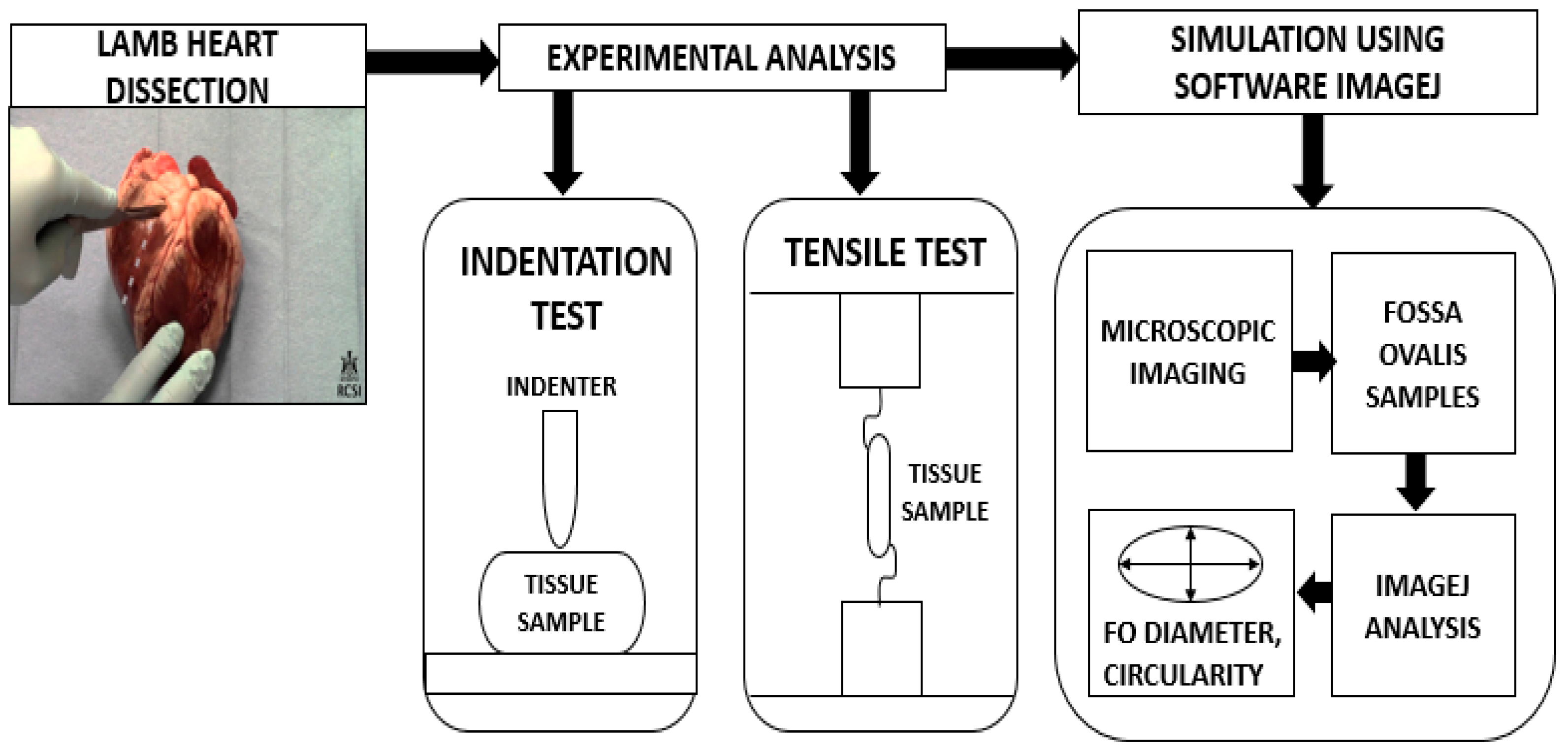
The size and dimensions of the lamb hearts were considered fixed variables in the mechanical tests. This ensured that the FO size in the sample did not considerably vary and influence the results, leading to inaccurate force readings. This was achieved by ensuring that the lambs were of similar body weight and their hearts were obtained on the same day. Only FO of similar dimensions was tested. The following section details the methodology adopted to analyze the mechanical behavior of fossa ovalis. This is schematically represented in
Figure 2.
2.1. Dissection of Lamb Hearts
For the purpose of experimental testing, lamb hearts (n = 20) were obtained from a local butcher. No animals were deliberately sacrificed for this experiment, and they were intended for use in the food market. Special consideration was given to the ethics of animal experimentation. Experiments with lamb hearts and the risks involved during research on heart samples were carefully handled by following laboratory protocols at the University of Galway, Ireland. A standard method of dissection was used. Prior to dissection, the anatomy of the fossa ovalis and surrounding landmarks were carefully studied. It requires more incisions with respect to the right atrium and left and right atrial appendages to gain access to the thinnest part of the interatrial septum. The fossa ovalis is located posteriorly, at the junction of the lower third and mid of the right atrium [
16]. The procedure involved is outlined below:
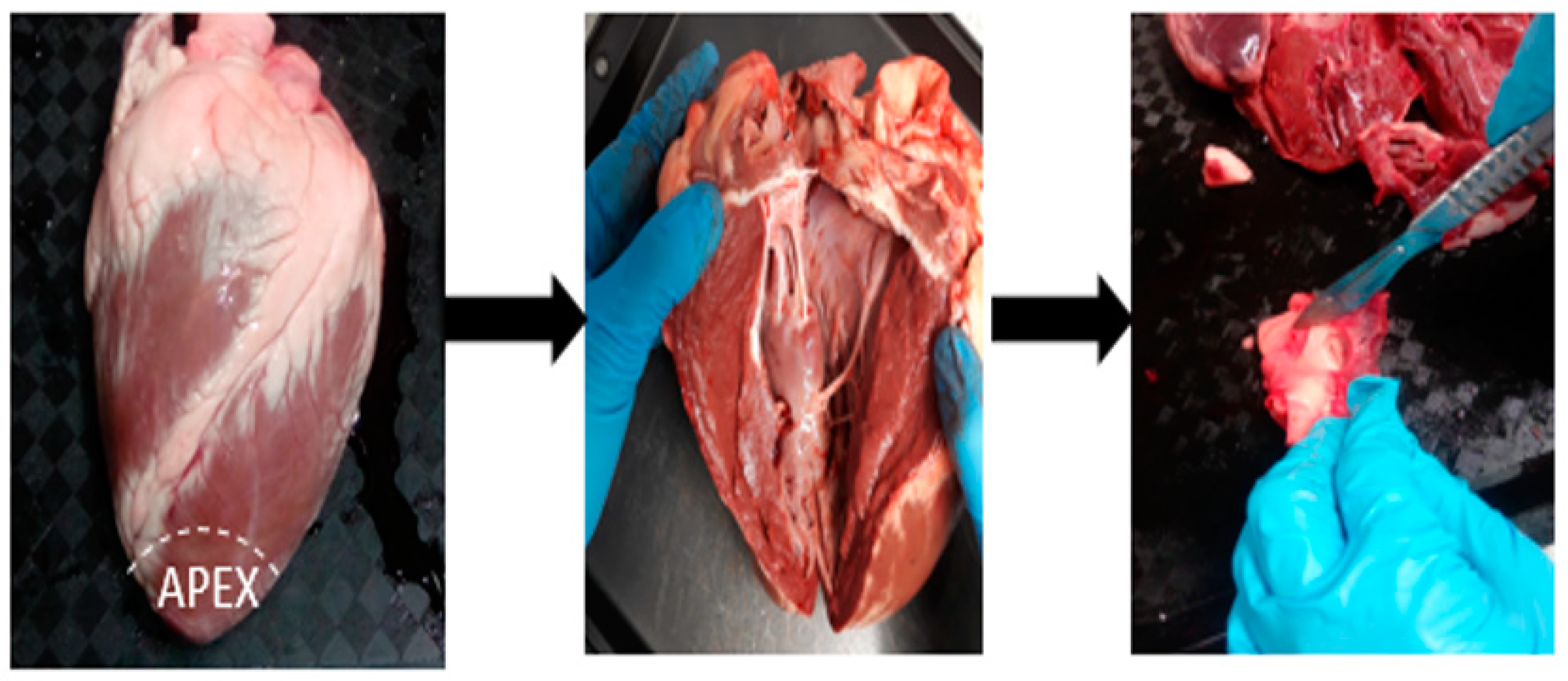
Step 1: The first step in dissection involves preparation of the dissection tray. The tray includes a scalpel, a pair of forceps, a blunt probe, and a pair of dissecting scissors.
Lamb hearts were obtained intact and washed free of blood before dissection. The hearts facing the anterior side were placed on the tray.
An incision was made at the apex (base) of the heart using a scalpel. The apex was cut off by making a circular incision to observe the thickness of the ventricular chambers. This helped in distinguishing the left and right ventricular chambers.
The left ventricular chamber appears to be thicker than the right, as the function of the left ventricle is to pump oxygenated blood to tissues all over the body.
Step 2: As the fossa ovalis is located posteriorly at the junction of the lower third and mid of the right atrium, an incision is made to cut off the right ventricle. This helps in achieving easy access to the right atrium, thus in turn the fossa ovalis.
Step 3: The heart was now observed from the posterior side and a small portion of tissue from both the right and left atrial appendage was cut off to view the interatrial septum. The inferior vena cava that lies posterior to the right atrium was also located. The mouth of the inferior vena cava directly connects to the fossa ovalis. Finally, the fossa ovalis was located and dissected.
The stages in the dissection process are illustrated in
Figure 3.
The diameters of fossa ovalis were measured using Vernier calipers. The diameters for all the samples varied from 8.2–15.0 mm. All the samples were then stored in phosphate buffer saline (Sigma-Aldrich) solution for 24 h [
17].
2.2. Indentation Test
Indentation can be performed in situ, thus enabling the test to be more physiological. This method is frequently used to evaluate the biomechanical properties of soft biological tissues [
18].
In this method, two different indenters were used: a steel (blunt) indenter with a hemispherical tip and a conventional Brockenbrough (BRK) needle with a sharp tip. BRK (St Jude Medicals) was cut 13.5 cm for easy grip into the tensile test machine (Zwick Roell Z005).
Figure 4 shows the two types of indenters used. A range of forces can act on varying surface areas of the FO tissue geometry in the human body. A steel (blunt) indenter corresponds to force applied to a large surface area of the FO and indents the tissue. Whereas Brockenbrough (BRK) needle corresponds to force applied to a small surface area of the FO and punctures the tissue. The two types of indenters were used to study the influence of blunt and sharp forces on FO, simulating the different force-to-surface area ratios on a lamb heart. Thus, it is vital to analyze the sequence of events and the impact between the punch state and puncture state. The same procedure was followed for the two types of indenters.
The fossa ovalis tissue samples were collected and placed on a plastic petri dish. The tissue samples were placed in such a way that the surface was perpendicular to the indenter. This limits the indentation to be uniaxial and limits any shear loading [
19]. FO was surrounded by tissue and was close in size to the Petri dish. This effectively prevented the sample from moving during the experimental test. No tissue movement or failure of the experiment was recorded during indentation tests as the resulting forces acted only at the center of the FO at a single contact point unlike the tensile tests, which hold uneven tissue structures at both ends (upper and lower grips) with uncertainty about resultant forces acting only at the center of the FO. The software used for testing was testXpert II (Zwick Roell Z005). The loading testing regime was programmed into the software. The test speed was maintained at 10 mm/min. [
16]. The maximum limit for force was set at 90 N.
Figure 3 illustrates indentation with two different types of indenters.
2.3. Tensile Testing of Fossa Ovalis
The test was carried out using Zwick Roell (Z005) tensile machine with a 100 N load cell. The procedure followed during the test is detailed in this section. The tissue sample was mounted onto the tensile machine and clamped at both ends using a 100 N load cell. It is not possible to directly grip the fossa ovalis due to its challenging anatomy. Therefore, the fossa ovalis tissue samples were dissected along its surrounding structures such as the atrial septum to obtain a proper grip.
One end of the tissue was clamped to the upper side of the machine using a 100 N load cell, while the other end of the tissue was immobilized to a fixed base (static fixture) in
Figure 5b. The tensile loading and relaxation testing regime were programmed into the software testXpert II followed by zero load and zero position in a sequence. The grip-to-grip separation at the start position was set as 20 mm. The speed for testing was maintained at 100 mm/min. The pre-load was maintained to be low and was set at 0.1 N. However, data were not recorded during the preload segment.
Figure 5a illustrates the experimental setup of the tensile test machine.
When the tissue samples were initially placed into the testing grips, they experienced small compressive forces. This would cause the samples to bend, thus producing inaccurate and inconsistent results. Therefore, the tensile test method establishes a small preload that helps to eliminate these compressive forces on the tissue samples and improves the quality of results. Fossa ovalis formed the cross-section of the sample and force was applied to rupture the tissue at the cross-section. The tissue samples were tested until rupture and the maximum rupture force was recorded by the software.
Figure 5b,c depicts the images of tissues captured at (b) preload and (c) rupture.
2.4. ImageJ Analysis to Measure Diameter of Fossa Ovalis
Fourteen fossa ovalis samples were imaged using a microscope (Nikon, SMZ1270). All the images were captured through NIS-Elements microscope imaging software at a scale of 500 px. ImageJ (version 1.47) was used to measure the diameter and circularity of FO directly from the photographed images of the samples. Prior to the analysis, the images were processed by improving their sharpness and contrast. A line tool was then used to calculate the diameter of the processed FO images. Circularity was measured using the equation below. The area and perimeter of the FO were obtained using the built-in options in ImageJ. A circularity value of 1.0 indicates that the FO is a perfect circle and a value close to 0.0, indicates that FO is an elongated shape.
The diameters of fossa ovalis measured using vernier calipers and obtained using ImageJ were compared.
Figure 6 illustrates images of two fossa ovalis samples captured using the microscope.
The data for five samples could not be measured due to the inconsistency of the sample sizes which produced inaccurate results. Therefore, fifteen lamb hearts were dissected successfully, and fossa ovalis tissue samples were obtained for the purpose of experimental testing.
3. Results
The size and dimension of the FO samples are considered fixed variables in the indentation and tensile test. Hence, all the FO samples should be of similar sizes. Twenty samples were obtained from twenty different lamb hearts. Due to biological differences between each lamb sample, five samples had vastly different dimensions and thicknesses. This means that it cannot be predicted what part of the tissue sample will rupture first during the test, creating anomalies in the data. For the tensile and indentation test, force should be applied at the same location on the tissue for all samples. Five out of twenty samples did not successfully rupture at their intended location in the tissue. Thus, these samples were discarded and not included in the results.
3.1. Indentation Test Results
There were notable differences in the indentation forces on fossa ovalis with two separate indenters. The steel indenter with a hemispherical tip requires a larger indentation force compared to the Brockenbrough needle. As the hemispherical indenter (blunt) is not capable of perforating the tissue, it only acts as a punch. The tissue deformation with the hemispherical indenter was observed to be reversible. On the contrary, the traditional Brockenbrough needle perforated the tissue to a little extent. After a few seconds of perforation, the needle started to bend, thus restricting complete perforation. The difference between the indentation forces for the two indenters is illustrated in
Figure 7.
The phases involved during indentation with hemispherical indenter involve contact with the tissue, tissue punch, and contact with the base upon which the tissue is placed. Initially, the tissue experiences small forces upon indentation followed by a sudden rise that indicates the punch state. The maximum force indicates the force at which the indenter virtually comes in contact with the base (Petri dish) upon which the sample is placed. The force at this point is recorded as the maximum force by the Zwick machine. On the other hand, indentation with the BRK needle involves different phases such as deformation, puncture, insertion, and bending. Initially, the tissue experiences deformation due to small forces followed by puncture with a small drop in the force value. This is then followed by an insertion phase that continues for a few seconds. After this time has elapsed, the bending phase begins, and the force starts decreasing. Here, the maximum force indicates the force at which the needle starts bending.
During the indentation test, the indenter acts perpendicular to the tissue. The direction in which the tissue is loaded is important to analyze the collagen fiber orientation. The collagen fibers are distributed randomly when the tissue is in a relaxed state. Upon indentation, collagen fiber reorientation occurs, as the loading, in this case, is perpendicular [
17].
3.2. Tensile Test Results
All 15 samples illustrated a normal failure pattern shown in
Figure 8. The mean (
Figure 8a) and maximum (
Figure 8b) force is plotted against the displacement of the tissue. The inset image in
Figure 8a indicates the standard deviation. The toe region indicates the initial phase where the force increases consecutively. At the toe region, uncrimping of the collagen fibrils occurs and this region lasts until all the crimpled collagen fibrils straighten. At the end of the toe region, the linear response of fossa ovalis is observed, as the collagen fibers respond linearly to the load. When the strain is large, the cross-links fail, thus resulting in collagen fibers sliding past one another. This leads to microscopic failure. However, if the strain is increased, it can lead to macroscopic failure [
13].
Storage modulus (or Young’s modulus) was calculated as 0.079 MPa. Thus, FO can withstand 0.079 MPa of force under tension before reaching its elastic limit.
3.3. ImageJ Analysis on Fossa Ovalis
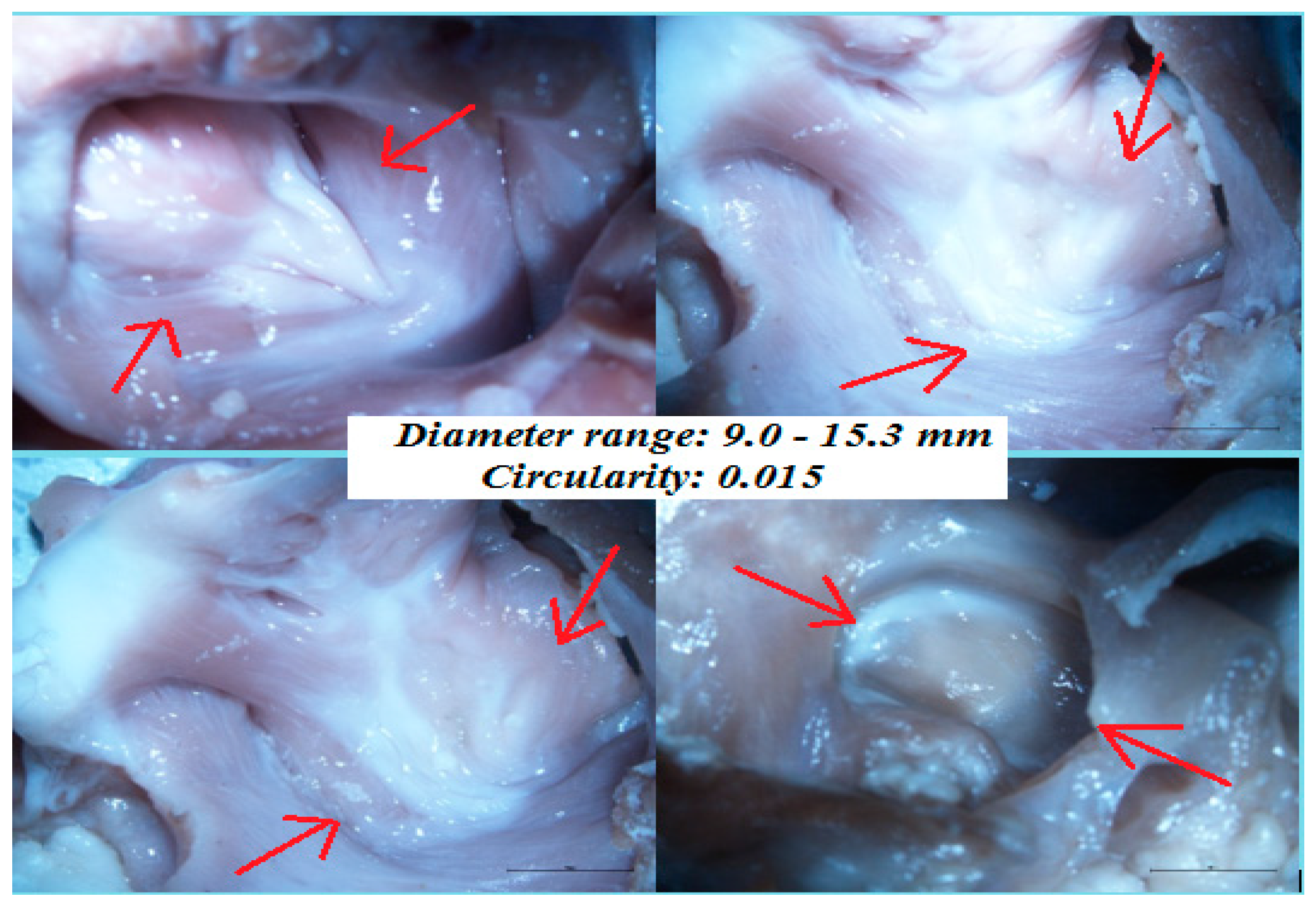
The diameters calculated using ImageJ were in the range 9.0–15.3 mm. The average diameter was calculated to be 12.5 mm.
Figure 9 illustrates images of fossa ovalis captured using a Nikon microscope. The mean circularity of 15 samples was 0.015, indicating an oval fossa.
4. Discussion
This research includes the methodology of fossa ovalis dissection. The dissection technique was practiced in the Human Biology Building at the University of Galway, Ireland. It is quite challenging to locate the fossa ovalis, especially in the lamb hearts that have very small and delicate internal structures. Unlike the other structures of the heart such as the heart valves, aorta, and left and right atrial appendages that can be easily located, access to the fossa ovalis requires many incisions as it lies on the interatrial septum, and towards the mid of the right atrium. Therefore, the purpose of the paper is to provide tips for the dissection of fossa ovalis, studying the advantages of analyzing the mechanical response of fossa ovalis tissue using two different methods: indentation and tensile testing.
A difference in collagen fiber orientation depending on the technique of loading was studied. Indentation provides a compressive load on the tissue sample that is totally different and opposite to the tensile (stretching) load. As the direction of loading is perpendicular in the case of the indentation test, fiber reorientation occurs and uncrimping of collagen fibers does not necessarily take place. On the other hand, a reduction in collagen fiber crimp and reorientation occurs when a load is applied in parallel to the preferred fiber direction [
20].
During a tensile test, fibroblasts tend to elongate under stretch and orient themselves along the direction of the uniaxial stretch. A study reported by G. M. Fomovsky et al. sought to develop an in vitro model system to establish the basic structure–function relationship and the development of structural and mechanical anisotropy in a well-controlled environment [
21]. In this study, cardiac and tendon fibroblasts were isolated from adult rats and were then used to populate 9 sets of gels which were constrained either uniaxially or biaxially for 72 h. Two sets of gels, one with adult cardiac fibroblasts (N = 6) and other acellular (N = 5) were constrained uniaxially to establish the development of structural anisotropy and to determine whether the gels require active matrix remodeling by fibroblasts. Results demonstrated structural anisotropy in the uniaxial group which was associated with mechanical anisotropy. Similarly, the biaxial group also demonstrated an association between structural isotropy and mechanical isotropy. When the collagen gels were constrained uniaxially, reorientation of collagen fibers occurred when the gel contracted in the unconstrained region [
21]. Similar biaxial tests on collagen gels have been performed previously, where collagen formed into tubes was constrained uniaxially and internal pressure was applied simultaneously [
22]. This study also proved the anisotropic mechanical behavior of collagen gels and its association with orthogonal cell orientation [
22].
This study deals with two different types of tests on fossa ovalis that differ in terms of tissue deformation and failure mechanism. The tensile test resulted in macroscopic failure due to tissue rupture. During the tensile test, the tissue samples exhibit necking, which further initiates rupture events. During the indentation test, deformation was observed to be reversible in the case of hemispherical indentation. A small compressive force acts on the tissue, which recovers its initial configuration when stress is released [
23]. On the other hand, indentation with the Brockenbrough needle resulted in plastic deformation that caused perforation of the tissue.
ImageJ analysis measured the diameter of the FO.
Table 1 represents a comparison of previous literature data to currently reported data.
In this study, the actual collagen fiber orientation is not known. Significant differences in mechanical properties of FO exist when loaded parallel and perpendicular to the direction of collagen fiber orientation. Previous studies use SALS on tissue-engineered pericardium composed of much thinner and transparent tissues. This methodology helped to determine the actual collagen fiber orientation mainly due to bio-prosthetic transparent tissues [
11]. This transparency is not well established in the fossa ovalis to determine the actual collagen orientation. Hence, different loading methods such as indentation and tensile testing are used to relate the changes in collagen orientation to the mechanical properties of FO. Previous studies on collagen fiber orientation provide an insight into the mechanical behavior of FO. This mechanical behavior is studied by loading FO using two different methods. Moreover, the puncture force is mainly dependent on the geometry of the needle (tapered/bevel/trocar) [
10,
23]. This validates the reason for indentation on fossa ovalis with two indenters of different geometries. Variations exist between previous data and current data for average puncture force with BRK needles of different sizes and tip geometries [
10,
23]. Other parameters that contribute to the variation in puncture forces include experiments on other animal hearts (porcine), indentation on different anatomical sites (porcine heart tissue), distinct methodologies involved during testing (suction device/linear actuators), and different test speeds (1 mm/s, 10 mm/s). In terms of FO shape, no significant differences were observed between the diameter range and shape of FO.
Limitations
Sample sizes should be consistent, despite the difficulty in maintaining the dimensions of cardiac tissues due to their inhomogeneity. This task is quite challenging as the interatrial septum consists of both the thinner and thicker regions. Moreover, it is important to grip all the samples at the same location when mounted on the tensile test machine. This is not practically achievable in the case of some biological samples, as the thickness of the septum varies from heart to heart. In the tensile test, FO was securely fixed at one end using a static fixture. A similar fixture was not used during the indentation test. However, the sample was almost the size of the Petri dish, which resisted FO from moving during the test. A fixture setup may be used to hold the tissue more effectively. During the test, one cannot predict what part of the sample will rupture initially as this depends on the thickness of the sample. Therefore, the plots for such tissue samples tend to show significant variation. Due to these limitations, five samples out of twenty failed during the test.
With respect to the tensile test experiments, only the uniaxial boundary condition was considered. A limitation of biaxial testing is that the system cannot record the actual region of interest loads. These could be lower than the applied loads measured by the load cells. To overcome challenges with such experimental methods, optical imaging methods could be considered to determine the strain in FO. Literature studies suggest using finite element methods to calculate a correction factor, which could be used to calculate the experimental stresses at the region of interest. A combination of experimental and finite element methods could be incorporated in future studies to accurately analyze the tissue’s mechanical behavior and address the challenges arising from boundary conditions [
24].
5. Conclusions
In this work, the mechanical aspects of fossa ovalis tissue were successfully studied. A methodology to identify and dissect fossa ovalis obscured inside the interatrial septum was developed. Different indenter geometries, i.e., blunt (hemispherical) and sharp (BRK) tips were shown to have varying effects on the deformation of fossa ovalis and thus the force results. Tensile tests were successfully performed to determine the average rupture force of FO. The force data for indentation and tensile test regime were reported and compared with data from previous studies. Challenges in clamping and mounting the fossa ovalis geometry on the tensile test machine were identified and solution strategies were implemented.
Studies on the heart samples were conducted both experimentally and using ImageJ. Experimental analysis was deemed to be time consuming, where the overall success and accuracy of the results depends on the quality of the tissue sample obtained, their dissection, and storage conditions. Computational modeling using the finite element analysis (FEA) is considered the primary analysis stage prior to developing a prototype to simulate the stress–strain and force–deflection response of the tissue sample. The computed forces can vary depending on the material properties of the tissue sample. FEA solver allows for flexibility in varying the tissue geometry as well as the material properties. Thus, the experimental analysis completed as part of this study forms a basis on which FEA solvers can be calibrated to simulate patient-specific or idealized fossa ovalis geometries subjected to various needle geometries and characteristics. This ultimately provides an insight into the anatomical characterization, mechanical forces, and tissue deformation—all of which are essential for a safe and efficacious trans-septal puncture.