Changes of Some Physicochemical Properties of Canola Oil by Adding n-Hexane and Ethanol Regarding Its Application as Diesel Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Physicochemical Properties of Canola Oil with Regard to Its Composition
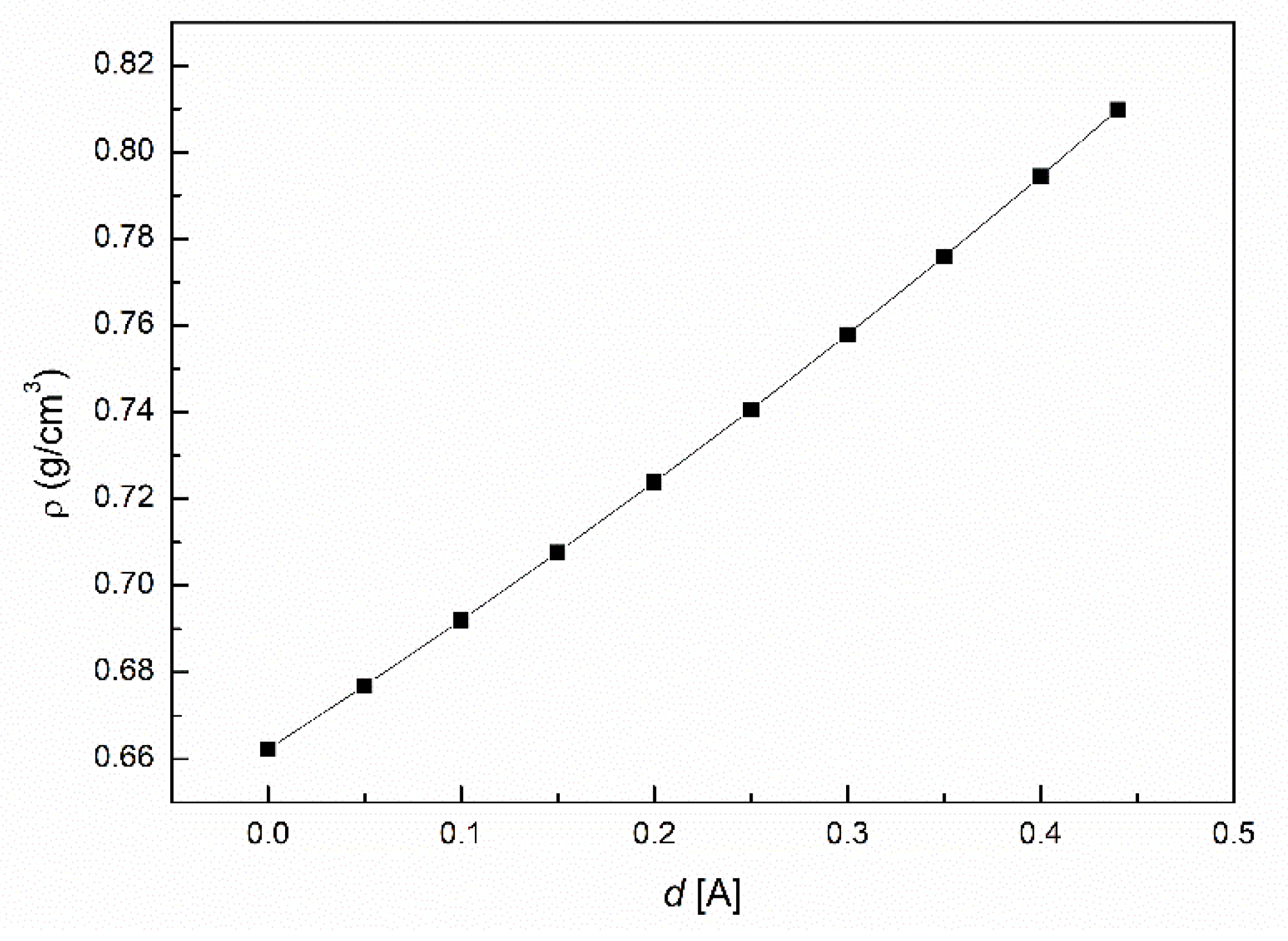
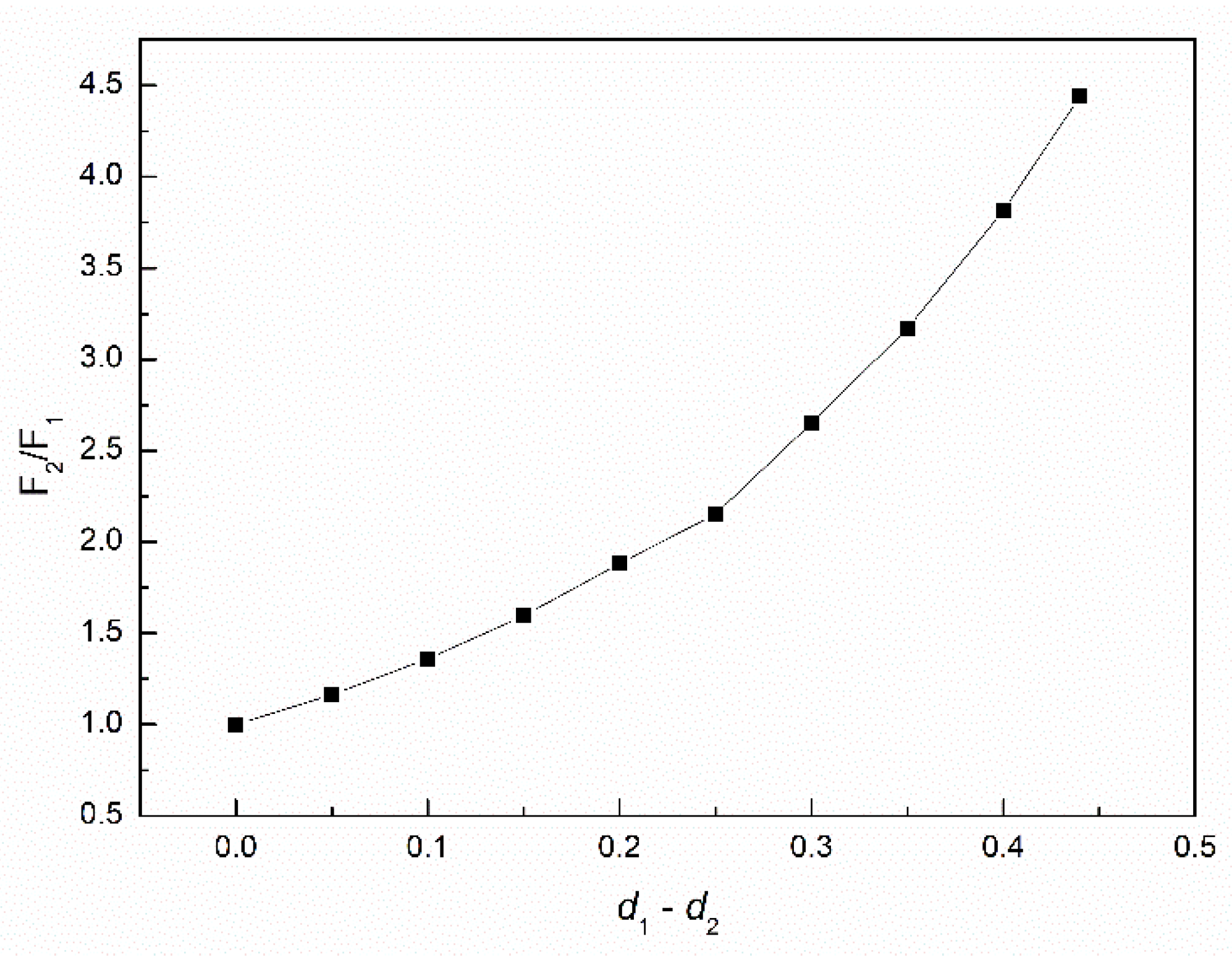
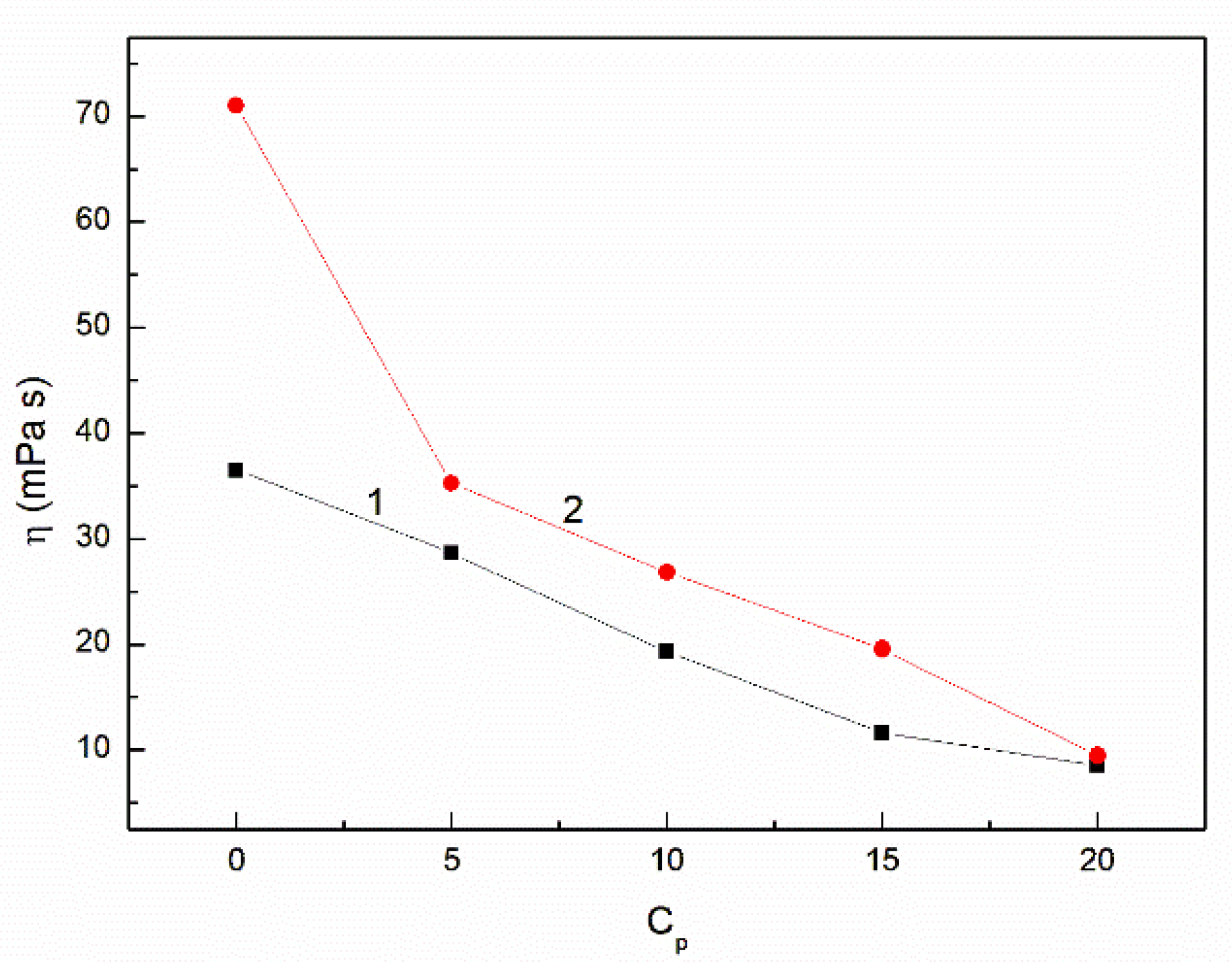
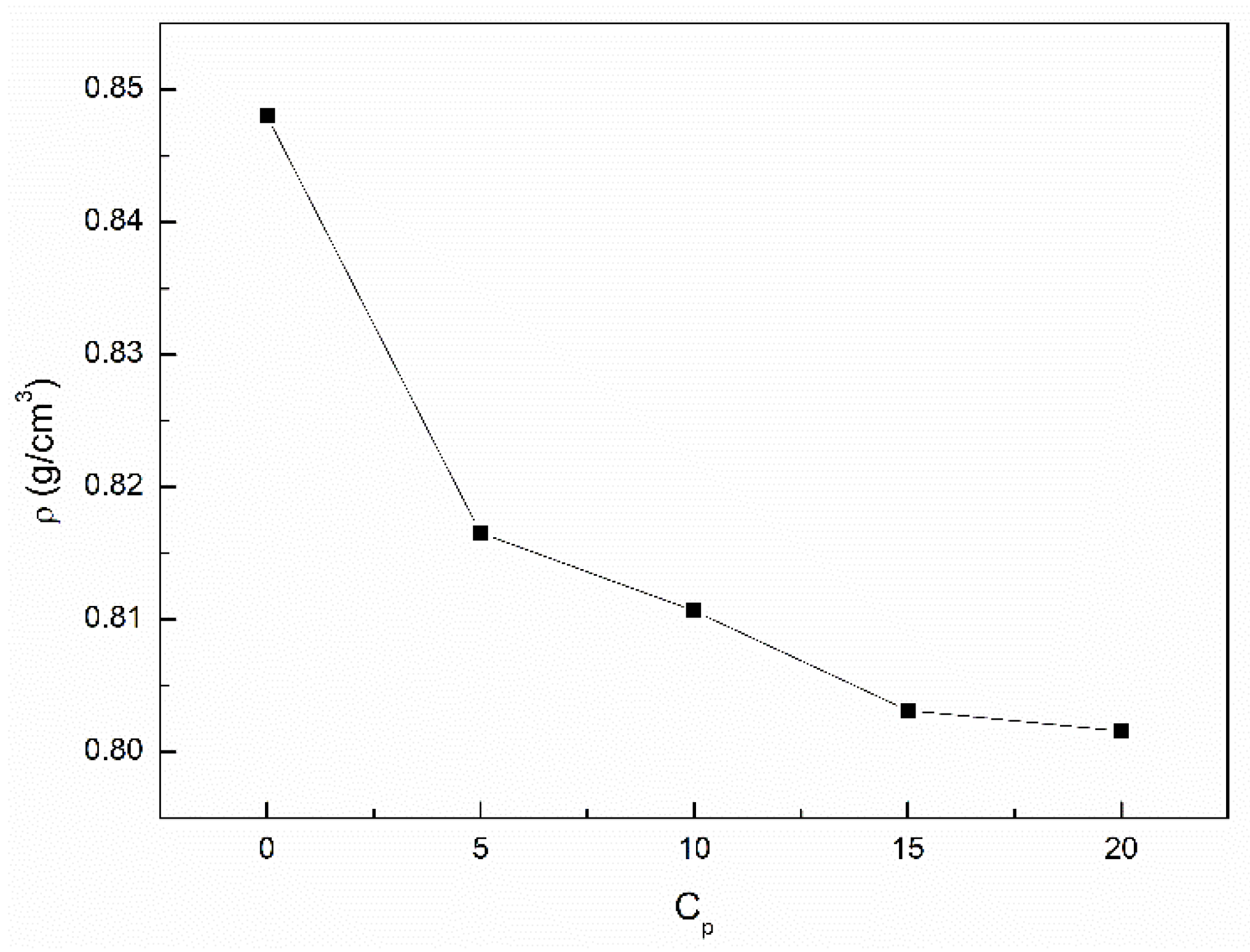
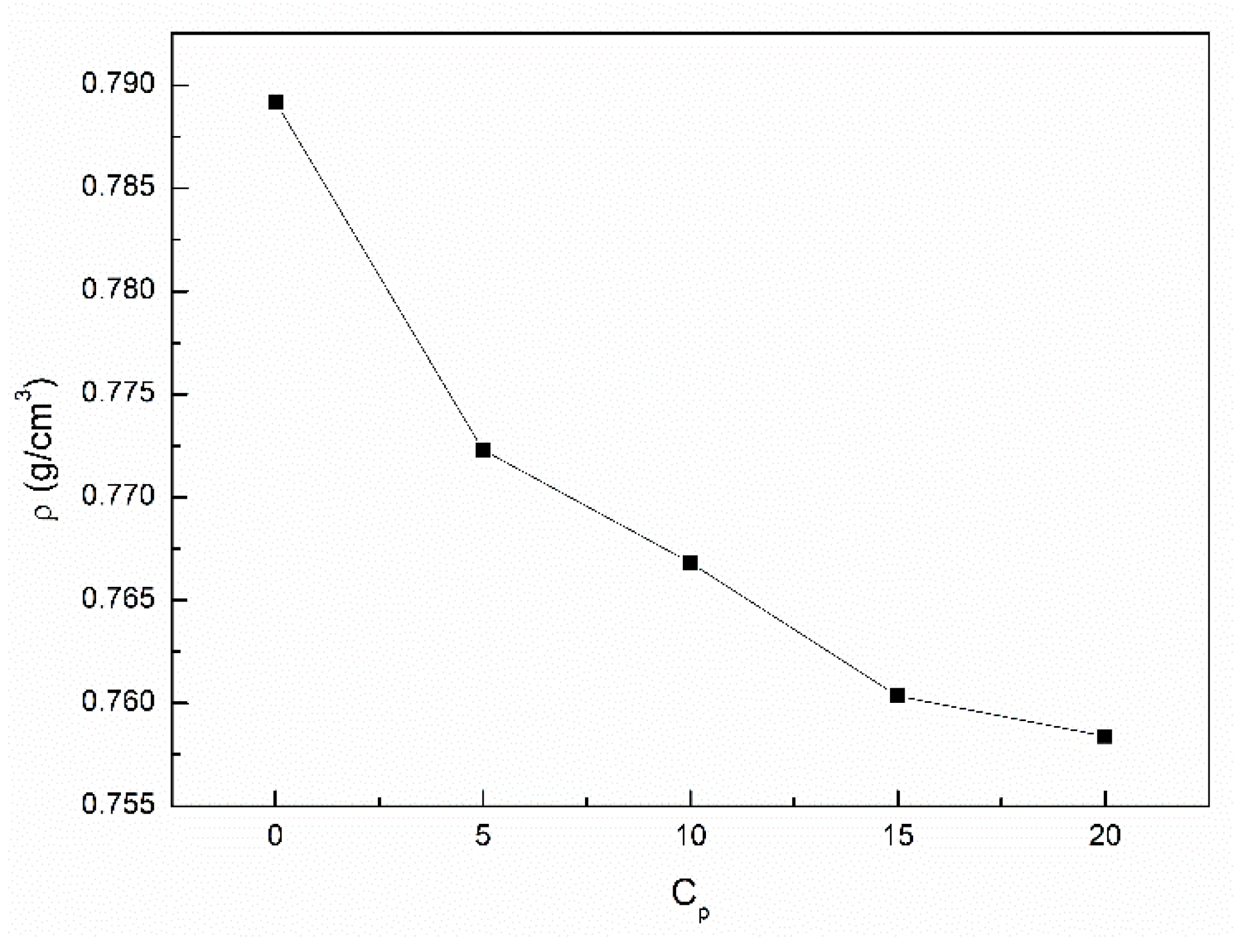
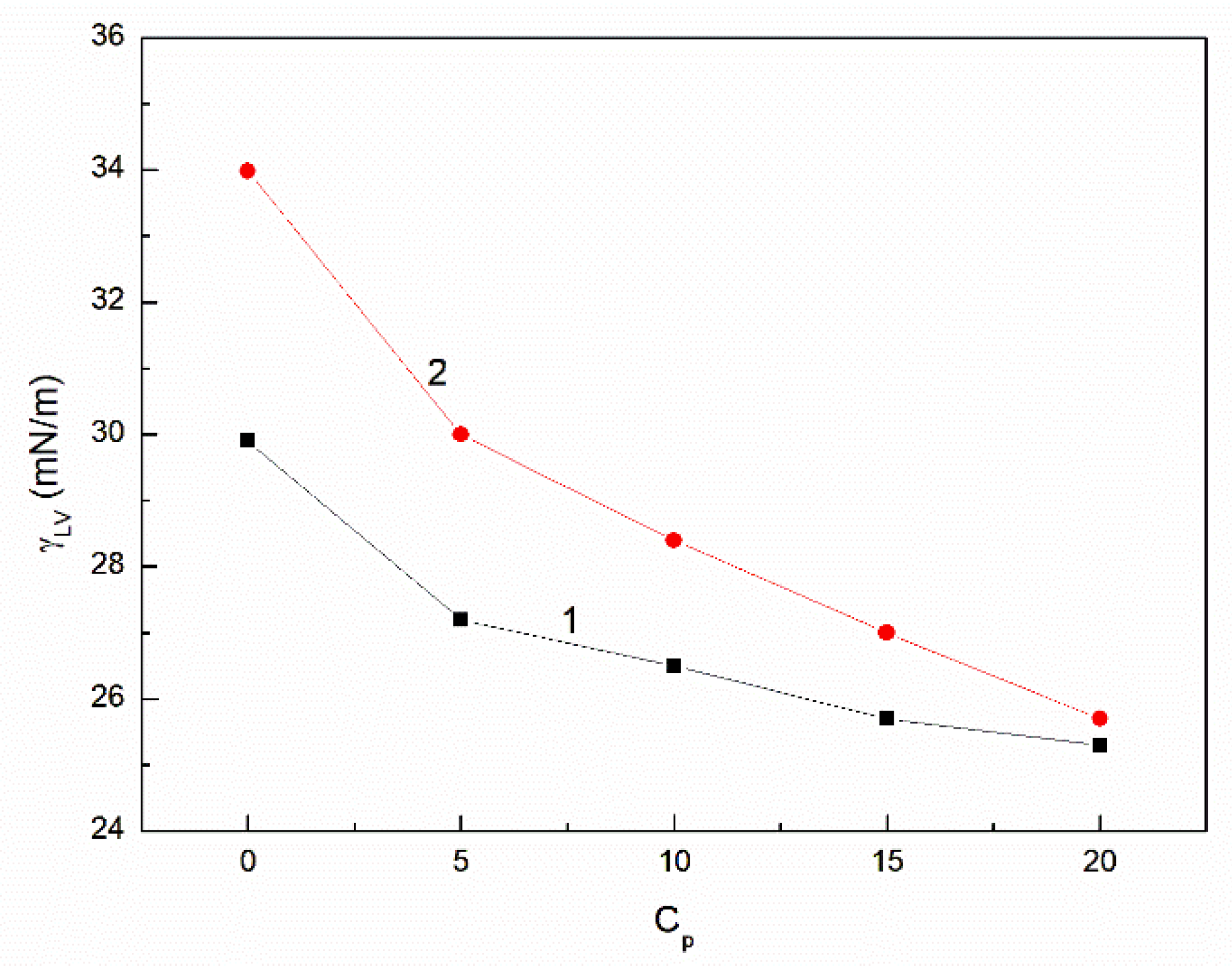
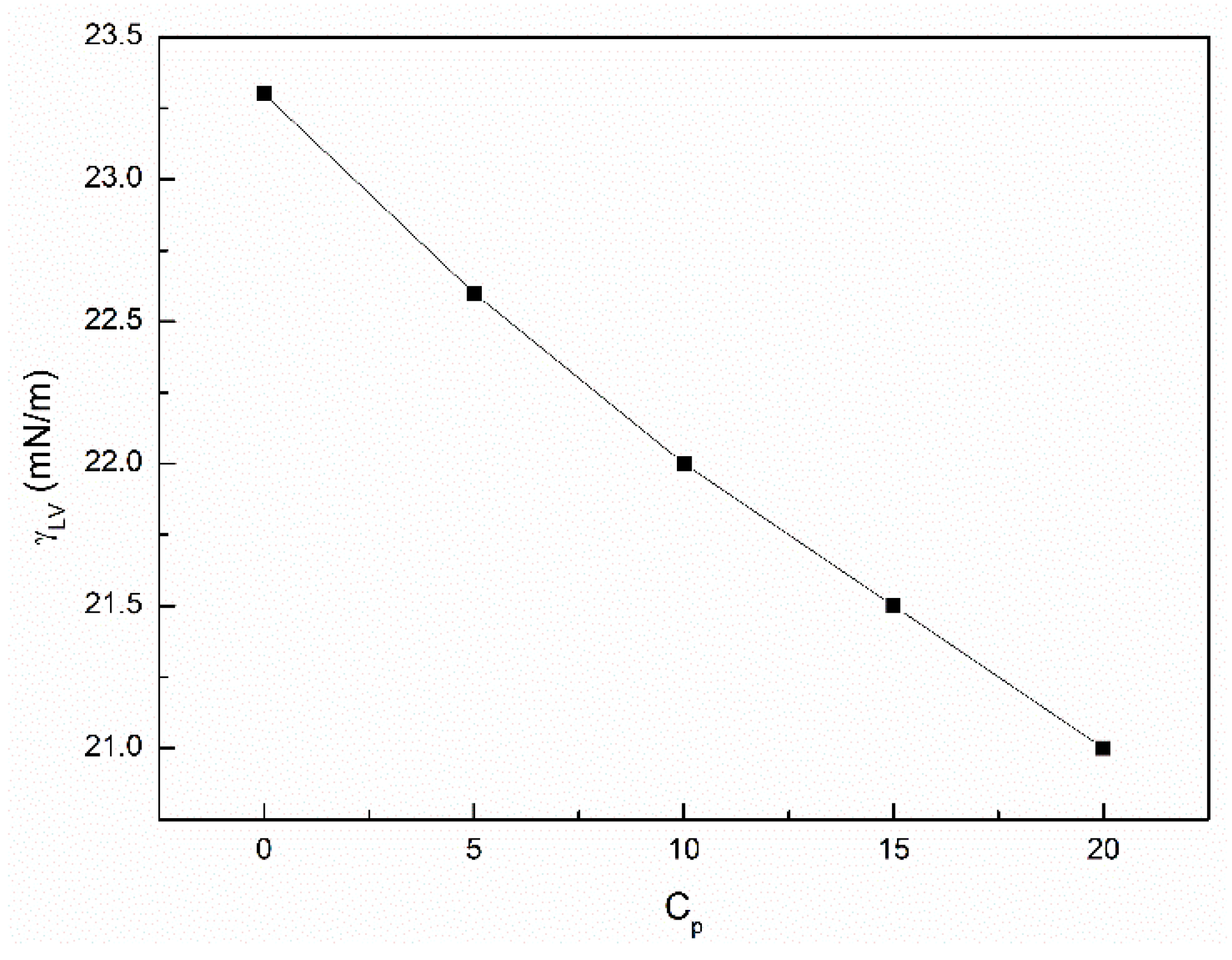
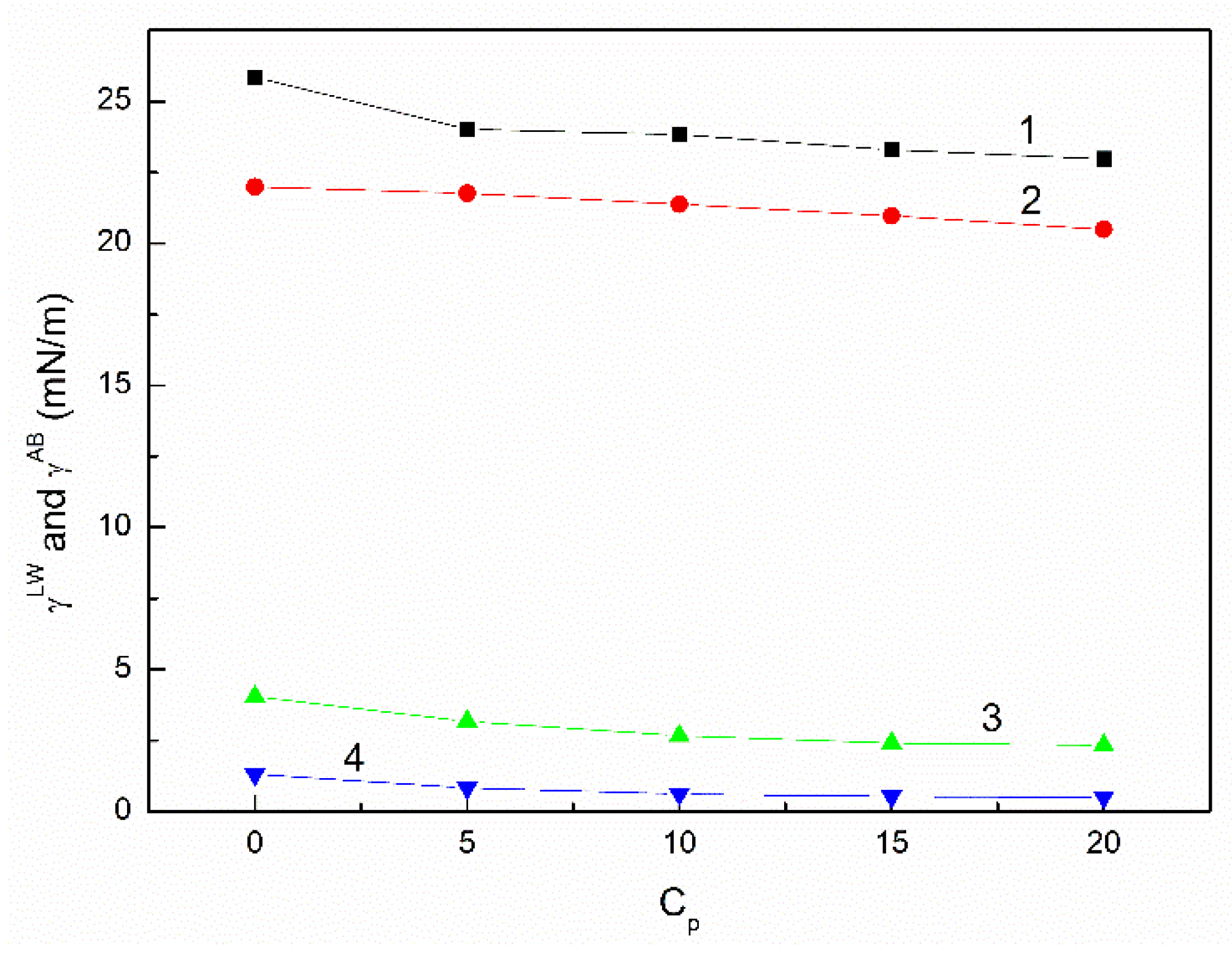
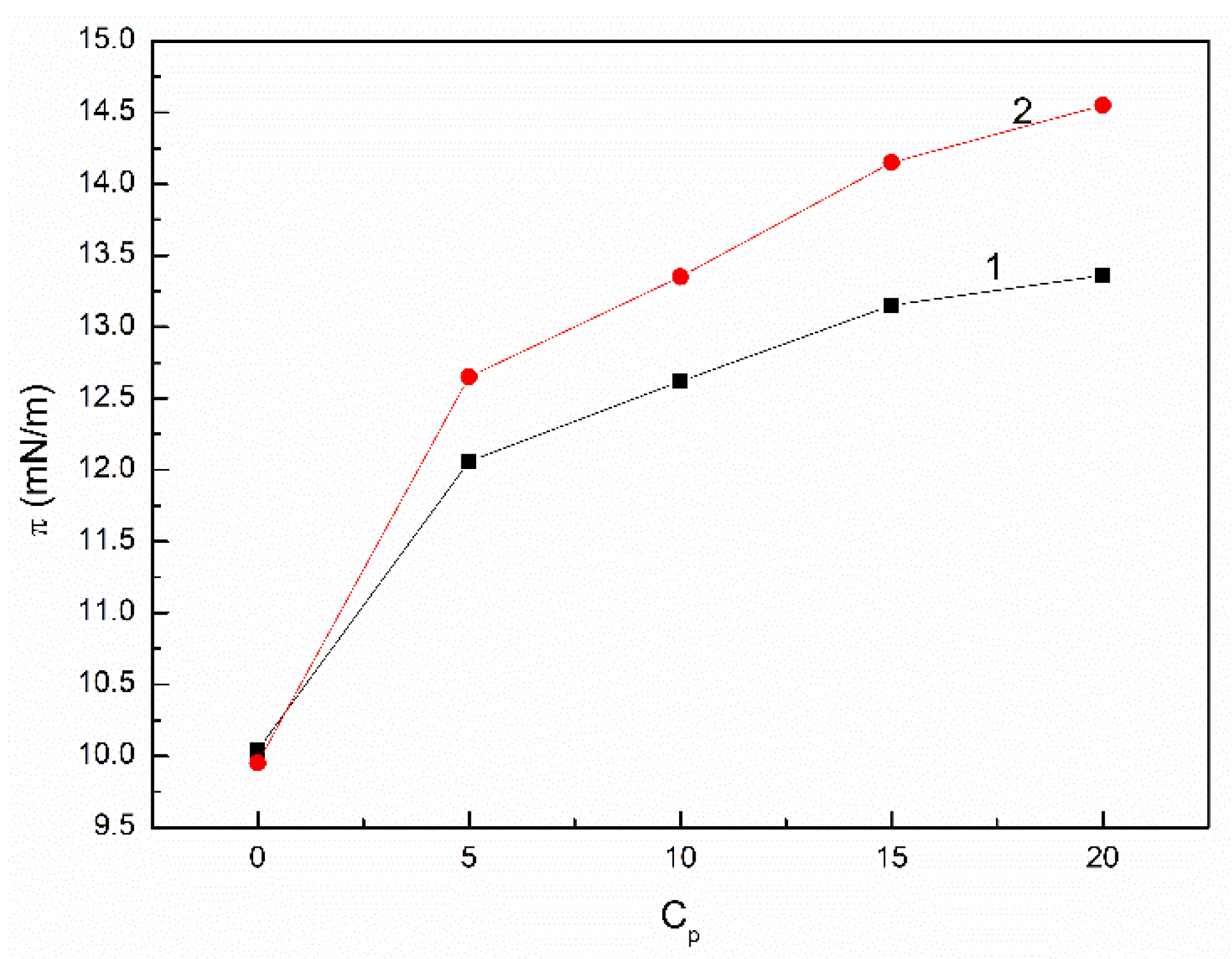
3.2. The Influence of n-Hexane and Ethanol on the Viscosity, Density and Surface Tension of Canola Oil
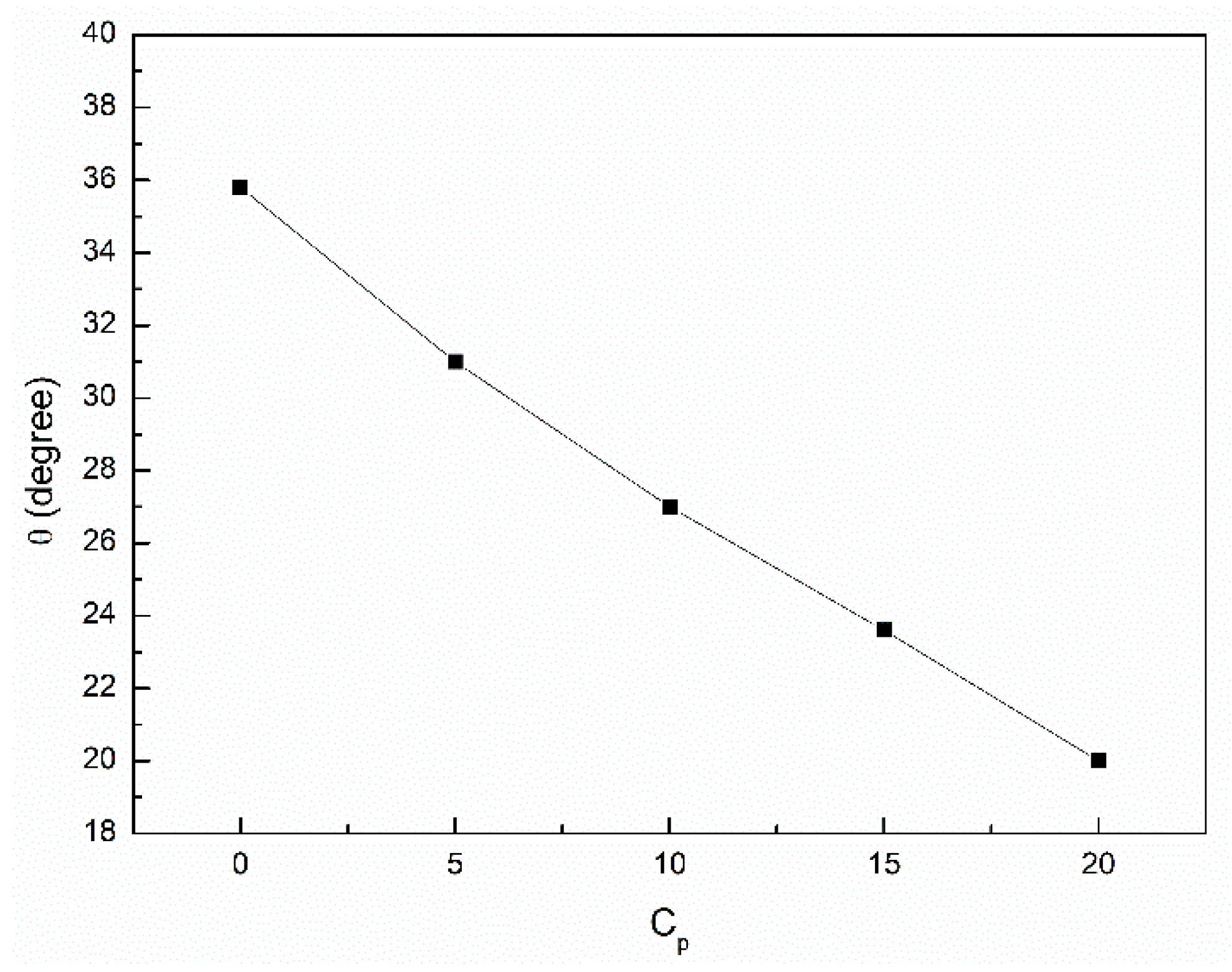
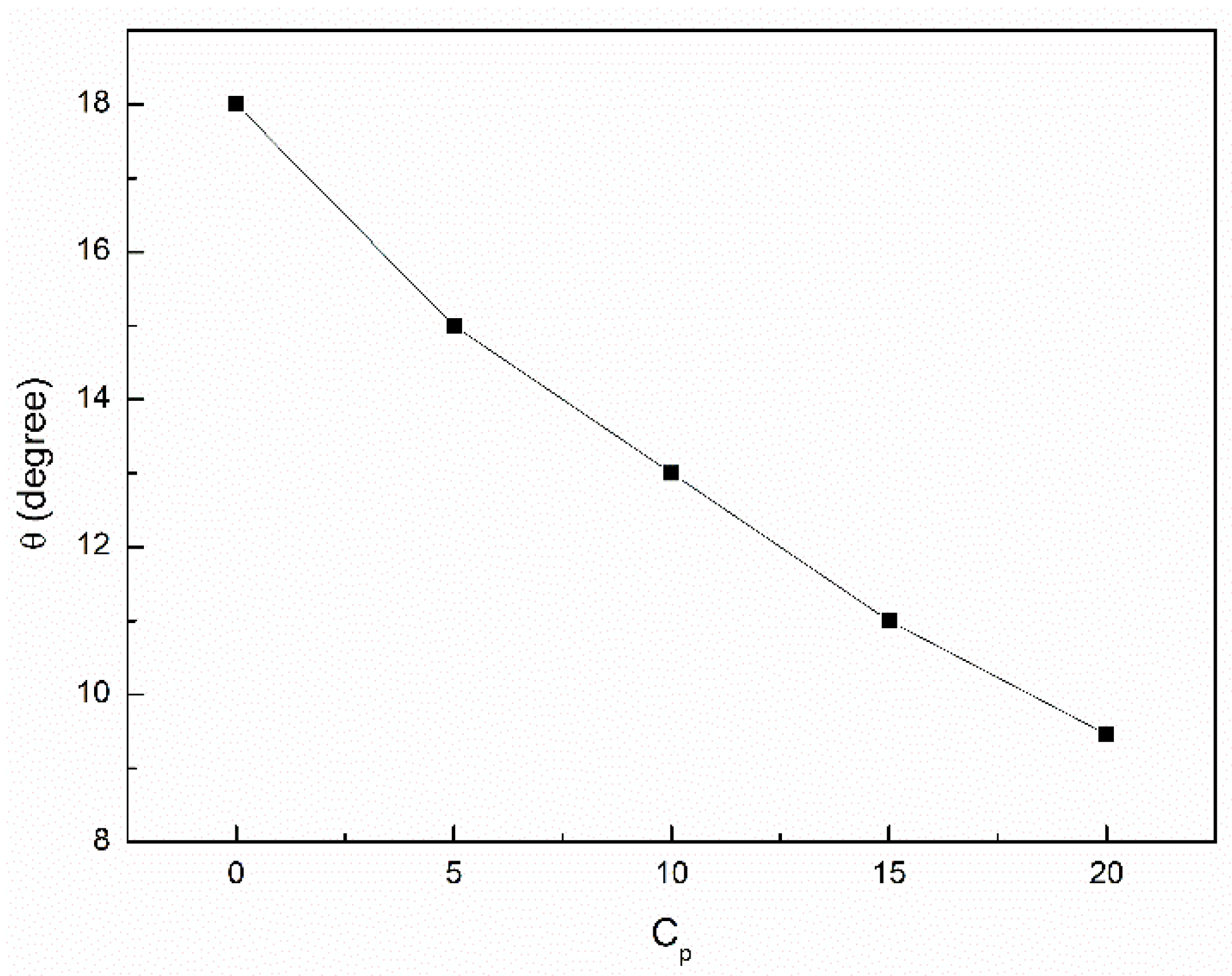
3.3. Wetting Properties of the Canola Oil Mixture with n-Hexane and Ethanol
4. Conclusions
- The surface tension of canola oil is higher than that of unsaturated and saturated fatty acids being its components, which can result from the greater density of particular chemical groups at the liquid-air interface in comparison to the particular components of canola oil.
- The surface tension of canola oil as well as its components results from the Lifshitz-van der Waals, electron-acceptor and electron-donor intermolecular interactions. However, the contribution of the Lifshitz-van der Waals intermolecular interactions to the surface tension is considerably greater than the acid-base one.
- The density and viscosity of canola oil similar to the surface tension are greater than those for the unsaturated and saturated fatty acids being the components of canola oil. This phenomenon can result from the decrease in the volume of canola oil in comparison to the sum of the volume of individual its components resulting from the increasing intermolecular interactions.
- The addition of n-hexane and ethanol to canola oil decreases its surface tension, density and viscosity to the larger extent than it results from the difference of surface tension, density and viscosity of canola oil and n-hexane. This phenomenon indicates that probably the n-hexane molecules increase the distance between the molecules of canola oil components.
- As a result of the ethanol contact with the canola oil mixture with n-hexane, there is a further decrease in the surface tension, density and viscosity of canola oil. This decrease is not proportional to the difference between the surface tension, density and viscosity of canola oil and ethanol. This effect results probably from the changes of the structure of the canola oil phase as well as the concentration of the components of canola oil solution at the solution-air interface in comparison to the bulk phase.
- At the concentration of n-hexane close to 20% volume the viscosity of the canola oil-n-hexane-ethanol mixture is close to that of the diesel fuel.
- The addition of n-hexane and ethanol to canola oil changes its wetting properties in relation to the hydrophobic and hydrophilic solids.
- To sum up it can be stated that the addition of n-hexane and ethanol at a given amount improves the canola oil physical properties regarding its possible application as a fuel in the diesel engine.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Backes, F.; Wachtmeister, G. Application of canola oil operation in a diesel engine with common rail system. Fuel 2015, 159, 141–149. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using canola oil biodiesel as an alternative fuel in diesel engines: A Review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef] [Green Version]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Abdul Adam, A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, B.K. A comprehensive review of biodiesel as an alternative fuel for compression ignition engine. Renew. Sustain. Energy Rev. 2016, 57, 799–821. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Progress in biodisel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Somidi, A.K.R.; Dalai, A.K. Chemical/structural modification of canola oil and canola biodiesel: Kinetic studies and biodegradability of the alkoxides. Lubricants 2017, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.C.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Application of canola oil biodiesel/diesel blends in a common rail diesel engine. Appl. Sci. 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.M.; Wang, W.; Bujold, J. Biodiesel production and comparison of emissions of a DI diesel engine fueled by biodiesel–diesel and canola oil–diesel blends at high idling operations. Appl. Energy 2013, 106, 198–208. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A. Biodiesel Production from Greenseed Canola Oil. Energy Fuels 2010, 24, 4652–4658. [Google Scholar] [CrossRef]
- Qi, D.H.; Bae, C.; Feng, Y.M.; Ji, C.C.; Bian, Y.Z. Preparation, characterization, engine combustion and emission characteristics of rapeseed oil based hybrid fuels. Renew. Energy 2013, 60, 98–106. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Tate, R.E.; Watts, K.C.; Allen, C.A.W.; Wilkie, K.I. The viscosities of three biodiesel fuels at temperatures up to 300 °C. Fuel 2006, 85, 1010–1015. [Google Scholar] [CrossRef]
- Longwic, R.; Sander, P. The course of combustion process under real conditions of work of a traction diesel engine supplied by mixtures of canola oil containing n-hexane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 421, 042050. [Google Scholar] [CrossRef] [Green Version]
- Górski, K.; Sander, P.; Longwic, R. The assessment of ecological parameters of diesel engine supplied with mixtures of canola oil with n-hexane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 421, 042025. [Google Scholar] [CrossRef]
- Longwic, R.; Sander, P.; Jańczuk, B.; Zdziennicka, A.; Szymczyk, K. Modification of canola oil physicochemical properties by hexane and ethanol with regards of its application in diesel engine. Energies 2021, 14, 4469. [Google Scholar] [CrossRef]
- Nadeem, M.; Rangkuti, C.; Anuar, K.; Haq, M.R.U.; Tan, I.B.; Shah, S.S. Diesel engine performance and emission evaluation using emulsified fuels stabilized by conventional and gemini surfactants. Fuel 2006, 85, 2111–2119. [Google Scholar] [CrossRef]
- Rezkia, B.; Essamlalib, Y.; Aadila, M.; Semlalc, N.; Zahouily, M. Biodiesel production from rapeseed oil and low free fatty acid waste cooking oil using a cesium modified natural phosphate catalyst. RSC Adv. 2020, 10, 41065–41077. [Google Scholar] [CrossRef]
- Singh, T.S.; Verma, T.N. Biodiesel production from Momordica charantia (L.): Extraction and engine characteristics. Energy 2019, 189, 116198. [Google Scholar] [CrossRef]
- Singh, T.S.; Verma, T.N. Taguchi design approach for extraction of methyl ester from waste cooking oil using synthesized CaO as heterogeneous catalyst: Response surface methodology optimization. Energy Convers. Manag. 2019, 182, 383–397. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, S.-A. Effects of emulsification variables on fuel properties of two- and three-phase biodiesel emulsions. Fuel 2007, 86, 210–217. [Google Scholar] [CrossRef]
- Ghazani, S.M.; García-Llatas, G.; Marangoni, A.G. Micronutrient content of cold-pressed, hot-pressed, solvent extracted and RBD canola oil: Implications for nutrition and quality. Eur. J. Lipid Sci. Technol. 2014, 116, 380–387. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.M.; Juhaimi, F.A. Some rape/canola seed oils: Fatty acid composition and tocopherols. Z. Nat. C J. Biosci. 2016, 71, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Eskin, N.A.M.; Przybylski, R. Rape Seed Oil/Canola in Encyclopaedia of Food Sciences and Nutrition, 2nd ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Rustan, A.C.; Drevon, C.A. Fatty Acids: Structures and Properties. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2005. [Google Scholar]
- Jańczuk, B.; Zdziennicka, A.; Wójcik, W. Relationship between wetting of Teflon by cetyltrimethylammonium bromide solution and adsorption. Eur. Polym. J. 1997, 33, 1093–1098. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- van Oss, C.J. Interfacial Forces in Aqueous Media, 1st ed.; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- van Oss, C.J.; Costanzo, P.M. Adhesion of anionic surfactants to polymer surfaces and low-energy materials. J. Adhes. Sci. Technol. 1992, 6, 477–487. [Google Scholar] [CrossRef]
- Vogel, A.I. Preparatyka Organiczna, 3rd ed.; WNT: Warszawa, Poland, 2006. [Google Scholar]
- Valeri, D.; Meirelles, A.J.A. Viscosities of fatty acids, triglycerides, and their binary mixtures. J. Am. Oil Chem. Soc. 1997, 74, 1221–1226. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Krawczyk, J.; Szymczyk, K.; Jańczuk, B. Components and parameters of liquids and some polymers surface tension at different temperature. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 864–875. [Google Scholar] [CrossRef]
- Zisman, W.A. Relation of the equilibrium contact angle to liquid and solid constitution. In Contact angle wettability and adhesion; Advances in Chemistry Series; Am Chem: Washington, DC, USA, 1964; Volume 43, pp. 1–51. [Google Scholar]
- Aguilera, A.F.; Rahkila, J.; Hemming, J.; Nurmi, M.; Torres, G.; Razat, T.; Tolvanen, P.; Eranen, K.; Leveneur, S.; Salmi, T. Epoxidation of tall oil catalyzed by an ion exchange resin under conventional heating and microwave irradiation. Ind. Eng. Chem. Res. 2020, 59, 10397–10406. [Google Scholar] [CrossRef]
- Jańczuk, B.; Mendez Sierra, J.A.; Gonzalez-Martın, M.L.; Bruque, J.M.; Wócjik, W. Properties of decylammonium chloride and cesium perfluorooctanoate at interfaces and standard free energy of their adsorption. J. Colloid Interface Sci. 1997, 192, 408–414. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J.; Keeler, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Good, R.J.; Elbing, E. Generalization of theory for estimation of interfacial energies. Ind. Eng. Chem. 1970, 62, 54–78. [Google Scholar] [CrossRef]
- Sahasrabudhe, S.N.; Rodriguez-Martinez, V.; O’Meara, M.; Farkas, B.E. Density, viscosity, and surface tension of five vegetable oils at elevated temperatures: Measurements and modeling. Int. J. Food Prop. 2017, 20, 1965–1981. [Google Scholar] [CrossRef] [Green Version]
- Fasina, O.O.; Colley, Z. Viscosity and specific heat of vegetable oils as a function of temperature: 35° to 185°. Int. J. Food Prop. 2008, 11, 738–746. [Google Scholar] [CrossRef]
- Anand, K.; Ranjan, A.; Mehta, P.S. Estimating the viscosity of vegetable oil and biodiesel fuels. Energy Fuels 2010, 24, 664–672. [Google Scholar] [CrossRef]
- Diamante, L.M.; Lan, T. Absolute viscosities of vegetable oils at different temperatures and shear rate range of 64.5 to 4835 s−1. J. Food Process. 2014, 234583. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.L. Vegetable oil as a diesel fuel: Status and research priorities. Am. Soc. Agric. Eng. 1986, 25, 1413–1422. [Google Scholar] [CrossRef]
- Yalcin, H.; Toker, S.O.; Dogan, M. Vegetable oil type and fatty acid composition on dynamic and steady shear rheology of vegetable oils. J. Oleo Sci. 2012, 61, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Klein, T.; Yan, S.; Cui, J.; Magee, J.W.; Kroenlein, K.; Rausch, M.H.; Koller, T.M.; Fröba, A.P. Liquid viscosity and surface tension of n-hexane, n-octane, n-decane, and n-hexadecane up to 573 K by surface light scattering. J. Chem. Eng. Data 2019, 64, 4116–4131. [Google Scholar] [CrossRef]
- Vázquez, G.; Alvarez, E.; Navaza, J.M. Surface tension of alcohol + water from 20 to 50 °C. J. Chem. Eng. Data 1995, 40, 611–614. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Szymczyk, K.; Jańczuk, B.; Longwic, R.; Sander, P. Adhesion of canola and diesel oils to some parts of diesel engine in the light of surface tension components and parameters of these substrates. Inter. J. Adhes. Adhes. 2015, 60, 23–30. [Google Scholar] [CrossRef]
- Zana, R. Aqueous surfactant–alcohol systems: A review. Adv. Colloid Interface Sci. 1995, 57, 1–64. [Google Scholar] [CrossRef]
- Yano, Y.F. Correlation between surface and bulk structures of alcohol–water mixtures. J. Colloid Interface Sci. 2005, 284, 255–259. [Google Scholar] [CrossRef]
- van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Colloid Interface Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley-Interscience: New York, NY, USA, 2004; pp. 34–178. [Google Scholar]
- Zdziennicka, A.; Jańczuk, B. Modification of adsorption, aggregation and wetting properties of surfactants by short chain alcohols. Adv. Colloid Interface Sci. 2020, 284, 102249. [Google Scholar] [CrossRef] [PubMed]
- Harkins, W.D. Films on liquids. In The Physical Chemistry of Surface Films; Reinhold: New York, NY, USA, 1952; pp. 94–198. [Google Scholar]



| Substance | % | [mN/m] | [mN/m] | [mN/m] | [g/cm3] | [mPa·s] |
|---|---|---|---|---|---|---|
| Oleic acid | 62 | 32.00 | 26.56 | 5.44 | 0.895 | 26.91 |
| Linoleic acid | 20 | 26.09 | 22.15 | 3.94 | 0.9012 | 27.20 |
| Alpha-linolenic acid | 12 | 30.15 | 25.65 | 4.50 | 0.9134 | 27.65 |
| Palmitic acid | 3 | 29.25 | 26.25 | 2.00 | 0.9052 | 7.60 at 70 °C |
| Stearic acid | 2 | 29.01 | 26.51 | 2.50 | 0.842 | 9.81 at 70 °C |
| Trans fat a | 0.5 | 27.51 | 25.00 | 2.51 | 0.8521 | 9.12 at 75 °C |
| Erucic acid | <0.1 | 28.12 | 26.12 | 2.00 | 0.8571 | 8.75 at 70 °C |
| n-heksane | 18.52 | 18.52 | 0.00 | 0.6601 | 0.3102 | |
| Ethanol | 23.25 | 21.45 | 1.80 | 0.7892 | 1.22 | |
| Canola oil | 33.98 | 32.51 | 1.47 | 0.9161 | 70.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longwic, R.; Sander, P.; Zdziennicka, A.; Szymczyk, K.; Jańczuk, B. Changes of Some Physicochemical Properties of Canola Oil by Adding n-Hexane and Ethanol Regarding Its Application as Diesel Fuel. Appl. Sci. 2023, 13, 1108. https://doi.org/10.3390/app13021108
Longwic R, Sander P, Zdziennicka A, Szymczyk K, Jańczuk B. Changes of Some Physicochemical Properties of Canola Oil by Adding n-Hexane and Ethanol Regarding Its Application as Diesel Fuel. Applied Sciences. 2023; 13(2):1108. https://doi.org/10.3390/app13021108
Chicago/Turabian StyleLongwic, Rafał, Przemysław Sander, Anna Zdziennicka, Katarzyna Szymczyk, and Bronisław Jańczuk. 2023. "Changes of Some Physicochemical Properties of Canola Oil by Adding n-Hexane and Ethanol Regarding Its Application as Diesel Fuel" Applied Sciences 13, no. 2: 1108. https://doi.org/10.3390/app13021108






