Abstract
Xanthones are secondary metabolites isolated from the peel of mangosteen showing medicinal potencies. Alpha-mangostin (α-MG) is the most plentiful xanthone, which has been reported to possess anti-inflammatory, anti-oxidant, and anti-bacterial activities. We aimed to investigate the anti-inflammatory effects of xanthones on LPS-treated hDPCs. Cell viability was determined using the WST-1 assay. The mRNA and protein expression profiles of inflammatory mediators were evaluated using quantitative real-time polymerase chain reaction (qPCR) and Western blot analysis. Anti-inflammatory effects were assessed using the Western blot analysis to examine underlying mechanisms. A one-way analysis of variance followed by Tukey’s post hoc test was used to determine statistically significant differences (p < 0.05). The study found no significant differences between the cytotoxic effects in the α-MG-treated groups and controls. The mRNA and protein expression levels of inflammatory markers in the α-MG treated groups decreased. α-MG significantly inhibited LPS-induced phosphorylation of proteins associated with the MAPK and NF-κB pathways. This study suggests that α-MG exerts anti-inflammatory effects by suppressing the MAPK and NF-κB signaling pathways in LPS-treated hDPCs.
1. Introduction
External stimuli, such as microorganisms and chemicals, can trigger inflammation in the human body [1]. Inflammatory responses activate host defense mechanisms, followed by the synthesis of local inflammatory mediators or reactive oxygen species and an increase in vascular permeability and tissue destruction via leukocyte migration [2]. The secretion of the inflammatory mediators, such as tumor necrosis factor (TNF)-α, nitric oxide (NO), interleukin (IL)-1β, IL-6, and IL-10, is the primary response to inflammation [3].
Natural extracts, especially those isolated from tropical plants, have contributed to developing therapeutic drugs against various human diseases [4]. Garcinia mangostana L. (mangosteen) has been used in therapeutics for several hundred years worldwide, especially in Southeast Asia [5]. Many studies have shown that mangosteen extracts contain secondary metabolites, such as xanthones. Xanthones, the major bioactive secondary metabolites, possess anti-inflammatory, anti-oxidant, cytotoxic, anti-histamine, and anti-microbial activities [3,5,6].
Alpha-mangostin (α-MG), the dominant xanthone found in the peel of mangosteen, has been reported to have anti-inflammatory effects due to its ability to decrease the expression of pro-inflammatory mediators. A previous study demonstrated that α-MG prevented lipopolysaccharide (LPS)-induced inflammation by inhibiting nuclear factor kappa B (NF-κB) signaling in RAW 264.7 macrophages in vitro and inhibited carrageenan-induced peritonitis in vivo [7]. Studies also showed that α-MG exerted inhibitory effects on tumor cells by downregulating the mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways [8,9,10]. However, the effect of α-MG on LPS-induced human dental pulp cells (hDPCs) has not been studied.
Several mechanisms have been proposed to explain the anti-inflammatory effects of α-MG: (1) anti-oxidant and radical scavenging activity; (2) modulation of inflammation-related cellular activities; (3) modulation of the activities of pro-inflammatory enzymes; (4) modulation of the production of other pro-inflammatory molecules; and (5) modulation of pro-inflammatory gene expression [11].
This study evaluated the potential of α-MG to control the levels of inflammatory mediators, such as IL-1β, IL-6intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in LPS-treated hDPCs and aimed to investigate the underlying anti-inflammatory mechanisms.
2. Materials and Methods
2.1. Reagents
α-MG (purity ≥ 98%) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St Louis, MO, USA). α-MG was dissolved in DMSO to prepare a 1 mg/mL stock solution and stored at −20 °C. All subsequent dilutions were performed using the cell culture medium.
2.2. Cell Culture
The experimental protocol of this study was approved by the Institutional Review Board of Chonnam National University Dental Hospital (CNUDH-2016-0016). HDPCs were isolated from freshly extracted caries-free supernumerary teeth (n = 10, 6–12 years old). All procedures were performed with the informed consent of each patient. The dental pulp tissues were immediately separated from the teeth under aseptic conditions after tooth extraction and washing with Dulbecco’s phosphate-buffered saline solution (DPBS, Welgene, Daegu, Korea). The pulp tissues were minced and placed in cell culture dishes. We cultured the tissue explants in alpha minimum essential medium (α-MEM (1X), Gibco Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco Invitrogen) and 1% antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin, Gibco Invitrogen) in a humidified atmosphere of 5% CO₂ at 37 °C. We used the cells between passage numbers three to six.
2.3. Cytotoxicity Assay
Cells were cultured in 96-well plates at a density of 1 × 104 cells per well and treated with various concentrations (1.5, 3, and 6 μg/mL) of α-MG for 24 h. Cell viability was analyzed using the WST-1 assay (EZ-Cytox assay kit, Daeil Lab Service, Seoul, Republic of Korea) according to the manufacturer’s recommendations. Briefly, 10 μL Ez-Cytox reagent was added to the medium. After cells were incubated at 37 °C for 3 h, absorbance was measured at 450 nm using a spectrophotometer (Thermo Scientific, Waltham, MA, USA). The relative cell viability was calculated as a percentage of the mean of the control.
2.4. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Cells were cultured in 6-well plates at a density of 2 × 105 cells per well and pre-incubated in α-MEM containing 10% FBS and antibiotics for 24 h. The LPS from Escherichia coli was used to induce the inflammation. Cells were pretreated with 1.5 and 3 μg/mL of α-MG for 1 h before stimulation with 1 μg/mL LPS for 24 h. Total RNA from each group was extracted using the TRIzol reagent (Gibco Invitrogen). Complementary DNA (cDNA) was synthesized using an AccessQuickTM RT-PCR system (Promega, Madison, WI, USA) and qPCR was performed using the QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA, USA) in triplicates, and Rotor-Gene 6000 (Corbett Research, Sydney, Australia). All quantified values were normalized to the endogenous control β-actin. Relative gene expression values were analyzed using the ΔΔCt method (as described previously) [12]. The primer sequences are detailed in Table 1.

Table 1.
Primer sequences used for real-time PCR.
2.5. Western Blot Analysis
Protein expression levels of inflammatory markers and activation of MAPK or NF-κB pathway were analyzed by Western blotting. hDPCs were cultured at a density of 3 × 10⁵ cells per dish on 60-mm cell culture plates and pretreated with 1.5 and 3 μg/mL α-MG for 1 h before stimulation with 1 μg/mL LPS for 24 h. Cells were washed twice with DPBS. After cell treatment, cells were lysed in protein lysis buffer (Cell Signaling Technology, Beverly, MA, USA). Protein concentrations were determined using the Lowry protein assay reagent kit (Bio-Rad Laboratories, Hercules, CA, USA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis were performed using polyvinylidene difluoride membranes. After blocking with blocking solution (5% nonfat dried skimmed milk, 0.01 mol/L DPBS, and 0.1% Tween 20 (PBST) [Biosesang, Sungnam, Republic of Korea]) at room temperature for 1 h, membranes were incubated with the following primary antibodies: anti-IL-1β (Thermo Fisher Scientific, Rockford, IL, USA), anti-IL-6 (Abcam, Cambridge, MA, UK), anti-ICAM (Abcam), anti-VCAM (Thermo Fisher Scientific), anti-ERK, anti-phospho-ERK, anti-p38, anti-phospho-p38, anti-JNK, anti-phospho-JNK, anti-AKT, and anti-phospho-Akt (Cell Signaling Technology) at 4 °C overnight. After washing membranes with PBST, membranes were incubated with secondary antibodies (horseradish peroxidase-conjugated anti-rabbit IgG, Sigma-Aldrich) at room temperature for 1 h. After washing with DPBS, a chemiluminescent HRP substrate (Millipore, Billerica, MA, USA) was used for detection, and protein signals were visualized with a chemiluminescence imaging system (Ez-capture; Atto, Tokyo, Japan).
2.6. Alkaline Phosphatase Staining
Cells were cultured at a density of 2 × 104 cells per well on 48-well plates and treated with 1 μg/mL LPS for 1 h. Next, the cells were incubated in an induction medium, with or without 1.5 and 3 μg/mL α-MG for 7 days. Fresh medium containing LPS (with or without α-MG) was replaced every 2 days. After 7 days of exposure, cells were washed with DPBS, fixed with 70% ethanol for 30 min, and rinsed with distilled water. Fixed cells were treated with 300 µL staining reagent per well (BCIP®/NBT Liquid substrate System; Sigma-Aldrich). Samples were photographed using a scanner (Epson, Seoul, Republic of Korea). For quantitative evaluation, the stains were treated with 10% (w/v) cetylpyridinium chloride (pH = 7.0) for 30 min. Absorbance was measured at a wavelength of 562 nm using the spectrophotometer.
2.7. Statistical Analysis
Each experiment was performed at least twice and consisted of triplicate independent tests. A one-way analysis of variance was performed, and Tukey’s post hoc test was used to determine any statistically significant differences. The SPSS 18.0 software program was used for analysis (SPSS, Chicago, IL, USA). Differences were considered significant at p < 0.05.
3. Results
3.1. Effects of LPS and α-MG on Cell Viability
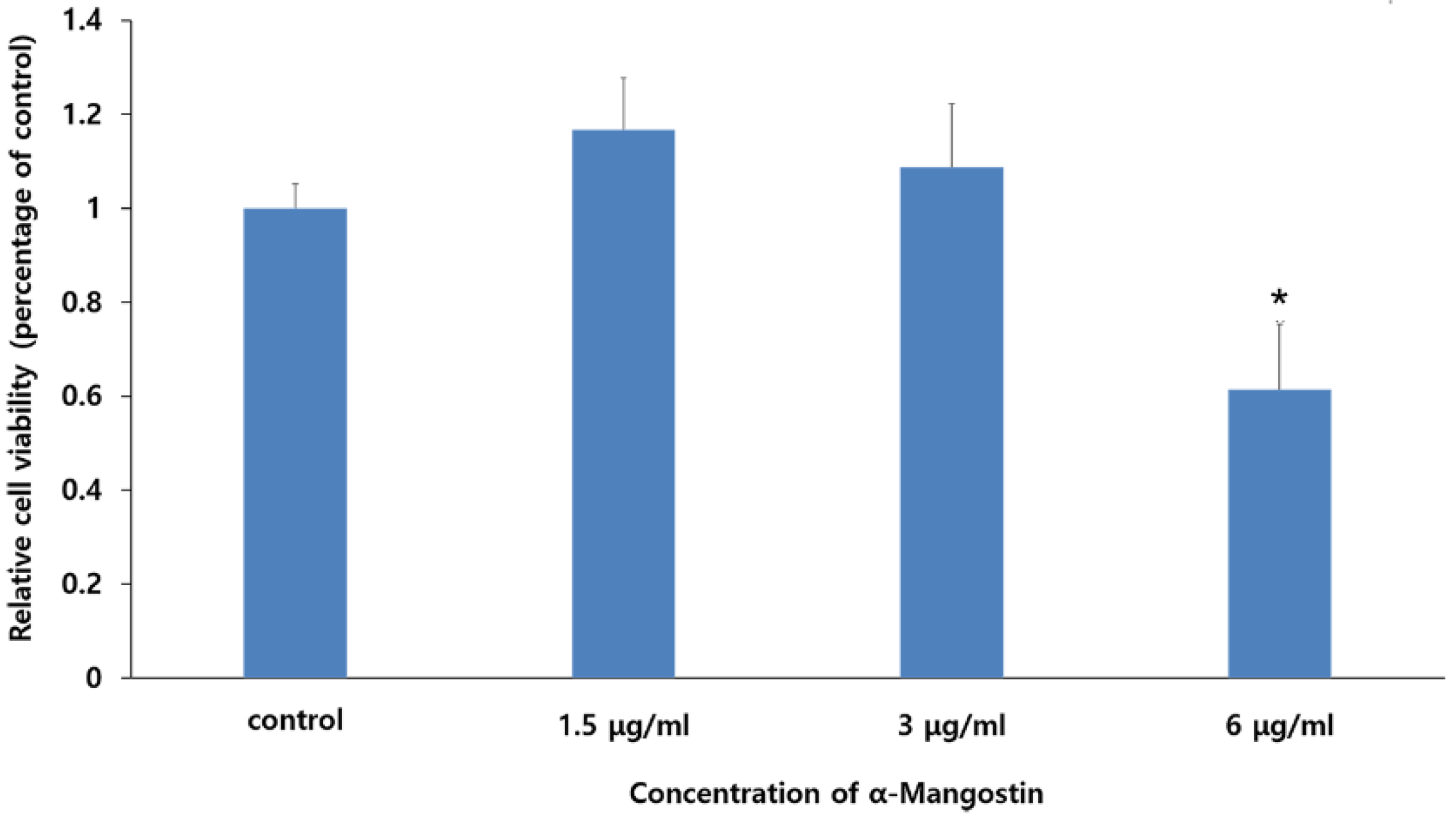
The WST-1 assay was performed to evaluate the cytotoxic effects of various concentrations of LPS and α-MG on hDPCs (Figure 1). We observed that treatment with 1 μg/mL LPS did not affect cell viability. Additionally, no cytotoxicity was observed for 0 to 3 μg/mL α-MG. However, α-MG concentrations of 6 μg/mL or more led to significant cell damage (p < 0.05). Consequently, α-MG concentrations of 1.5 and 3 μg/mL were applied for all subsequent experiments.
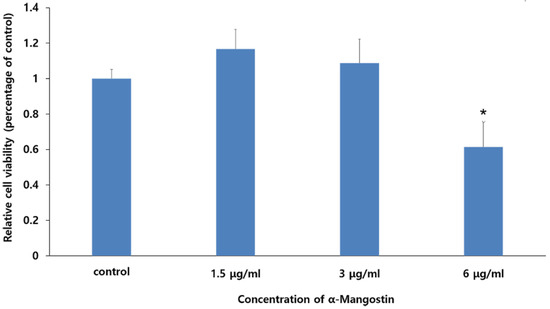
Figure 1.
The effect of α-MG on the viability of hDPCs. Results are expressed as relative cell viabilities depending on the different concentrations of α-MG. Cell viability significantly decreased in the 6 μg/mL α-MG treated group (* p < 0.05 compared to the control group).
3.2. Effects of α-MG on LPS-Induced mRNA and Protein Expression of Inflammatory Mediators in hDPCs
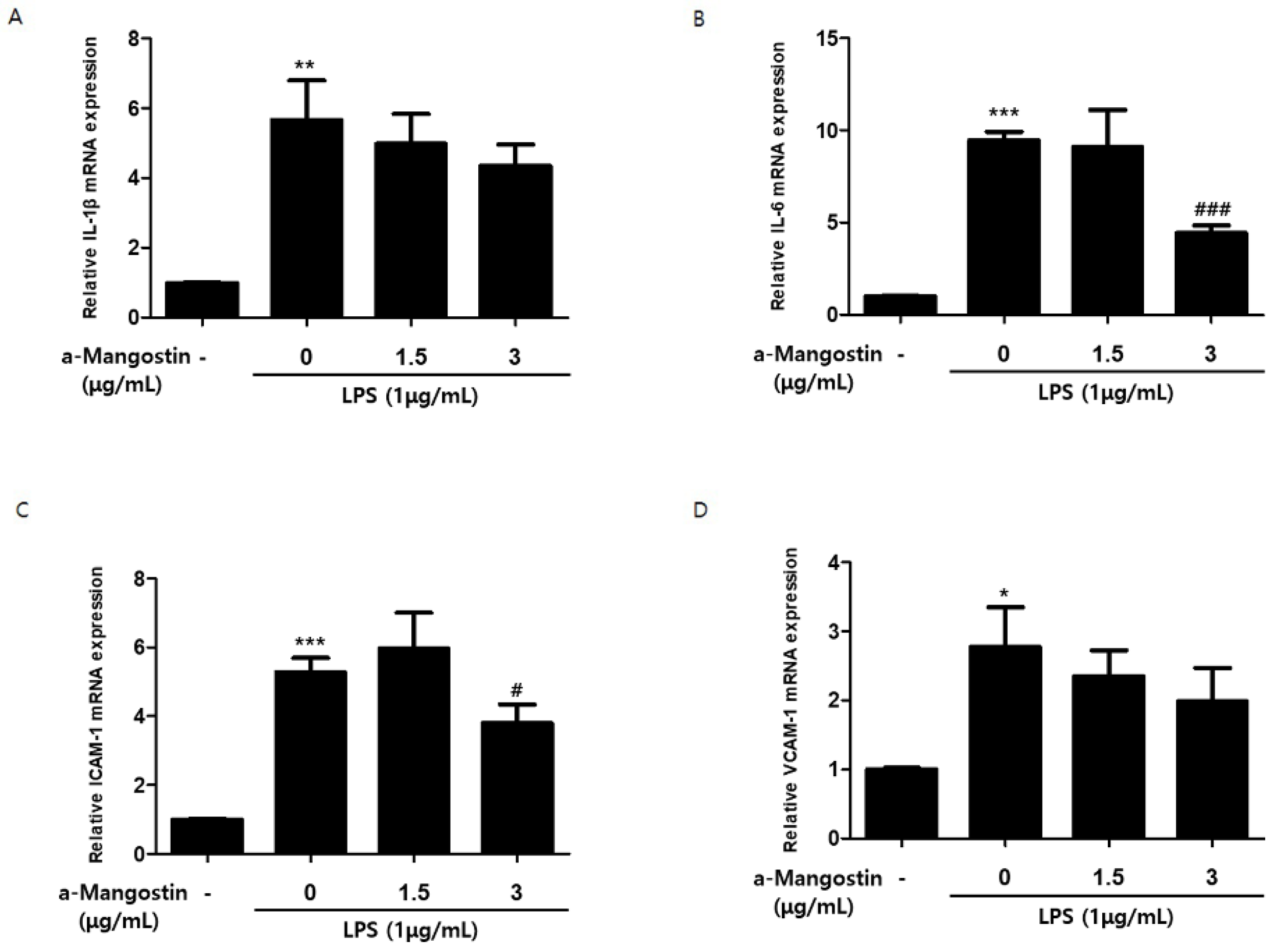
The mRNA expression of inflammatory mediators was measured by qPCR to evaluate the effects of α-MG on the inflammatory response. As shown in Figure 2, the mRNA expression of all inflammatory mediators increased significantly upon stimulation with 1 μg/mL LPS. However, pretreatment with 3 μg/mL α-MG resulted in significant suppression of the increase in IL-6 and ICAM-1 (p < 0.05) (Figure 2B,C). α-MG pretreatment also attenuated IL-1β and VCAM-1 in a concentration-dependent manner, but there were no significant differences (p > 0.05) (Figure 2A,D).
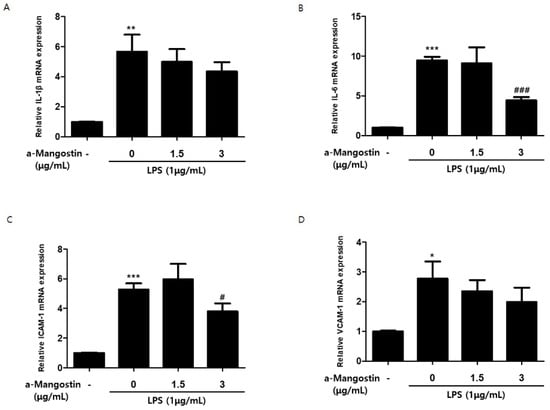
Figure 2.
The effect of α-MG on the mRNA expression of LPS-induced inflammatory mediators analyzed by qPCR. Relative mRNA expression levels of (A) IL-1β, (B) IL-6, (C) ICAM-1, and (D) VCAM-1 were normalized by β-actin (* p < 0.05, ** p < 0.01 and *** p < 0.001 compared to the control group, # p < 0.05 and ### p < 0.001 compared to the LPS-only treated group).
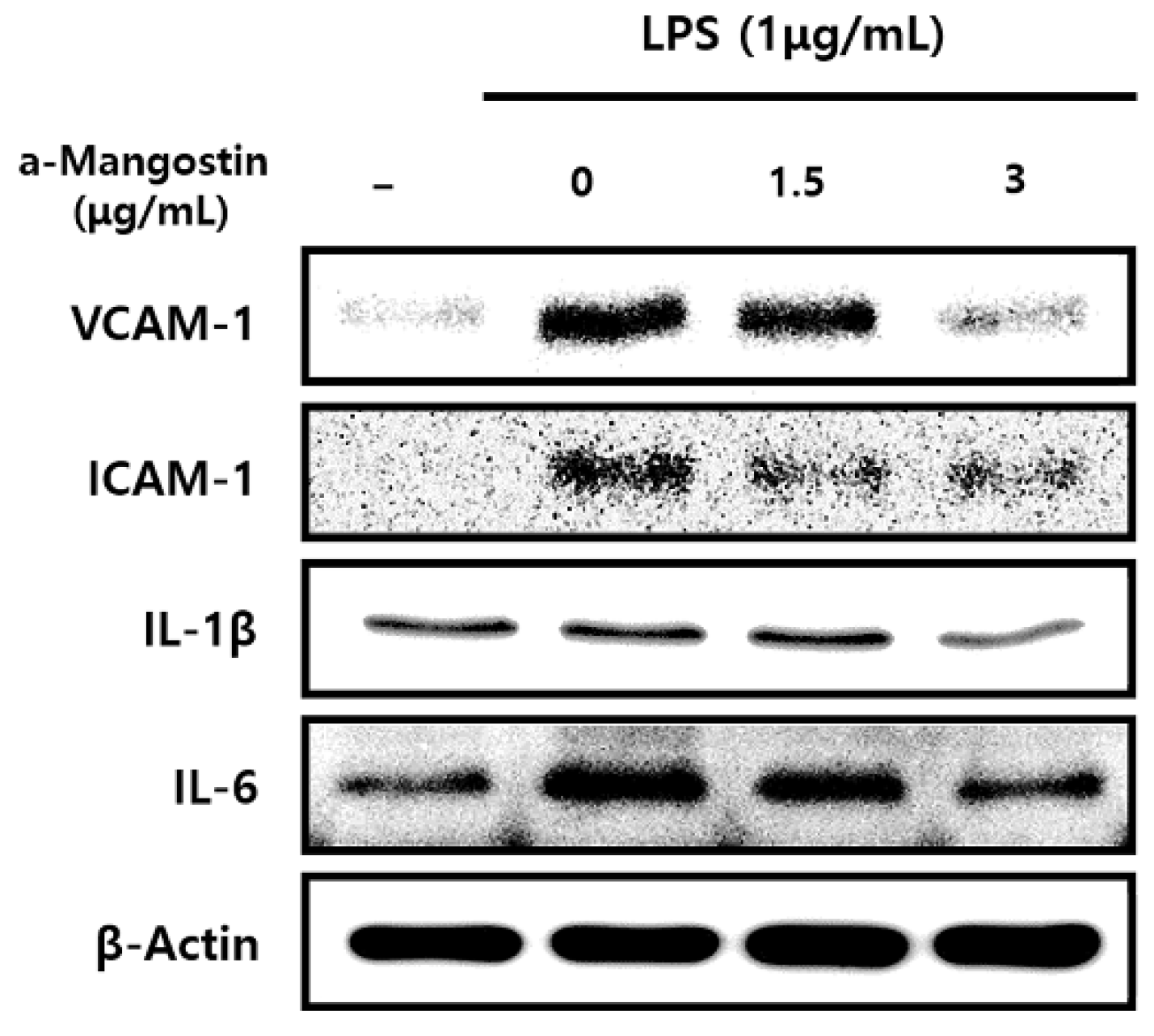
Consistent with the results of mRNA expression, the protein expression levels of inflammatory mediators significantly increased by LPS; however, α-MG pretreatment attenuated this increase in a dose-dependent manner (p < 0.05) (Figure 3).
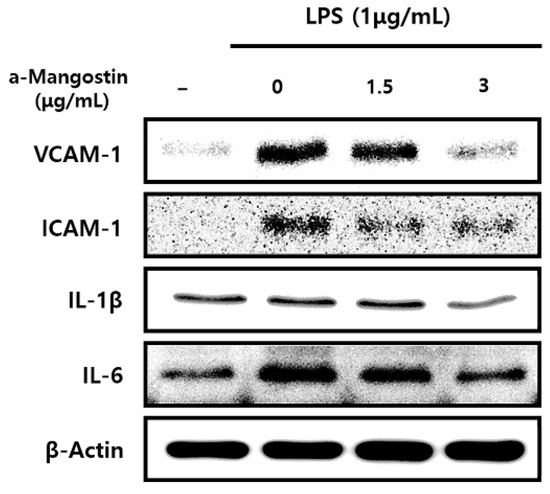
Figure 3.
The effect of α-MG on protein expressions of inflammatory mediators after LPS treatment using Western blot analysis. The expression of IL-1β, IL-6, ICAM-1, and VCAM-1 proteins markedly decreased after pretreatment with 3 μg/mL α-MG.
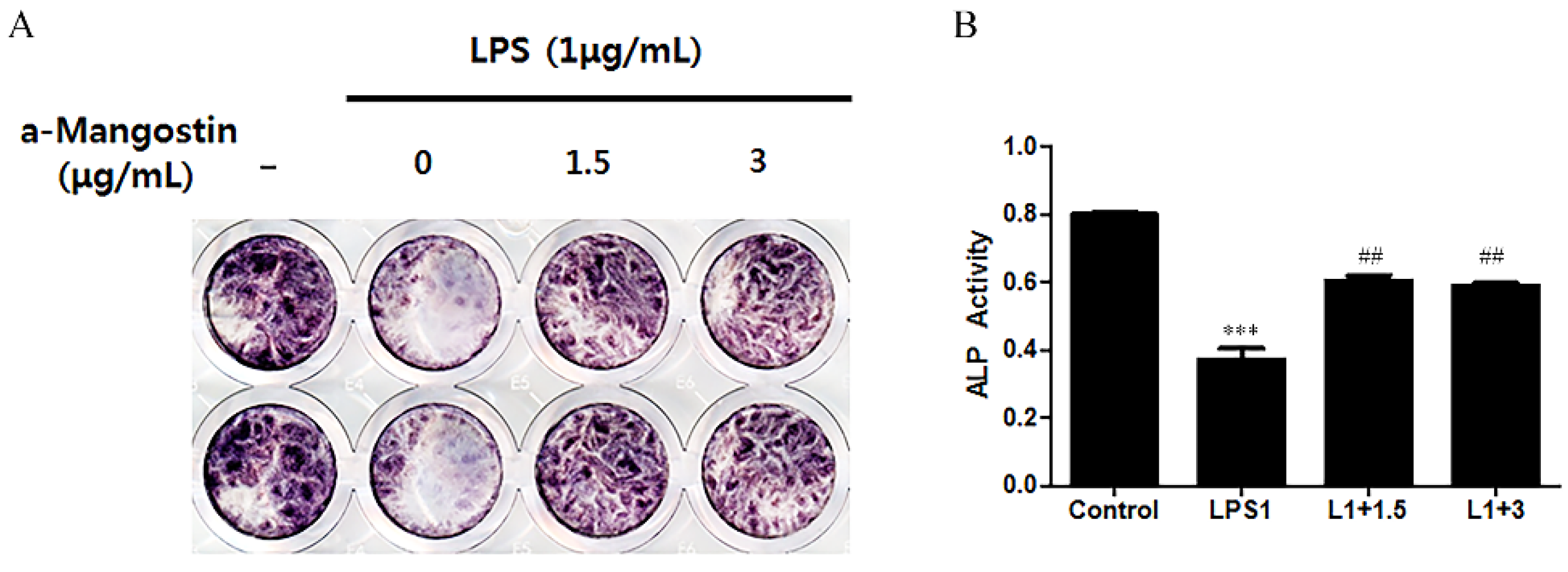
3.3. Effects of α-MG on ALP Activity
ALP activity was examined by ALP staining (Figure 4) to ascertain if α-MG could affect the odontoblastic differentiation of hDPCs. LPS significantly downregulated ALP activity compared to the non-LPS treated control (p < 0.05). This result was reversed when the cells were treated with either 1.5 or 3 μg/mL α-MG. The α-MG treated cells showed higher ALP activity than the LPS-only treated group (p < 0.05).
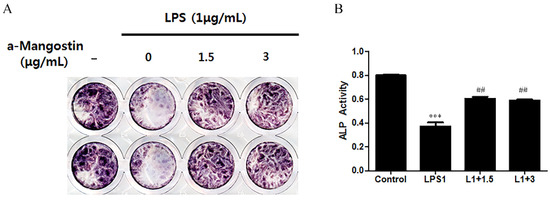
Figure 4.
The effect of α-MG on the expression of ALP using ALP staining. (A) Image of ALP staining and (B) Quantification of ALP staining. α-MG increased the ALP activity at concentrations of 1.5 and 3 μg/mL compared to the LPS-only treated group (*** p < 0.001 compared to the control group, ## p < 0.01 compared to the LPS-only treated group).
3.4. Effects of α-MG on MAPK and NF-κB Signaling Pathways in hDPCs
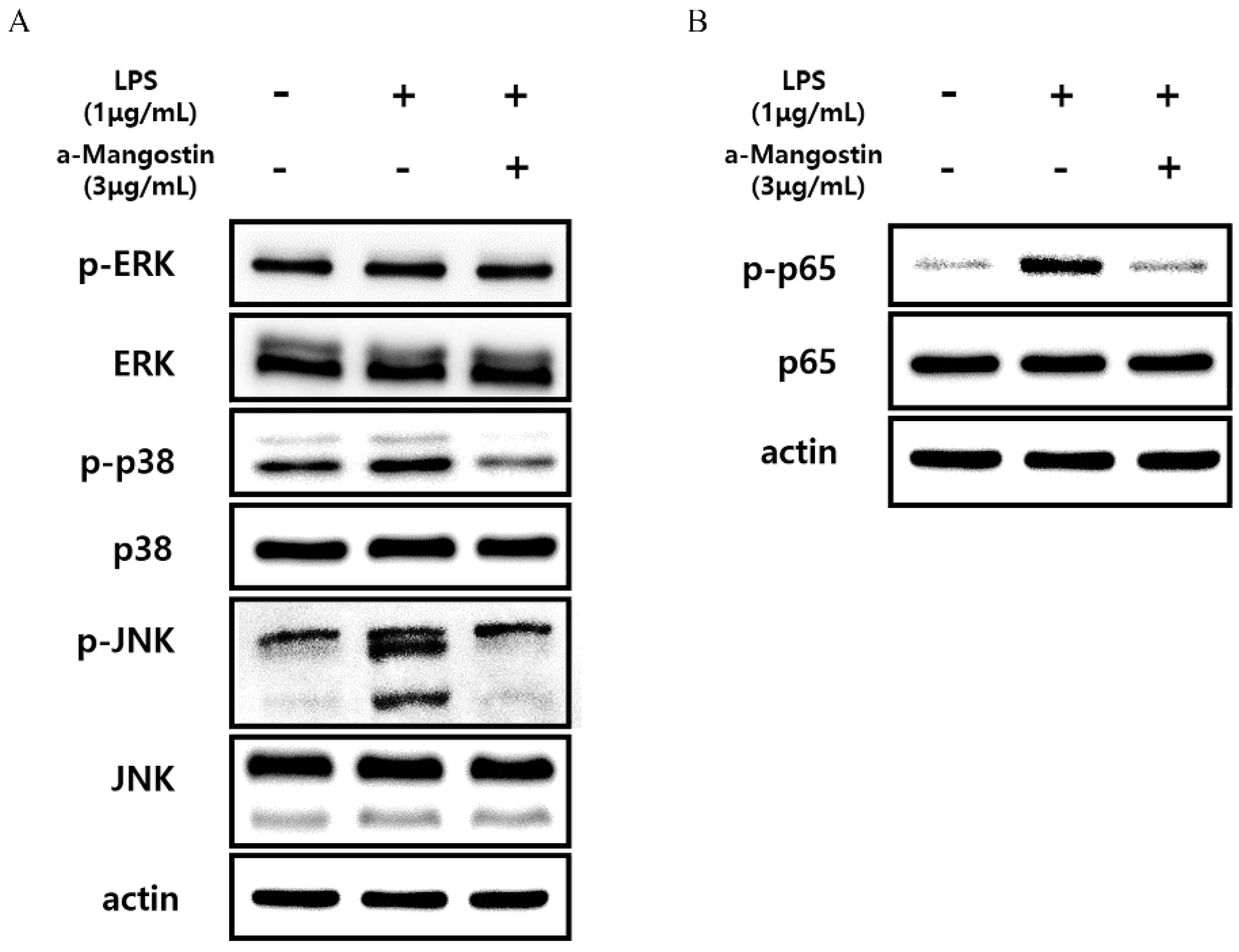
Western blot analysis was performed, and phosphorylation of proteins related to the MAPK and NF-kB signaling pathways was examined to elucidate the molecular mechanisms by which α-MG inhibits LPS-induced inflammatory responses in hDPCs. The expression levels of phosphorylated ERK, JNK, and p38 increased in the LPS-treated group compared to untreated controls. However, treatment with 3 μg/mL α-MG inhibited the increased p-ERK, p-JNK, and p-p38 levels in LPS-treated cells (Figure 5A). Furthermore, the upregulation of phosphorylated p65, induced by LPS, was notably suppressed after treatment with 3 μg/mL α-MG (Figure 5B). These results suggest that α-MG may affect the MAPK and NF-kB signaling pathways by suppressing the phosphorylation of ERK, JNK, p38, and p65.
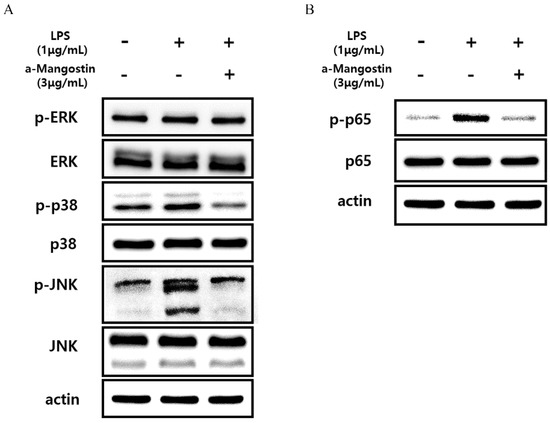
Figure 5.
Western blot analysis monitoring the effect of α-MG on the activation of the MAPK and NF-kB signaling pathways in LPS-treated cells. The expression levels of (A) p-ERK, p-p38, p-JNK, and (B) p-p65 were evaluated.
4. Discussion
α-MG is known to possess diverse biological properties. Many studies have revealed the inhibitory effects of α-MG on inflammation using human cell lines, such as adipocytes [13], macrophage-like U397 cells [14,15], and macrophage-like THP-1 cells [16]. To the best of our knowledge, there are no studies investigating the inhibitory effects of α-MG on LPS-treated hDPCs. Therefore, we hypothesized that α-MG could have similar anti-inflammatory effects on hDPCs.
We also examined the cytotoxic effects of LPS and α-MG on hDPCs. The results showed that non-cytotoxic concentrations of LPS increased mRNA and protein expressions of IL-1β and IL-6. However, pretreating cells with non-cytotoxic concentrations of α-MG (especially with 3 μg/mL) caused a decrease in the upregulation of mRNA and protein expression in hDPCs stimulated with LPS.
Consistent with our results, a previous study showed that α-MG suppressed LPS-induced IL-6 gene expression in macrophage-like cells [13] and adipocytes [17]. Likewise, α-MG also blocked the activation of LPS-induced RAW 264.7 cells and inhibited the secretions of inflammatory mediators, such as NO, COX-2, IL-1β andIL-6 [18].
Meanwhile, cell adhesion molecules involved in cell–cell or cell–extracellular matrix binding have been associated with immune cell activation [19], leukocyte migration [20], and cellular mechanisms of growth or apoptosis. Of the CAMs, ICAM-1 and VCAM-1 attract mononuclear cells and neutrophils [21] to inflamed sites to induce inflammatory responses. This study also examined whether α-MG suppressed the expressions of ICAM-1 and VCAM-1 in hDPCs after LPS treatment. The results of the present study show that LPS stimulation led to an increase in the mRNA and protein expression of ICAM-1 and VCAM-1. Furthermore, pretreatment with α-MG (especially with 3 μg/mL α-MG) prevented this upregulation in ICAM-1 and VCAM-1 expression. These results indicate that α-MG could attenuate the gene expression and secretion of inflammatory mediators, such as IL-1β, IL-6, ICAM-1, and VCAM-1 in hDPCs, and the most effective and non-cytotoxic concentration was 3 μg/mL.
Previous studies reported that α-MG inhibited LPS-induced inflammatory responses via several intracellular signaling pathways, such as the MAPK [13,14,15] and NF-κB [22,23] pathways. This study found that treating LPS-stimulated hDPCs with 3 μg/mL α-MG inhibited the phosphorylation of MAPK-related proteins, such as ERK, JNK, and p38, implying that α-MG could exert its anti-inflammatory effects by suppressing the MAPK pathway. Moreover, there was a decrease in the phosphorylated p65 levels after α-MG treatment, indicating that α-MG could also partially influence the NF-κB pathway. Further studies are needed to clarify underlying molecular mechanisms.
The results from the present study also highlight the positive effects of α-MG on ALP activity. Increased ALP levels are relevant to the odontoblastic differentiation of hDPCs [24]. Consequently, α-MG may affect the differentiating hDPCs and possibly promote mineralization. Follow-up experiments and in vivo studies are needed to support the use of α-MG for treating inflammatory conditions in the human dental pulp.
5. Conclusions
In conclusion, α-MG attenuates the LPS-induced release of proinflammatory factors, such as IL-1β, IL-6, ICAM-1, and VCAM-1 in hDPCs by suppressing the activation of MAPK completely and NF-kB signaling pathways partially. Therefore, α-MG could be considered a therapeutic method for vital pulp therapy and regenerative endodontics.
Author Contributions
Conceptualization, B.-N.L. and W.-M.O.; methodology, Y.-S.K. and B.-N.L.; software, J.-T.K. and J.-H.J.; validation, Y.-S.K., Y.-C.H. and B.-N.L.; formal analysis, J.-T.K., B.-N.L. and J.-H.J.; investigation, W.-M.O., Y.-C.H. and B.-N.L.; resources, Y.-S.K.; data curation, J.-H.J., J.-T.K. and Y.-C.H.; writing—original draft preparation, Y.-S.K. and B.-N.L.; writing—review and editing, B.-N.L. and W.-M.O.; visualization, Y.-C.H. and B.-N.L.; supervision, B.-N.L. and W.-M.O.; project administration, Y.-C.H., W.-M.O. and B.-N.L.; funding acquisition, J.-T.K., Y.-C.H. and B.-N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (Nos. 2019R1A5A2027521, 2022R1C1C1007851, 2022R1A2C1010249 and 2022R1A4A1029312).
Institutional Review Board Statement
The experimental protocol of this study was approved by the Institutional Review Board of Chonnam National University Dental Hospital (CNUDH-2016-0016).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maślińska, D.; Gajewski, M. Some aspects of the inflammatory process. Folia Neuropathol. 1998, 36, 199–204. [Google Scholar] [PubMed]
- Shingala, Z.; Chauhan, B.; Baraiya, J. A review on medicinal plants as a source of anti-inflammatory agents. J. Pharmacogn. Phytochem. 2021, 10, 364–371. [Google Scholar] [CrossRef]
- Lam, D.; Harris, D.; Qin, Z. Inflammatory mediator profiling reveals immune properties of chemotactic gradients and macrophage mediator production inhibition during thioglycollate elicited peritoneal inflammation. Mediat. Inflamm. 2013, 2013, 931562. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Boyd, M.R.; Khanna, R.; Newman, D.J.; Sausville, E.A. Natural product drug discovery and development. In Phytochemicals in Human Health Protection, Nutrition, and Plant Defense; Springer: Boston, MA, USA, 1999; pp. 1–29. [Google Scholar]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J.; Snader, K.M. Natural products in drug discovery and development. J. Nat. Prod. 1997, 60, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Syam, S.; Bustamam, A.; Abdullah, R.; Sukari, M.A.; Hashim, N.M.; Mohan, S.; Looi, C.Y.; Wong, W.F.; Yahayu, M.A.; Abdelwahab, S.I. β Mangostin suppress LPS-induced inflammatory response in RAW 264.7 macrophages in vitro and carrageenan-induced peritonitis in vivo. J. Ethnopharmacol. 2014, 153, 435–445. [Google Scholar] [CrossRef]
- Hung, S.-H.; Shen, K.-H.; Wu, C.-H.; Liu, C.-L.; Shih, Y.-W. α-Mangostin suppresses PC-3 human prostate carcinoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen expression through the JNK signaling pathway. J. Agric. Food Chem. 2009, 57, 1291–1298. [Google Scholar] [CrossRef]
- Hsieh, S.-C.; Huang, M.-H.; Cheng, C.-W.; Hung, J.-H.; Yang, S.-F.; Hsieh, Y.-H. α-Mangostin induces mitochondrial dependent apoptosis in human hepatoma SK-Hep-1 cells through inhibition of p38 MAPK pathway. Apoptosis 2013, 18, 1548–1560. [Google Scholar] [CrossRef]
- Shih, Y.-W.; Chien, S.-T.; Chen, P.-S.; Lee, J.-H.; Wu, S.-H.; Yin, L.-T. α-Mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via αvβ3 integrin/FAK/ERK and NF-κB signaling pathway in human lung adenocarcinoma A549 cells. Cell Biochem. Biophys. 2010, 58, 31–44. [Google Scholar] [CrossRef]
- Bellik, Y.; Boukraâ, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: An update. Molecules 2013, 18, 322–353. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Kalpravidh, R.W.; Chitchumroonchokchai, C.; Chuang, C.-C.; West, T.; Kennedy, A.; McIntosh, M. Xanthones from mangosteen prevent lipopolysaccharide-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J. Nutr. 2009, 139, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Kalpravidh, R.W.; Chuang, C.-C.; Overman, A.; Martinez, K.; Kennedy, A.; McIntosh, M. Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. J. Nutr. 2010, 140, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lee, L.-T.; Hu, N.-Y.; Huange, K.-K.; Shih, Y.-C.; Munekazu, I.; Li, J.-M.; Chou, T.-Y.; Wang, W.-H.; Chen, T.-S. Effects of alpha-mangostin on the expression of anti-inflammatory genes in U937 cells. Chin. Med. 2012, 7, 19. [Google Scholar] [CrossRef]
- Gutierrez-Orozco, F.; Chitchumroonchokchai, C.; Lesinski, G.B.; Suksamrarn, S.; Failla, M.L. α-Mangostin: Anti-inflammatory activity and metabolism by human cells. J. Agric. Food Chem. 2013, 61, 3891–3900. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef]
- Widowati, W.; Darsono, L.; Suherman, J.; Fauziah, N.; Maesaroh, M.; Erawijantari, P.P. Anti-inflammatory effect of mangosteen (Garcinia mangostana L.) peel extract and its compounds in LPS-induced RAW264. 7 cells. Nat. Prod. Sci. 2016, 22, 147–153. [Google Scholar] [CrossRef]
- Springer, T.A. Adhesion receptors of the immune system. Nature 1990, 346, 425–434. [Google Scholar] [CrossRef]
- Springer, T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995, 57, 827–872. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Weissmann, G. The adhesion molecules of inflammation. Arthritis Rheumatol. 1993, 36, 147–157. [Google Scholar] [CrossRef]
- Zou, W.; Yin, P.; Shi, Y.; Jin, N.; Gao, Q.; Li, J.; Liu, F. A Novel Biological Role of α-Mangostin via TAK1–NF-κB Pathway against Inflammatory. Inflammation 2019, 42, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Zou, W.; Li, J.; Jin, N.; Gao, Q.; Liu, F. Using high-throughput sequencing to explore the anti-inflammatory effects of α-mangostin. Sci. Rep. 2019, 9, 15626. [Google Scholar] [CrossRef] [PubMed]
- Mucuk, G.; Sepet, E.; Erguven, M.; Ekmekcı, O.; Bılır, A. 1, 25-Dihydroxyvitamin D3 stimulates odontoblastic differentiation of human dental pulp-stem cells in vitro. Connect. Tissue Res. 2017, 58, 531–541. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).