Effects of Xanthan Gum Biopolymer on Soil Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
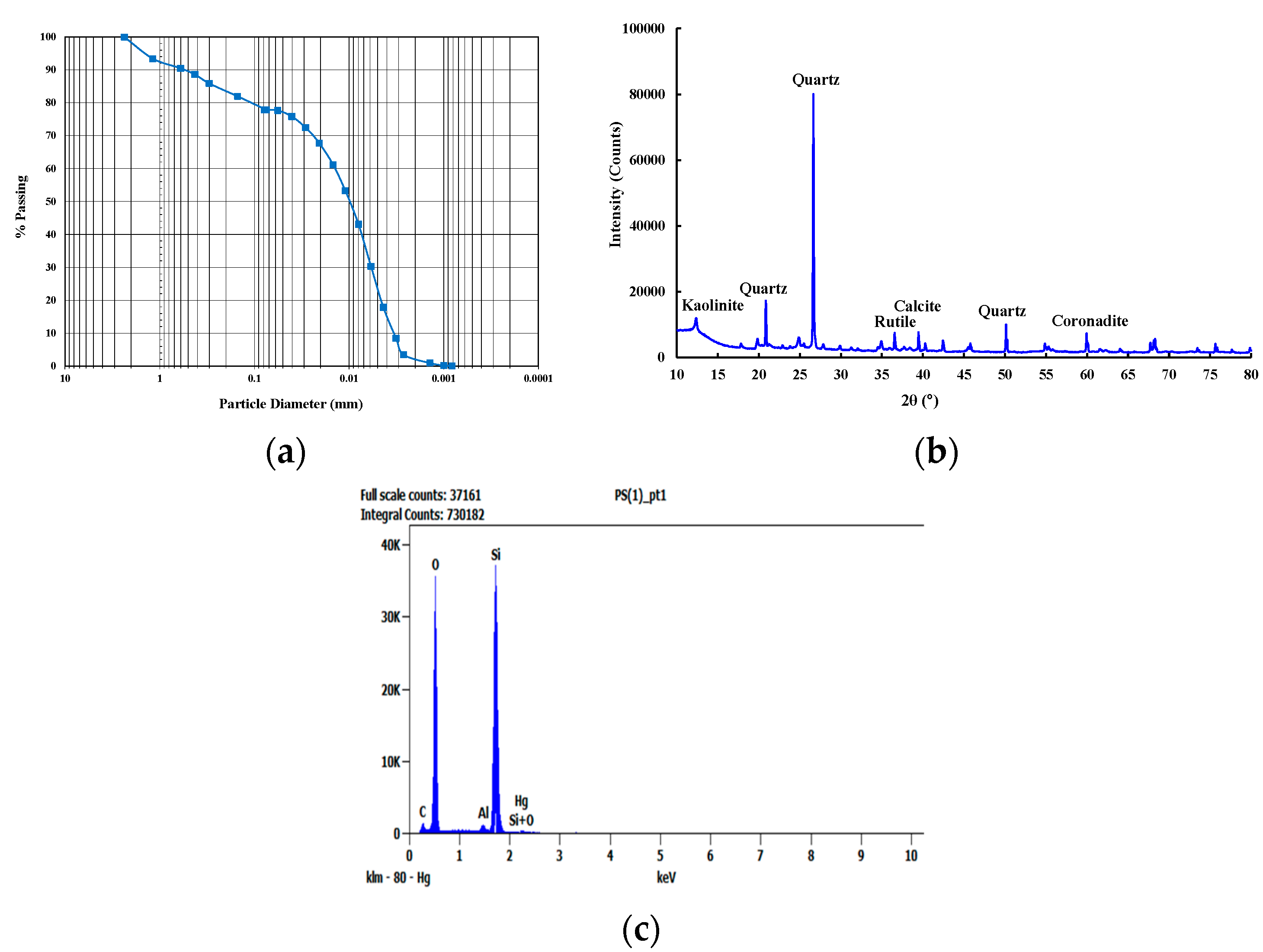
2.1. Materials
2.2. Specimen Preparation
2.3. Experimental Program
3. Results and Discussions
3.1. Atterberg Limits
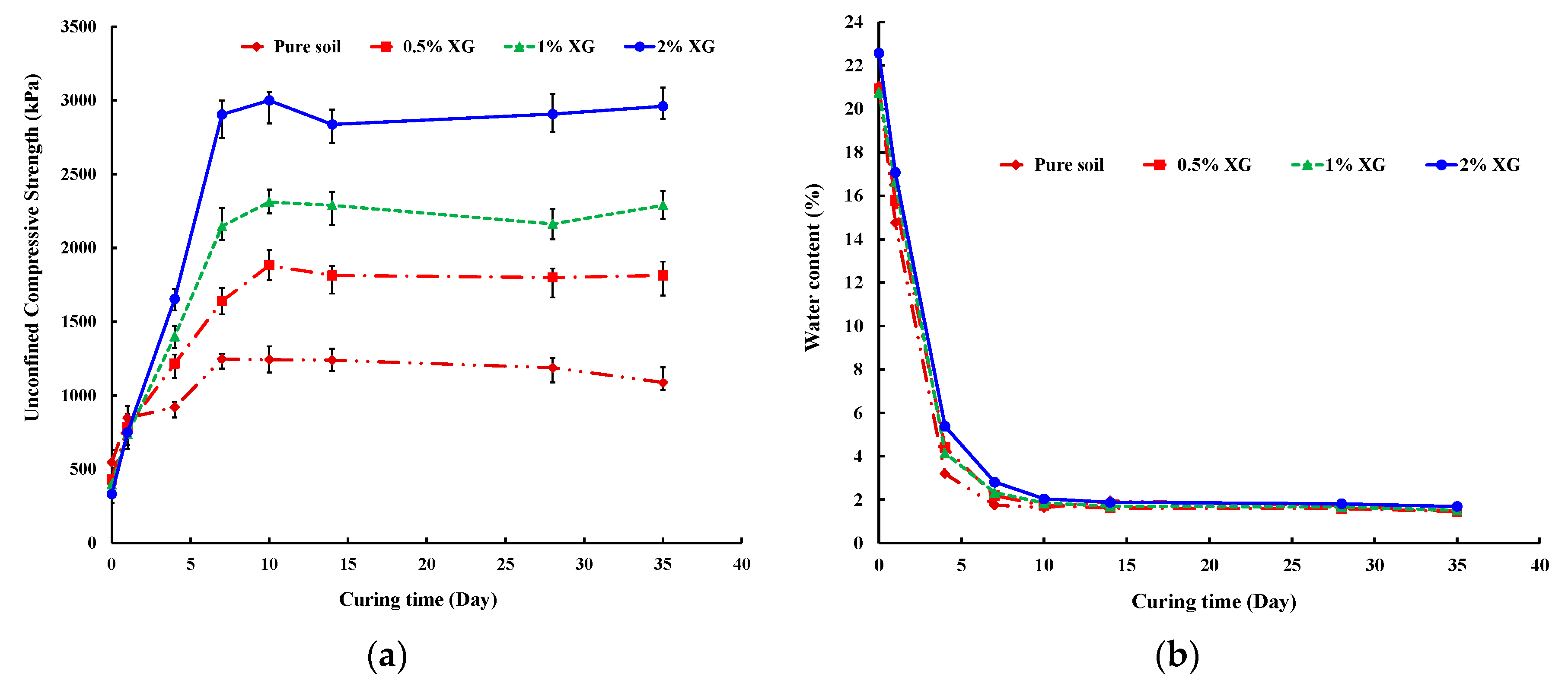
3.2. Unconfined Compressive Strength (UCS)
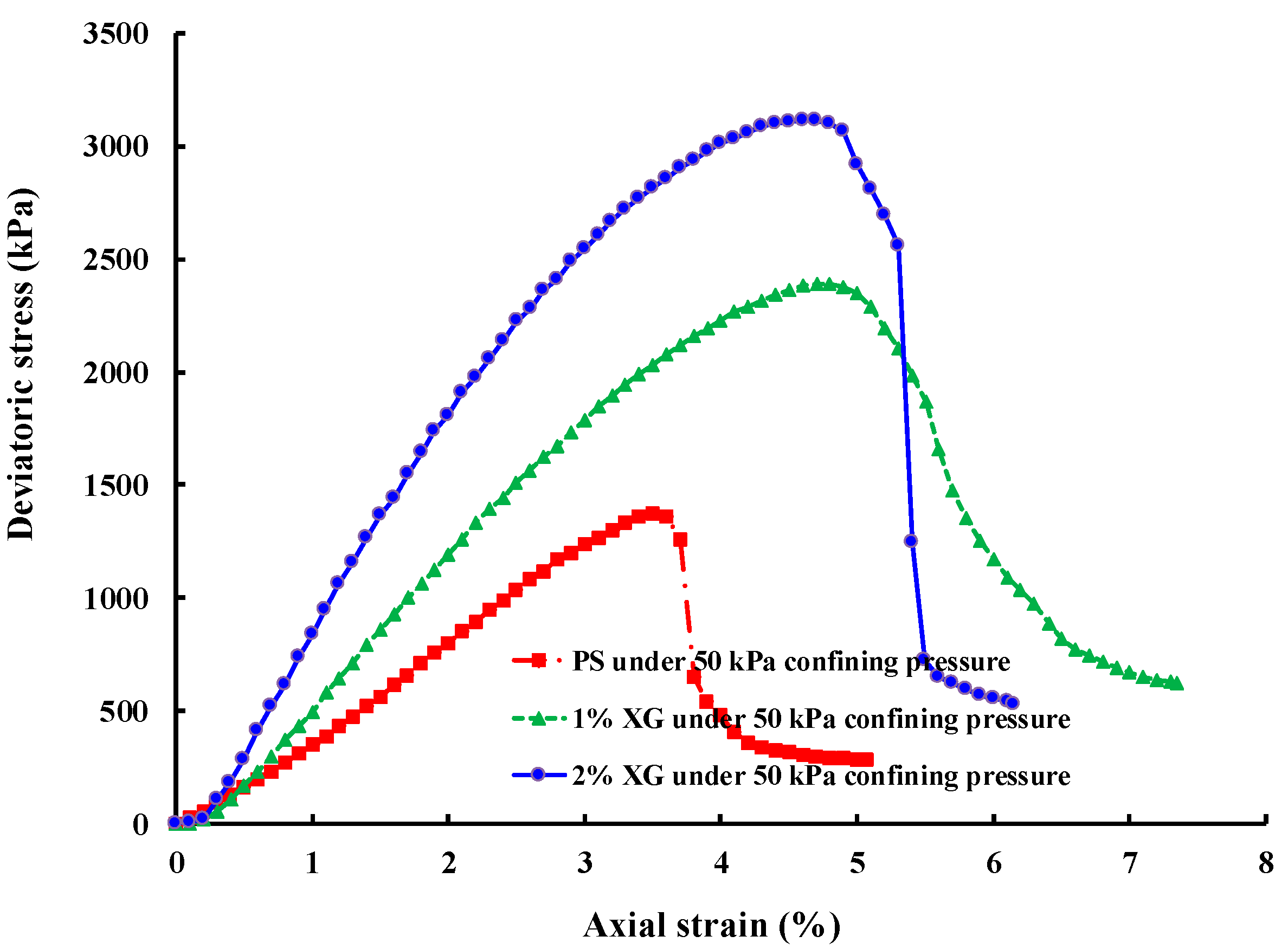
3.3. Shear Strength
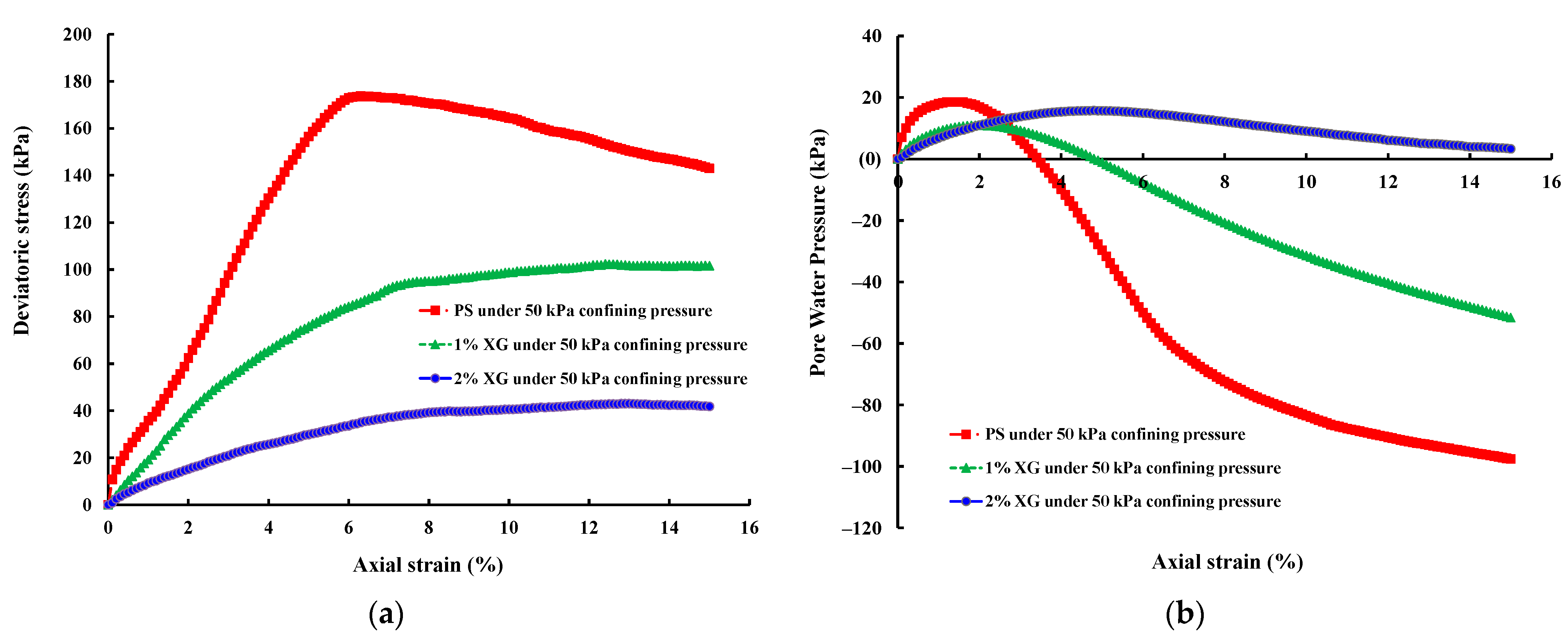
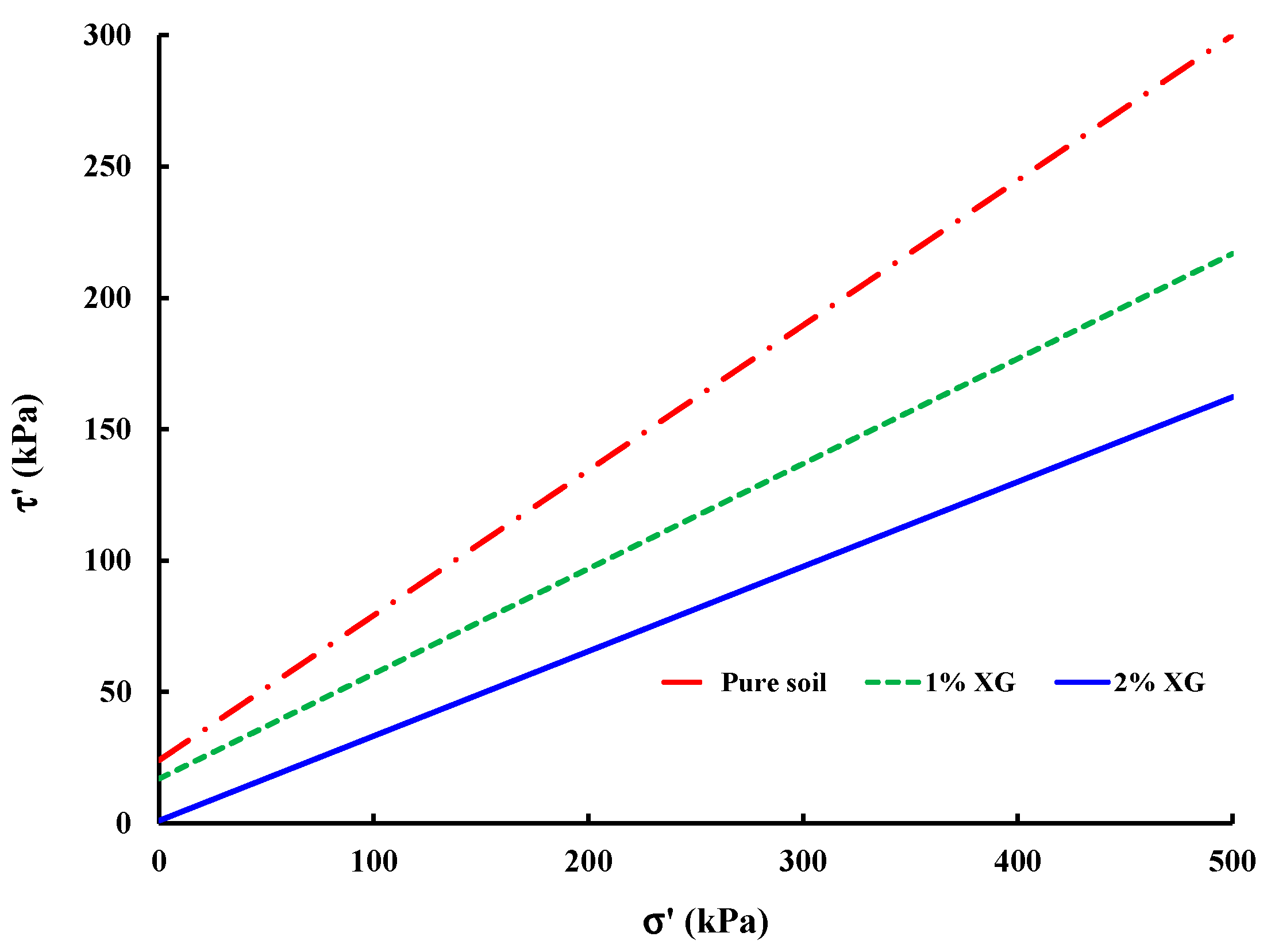
3.3.1. Saturated Sample (CU Test)
3.3.2. Dry Sample (UU Test)
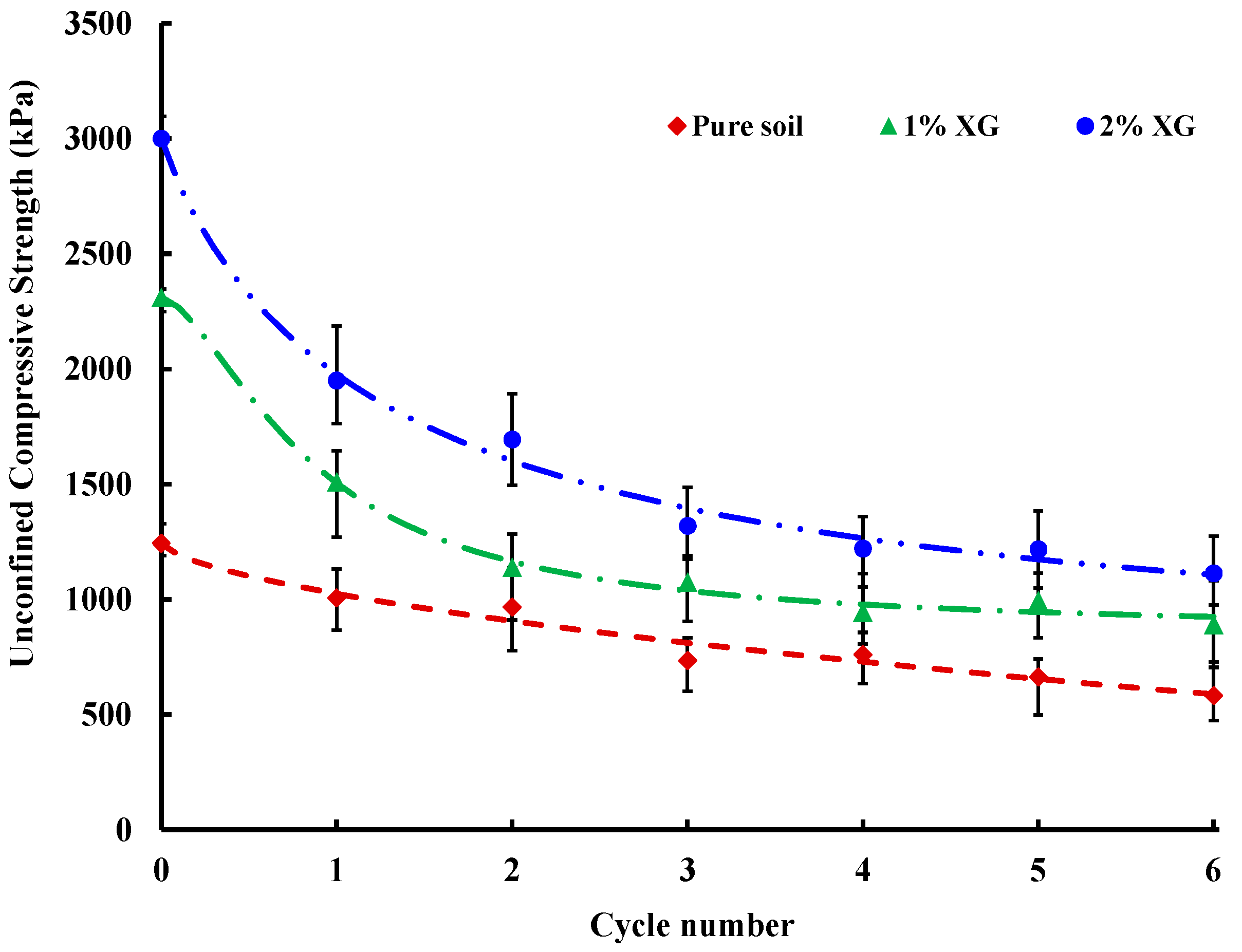
3.4. Wetting and Drying Cycles
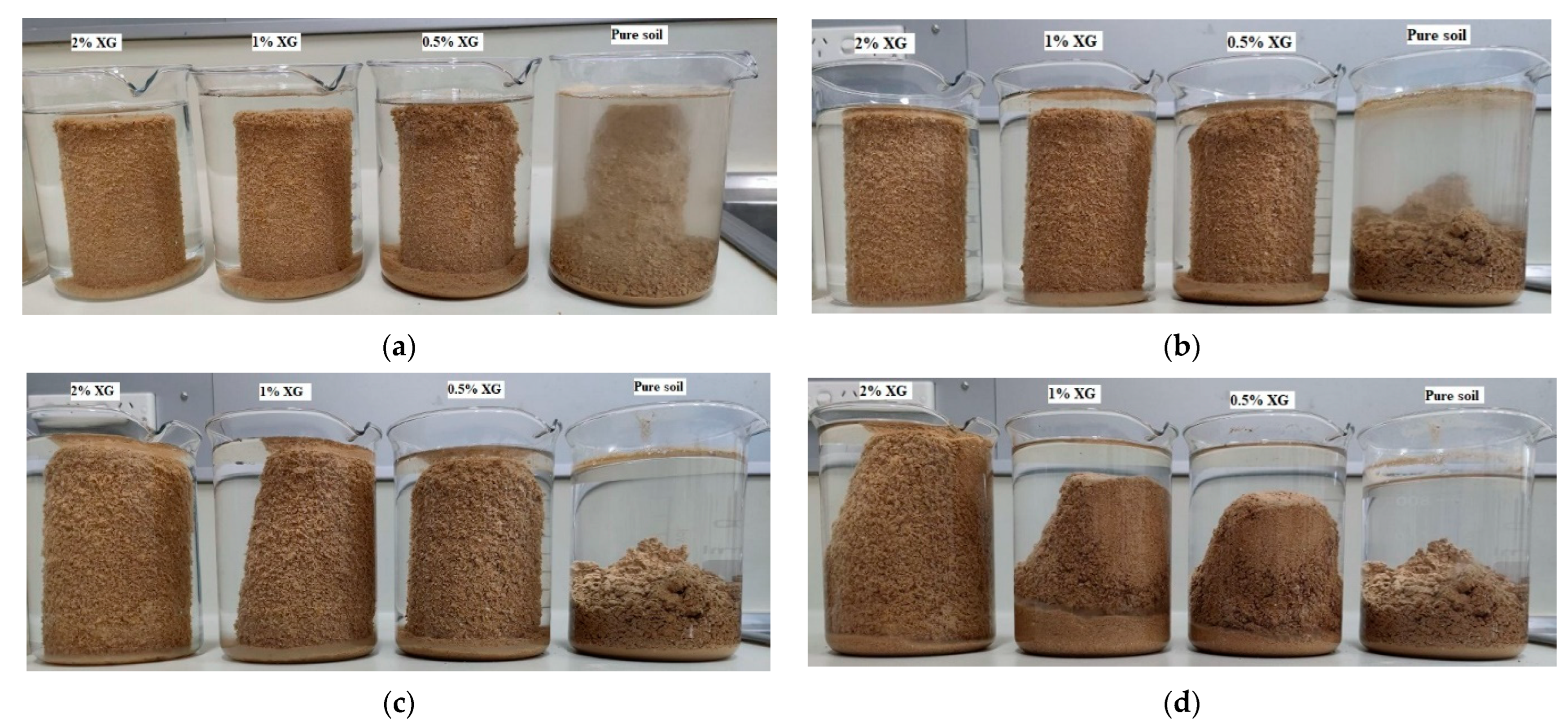
3.5. Moisture Susceptibility
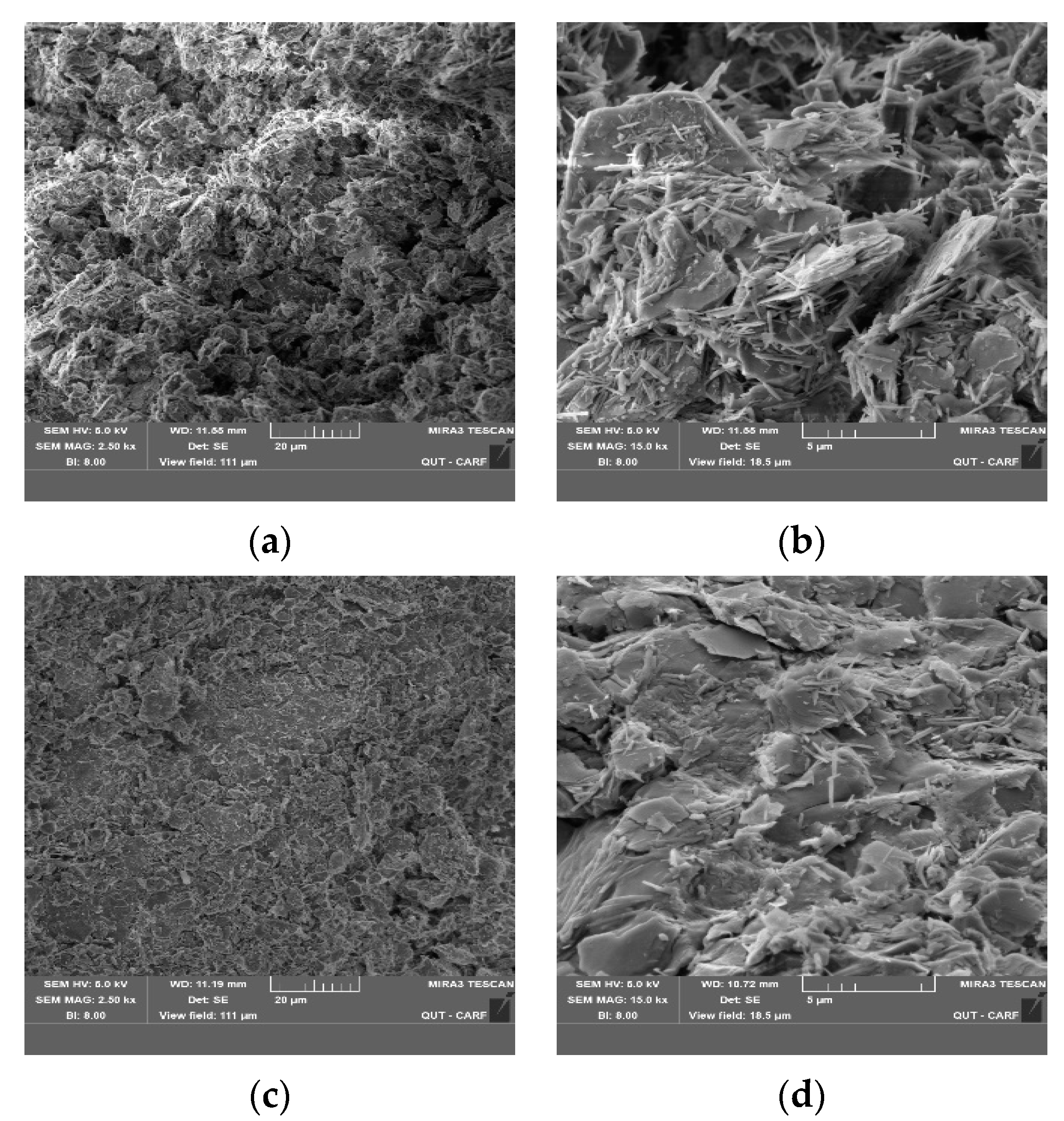
3.6. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef] [Green Version]
- Niaounakis, M. Biopolymers: Applications and Trends; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Soldo, A.; Miletic, M. Durability against Wetting-Drying Cycles of Sustainable Biopolymer-Treated Soil. Polymers 2022, 14, 4247. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, S.; Cai, G.; Puppala, A.J. Experimental investigation of thermal and mechanical properties of lignin treated silt. Eng. Geol. 2015, 196, 1–11. [Google Scholar] [CrossRef]
- Ta’Negonbadi, B.; Noorzad, R. Stabilization of clayey soil using lignosulfonate. Transp. Geotech. 2017, 12, 45–55. [Google Scholar] [CrossRef]
- Smitha, S.; Rangaswamy, K. Effect of Biopolymer Treatment on Pore Pressure Response and Dynamic Properties of Silty Sand. J. Mater. Civ. Eng. 2020, 32, 04020217. [Google Scholar] [CrossRef]
- Chang, I.; Cho, G.-C. Strengthening of Korean residual soil with β-1, 3/1, 6-glucan biopolymer. Constr. Build. Mater. 2012, 30, 30–35. [Google Scholar] [CrossRef]
- Soldo, A.; Miletić, M.; Auad, M.L. Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci. Rep. 2020, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Fatehi, H.; Ong, D.; Yu, J.; Chang, I. Biopolymers as Green Binders for Soil Improvement in Geotechnical Applications: A Review. Geosciences 2021, 11, 291. [Google Scholar] [CrossRef]
- Hataf, N.; Ghadir, P.; Ranjbar, N. Investigation of soil stabilization using chitosan biopolymer. J. Clean. Prod. 2018, 170, 1493–1500. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Lee, S.-W.; Cho, G.-C. Strength durability of gellan gum biopolymer-treated Korean sand with cyclic wetting and drying. Constr. Build. Mater. 2017, 143, 210–221. [Google Scholar] [CrossRef]
- Ham, S.-M.; Chang, I.; Noh, D.-H.; Kwon, T.-H.; Muhunthan, B. Improvement of Surface Erosion Resistance of Sand by Microbial Biopolymer Formation. J. Geotech. Geoenviron. Eng. 2018, 144, 06018004. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Hao, G.-L.; Chen, J.-Q.; Ma, L.; Geng, X.-Y. The Optimisation Analysis of Sand-Clay Mixtures Stabilised with Xanthan Gum Biopolymers. Sustainability 2021, 13, 3732. [Google Scholar] [CrossRef]
- Chen, C.; Wei, K.; Gu, J.; Huang, X.; Dai, X.; Liu, Q. Combined Effect of Biopolymer and Fiber Inclusions on Unconfined Compressive Strength of Soft Soil. Polymers 2022, 14, 787. [Google Scholar] [CrossRef] [PubMed]
- Ta’Negonbadi, B.; Noorzad, R. Physical and geotechnical long-term properties of lignosulfonate-stabilized clay: An experimental investigation. Transp. Geotech. 2018, 17, 41–50. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, S.; Zhan, H.; Ma, C.; Cai, G. Durability of silty soil stabilized with recycled lignin for sustainable engineering materials. J. Clean. Prod. 2020, 248, 119293. [Google Scholar] [CrossRef]
- ASTM D422-63; Standard Test Method for Particle-Size Analysis of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D4318-17; Standard Test Methods for Liquid Limit, Plastic Limit and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D2487-17; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D854-14; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D698-12; Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D2166-06; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2010.
- Mendonça, A.; Morais, P.V.; Pires, A.C.; Chung, A.P.; Oliveira, P.J.V. Reducing Soil Permeability Using Bacteria-Produced Biopolymer. Appl. Sci. 2021, 11, 7278. [Google Scholar] [CrossRef]
- Mendonça, A.; Morais, P.V.; Pires, A.C.; Chung, A.P.; Oliveira, P.V. A Review on the Importance of Microbial Biopolymers Such as Xanthan Gum to Improve Soil Properties. Appl. Sci. 2021, 11, 170. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Prasidhi, A.K.; Cho, G.-C. Effects of Xanthan gum biopolymer on soil strengthening. Constr. Build. Mater. 2015, 74, 65–72. [Google Scholar] [CrossRef]
- Wiszniewski, M.; Skutnik, Z.; Biliniak, M.; Cabalar, A.F. Some geomechanical properties of a biopolymer treated medium sand. Ann. Wars. Univ. Life Sci. SGGW Land Reclam. 2017, 49, 201–212. [Google Scholar] [CrossRef]
- Soldo, A.; Miletić, M. Study on Shear Strength of Xanthan Gum-Amended Soil. Sustainability 2019, 11, 6142. [Google Scholar] [CrossRef]
- Cho, G.-C.; Chang, I. Cementless Soil Stabilizer–Biopolymer. In Proceedings of the 2018 World Congress on Advances in Civil, Environmental & Materials Research (ACEM18) Songdo Convensia, Incheon, Republic of Korea, 27–31 August 2018. [Google Scholar]
- Khatami, H.R.; O’Kelly, B.C. Improving Mechanical Properties of Sand Using Biopolymers. J. Geotech. Geoenviron. Eng. 2013, 139, 1402–1406. [Google Scholar] [CrossRef] [Green Version]
- Ayeldeen, M.; Negm, A.; El-Sawwaf, M.; Kitazume, M. Enhancing mechanical behaviors of collapsible soil using two biopolymers. J. Rock Mech. Geotech. Eng. 2017, 9, 329–339. [Google Scholar] [CrossRef]



| Soil Reference | Liquid Limit, LL (%) | Plastic Limit, PL (%) | Plasticity Index, PI (%) |
|---|---|---|---|
| Pure soil | 38.0 | 26.9 | 11.1 |
| 0.5% XG | 59.6 | 28.5 | 31.1 |
| 1% XG | 62.2 | 29.6 | 32.6 |
| 2% XG | 64.7 | 30.7 | 34.0 |
| Soil Reference | Effective Cohesion, c′ (kPa) | Effective Internal Friction Angle, ϕ′ (°) |
|---|---|---|
| Pure soil | 24 | 28.9 |
| 1% XG | 17 | 21.8 |
| 2% XG | 1 | 18.0 |
| Soil Reference | Cohesion, c (kPa) | Internal Friction Angle, ϕ (°) |
|---|---|---|
| Pure soil | 280 | 41.0 |
| 1% XG | 580 | 36.6 |
| 2% XG | 970 | 25.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagheri, P.; Gratchev, I.; Rybachuk, M. Effects of Xanthan Gum Biopolymer on Soil Mechanical Properties. Appl. Sci. 2023, 13, 887. https://doi.org/10.3390/app13020887
Bagheri P, Gratchev I, Rybachuk M. Effects of Xanthan Gum Biopolymer on Soil Mechanical Properties. Applied Sciences. 2023; 13(2):887. https://doi.org/10.3390/app13020887
Chicago/Turabian StyleBagheri, Pouyan, Ivan Gratchev, and Maksym Rybachuk. 2023. "Effects of Xanthan Gum Biopolymer on Soil Mechanical Properties" Applied Sciences 13, no. 2: 887. https://doi.org/10.3390/app13020887
APA StyleBagheri, P., Gratchev, I., & Rybachuk, M. (2023). Effects of Xanthan Gum Biopolymer on Soil Mechanical Properties. Applied Sciences, 13(2), 887. https://doi.org/10.3390/app13020887











