4-Hexylresorcinol and Its Effects on Circumvallate Papillae Taste Buds in Diabetic and Healthy Rats: An Initial Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
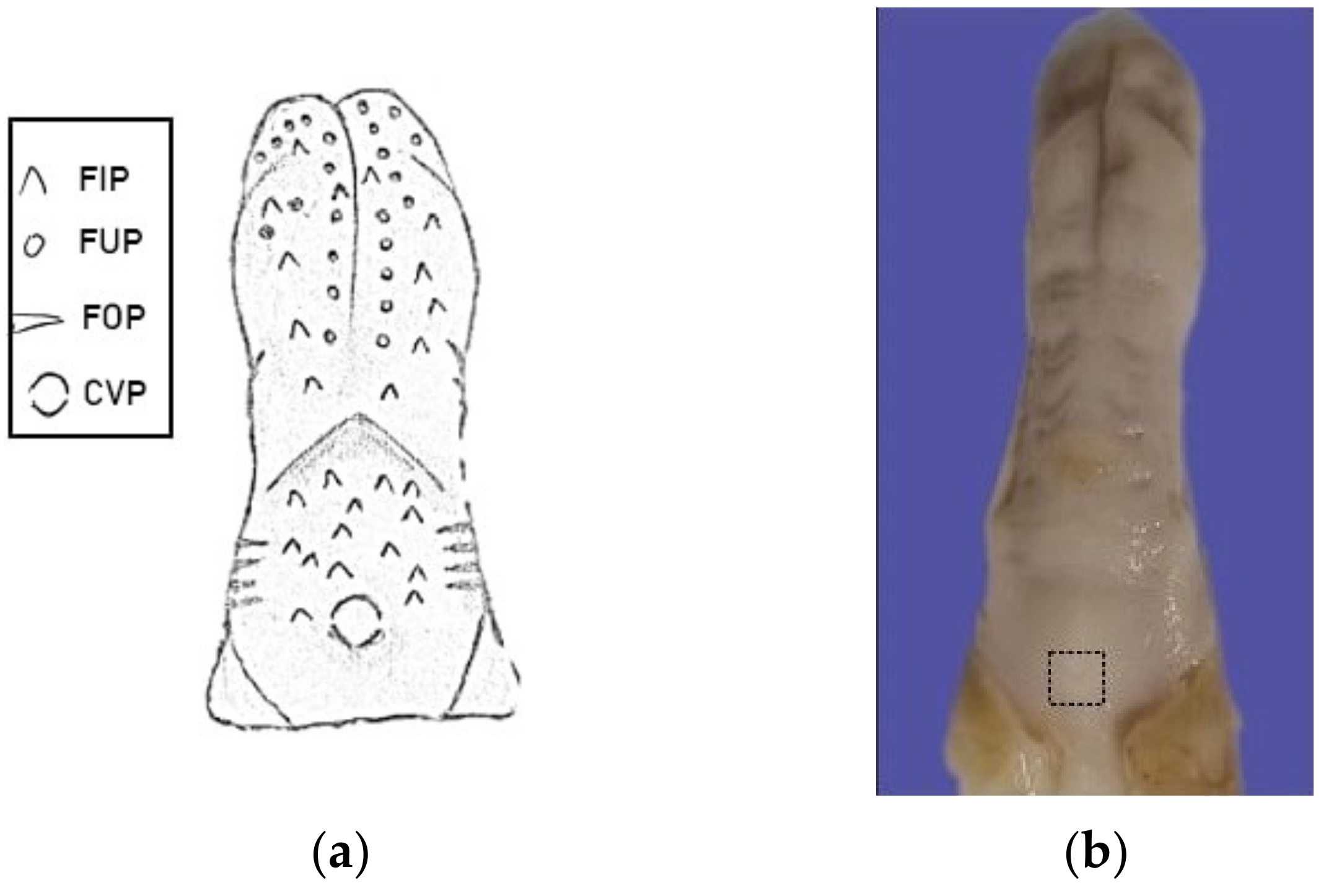
2.2. Sample Collection and Preparation
2.3. Histologic Examination
2.3.1. Hematoxylin and Eosin (H&E) Staining
2.3.2. Immunohistochemical (IHC) Staining
2.3.3. TUNEL Assay
2.3.4. Immunoprecipitation High-Performance Liquid Chromatography (IP-HPLC)
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Risso, D.; Drayna, D.; Morini, G. Alteration, reduction and taste loss: Main causes and potential implications on dietary habits. Nutrients 2020, 12, 3284. [Google Scholar] [CrossRef] [PubMed]
- Maheswaran, T.; Abikshyeet, P.; Sitra, G.; Gokulanathan, S.; Vaithiyanadane, V.; Jeelani, S. Gustatory dysfunction. J. Pharm. Bioallied Sci. 2014, 6, S30. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.R.; Meghana, I.S.S.; Thomas, B.; Shenoy, N.; Bhandary, R. Diabetes mellitus and potential oral complications—A review. Rom. J. Diabetes Nutr. Metab. Dis. 2021, 28, 453–462. [Google Scholar]
- Catamo, E.; Tornese, G.; Concas, M.P.; Gasparini, P.; Robino, A. Differences in taste and smell perception between type 2 diabetes mellitus patients and healthy controls. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 193–200. [Google Scholar] [CrossRef]
- Pugnaloni, S.; Alia, S.; Mancini, M.; Santoro, V.; Di Paolo, A.; Rabini, R.A.; Fiorini, R.; Sabbatinelli, J.; Fabri, M.; Mazzanti, L.; et al. A study on the relationship between type 2 diabetes and taste function in patients with good glycemic control. Nutrients 2020, 12, 1112. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Hack, C.; Yu, C.; Rennick, I.; Ohanian, J.; Dezan, M.; Mott, N.; Manibo, R.; Tucker, R.M. Alterations in sweet taste function in adults with diabetes mellitus: A systematic review and potential implications. Crit. Rev. Food Sci. Nutr. 2023, 63, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, P.; Gouveris, H.; Kekes, G.; Maurer, J. Electrogustometry thresholds, tongue tip vascularization, and density and morphology of the fungiform papillae in diabetes. B-ENT 2014, 10, 271–278. [Google Scholar]
- Liang, Z.; Wilson, C.E.; Teng, B.; Kinnamon, S.C.; Liman, E.R. The proton channel OTOP1 is a sensor for the taste of ammonium chloride. Nat. Commun. 2023, 14, 6194. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Macpherson, L.J.; Parada, C.A.; Zuker, C.S.; Ryba, N.J.P. Rewiring the taste system. Nature 2017, 548, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.J.; Larson, E.D.; Shechtman, L.A.; Trahan, G.D.; Gaillard, D.; Fellin, T.J.; Scott, J.K.; Jones, K.L.; Barlow, L.A. Onset of taste bud cell renewal starts at birth and coincides with a shift in SHH function. eLife 2021, 10, e64013. [Google Scholar] [CrossRef]
- Cheng, B.; Pan, S.; Liu, X.; Zhang, S.; Sun, X. Cell apoptosis of taste buds in circumvallate papillae in diabetic rats. Exp. Clin. Endocrinol. Diabetes 2011, 119, 480–483. [Google Scholar] [CrossRef]
- Lee, A.A.; Owyang, C. Sugars, sweet taste receptors, and brain responses. Nutrients 2017, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Catamo, E.; Robino, A.; Tinti, D.; Dovc, K.; Franceschi, R.; Giangreco, M.; Gasparini, P.; Barbi, E.; Cauvin, V.; Rabbone, I.; et al. Altered taste function in young individuals with type 1 diabetes. Front. Nutr. 2022, 8, 797920. [Google Scholar] [CrossRef] [PubMed]
- El-Haddad, K.; El-Faramawy, N. Effects of dose-dependent response to gamma radiation on circumvallate papilla by expression of caspase-3 in vivo. Saudi Dent. J. 2021, 33, 869–876. [Google Scholar] [CrossRef]
- Ichimori, Y.; Ueda, K.; Okada, H.; Honma, S.; Wakisaka, S. Histochemical changes and apoptosis in degenerating taste buds of the rat circumvallate papilla. Arch. Histol. Cytol. 2009, 72, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hevér, H.; Altdorfer, K.; Zelles, T.; Batbayar, B.; Fehér, E. Innervation of papilla vallata in diabetic rats. Orvosi Hetilap 2013, 154, 443–448. [Google Scholar] [CrossRef]
- Zavyalov, O.; Galimzhan, D.; Marina, K. Effect of feeding bioactive compounds identified from plant extracts (4-hexylresorcinol, 7-hydroxycoumarin, and gamma-octalactone) on the productivity and quality of broiler meat. Vet. World 2022, 15, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-G. 4-Hexylresorcinol: Pharmacologic chaperone and its application for wound healing. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 5. [Google Scholar] [CrossRef]
- Lee, I.S.; Chang, J.H.; Kim, D.W.; Kim, S.G.; Kim, T.W. The effect of 4-hexylresorinol administration on NAD+ level and SIRT activity in Saos-2 cells. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 39. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yu, M.; Li, D.; Zhao, G.; Zhang, M.; Wang, Z.; Liu, Y.; Zhou, D. Effects of non-enzymatic browning and lipid oxidation on color of ready-to-eat abalone during accelerated storage and its control. Foods 2023, 12, 1514. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Cheng, X.; Ni, B.; Zhang, F.; Hu, Y.; Zhao, J. High glucose-induced oxidative stress mediates apoptosis and extracellular matrix metabolic imbalances possibly via p38 MAPK activation in rat nucleus pulposus cells. J. Diabetes Res. 2016, 2016, 3765173. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, J.; Che, X.; Choi, J.; Jang, I.; Kim, S. Effects of 4-hexylresorcinol on Facial Skeletal Development in Growing Rats: Considerations for Diabetes. Korean J. Orthod. 2023, in press. Available online: https://e-kjo.org/journal/view.html?uid=2101&vmd=Full (accessed on 22 October 2023).
- Davydova, L.; Tkach, G.; Tymoshenko, A.; Moskalenko, A.; Sikora, V.; Kyptenko, L.; Lyndin, M.; Muravskyi, D.; Maksymova, O.; Suchonos, O. Anatomical and morphological aspects of papillae, epithelium, muscles, and glands of rats’ tongue: Light, scanning, and transmission electron microscopic study. Interv. Med. Appl. Sci. 2017, 9, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Kim, D.-W.; Oh, J.-H.; Lee, S.K.; Choi, J.-Y.; Kim, S.-G.; Kim, T.-W. Effects of 4-hexylresorcinol on craniofacial growth in rats. Int. J. Mol. Sci. 2021, 22, 8935. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Kim, D.-W.; Lee, S.K.; Kim, S.-G. Effects of 4-hexylresorcinol administration on the submandibular glands in a growing rat model. Head Face Med. 2022, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.F.V.E.; Padín-Iruegas, M.E.; Caponio, V.C.A.; Lorenzo-Pouso, A.I.; Saavedra-Nieves, P.; Chamorro-Petronacci, C.M.; Suaréz-Peñaranda, J.; Pérez-Sayáns, M. Caspase 3 and cleaved caspase 3 expression in tumorogenesis and its correlations with prognosis in head and neck cancer: A systematic review and meta-analysis. Int. J. Mol. Sci. 2022, 23, 11937. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Bansho, S.; Ikukawa, A.; Kubota, T.; Ohishi, A.; Nagasawa, K. Expression profile of the zinc transporter ZnT3 in taste cells of rat circumvallate papillae and its role in zinc release, a potential mechanism for taste stimulation. Eur. J. Histochem. 2022, 66, PMC9693774. [Google Scholar] [CrossRef]
- De Carli, L.; Gambino, R.; Lubrano, C.; Rosato, R.; Bongiovanni, D.; Lanfranco, F.; Broglio, F.; Ghigo, E.; Bo, S. Impaired taste sensation in type 2 diabetic patients without chronic complications: A case-control study. J. Endocrinol. Investig. 2018, 41, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Kusakabe, Y.; Hashido, K.; Hino, A.; Ooki, M.; Harada, S. The glossopharyngeal nerve controls epithelial expression of Sprr2a and Krt13 around taste buds in the circumvallate papilla. Neurosci. Lett. 2014, 580, 147–152. [Google Scholar] [CrossRef]
- Meng, L.; Jiang, X.; Ji, R. Role of neurotrophin in the taste system following gustatory nerve injury. Metab. Brain Dis. 2015, 30, 605–613. [Google Scholar] [CrossRef]
- Hiroyuki, O.; Amano, K.; Morita, T.; Miura, T.; Mori, N.; Tatara, R.; Kessoku, T.; Matsuda, Y.; Tagami, K.; Mori, M.; et al. Impact of taste/smell disturbances on dietary intakes and cachexia-related quality of life in patients with advanced cancer. Support. Care Cancer 2023, 31, 141. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Haque, M. Oral health messiers: Diabetes mellitus relevance. Diabetes Metab. Syndr. Obes. 2021, 14, 3001–3015. [Google Scholar] [CrossRef] [PubMed]
- Braud, A.; Boucher, Y. Taste disorder’s management: A systematic review. Clin. Oral Investig. 2020, 24, 1889–1908. [Google Scholar] [CrossRef]
- Tamel Selvan, K.; Goon, J.A.; Makpol, S.; Tan, J.K. Therapeutic potentials of microalgae and their bioactive compounds on diabetes mellitus. Mar. Drugs 2023, 21, 462. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, Q.; Zhang, X.; Yan, Y.; Wang, X. Allicin alleviates diabetes mellitus by inhibiting the formation of advanced glycation end products. Molecules 2022, 27, 8793. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Q.; Chai, W.-M.; Xia, S.-S.; Yu, Z.-Y.; Wei, Q.-M. Inhibitory potential of 4-hexylresorcinol against α-glucosidase and non-enzymatic glycation: Activity and mechanism. J. Biosci. Bioeng. 2021, 131, 241–249. [Google Scholar] [CrossRef]
- Olmos, Y.; Sánchez-Gómez, F.J.; Wild, B.; García-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid. Redox Signal 2013, 19, 1507–1521. [Google Scholar] [CrossRef]
- Tseng, A.H.; Wu, L.H.; Shieh, S.S.; Wang, D.L. SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem. J. 2014, 464, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; Nagui, D.A. Effect of Quercetin on filiform and fungiform papillae of the tongue of albino rats with induced diabetes. Egypt. Dent. J. 2020, 66, 197–211. [Google Scholar] [CrossRef]
- Welcome, M.O.; Dogo, D.; Mastorakis, N.E. Cellular mechanisms and molecular pathways linking bitter taste receptor signalling to cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction in heart diseases. Inflammopharmacology 2023, 31, 89–117. [Google Scholar] [CrossRef]
- Kayode, O.T.; Bello, J.A.; Oguntola, J.A.; Kayode, A.A.A.; Olukoya, D.K. The interplay between monosodium glutamate (MSG) consumption and metabolic disorders. Heliyon 2023, 9, e19675. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Sánchez, E.; Peña-Montes, D.; Sánchez-Duarte, S.; Saavedra-Molina, A.; Sánchez-Duarte, E.; Montoya-Pérez, R. Effects of apocynin on heart muscle oxidative stress of rats with experimental diabetes: Implications for mitochondria. Antioxidants (Basel) 2021, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef]
- Yu, J.H.; Shin, M.-S.; Lee, J.R.; Choi, J.H.; Koh, E.H.; Lee, W.J.; Park, J.-Y.; Kim, M.-S. Decreased sucrose preference in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2014, 104, 214–219. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaida, D.; Park, Y.-W.; Kim, S.-G. 4-Hexylresorcinol and Its Effects on Circumvallate Papillae Taste Buds in Diabetic and Healthy Rats: An Initial Investigation. Appl. Sci. 2023, 13, 11617. https://doi.org/10.3390/app132111617
Gaida D, Park Y-W, Kim S-G. 4-Hexylresorcinol and Its Effects on Circumvallate Papillae Taste Buds in Diabetic and Healthy Rats: An Initial Investigation. Applied Sciences. 2023; 13(21):11617. https://doi.org/10.3390/app132111617
Chicago/Turabian StyleGaida, Dhouha, Young-Wook Park, and Seong-Gon Kim. 2023. "4-Hexylresorcinol and Its Effects on Circumvallate Papillae Taste Buds in Diabetic and Healthy Rats: An Initial Investigation" Applied Sciences 13, no. 21: 11617. https://doi.org/10.3390/app132111617
APA StyleGaida, D., Park, Y.-W., & Kim, S.-G. (2023). 4-Hexylresorcinol and Its Effects on Circumvallate Papillae Taste Buds in Diabetic and Healthy Rats: An Initial Investigation. Applied Sciences, 13(21), 11617. https://doi.org/10.3390/app132111617






