Comparison of the Bioactive and Bacteriostatic Performance of Different Alginate-Based Dental Prosthetic Impression Materials with and without Zirconium Phosphate-Based Ion Exchange Resin Containing Silver: An In Vitro Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alginate Impression Materials
2.3. Testing Methods
2.3.1. IR Infrared Spectroscopy
2.3.2. Thermogravimetric Analysis
2.3.3. Optical Microscopy
2.3.4. Material Setting Tests
2.3.5. Detail Reproduction
2.3.6. Compatibility with Plaster
2.3.7. Contraction Measurement
2.3.8. Compressive Strength
2.3.9. Tearing Strength
2.3.10. Elastic Recovery
2.3.11. Compressive Strain
2.3.12. Determination of the Microbiological Activity of Alginates against Streptococcus mutans
2.3.13. Statistical Analysis
3. Results and Discussion
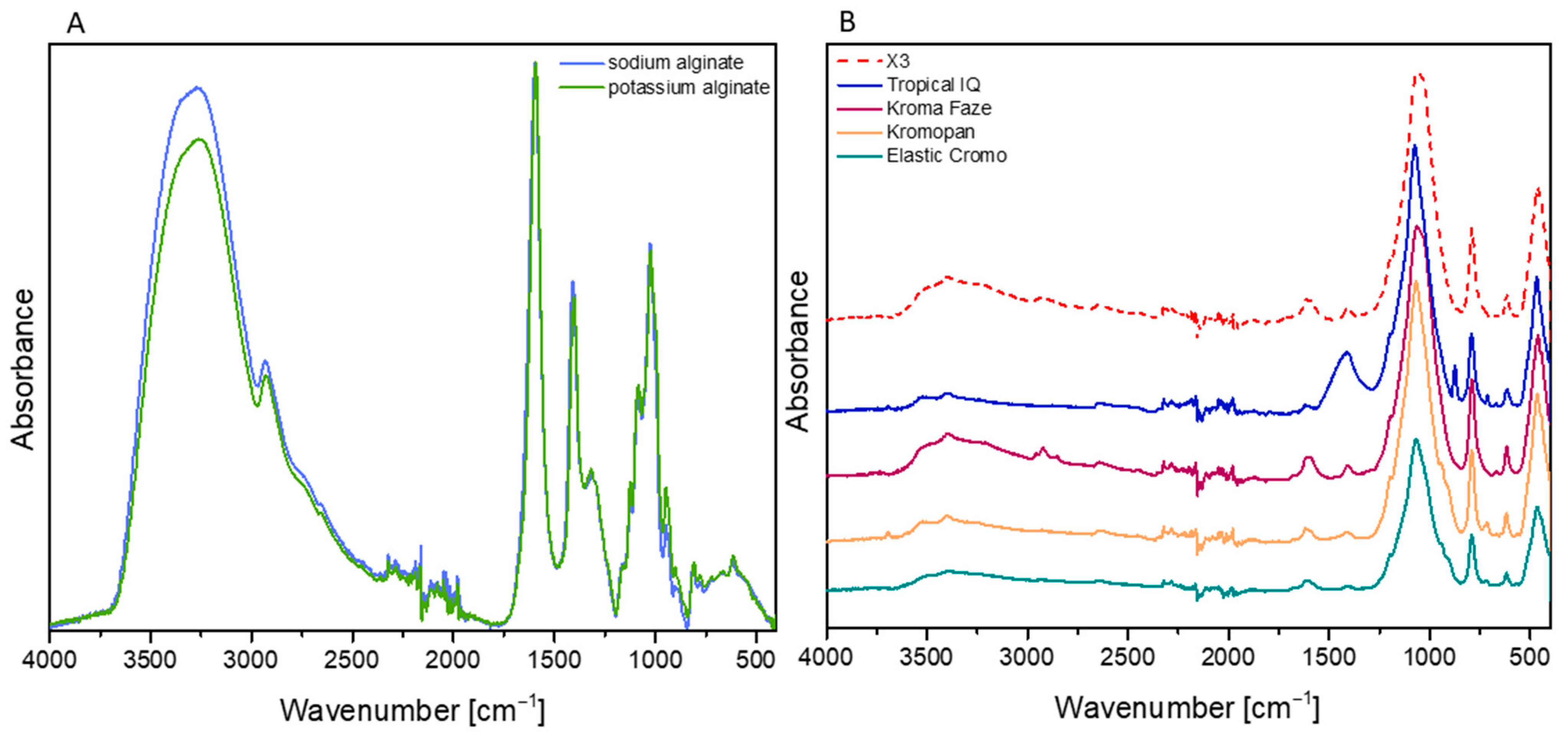
3.1. FT-IR Spectroscopy
3.2. Thermogravimetry
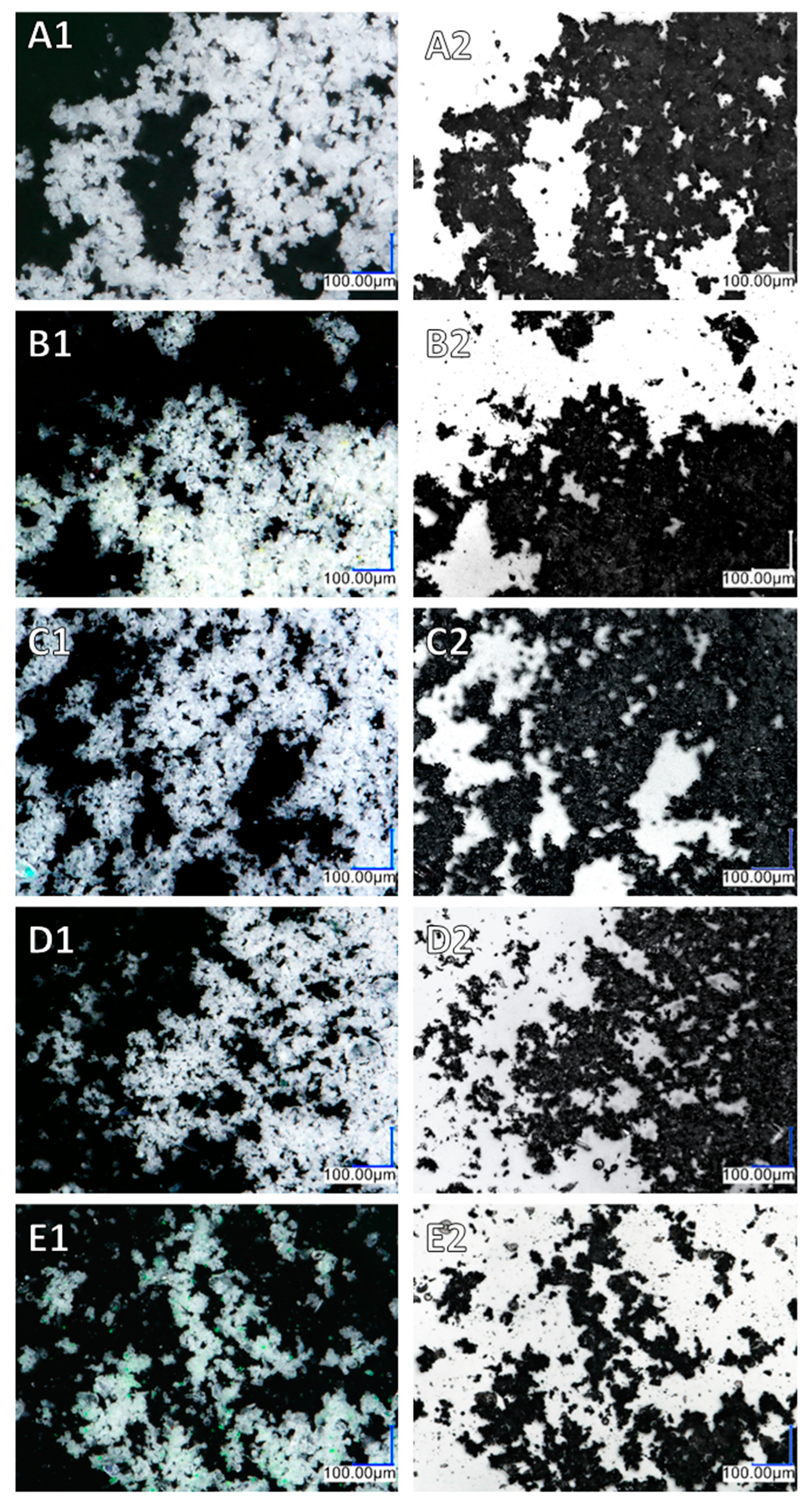
3.3. Optical Microscopy
3.4. Material Setting Properties and Detail Reproduction
3.5. Mechanical Properties
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivakumar, A.; Thangaswamy, V.; Ravi, V. Treatment planning in conservative dentistry. J. Pharm. Bioallied. Sci. 2012, 4, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Vrbova, R.; Bradna, P.; Bartos, M.; Roubickova, A. The effect of disinfectants on the accuracy, quality and surface structure of impression materials and gypsum casts: A comparative study using light microscopy, scanning electron microscopy and micro computed tomography. Dent. Mater. J. 2020, 39, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.W.; Chaturvedi, S.; Naqash, T.A.; Ahmed, A.R.; Das, G.; Rana, M.H.; Abdelmonem, A.M. Influence of time, temperature and humidity on the accuracy of alginate impressions. J. Ayub. Med. Coll. Abbottabad. 2020, 32 (Suppl. S1), S659–S667. [Google Scholar]
- Arqoub, M.; Rabi, T.; Arandi, N. Dental impression materials in prosthodontics: An overview for the general dentist. Int. J. Prev. Clin. Dent. Res. 2018, 5, 21–23. [Google Scholar] [CrossRef]
- Saniour, S.H.S.; Abd El-Ghaffar, M.A.; Fath El-Bab, I.I.; Saba, D.A. Effect of composition of alginate impression material on “recovery from deformation”. J. Am. Sci. 2011, 7, 443–448. [Google Scholar]
- ISO 21563; 2013 Dentistry: Hydrocolloid Impression Materials. International Organization for Standardization: Geneva, Switzerland, 2013.
- Ibrahem, F.; Giugliano, T.; Ruff, R.R.; Choi, M. Digital Analysis of the Dimensional Change of an Irreversible Hydrocolloid Impression Material (Alginate) with Varying Storage Times. Prim. Dent. J. 2022, 11, 86–91. [Google Scholar] [CrossRef]
- Bitencourt, S.B.; Catanoze, I.A.; Silva, E.V.F.D.; Turcio, K.H.L.; Santos, D.M.D.; Brandini, D.A.; Goiato, M.C.; Guiotti, A.M. Extended-pour and conventional alginates: Effect of storage time on dimensional accuracy and maintenance of details. Dental. Press J. Orthod. 2021, 26, e2119251. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, L.; Lin, C.; Fan, Z.; Liu, B. The influence of the negative ion powder on the properties of alginate impression materials. Dent. Mater. J. 2019, 38, 522–527. [Google Scholar] [CrossRef]
- Raszewski, Z.; Nowakowska-Toporowska, A.; Weżgowiec, J.; Nowakowska, D. Effect of water quantity and quality on the properties of alginate impression materials. Dent. Med. Probl. 2018, 55, 43–48. [Google Scholar] [CrossRef]
- Gupta, R.; Brizuela, M. Dental Impression Materials. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Laino, L.; Troiano, G.; Amoroso, G.; Crimi, S.; Matarese, M.; D’Amico, C.; Nastro Siniscalchi, E.; et al. Alginate Materials and Dental Impression Technique: A Current State of the Art and Application to Dental Practice. Mar Drugs. 2018, 17, 18. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Cornejo-Ríos, E.; Tosco, V.; Monterubbianesi, R.; Mancino, S.; Eid, A.; Mancino, D.; et al. Disinfection Procedures and Their Effect on the Microorganism Colonization of Dental Impression Materials: A Systematic Review and Meta-Analysis of In Vitro Studies. Bioengineering 2022, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Daneu, G.D.; Vasconcelos, J.B.; Oltramari, P.V.; de Almeida, M.R.; Guiraldo, R.D.; Fernandes, T.M. Dimensional stability of alginate moulds scanned at different storage times. Acta Odontol. Latinoam. 2020, 33, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Benakatti, V.B.; Patil, A.P.; Sajjanar, J.; Shetye, S.S.; Amasi, U.N.; Patil, R. Evaluation of antibacterial effect and dimensional stability of self-disinfecting irreversible hydrocolloid: An in vitro study. J. Contemp. Dent. Pract. 2017, 18, 887–892. [Google Scholar] [CrossRef]
- Babiker, G.H.; Khalifa, N.; Alhajj, M.N. Dimensional accuracy of alginate impressions using different methods of disinfection with varying concentrations. Compend. Contin. Educ. Dent. 2018, 39, e17–e20. [Google Scholar] [PubMed]
- Mushtaq, M.A.K.M. An overview of dental impression disinfection techniques—A literature review. J. Pak. Dent. Assoc. 2018, 27, 207–212. [Google Scholar] [CrossRef]
- Al Shikh, A.; Milosevic, A. Effectiveness of Alcohol and Aldehyde Spray Disinfectants on Dental Impressions. Clin. Cosmet. Investig. Dent. 2020, 12, 25–30. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, J.; Xu, Y.; Shi, Z.; Wang, Y.; Zhang, L.; Fu, B. Disinfection efficacy of sodium hypochlorite and glutaraldehyde and their effects on the dimensional stability and surface properties of dental impressions: A systematic review. Peer J. 2023, 11, e14868. [Google Scholar] [CrossRef]
- Mc Neill, M.R.; Coulter, W.A.; Hussey, D.L. Disinfection of irreversible hydrocolloid impressions: A comparative study. Int. J. Prosthodont. 1992, 5, 563–567. [Google Scholar]
- Ginjupalli, K.; Alla, R.K.; Tellapragada, C.; Gupta, L.; Upadhya Perampalli, N. Antimicrobial activity and properties of irreversible hydrocolloid impression materials incorporated with silver nanoparticles. J. Prosthet. Dent. 2016, 115, 722–728. [Google Scholar] [CrossRef]
- Singer, L.; Bourauel, C. Mechanical and Physical Properties of an Experimental Chemically and Green-Nano Improved Dental Alginate after Proven Antimicrobial Potentials. Gels 2023, 9, 429. [Google Scholar] [CrossRef]
- Manikyamba, Y.J.B.; Rama Raju, A.V.; Suresh Sajjan, M.C.; Bhupathi, P.A.; Rao, B.D.; Raju, J.V. An evaluation of antimicrobial potential of irreversible hydrocolloid impression material incorporated with chitosan. J. Indian Prosthodont. Soc. 2020, 20, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Omidkhoda, M.; Hasanzadeh, N.; Soleimani, F.; Shafaee, H. Antimicrobial and physical properties of alginate impression material incorporated with silver nanoparticles. Dent. Res. J. 2019, 16, 372–376. [Google Scholar]
- Nia, A.F.; Ataei, M.; Zeighami, H. A comparative study on the antimicrobial activity of irreversible hydrocolloid mixed with silver nanoparticles and chlorhexidine. Dent. Res. J. 2020, 17, 120–125. [Google Scholar]
- Hamedi Rad, F.; Ghaffari, T.; Safavi, S.H. In vitro evaluation of dimensional stability of alginate impressions after disinfection by spray and immersion methods. J. Dent. Res. Dent. Clin. Dent. Prospect 2010, 4, 130–135. [Google Scholar] [CrossRef]
- Ismail, H.A.; Mahross, H.Z.; Shikho, S. Evaluation of dimensional accuracy for different complete edentulous impressions immersed in different disinfectant solutions. Eur. J. Dent. 2017, 11, 242–249. [Google Scholar] [CrossRef]
- Hudecki, A.; Pawlyta, M.; Dobrzański, L.A.; Chladek, G. Examination of the surface properties of ceramic micro and nanoparticles. J. Achiev. Mater. Manuf. Eng. 2013, 61, 257–262. [Google Scholar]
- Qin, Y. Silver-containing alginate fibres and dressings. Int. Wound J. 2005, 2, 172–176. [Google Scholar] [CrossRef]
- AlphaSan Characteristic. Available online: https://www.milliken.com/en-us/businesses/chemical/product/alphasan (accessed on 28 September 2023).
- Guiraldo, R.D.; Moreti, A.F.; Martinelli, J.; Berger, S.B.; Meneghel, L.L.; Caixeta, R.V.; Sinhoreti, M.A. Influence of alginate impression materials and storage time on surface detail reproduction and dimensional accuracy of stone models. Acta Odontol. Latinoam. 2015, 28, 156–161. [Google Scholar] [CrossRef]
- ISO 21563:2021; Dentistry—Hydrocolloid Impression Materials. ISO: Geneva, Switzerland, 2021.
- Abdelraouf, R.M. Chemical analysis and microstructure examination of extended-pour alginate impression versus conventional one (characterization of dental extended-pour alginate). Int. J. Polym. Mater. Polym. Biomater. 2017, 67, 612–618. [Google Scholar] [CrossRef]
- Sharif, R.A.; Abdelaziz, K.M.; Alshahrani, N.M. The accuracy of gypsum casts obtained from the disinfected extended-pour alginate impressions through prolonged storage times. BMC Oral. Health 2021, 21, 296. [Google Scholar] [CrossRef]
- Walker, M.P.; Burckhard, J.; Mitts, D.A.; Williams, K.B. Dimensional change over time of extended-storage alginate impression materials. Angle Orthod. 2010, 80, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Köhler, B.; Bratthall, D. Practical method to facilitate estimation of Streptococcus mutans levels in saliva. J. Clin. Microbiol. 1979, 9, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Odusote, J.K.; Danyuo, Y.; Baruwa, A.D.; Azeez, A.A. Synthesis and characterization of hydroxyapatite from bovine bone for production of dental implants. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019836829. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.; Thakur, A. Thermal stability and kinetics of sodium alginate and lignosulphonic acid blends. Iran. J. Mater. Sci. Eng. 2019, 15, 53–59. [Google Scholar]
- Liu, Y.; Zou, C.; Li, C.; Lin, L.; Chen, W. Evaluation of β-cyclodextrin–polyethylene glycol as green scale inhibitors for produced-water in shale gas well. Desalination 2016, 377, 28–33. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Wu, J.; Luo, J.; Wang, Y.; Li, Q. Synthesis of a novel injectable alginate impression material and impression accuracy evaluation. Hua Xi Kou Qiang Yi Xue Za Zhi 2022, 40, 662–667. [Google Scholar] [CrossRef]
- Abdelraouf, R.M.; Bayoumi, R.E.; Hamdy, T.M. Effect of Powder/Water Ratio Variation on Viscosity, Tear Strength and Detail Reproduction of Dental Alginate Impression Material (In Vitro and Clinical Study). Polymers 2021, 13, 2923. [Google Scholar] [CrossRef]
- Nallamuthu, N.A.; Braden, M.; Patel, M.P. Some aspects of the formulation of alginate dental impression materials—Setting characteristics and mechanical properties. Dent. Mater. 2012, 28, 756–762. [Google Scholar] [CrossRef]
- Lemon, J.C.; Okay, D.J.; Powers, J.M.; Martin, J.W.; Chambers, M.S. Facial moulage: The effect of a retarder on compressive strength and working and setting times of irreversible hydrocolloid impression material. J. Prosthet. Dent. 2003, 90, 276–281. [Google Scholar] [CrossRef]
- Yin, I.X.; Yu, O.Y.; Zhao, I.S. Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch. Oral. Biol. 2019, 102, 106–112. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Heo, D.N.; Lee, D. One-step fabrication of AgNPs embedded hybrid dual nanofibrous oral wound dressings. J. Biomed. Nanotechnol. 2016, 12, 2041–2050. [Google Scholar] [CrossRef] [PubMed]








| Name Manufacturer | Composition [a] | Lot Number | Colour Change Under Working [b] | Mixing Ratio Powder/Liquid [g/mL] Mixing Time [s] |
|---|---|---|---|---|
| Kromopan Lascod, Florence, Italy | Diatomaceous earth [60–80%], alginate, sodium phosphates, gypsum, potassium hexafluorotitanate [1–2.5%] flavours | 0173310184 | Violet to white | 9/20 [g/mL] 45 [s] |
| Tropical IQ Lascod, Florence, Italy | Diatomaceous earth [60–80%], alginate, sodium phosphates, gypsum, potassium hexafluorotitanate [1–2.5%] flavours | 0172371112 | Red to orange to yellow | 10, 5/20 [g/mL] 45 [s] |
| Elastic Cromo SpofaDental, Jicin, Czech Republic | Diatomaceous earth, sodium alginate, sodium phosphates, gypsum, potassium hexafluorotitanate flavours | 10043390 | Violet to white | 9/20 [g/mL] 30 [s] |
| KromaFaze Kerr, Orange, USA | Diatomaceous earth, potassium alginate, sodium phosphates, gypsum, potassium hexafluorotitanate flavours | 9888019 | Blue to white | 7, 5/16 [g/mL] 30 [s] |
| X1 Developed material with 0.25% AlphSan | Table 2 | N/A | No colour changes green colour | 6/16 [g/mL] 30 [s] |
| X2 Developed material with 0.25% AlphSan | Table 2 | N/A | No colour changes green colour | 6/16 [g/mL] 30 [s] |
| X3 Developed material with 0.25% AlphSan | Table 2 | N/A | No colour changes green colour | 6/16 [g/mL] 30 [s] |
| Material | Concentration [wt%] | Producer |
|---|---|---|
| Mineral oil Dratex | 0.80 | Texas oil (Beamont, TX, USA) |
| Color N.V GWT-11 Green | 1.50 | Radiant (Houthalen, Belgium) |
| Kimica Algin 2G-200W Kimica (Potassium Alginate) | 11.20 | Kimica (Tokyo, Japan) |
| K2TiF6 | 2.37 | Merck (Praha, Czech Republic) |
| Celite 281 | 75.25 | Merck (Praha, Czech Republic) |
| ZnO | 1.50 | Merck (Praha, Czech Republic) |
| Gypsum dihydrate | 6.25 | Merck (Praha, Czech Republic) |
| Na4P2O7 | 0.50 | Merck (Praha, Czech Republic) |
| MgO | 0.50 | Merck (Praha, Czech Republic) |
| Material | Tonset1 [°C] | Tmax1 [°C] | Tonset2 [°C] | Tmax2 [°C] | Residual Mass [%] |
|---|---|---|---|---|---|
| Sodium alginate | 48.1 | 66.3 | 230.6 | 244.9 | 31.1 |
| Potassium Alginate | 47.6 | 66.3 | 230.8 | 242.9 | 31.8 |
| Kroma Faze | 96.7 | 105.6 | 231.5 | 244.6 | 86.6 |
| Tropical IQ | 85.3 | 98.2 | 224.7 | 243.3 | 80.6 |
| Kromopan | 91.8 | 102.7 | 229.9 | 244.5 | 88.5 |
| Elastic Cromo | 93.7 | 104.6 | 226.3 | 240.2 | 87.7 |
| X3 | 95.7 | 107.5 | 229.8 | 243.5 | 86.6 |
| Material | Setting Time [s] | Contraction after 24 h [%] |
|---|---|---|
| Kromopan | 132.2 ± 2.8 [a] | 0.83 ± 0.03 [a] |
| Tropical IQ | 107.4 ± 2.1 [a] | 1.14 ± 0.1 |
| Elastic Cromo | 143.2 ± 2.4 [a] | 0.90 ± 0.09 [a] |
| KromaFaze | 147.4 ± 1.7 [a] | 1.02 ± 0.08 [a] |
| X1 | 131.2 ± 1.3 | 1.10 ± 0.06 [a] |
| X2 | 127.2 ± 2.6 [b] | 1.22 ± 0.08 [a] |
| X3 | 131.6 ± 1.1 | 1.08 ± 0.06 [a] |
| Material | Compressive Strength [MPa] | Elastic Recovery [%] | Strain in Compression [%] | Tearing Strength [MPa] |
| Kromopan | 1.04 ± 0.018 [a] | 97.28 ± 0.13 [a] | 13.96 ± 0.29 [a] | 0.54 ± 0.02 [a] |
| Tropical IQ | 0.87 ± 0.016 | 96.88 ± 0.2 [a] | 11.22 ± 0.13 [a] | 0.50 ± 0.02 |
| Elastic Cromo | 1.07 ± 0.028 [a] | 97.07 ± 0.13 | 13.51 ± 0.3 [a] | 0.54 ± 0.02 |
| KromaFaze | 0.84 ± 0.033 | 96.95 ± 0.1 [a] | 11.76 ± 0.07 [a] | 0.57 ± 0.02 |
| X1 | 0.85 ± 0.015 | 96.92 ± 0.11 [a] | 12..49 ± 0.29 | 0.55 ± 0.01 |
| X2 | 0.85 ± 0.016 | 96.97 ± 0.11 [a] | 11.76 ± 0.33 [b] | 0.47 ± 0.02 [a] |
| X3 | 0.84 ± 0.008 [a] | 96.67 ± 0.07 [a] | 11.32 ± 0.26 | 0.44 ± 0.01 [a] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raszewski, Z.; Mikulewicz, M.; Brząkalski, D.; Pakuła, D.; Przekop, R.E. Comparison of the Bioactive and Bacteriostatic Performance of Different Alginate-Based Dental Prosthetic Impression Materials with and without Zirconium Phosphate-Based Ion Exchange Resin Containing Silver: An In Vitro Study. Appl. Sci. 2023, 13, 11639. https://doi.org/10.3390/app132111639
Raszewski Z, Mikulewicz M, Brząkalski D, Pakuła D, Przekop RE. Comparison of the Bioactive and Bacteriostatic Performance of Different Alginate-Based Dental Prosthetic Impression Materials with and without Zirconium Phosphate-Based Ion Exchange Resin Containing Silver: An In Vitro Study. Applied Sciences. 2023; 13(21):11639. https://doi.org/10.3390/app132111639
Chicago/Turabian StyleRaszewski, Zbigniew, Marcin Mikulewicz, Dariusz Brząkalski, Daria Pakuła, and Robert E. Przekop. 2023. "Comparison of the Bioactive and Bacteriostatic Performance of Different Alginate-Based Dental Prosthetic Impression Materials with and without Zirconium Phosphate-Based Ion Exchange Resin Containing Silver: An In Vitro Study" Applied Sciences 13, no. 21: 11639. https://doi.org/10.3390/app132111639






