Protective Effect of Iris germanica L. Rhizome-Derived Exosome against Oxidative-Stress-Induced Cellular Senescence in Human Epidermal Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
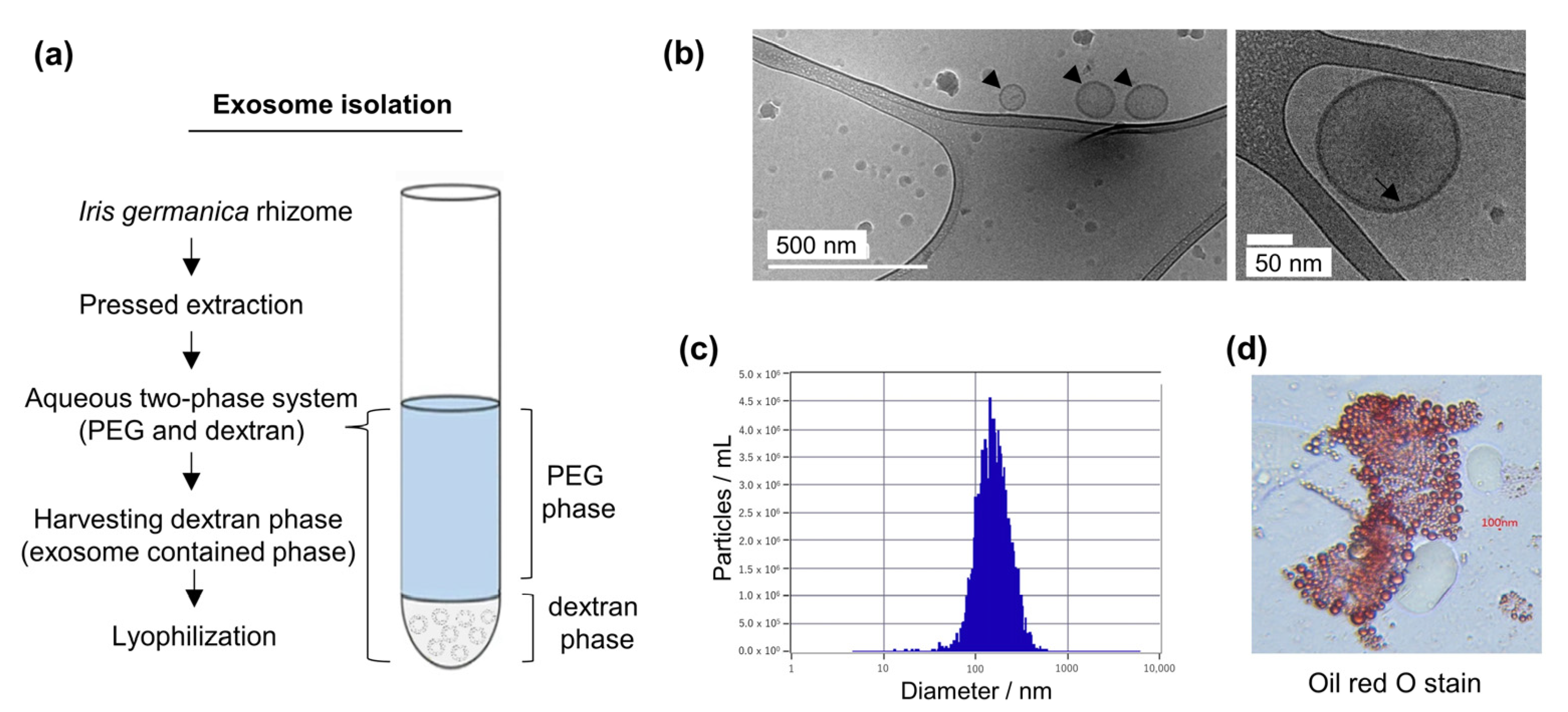
2.1. Isolation and Characterization of Iris germanica L. Rhizome-Derived Exosomes (Iris-Exosomes)
2.1.1. Cryo-TEM Analysis
2.1.2. Nanoparticle Tracking Analysis (NTA)
2.1.3. Oil Red O Staining
2.2. Cell Culture and Reagents
2.3. In Vitro DPPH and ABTS Radical Scavenging Assays
2.4. Cell Viability Assay
2.5. Intracellular ROS Measurement Assay
2.6. Cellular-Senescence-Analysis-Associated SA-β-Galactosidase Activity
2.7. Polymerase Chain Reaction (PCR) and Quantitative Reverse Transcription PCR (RT-qPCR)
2.8. Immunoblot Analysis
2.9. Keratinocyte Differentiation
2.10. Wound-Healing Assay
2.11. Statistical Analysis
3. Results
3.1. Purification and Characterization of Iris germanica L. Rhizome-Derived Exosome
3.2. Iris-Exosome Protects nHEKs from the Loss of Cell Viability Caused by Oxidative Stress
3.3. Iris-Exosome Reduces Intracellular ROS Levels in nHEKs
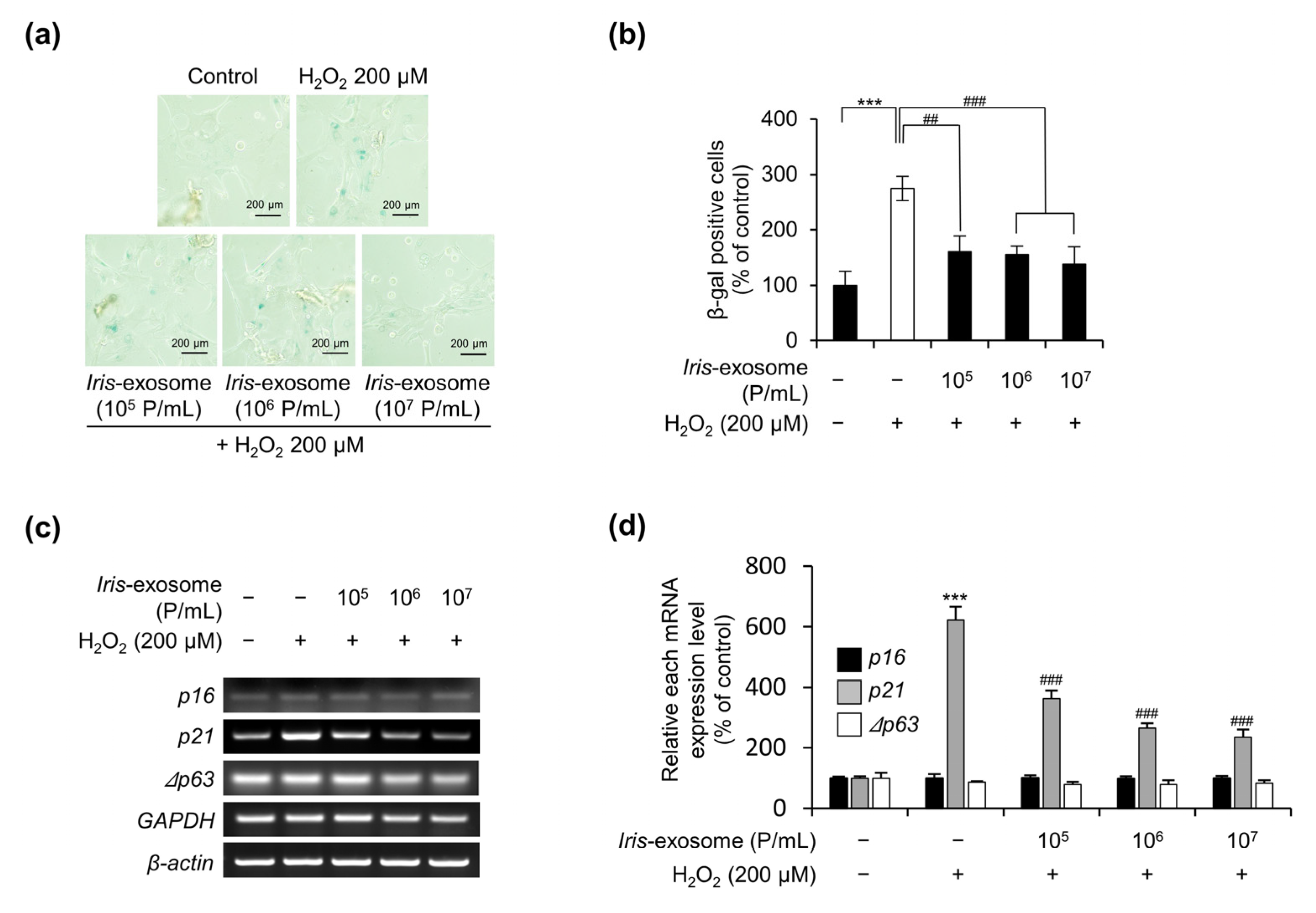
3.4. Iris-Exosome Is Capable of Attenuating Cellular Senescence Induced by H2O2
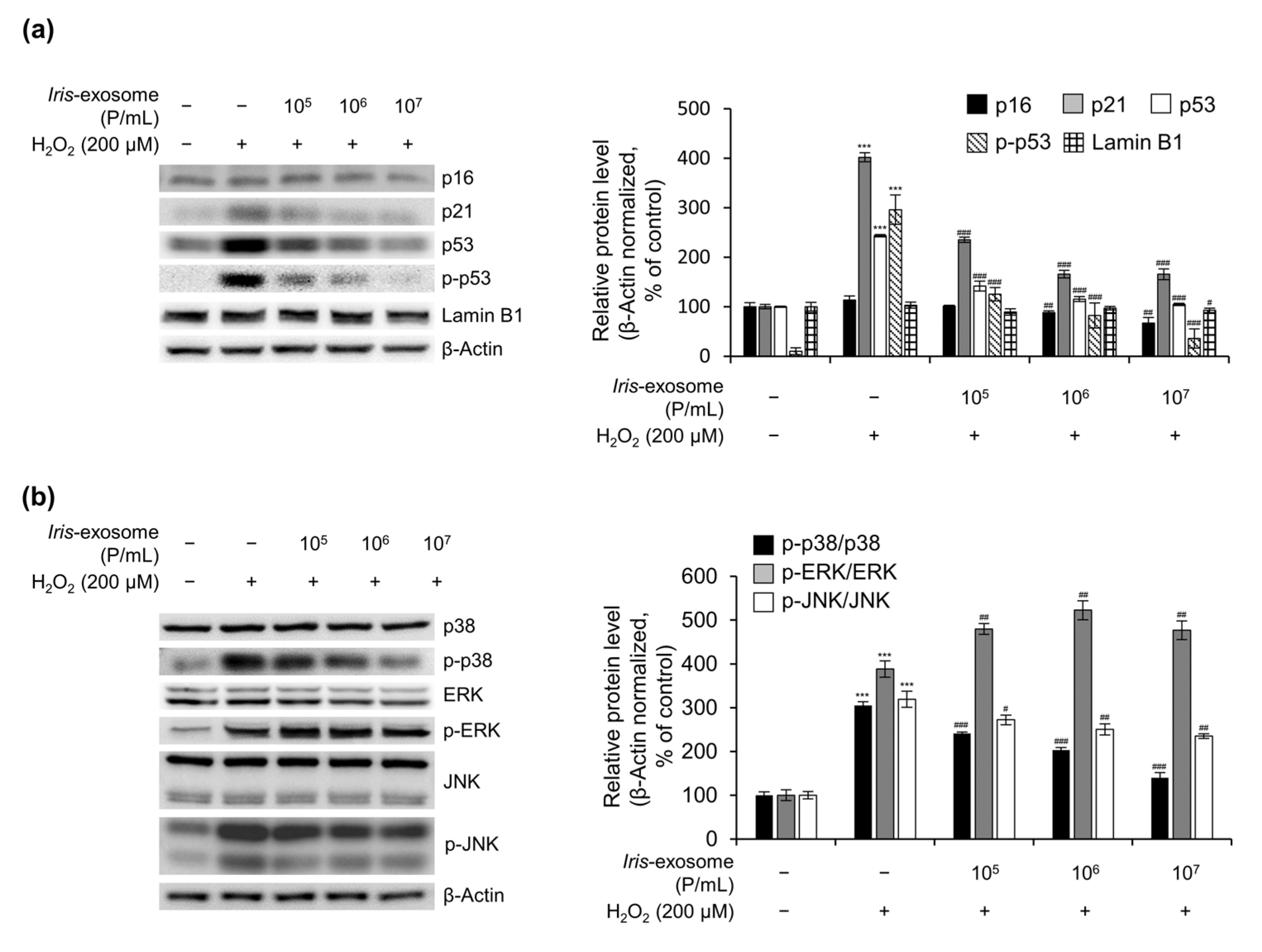
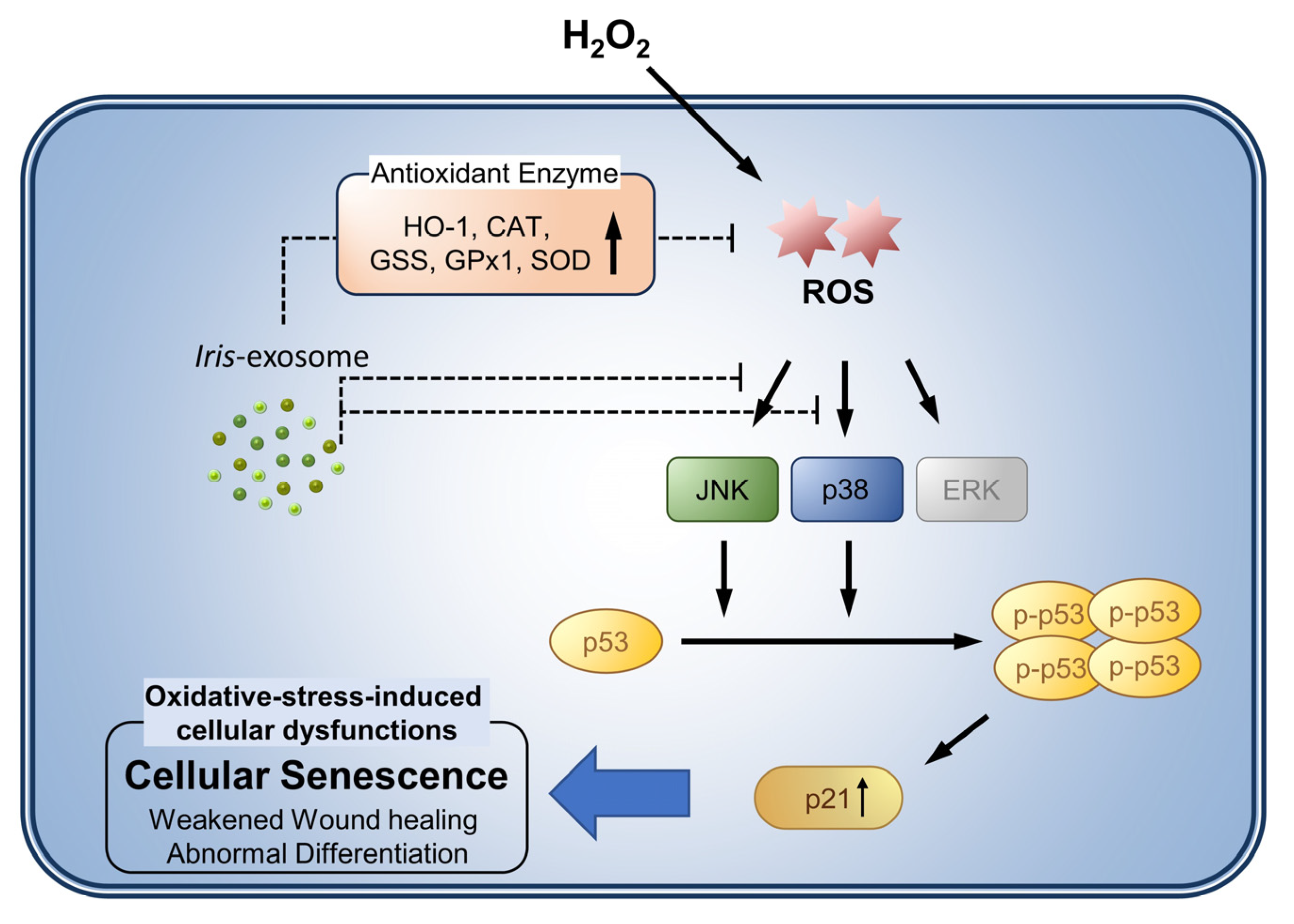
3.5. Iris-Exosome Inhibits H2O2-Induced Cellular Oxidative Stress via p38 Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway
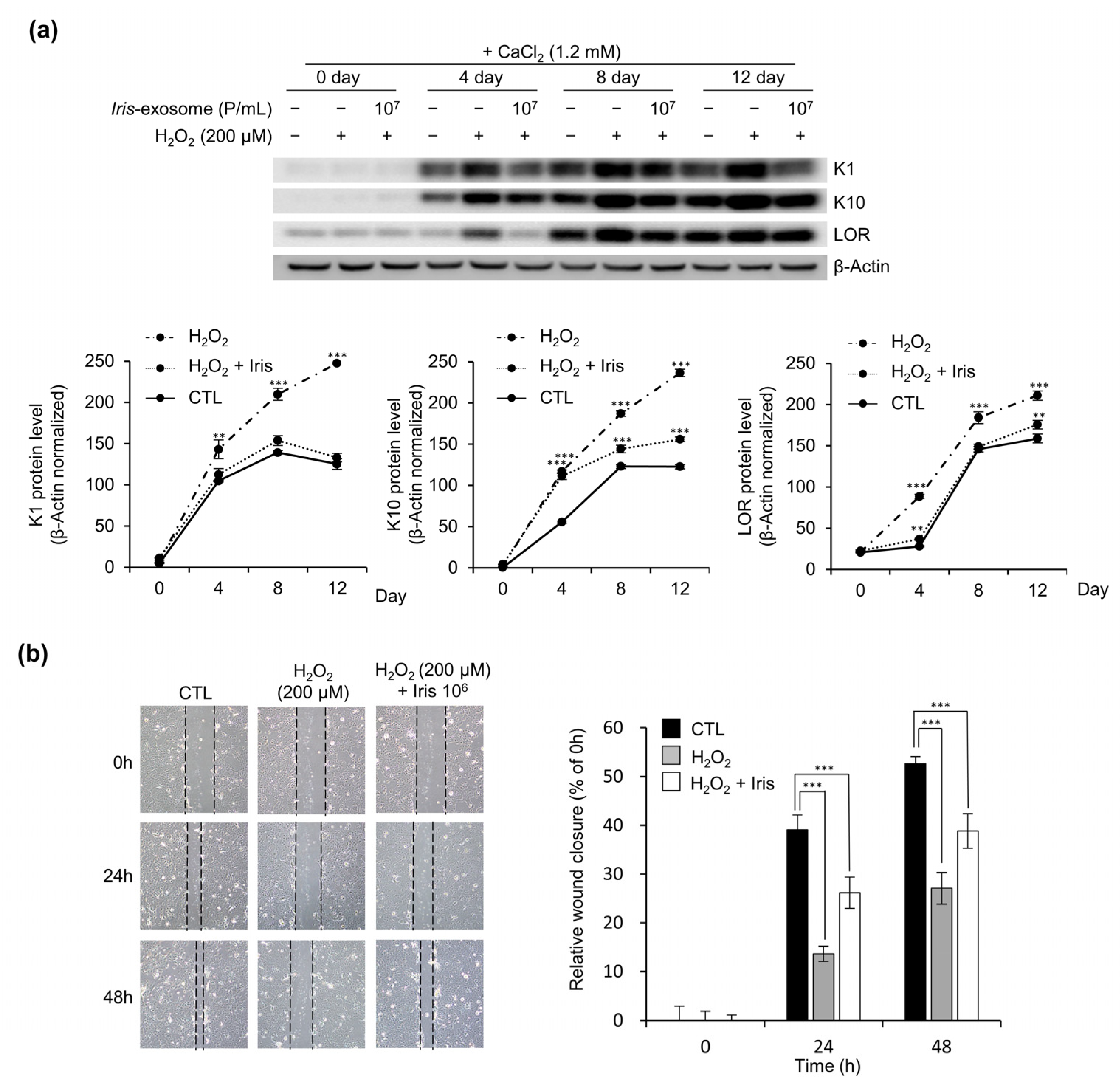
3.6. Iris-Exosome Improves Oxidative-Stress-Induced Impairment of Keratinocyte Barrier Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell. Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef]
- Karampoga, A.; Tzaferi, K.; Koutsakis, C.; Kyriakopoulou, K.; Karamanos, N. Exosomes and the extracellular matrix: A dynamic interplay in cancer progression. Int. J. Dev. Biol. 2022, 66, 97–102. [Google Scholar] [CrossRef]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell. Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Vahabi, A.; Rezaie, J.; Hassanpour, M.; Panahi, Y.; Nemati, M.; Rasmi, Y.; Nemati, M. Tumor Cells-derived exosomal CircRNAs: Novel cancer drivers, molecular mechanisms, and clinical opportunities. Biochem. Pharmacol. 2022, 200, 115038. [Google Scholar] [CrossRef]
- Yang, G.H.; Lee, Y.B.; Kang, D.; Choi, E.; Nam, Y.; Lee, K.H.; You, H.J.; Kang, H.J.; An, S.H.; Jeon, H. Overcome the barriers of the skin: Exosome therapy. Biomater. Res. 2021, 25, 22. [Google Scholar] [CrossRef]
- Kwack, M.H.; Seo, C.H.; Gangadaran, P.; Ahn, B.C.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 2019, 28, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, M.; Sarra, A.; D’Oria, V.; Mura, F.; Bordi, F.; Postorino, P.; Fratantonio, D. Blueberry-Derived Exosome-Like Nanoparticles Counter the Response to TNF-α-Induced Change on Gene Expression in EA.hy926 Cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Meetham, P.; Kanlayavattanakul, M.; Lourith, N. Development and clinical efficacy evaluation of anti-greasy green tea tonner on facial skin. Rev. Bras. Farmacogn. 2018, 28, 214–217. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, S.; Zhu, Y.; Wang, R.; Wang, J. Amelioration of colitis progression by ginseng-derived exosome-like nanoparticles through suppression of inflammatory cytokines. J. Ginseng Res. 2023, 47, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Park, H.M.; Kim, Y.B.; Kim, H.; Kim, N.; Do, J.H.; Kang, C.; Cho, Y.; Kim, S.Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J. Ethnopharmacol. 2009, 123, 446–451. [Google Scholar] [CrossRef]
- Cho, J.H.; Hong, Y.D.; Kim, D.; Park, S.J.; Kim, J.S.; Kim, H.-M.; Yoon, E.J.; Cho, J.-S. Confirmation of plant-derived exosomes as bioactive substances for skin application through comparative analysis of keratinocyte transcriptome. Appl. Biol. Chem. 2022, 65, 8. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Baumann, L. Skin ageing and its treatment. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, J.; Kurokawa, T.; Tanizaki, H.; Moriwaki, S. The evaluation of oxidative stress in patients with psoriasis vulgaris and atopic dermatitis by measuring the urinary level of 8-hydroxy-2′-deoxyguanosine. J. Cutan. Immunol. Allergy 2019, 2, 163–168. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, J.E.; Kim, H.-S. Caffeic acid induces keratinocyte differentiation by activation of PPAR-α. J. Pharm. Pharmacol. 2014, 66, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.R.; Tan, C.L.; Chin, T.; Morenc, M.; Ho, C.Y.; Rovito, H.A.; Quek, L.S.; Soon, A.L.; Lim, J.S.; Dreesen, O. Nicotinamide prevents UVB-and oxidative stress-induced photoaging in human primary keratinocytes. J. Investig. Dermatol. 2022, 142, 1670–1681.e12. [Google Scholar] [CrossRef]
- Zhao, F.; Lang, H.; Wang, Z.; Zhang, T.; Zhang, D.; Wang, R.; Lin, X.; Liu, X.; Shi, P.; Pang, X. Human novel microRNA Seq-915_x4024 in keratinocytes contributes to skin regeneration by suppressing scar formation. Mol. Ther. Nucleic Acids 2019, 14, 410–423. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Glasauer, A.; Hoover, P.; Yang, S.; Blatt, H.; Mullen, A.R.; Getsios, S.; Gottardi, C.J.; DeBerardinis, R.J.; Lavker, R.M. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci. Signal. 2013, 6, ra8. [Google Scholar] [CrossRef]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: A single-blind case-control clinical study. Oxidative Med. Cell. Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Asad, M.H.H.B.; Akram, M.R.; Kalsoom Khan, A.; Malik, A.; Chen, C.; Murtaza, G. Anti-Aging Potential of Phytoextract Loaded-Pharmaceutical Creams for Human Skin Cell Longetivity. Oxidative Med. Cell. Longev. 2015, 2015, 709628. [Google Scholar] [CrossRef]
- de Szalay, S.; Wertz, P.W. Protective Barriers Provided by the Epidermis. Int. J. Mol. Sci. 2023, 24, 3145. [Google Scholar] [CrossRef]
- Brüggen, M.C.; Stingl, G. Subcutaneous white adipose tissue: The deepest layer of the cutaneous immune barrier. J. Dtsch. Dermatol. Ges. 2020, 18, 1225–1227. [Google Scholar] [CrossRef]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef] [PubMed]
- Zingkou, E.; Pampalakis, G.; Sotiropoulou, G. Keratinocyte differentiation and proteolytic pathways in skin (patho) physiology. Int. J. Dev. Biol. 2021, 66, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Woodley, D.T.; Wysong, A.; DeClerck, B.; Chen, M.; Li, W. Keratinocyte Migration and a Hypothetical New Role for Extracellular Heat Shock Protein 90 Alpha in Orchestrating Skin Wound Healing. Adv. Wound Care 2015, 4, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Phenotypic and lipidomic characterization of primary human epidermal keratinocytes exposed to simulated solar UV radiation. J. Dermatol. Sci. 2018, 92, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Shin, K.O.; Muraoka, S.; Choi, Y.; Park, J.H.; Park, S.H.; Hwang, J.T.; Park, K.; Uchida, Y. The Epidermal Environment’s Influence on the Dermal Environment in Response to External Stress. Skin Pharmacol. Physiol. 2023, 36, 149–159. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Taverne, Y.J.; Bogers, A.J.; Duncker, D.J.; Merkus, D. Reactive oxygen species and the cardiovascular system. Oxidative Med. Cell. Longev. 2013, 2013, 862423. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Ahmed, E.K.; Rogowska-Wrzesinska, A.; Roepstorff, P.; Bulteau, A.L.; Friguet, B. Protein modification and replicative senescence of WI-38 human embryonic fibroblasts. Aging Cell 2010, 9, 252–272. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. The cornified envelope: A first line of defense against reactive oxygen species. J. Investig. Dermatol. 2011, 131, 1409–1411. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Heard, C.M.; Garcia-Pardo, J. Bioactive Molecules from Plants: Discovery and Pharmaceutical Applications. Pharmaceutics 2022, 14, 2116. [Google Scholar] [CrossRef]
- Kiasi, Y.; Forouzeh, M.R.; Mirdeilami, S.Z.; Niknahad-Gharmakher, H. Ethnobotanical Study on the Medicinal Plants in Khosh Yeilagh Rangeland, Golestan Province, Iran. 2020. Available online: https://www.researchsquare.com/article/rs-103978/v1 (accessed on 18 October 2023).
- Nguyen, T.S.; Xia, N.H.; Van Chu, T.; Van Sam, H. Ethnobotanical study on medicinal plants in traditional markets of Son La province, Vietnam. For. Soc. 2019, 3, 171–192. [Google Scholar] [CrossRef]
- Rokaya, M.B.; Münzbergová, Z.; Timsina, B. Ethnobotanical study of medicinal plants from the Humla district of western Nepal. J. Ethnopharmacol. 2010, 130, 485–504. [Google Scholar] [CrossRef]
- Askin, H.; Yilmaz, B.; Gulcin, I.; Taslimi, P.; Bakirci, S.; Yildiz, M.; Kandemir, N. Antioxidant Activity of the Aqueous Extract of Iris taochia and Identification of its Chemical Constituents. Indian J. Pharm. Sci. 2018, 80, 802–812. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Gašić, U.M.; Pešić, M.B.; Stanojević, S.P.; Barać, M.B.; Mačukanović-Jocić, M.P.; Avramov, S.N.; Tešić, Ž.L. Phytochemical analysis and total antioxidant capacity of rhizome, above-ground vegetative parts and flower of three Iris species. Chem. Biodivers. 2019, 16, e1800565. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, K.; Hdayed, I.; Tabcheh, M.; Abdel-Razzak, Z.; El-Bitar, H. Antioxidant properties of the Lebanese plant Iris x germanica L. crude extracts and antagonism of chlorpromazine toxicity on Saccharomyces cerevisiae. Drug Chem. Toxicol. 2022, 45, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-S.; Hong, S.-W.; Lee, J.-G.; Lee, Y.-M.; Kim, D.-W.; Kim, J.-E.; Jung, D.-J.; An, S.-K.; Hong, N.-J.; Kim, D. An ethanol extract of Iris nertschinskia induces p53-dependent apoptosis in the MCF7 human breast cancer cell line. Int. J. Mol. Med. 2011, 27, 401–405. [Google Scholar] [PubMed]
- Uzair, A.; Bakht, J.; Iqbal, A.; Naveed, K.; Ali, N. In vitro antimicrobial activities of different solvent extracted samples from Iris germanica. Pak. J. Pharm. Sci. 2016, 29, 145–150. [Google Scholar] [PubMed]
- Amin, H.I.M.; Hussain, F.H.S.; Najmaldin, S.K.; Thu, Z.M.; Ibrahim, M.F.; Gilardoni, G.; Vidari, G. Phytochemistry and Biological Activities of Iris Species Growing in Iraqi Kurdistan and Phenolic Constituents of the Traditional Plant Iris postii. Molecules 2021, 26, 264. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Sieniawska, E.; Widelski, J.; Urjin, O.; Głowniak, P.; Skalicka-Woźniak, K. Major secondary metabolites of Iris spp. Phytochem. Rev. 2015, 14, 51–80. [Google Scholar] [CrossRef]
- Roger, B.; Jeannot, V.; Fernandez, X.; Cerantola, S.; Chahboun, J. Characterisation and quantification of flavonoids in Iris germanica L. and Iris pallida Lam. resinoids from Morocco. Phytochem. Anal. 2012, 23, 450–455. [Google Scholar] [CrossRef]
- Rahman, A.U.; Nasim, S.; Baig, I.; Jalil, S.; Orhan, I.; Sener, B.; Choudhary, M.I. Anti-inflammatory isoflavonoids from the rhizomes of Iris germanica. J. Ethnopharmacol. 2003, 86, 177–180. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Al-Musayeib, N.M. New constituents from the rhizomes of Egyptian Iris germanica L. Molecules 2012, 17, 2587–2598. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Zayed, M.F.; Ross, S.A. 8-Hydroxyirilone 5-methyl ether and 8-hydroxyirilone, new antioxidant and α-amylase inhibitors isoflavonoids from Iris germanica rhizomes. Bioorg. Chem. 2017, 70, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, E.-J.; Lee, J.H.; Park, I.-C.; Lee, S.-J.; Hahn, H.J.; Ahn, K.J.; An, S.; An, I.-S.; Cha, H.J. Oridonin protects HaCaT keratinocytes against hydrogen peroxide-induced oxidative stress by altering microRNA expression. Int. J. Mol. Med. 2014, 33, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Pelle, E.; Mammone, T.; Maes, D.; Frenkel, K. Keratinocytes act as a source of reactive oxygen species by transferring hydrogen peroxide to melanocytes. J. Investig. Dermatol. 2005, 124, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Sritularak, B.; Likhitwitayawuid, K. New bisbibenzyls from Dendrobium falconeri. Helv. Chim. Acta 2009, 92, 740–744. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Lu, T.; Finkel, T. Free radicals and senescence. Exp. Cell Res. 2008, 314, 1918–1922. [Google Scholar] [CrossRef]
- Wang, A.S.; Ong, P.F.; Chojnowski, A.; Clavel, C.; Dreesen, O. Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci. Rep. 2017, 7, 15678. [Google Scholar] [CrossRef]
- Waaijer, M.E.; Parish, W.E.; Strongitharm, B.H.; van Heemst, D.; Slagboom, P.E.; de Craen, A.J.; Sedivy, J.M.; Westendorp, R.G.; Gunn, D.A.; Maier, A.B. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 2012, 11, 722–725. [Google Scholar] [CrossRef]
- Valieva, Y.; Ivanova, E.; Fayzullin, A.; Kurkov, A.; Igrunkova, A. Senescence-Associated β-Galactosidase Detection in Pathology. Diagnostics 2022, 12, 2309. [Google Scholar] [CrossRef]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.D. P16INK4A-More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Ressler, S.; Bartkova, J.; Niederegger, H.; Bartek, J.; Scharffetter-Kochanek, K.; Jansen-Dürr, P.; Wlaschek, M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Ravelojaona, V.; Robert, A.M.; Robert, L. Expression of senescence-associated beta-galactosidase (SA-beta-Gal) by human skin fibroblasts, effect of advanced glycation end-products and fucose or rhamnose-rich polysaccharides. Arch. Gerontol. Geriatr. 2009, 48, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Lee, H.L.; Jeong, J.H.; Yoon, Y.E.; Lee, I.-R.; Kim, J.M.; Wu, C.; Lee, S.-J. OR2AT4, an ectopic olfactory receptor, suppresses oxidative stress-induced senescence in human keratinocytes. Antioxidants 2022, 11, 2180. [Google Scholar] [CrossRef]
- Sasaki, M.; Kajiya, H.; Ozeki, S.; Okabe, K.; Ikebe, T. Reactive oxygen species promotes cellular senescence in normal human epidermal keratinocytes through epigenetic regulation of p16INK4a. Biochem. Biophys. Res. Commun. 2014, 452, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Wattanapitayakul, S.K.; Chularojmontri, L.; Schäfer-Korting, M. Ultraviolet B irradiation-induced keratinocyte senescence and impaired development of 3D epidermal reconstruct. Acta Pharm. 2021, 71, 293–303. [Google Scholar] [CrossRef]
- Ido, Y.; Duranton, A.; Lan, F.; Cacicedo, J.M.; Chen, T.C.; Breton, L.; Ruderman, N.B. Acute activation of AMP-activated protein kinase prevents H2O2-induced premature senescence in primary human keratinocytes. PLoS ONE 2012, 7, e35092. [Google Scholar] [CrossRef]
- Dorion, S.; Landry, J. Activation of the mitogen-activated protein kinase pathways by heat shock. Cell Stress Chaperones 2002, 7, 200–206. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Banerjee, P.; Nikolakaki, E.; Dai, T.; Rubie, E.A.; Ahmad, M.F.; Avruch, J.; Woodgett, J.R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 1994, 369, 156–160. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.; Chung, H.-T.; Pae, H.-O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar]
- Wang, L.; Xie, Y.; Yang, W.; Yang, Z.; Jiang, S.; Zhang, C.; Zhang, G. Alfalfa polysaccharide prevents H2O2-induced oxidative damage in MEFs by activating MAPK/Nrf2 signaling pathways and suppressing NF-κB signaling pathways. Sci. Rep. 2019, 9, 1782. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, Y.M.; Song, S.; Lee, Y.Y.; Yeum, K.J. Black soybeans protect human keratinocytes from oxidative stress-induced cell death. Food Sci. Nutr. 2018, 6, 2423–2430. [Google Scholar] [CrossRef]
- Park, G.B.; Choi, Y.; Kim, Y.S.; Lee, H.-K.; Kim, D.; Hur, D.Y. ROS-mediated JNK/p38-MAPK activation regulates Bax translocation in Sorafenib-induced apoptosis of EBV-transformed B cells. Int. J. Oncol. 2014, 44, 977–985. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Jiang, K.; Liu, J.; Liu, Z. Dural effects of oxidative stress on cardiomyogenesis via Gata4 transcription and protein ubiquitination. Cell Death Dis. 2018, 9, 246. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, A.S.; Choi, I. Melandrii Herba extract attenuates H2O2-induced neurotoxicity in human neuroblastoma SH-SY5Y cells and Scopolamine-induced memory impairment in mice. Molecules 2017, 22, 1646. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.I.; Borodkina, A.V.; Nikolsky, N.N.; Burova, E.B. Relationship between p53/p21/Rb and MAPK signaling pathways in human endometrium-derived stem cells under oxidative stress. Tsitologiia 2015, 57, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol. Ther. 2004, 3, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Thornton, T.M.; Rincon, M. Non-classical p38 map kinase functions: Cell cycle checkpoints and survival. Int. J. Biol. Sci. 2009, 5, 44. [Google Scholar] [CrossRef]
- Shi, T.; Dansen, T.B. Reactive oxygen species induced p53 activation: DNA damage, redox signaling, or both? Antioxid. Redox Signal. 2020, 33, 839–859. [Google Scholar] [CrossRef]
- Hanson, R.L.; Batchelor, E. Coordination of MAPK and p53 dynamics in the cellular responses to DNA damage and oxidative stress. Mol. Syst. Biol. 2022, 18, e11401. [Google Scholar] [CrossRef]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Kanta, J. The role of hydrogen peroxide and other reactive oxygen species in wound healing. Acta Medica 2011, 54, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.E.K.; Halliwell, B. Effects of hydrogen peroxide in a keratinocyte-fibroblast co-culture model of wound healing. Biochem. Biophys. Res. Commun. 2012, 423, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.E.K.; Ho, R.; Halliwell, B. Mechanism of hydrogen peroxide-induced keratinocyte migration in a scratch-wound model. Free. Radic. Biol. Med. 2011, 51, 884–892. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Lee, H.-J.; Yoon, E.-J.; Lee, H.; Ji, Y.; Kim, Y.; Park, S.-J.; Kim, J.; Bae, S. Protective Effect of Iris germanica L. Rhizome-Derived Exosome against Oxidative-Stress-Induced Cellular Senescence in Human Epidermal Keratinocytes. Appl. Sci. 2023, 13, 11681. https://doi.org/10.3390/app132111681
Kim J-S, Lee H-J, Yoon E-J, Lee H, Ji Y, Kim Y, Park S-J, Kim J, Bae S. Protective Effect of Iris germanica L. Rhizome-Derived Exosome against Oxidative-Stress-Induced Cellular Senescence in Human Epidermal Keratinocytes. Applied Sciences. 2023; 13(21):11681. https://doi.org/10.3390/app132111681
Chicago/Turabian StyleKim, Ji-Seon, Hyun-Jeong Lee, Eun-Jeong Yoon, Hyunsang Lee, Youngeun Ji, Youngseok Kim, Si-Jun Park, Junoh Kim, and Seunghee Bae. 2023. "Protective Effect of Iris germanica L. Rhizome-Derived Exosome against Oxidative-Stress-Induced Cellular Senescence in Human Epidermal Keratinocytes" Applied Sciences 13, no. 21: 11681. https://doi.org/10.3390/app132111681




