Abstract
Mediterranean olive cultivation faces challenges in the global environmental change context. Pests and diseases caused by arthropods such as Bactrocera oleae, Prays oleae, and certain vectors of Xylella fastidiosa are expected to increase and spread in part due to this global scenario. The control of these arthropods has relied on synthetic pesticides, the misuse of which has led to pest population resistance and concerns about their negative impacts on biodiversity and global health. Integrated pest management (IPM) methods have emerged through the careful consideration of all available control techniques and the subsequent integration of appropriate measures that discourage the development of pest populations. This paper reviews the IPM guidelines for olive cultivation, prioritizing the use of biological control methods, and the integration of genetics and biotechnology, which bring precision, efficacy, and safety. It evidences the importance of genetic analysis in pest populations, pesticide resistance and in the contributions of predators to pest control. Advances in formulations and delivery systems for pesticides such as Bacillus thuringiensis, plant-incorporated protectants, improved SIT techniques, and the specific efficacy of biologicals pesticides are covered. Finally, this paper explores promising tools such as RNAi and gene drive while recognizing the ethical, environmental, and regulatory challenges associated with their use. Shortly, these innovations have the potential to reduce the environmental impacts of pests while ensuring the long-term viability of the olive industry.
1. Introduction
The historical significance of the wild olive tree stretches back to the Neolithic period, where it was recognized for its diverse applications, including the extraction of essential oils, its use as a lamp fuel, and its use as a source of wood [1]. However, it was during its domestication that the olive tree truly blossomed, becoming a pervasive and integral part of human culture approximately 6000 to 5500 years ago [2,3,4,5,6]. Today, the olive tree (Olea europaea L., Lamiales, Oleaceae) stands as an iconic species within the Mediterranean Basin and represents one of the world’s most economically significant agricultural crops.
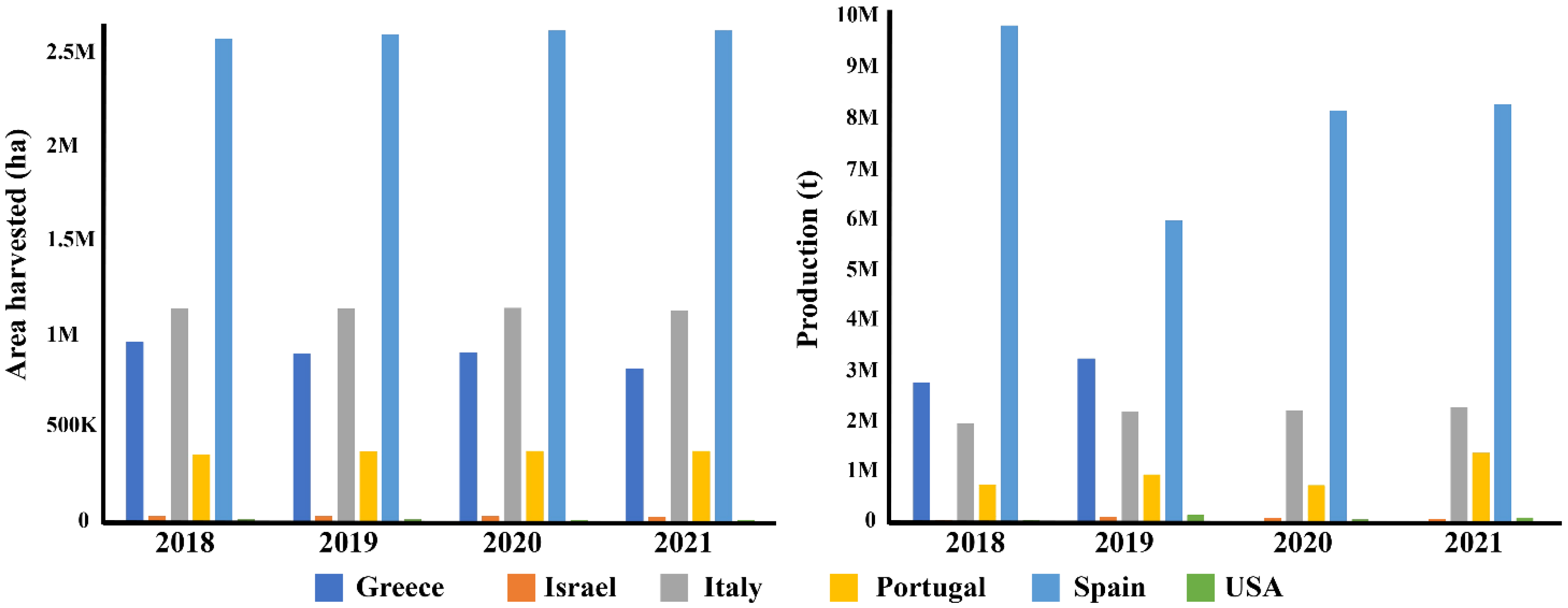
With 48.8% of the global olive crop concentrated within the Mediterranean region, spanning over nine million hectares of olive groves, it serves as a cornerstone of socio-economic and cultural life within this diverse geographic expanse [7]. In 2021, the olive harvest exceeded an impressive 23 million tonnes, with a substantial 63.7% originating from Mediterranean countries [8,9]. Spain emerges as the global leader in both olive oil and table olive production (Figure 1), commanding 45% of the world’s output, cultivated across more than 2.6 million hectares [10].
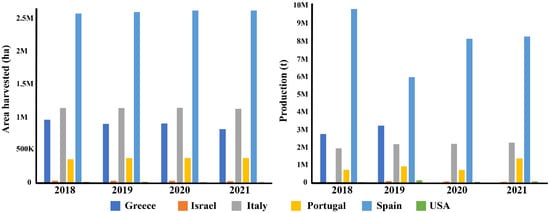
Figure 1.
Charts and updated data (2021) on Mediterranean and world olive production. Europe produced 14,701,594.88 t from 5,045,169 ha of cultivation. Worldwide, 23,054,310.6 t was obtained from 10,338,179 ha of cultivation [8]. ha = hectare; t = tonnes.
Beyond its economic importance, the olive tree holds a profound social significance. It contributes significantly to job creation and plays a pivotal role in sustaining rural populations. Globally renowned, olive oil is recognized as a fundamental component of the Mediterranean dietary model, acknowledged as a UNESCO Intangible Cultural Heritage since 2013 [11]. The surge in olive oil tourism offers an immersive experience, deepening the understanding of this essential Mediterranean staple, and fosters sustainable rural tourism, aiding in the promotion of olive oil and related products for olive farmers [12].
Furthermore, olive cultivation exerts a substantial environmental impact, particularly with regard to biodiversity, carbon sequestration, and soil conservation. With its remarkable temporal stability, high genetic variability, and intricate biotic communities, olive cultivation creates invaluable agricultural landscapes [13]. It remains the most widely cultivated perennial crop in the Mediterranean region, well suited to its climate, which is characterized by high temperatures and hydric stress. However, amid the backdrop of global environmental change, the Mediterranean Basin confronts mounting challenges, including heightened temperatures, prolonged drought, and increasingly extreme environmental conditions, all of which may disrupt the phenological cycle of the olive tree [7,14,15].
The confluence of global environmental change intensified global trade and travel, and the introduction of new plant species to novel regions fuelled the proliferation of economically consequential insect pests. These pests impose ecological disruptions, colossal financial losses, and substantial expenditures on prevention, quarantine, and control measures. Alarming statistics reveal that up to 40% of global crop production is annually lost to pests, with plant diseases exacting an annual toll of over USD 220 billion on the global economy, while invasive insects contribute a minimum of USD 70 billion and play significant roles in biodiversity loss [16].
To mitigate these losses, international cooperation, early pest detection, and the sustainable use of pesticides are imperative. Integrated pest management (IPM) emerges as a comprehensive strategy, incorporating biological, cultural, biotechnological, or physical methods to control pests whenever feasible, all while safeguarding crops, plant health, and global well-being. IPM, an ecosystem approach to crop production and protection, combines various management strategies and practices to nurture healthy crops while minimizing pesticide reliance [17]. Endorsed by international organizations such as the FAO and the European Union, IPM has gained further impetus through the One Health approach, especially in the wake of the COVID-19 pandemic ([17,18] and references therein present results from case studies that have implemented an integrated use of different approaches).
The global challenge posed by insect pests continues to escalate in the current global context. This review synthesizes the latest information on IPM guidelines for olive cultivation, prioritizing the use of biological methods such as the use of predators, parasitoids, and pathogens to control pests—including Bactrocera oleae, Prays oleae, and Cicadellidae (vectors of Xyllela)—and the integration of genetics and biotechnology. These sciences bring precision, efficacy, and safety in comprehending and managing olive pests with a limited environmental impact.
2. Main Arthropod Pests/Vectors in Olive Groves
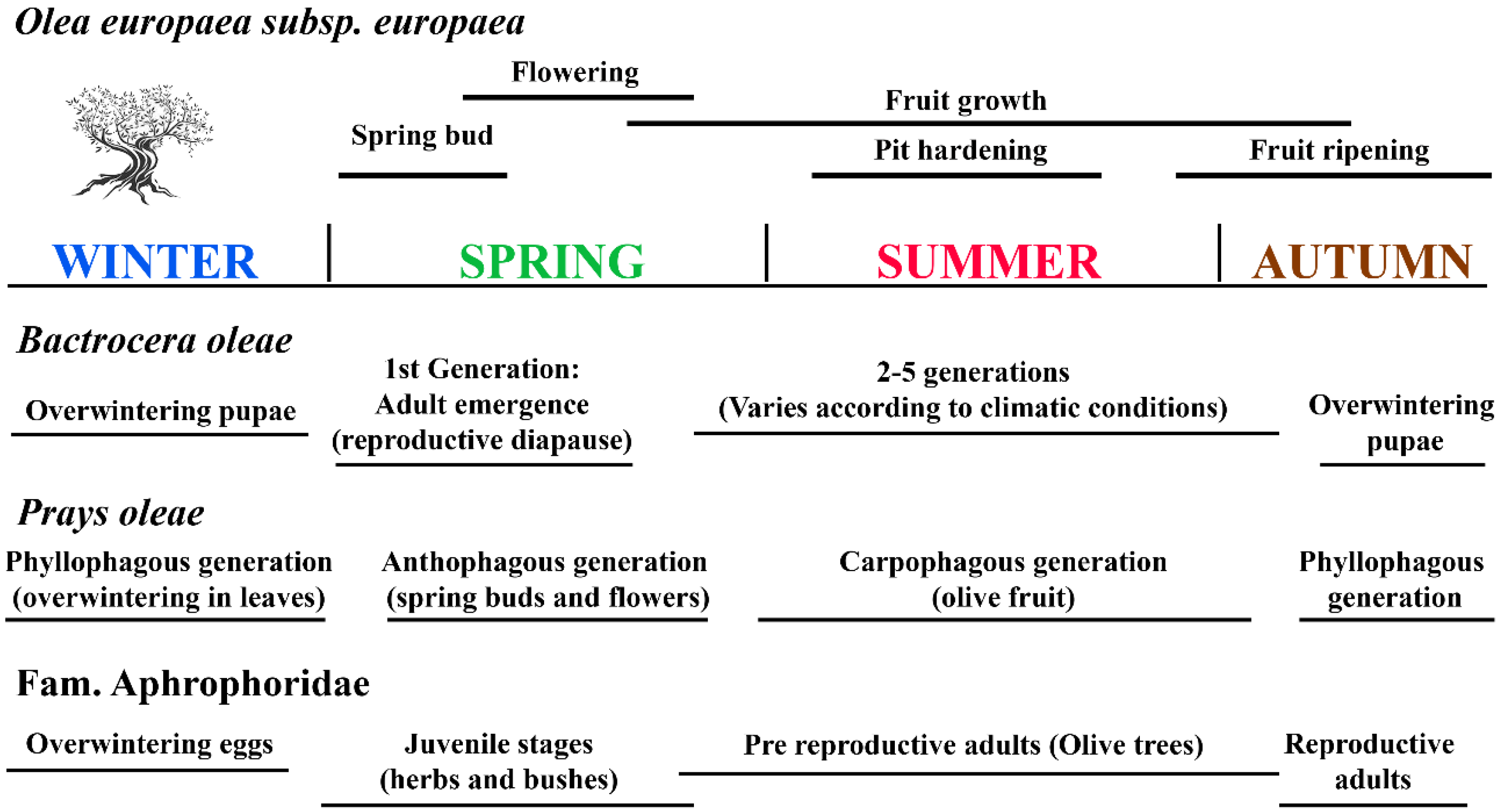
This section provides a brief description of the two most important pests of olive trees, which are widespread in the Mediterranean and cause serious losses by reducing the size and number of olive fruits [19,20,21,22]. The Cicadellidae vectors of the bacterium Xylella fastidiosa have also been included. Figure 2 schematises the life cycles of these two main olive pests and vectors of Xylella in close relation to olive fruit development and olive phenology.
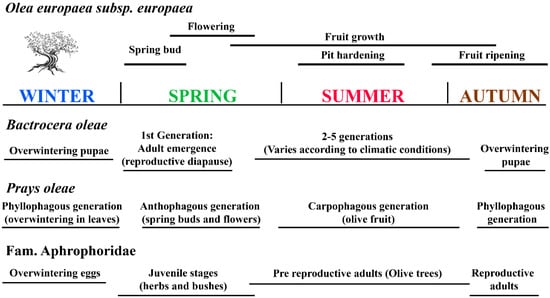
Figure 2.
Life cycles of the two main olive pests and of vectors of Xylella in parallel with olive fruit development and olive phenology.
2.1. Bactrocera (Dacus) oleae
The family Tephritidae consists of flies commonly known as “fruit flies”. It is one of the most numerous Diptera families (nearly 5000 species are currently recognised) and is the most economically relevant. The larvae of most species develop in the seed-bearing organs of plants and a large number of them attack commercial fruits [23,24,25,26].
The species Bactrocera oleae (Rossi 1790), also known as the olive fruit fly (olf), belongs to this family and is the most important pest of olive trees. There appear to be references to olive infestations in the eastern part of the Mediterranean Basin from as early as the 3rd century BC [27]. However, the first reliable reference is that of Pliny (1st century AD), who speaks of a “vermiculatio” of olives that affected their production for many years [28].
The only natural hosts of the olive fruit fly are the fruits of plants of the genus Olea. In general, females prefer varieties with large olives for oviposition. However, they are capable of infesting all olive varieties, including wild varieties. Most species of invasive Tephritidae are established in only 5 or a few countries, but Bactrocera oleae has been detected in 34 countries [29]. This insect is distributed throughout the Mediterranean region and the Middle East. It is also found in Southern and Eastern Africa, India, and Pakistan, and since the end of the 20th century, the olive fly has also been reported in California and Mexico [19].
In the Mediterranean Basin, this species has between two and five generations per year during the summer and early autumn, in parallel with the growth and ripening of the olives [30,31]. The damage caused by this Diptera is linked to the feeding habits of the larvae, which are strictly monophagous and feed on the olive pulp [27]. The females lay eggs under the epidermis, and when they hatch, the larvae have direct access to the fruit. If the olive tree is grown for the production of table olives, the mere presence of the oviposition mark alone will result in the fruit being discarded. If the production is intended for the production of olive oil, several types of damage can be considered. The most important of these are the premature drop of the infested fruit, the destruction of the pulp caused by the larvae developing inside the olives, and the increased acidity and other side effects associated with the development of the larvae, including infections resulting from the hole in the skin caused by the oviposition and the galleries resulting from the feeding of the larvae. An infestation level of more than 1% prevents the fruits from being used as table olives and an infestation level of more than 10% also prevents them from being used for oil production, causing olive oil yield losses of up to 11.5% and 18% [32].
2.2. Prays oleae
The olive moth Prays oleae (Bernard 1788) is the second most economically important pest of olive trees in the world’s olive-growing regions, after the olf [8]. This species has three generations, phyllophagous, anthophagous, and carpophagous; therefore, it attacks the olive trees at different levels, leaves, flowers, and fruits [33,34] (Figure 2). The first generation, the phyllophagous, is the longest (from autumn to spring), in which the larvae seek shelter from the harsh winters in the leaf mines. Around April, they emerge and begin the anthophagous generation (the shortest), in which they feed on the floral buttons. It has been estimated that one larva of this generation can destroy between 20 and 30 flowers [35]. Finally, the carpophagous generation enters the fruit during the summer period, where the larvae feed on the olive seeds [36,37,38]. This is considered to be the most damaging generation of the olive moth, causing premature fruit drop and reduced productivity. The 28-year study by Ramos et al. conducted in southern Spain (Andalusia) showed that this pest can reduce olive production by 50–60%, with heavy infestations occurring approximately every three years, causing 40% premature fruit drop and consequently significant losses [33].
2.3. Fam. Aphrophoridae
The arrival of the bacterium Xylella fastidiosa (Wells et al. 1987) in Europe in 2013 has posed another challenge to the agricultural economies of Mediterranean countries. This bacterium grows very slowly in microbiological culture media (fastidiosa), and it lives in the xylem of host plants (Xylella), causing a disease that seriously damages crops of high economic interest such as olive trees. The first outbreak of “Olive quick decline syndrome” (OQDS) was reported in Apulia (Italy) in 2013. This is a disease that can be asymptomatic in its early stages, but usually causes leaf scorching, extensive dieback, and eventually the death of the entire affected plant. Since then, the Aphrophoridae species Philaenus spumarius (L. 1758), Philaenus italosignus, and Neophilaenus campestris (Fallen 1805) have been catalogued as vectors of the bacterium Xylella fastidiosa in the Mediterranean area [10,39,40,41]. P. spumarius, the meadow spittlebug, is the main vector of Xylella fastidiosa in Europe of the above species in Europe. These xylem sap-feeding aphrophorids are ubiquitous and highly polyphagous species that can feed on a wide variety of plant species, mainly herbs and buses, but also olive trees [42]. The acquisition of X. fastidiosa involves its attachment to the foregut of adults from source plants, which transmit the bacteria to other plant species during the feeding process [43,44]. The eggs overwinter in the soil until early spring, when nymphal stages begin to develop in suitable herbs, preferably with a basal leaf rosette or with closely adjacent leaf and stem surfaces [45,46] (Figure 2). Between spring and summer, these species have long relative adult life intervals, which end in autumn with the oviposition of the females [47].
In general, the damage caused by vectors is often greater than that caused by insect pests. The single inoculation of the vectored pathogen continues to inflict damage throughout the plant and over time. In contrast, pest species attack an organ or structure at specific moments [47]. Due to the lethal consequences of X. fastidiosa infection, the management of this bacterium is currently based on monitoring and controlling its vectors, as there are no effective tools to combat this plant pathogen. Existing management measures are focused on controlling the vectors at their different stages: eggs [48], juveniles, and adults [49].
In addition to the enormous direct losses caused by the attack by X. fastidiosa, there are also the losses caused by the control measures, which include the eradication of all plants within a 100 m radius of the infested plant and heavy treatments with phytosanitary products throughout the eradication area [50]. It has been estimated that a full spread of X. fastidiosa could eventually cost the EU more than EUR 5.5 billion per year in production losses, with potential export losses of EUR 0.7 billion per year. If Xylella were to spread across the EU, it could affect over 70% of the production value of EU olive trees over 30 years old and could affect 35% of younger trees ([51] and references therein).
3. Pest Management. EFSA Regulations in the European Context and National Management Plans
Fly B. oleae and the lepidopteran P. oleae are currently the main olive pests in the Mediterranean region, and both must be the main target of management. Since the middle of the 20th century, B. oleae has been controlled by the use of synthetic insecticides such as Dichloro-Diphenyl Trichloroethane (DTT) and Organophosphates (OPs) [52]. Currently, pyrethroids, organophosphates, or neonicotinoids are the approved pesticides to control these pests in olive crops [10]. The misuse and abuse of chemical products in recent decades has led to increases in the distribution and frequency of alleles, conferring resistance to these products in natural pest populations in this area, as has been documented [53,54,55,56]. Such occurrences are a sign of insecticide failure.
Similarly, the moth pest has traditionally been controlled with insecticides such as pyrethroids, neonicotinoids, and organophosphates, sometimes combined with the release of natural enemies [10]. However, effectiveness is compromised because after the eggs hatch, carpophagous and anthophagous larvae live in the buds, flowers, and fruits [57].
The control of olive crop pests has become a growing and widespread challenge, and all of the indicators suggest that it will increase in the coming years. Indeed, global environmental change would affect their survival, fecundity, and dispersal, among other aspects [20]. It is therefore expected that the number of treatments or doses of agrochemicals will increase. Traditional methods of control have clearly proven to be inadequate, with undesirable and worrying side effects. They have negative impacts on the environment through pollution and the loss of biodiversity [58,59] due to their lack of specificity.
A summary of the plant protection products currently used in olive groves is shown in Supplementary Material S1. Although the use of these phytosanitary products is highly regulated at the European level [60] and at the national level [61,62], concerns about the harmful effects of pesticides on the environment, human health, and wildlife have led to research and support for integrated pest management (IPM) strategies in this changing world.
The European Commission’s Directorate-General for Agriculture and Rural Development has identified eight basic principles for the IMP approach. To prevent or reduce the presence of harmful organisms, the strategies focus on biological control (natural enemies of the pest), safeguarding the beneficial organisms, the use of semiochemicals (such as pheromones), hygiene measures, balanced fertilisation and irrigation, appropriate cultivation techniques, increasing crop diversity (intercropping or mixing cultivars), cultural control (such as crop rotation), cultivar selection, resistance/tolerance, the use of certified plant material, and, as a last option, chemical control. Crop rotation, the practice of growing different crops in succession on the same land, usually following a specific sequence or pattern, can reduce pest populations by interrupting their life cycles, depriving them of their preferred hosts, or exposing them to unfavourable conditions. It can also improve soil fertility, suppress weeds, help control insects, and break disease cycles [63,64]. In terms of selecting crop varieties that are best suited to local growing conditions and pest pressures, they can affect pest avoidance by influencing the phenology, morphology, physiology, or chemistry of the crop [65,66]. The planting date and density can affect pest avoidance by altering the crop’s growth stage, canopy structure, microclimate, or resource availability. Some pests may be more active or damaging at certain stages of crop development, such as seedling, flowering, or fruiting. The planting date and density can also affect the synchronization or asynchronization of crop and pest phenology, which may result in escape or the exposure to pest attack [67,68,69]. Fertilization and irrigation can affect pest avoidance by influencing a crop’s vigour, quality, and stress level. Excessive or deficient fertilization and irrigation can make the crop more susceptible to pest attack by reducing its resistance or inducing physiological disorders. Optimal fertilization and irrigation can enhance the crop’s health and productivity by providing adequate resources for growth and defence [70,71,72,73]. The use of certified plant material is relevant to avoid subsequent problems related to sucking and biting insects or pathogens producing systemic infections, which may stay asymptomatic on the plant for several months or years after planting until symptoms occur, as is the case with X. fastidiosa [51]
It is important to emphasise that the combination of many of them often has a greater effect on the IPM than individual ones. Likewise, IPM can benefit the landscape by enhancing its aesthetic, functional, and ecological values. It can also improve plant health and vigour, increase biodiversity and ecosystem resilience, reduce pollution and the contamination of soil and water resources, and save money and time by avoiding unnecessary or ineffective treatments.
The European Directive on the Sustainable Use of Pesticides established the framework for action to achieve the sustainable use of plant protection products [60] and promoted the creation of National Plans to develop strategies in IPM practices, according to Chapter III of the Royal Decree 1311/2012 of 14 September in Spain; for example, the Ministry of Agriculture, Fisheries and Food (MAPA) has developed 38 crop-specific guidelines [74], including a specific one for olive groves, called the Integrated Guide to Olive Grove Pests [75].
Furthermore, in 2018, the European Commission launched the Focus Group (FG) on Olive Tree Pests and Diseases as part of the activities of the European Innovation Partnership on Agricultural Productivity and Sustainability (EIP-AGRI). The question was how to increase the sustainability of olive cultivation, taking into account the risks posed by pests and diseases for which they produced a detailed report after a year of work. The FG considered that for the main pests and diseases, an IPM approach should be practiced in order to obtain a reliable level of plant protection, considering that IPM relies on knowledge, prevention, and observation. They developed a series of recommendations. Among them were innovation projects at the local level though operational groups: the development and adoption of new technologies of pest/pathogen monitoring techniques, the optimisation of cover crops, the quantification of the effect of agroecological principles/green infrastructure on pest and disease control, increasing the knowledge of farmers on the use of agroecological principles oriented to pests and disease control, or the use of experimental and demonstration farms and field networks to test biocontrol agents for disease control. Likewise, they recommended further research on topics such as the effects of climate change on olive pests and pathogens to assess or predict the potential effects of climatic change on the distribution and incidence of olive pests and pathogens, the development of new systems for pest and disease monitoring, and the use of new biotechnological phytosanitary tools (semiochemicals, attractants, deterrents, repellents, etc.) to control olive pests [51].
A recent review of how IPM has evolved over time addressed some of the shortcomings of this approach [76]. It highlights some issues such as the modest reductions or increases in the quantities of pesticides used; the hodgepodge of definitions and interpretations of IPM; the gap between IPM concepts, practices, and policies; the insufficient involvement of farmers in the development of IPM technologies and the frequent lack of basic understanding of the ecological concepts underlying IPM; or pest control that is too often based on the systematic and widespread use of synthetic pesticides. Indeed, despite the National Plan Actions, the European normative, and the promising results of awareness-raising campaigns, banned insecticides such as glyphosate have been detected in drinking water in Andalusia (Spain) during the 2020s [77].
Information dissemination at the national and European levels of all existing IPM strategies is decisive to improve the applications of IPM strategies. The European Commission has recently published a toolbox of good practices to reduce the use of chemical pesticides. It consists of a database overview of the current available PM methods, together with a study assessing their effectiveness and prospects for their further implementation, including 1300 examples of practices, techniques, and technologies [18]. This overview shows the great diversity in the implementation options of IPM across EU countries. Likewise, a second work looks at the current IPM practices and their potential to help reduce the dependence on chemical pesticides, their costs for implementation, and their overall effectiveness [78]. This survey revealed that the perceived inadequate number of viable and affordable approaches to conventional practices remains the main barrier to reducing our dependence on pesticides. These data underline the importance of improving the information and promotion of IPM treatments to inform farmers of the potential benefits of their effective uses in their olive crops.
4. Biological Control and the Three Ps: Predation, Parasitism, and Pathogens
As said, the European Directives and the National Action Plans led to the development of different guides for IPM based on culture. Because of pesticide concerns, a special emphasis has been put on the potential benefits of biological control. Natural enemies can be used to regulate and reduce insect pest population levels [79]. The main objective is to reduce pest populations in the stages where the pests are more susceptible to be predated or parasitised [80].
Without attempting to provide an exhaustive list of possibilities, these organisms can be divided into three different categories:
- Predators: These organisms feed directly on pests, thereby reducing their populations.
- Parasitoids: Parasitoids are organisms that lay their eggs inside or on the bodies of pests. The emerging larvae feed on the pest, eventually killing it.
- Pathogens: These are microorganisms, such as bacteria, fungi, or viruses, which infect and cause diseases in pests. By spreading among pest populations, they significantly reduce their numbers.
The essence of biological control is to promote the ecosystem services provided by the natural enemies that are present in each agroecosystem. However, the olive agroecosystem is often a complex landscape where the arthropod community of natural enemies varies according to the environmental conditions, land uses, and agricultural practices. For instance, soil management practices related to the agricultural intensification of the olive groves in Mediterranean countries has greatly affected biodiversity [81]. Among other facts, arable fields or specialization in few crops simplifies the landscape and reduces the possibility of having a great number of species [82]. This, joined with the use of herbicides to destroy natural vegetation, which often creates a microclimate and retains humidity, eliminates natural niches for the arthropod community. Thus, a lower abundance of pest species is related to landscape complexity in olive groves due to its effect on promoting different biocontrol organisms and the general biodiversity of arthropods [83,84,85]. Olive groves with lower cover crop and no heterogeneous surrounding habitats decrease the probability of promoting the biological control of B. oleae [86]. So, preserving habitats for natural enemies—protecting them from perturbations derived from crop cultivation, crop harvesting, phytosanitary applications, or weather variations—will guarantee the presence of the local arthropod species overwintering near the crop fields, preventing pest outbreaks during early spring [87]. Indeed, habitat management often implies the manipulation of the plant composition, increasing its diversity in order to increase the abundance of natural enemies by providing food sources (such as nectar or pollen), mating sites, and shelter, and as a consequence, regulating insect pest populations [79]. A table listing some potential biological control organisms in olive crops is shown in Supplementary Material S1 and is commented on below.
4.1. Predation
In Mediterranean olive cultivars, the main potential groups that predate pest species are Formicidae, Araneae, Forficulidae, Staphilinidae, Carabidae, and Scolopendromorpha [88,89,90,91].
Among the measures proposed in the management guides for the olf, the edaphic predatory arthropods community could contribute to the reduction in the population levels of the olive fly during the autumn and winter months [89]. As stated above (Figure 2), the larvae of the late generations in the autumn pupate in the soil, where they spend the winter buried and where they are more vulnerable to being predated; therefore, the number of adults that reach the reproductive period in spring decreases significantly [16].
The roles of carabids and their relative abundances in Spanish groves were tested in functional response experiments, revealing that the species Pterostichus globosus is a better candidate than Orthomus barbarus—the most abundant carabid species in the southeast region of Madrid—for the biological control of olf [71]. P. globosus was also suggested as a more efficient predator than Calathus granatensis in Portuguese cultures [88]. In both cases, the adult size of the carabid species was relevant in the predation rates. In the same line, predation by coleoptera upon B. oleae was also assessed in carabids and staphilinids picked up in olive groves from Tuscany (Italy) [92,93]. Many studies have also been performed analysing the role of spiders in IPM given that they are an abundant and generalist group in olive agroecosystems that would contribute to the regulation of olf populations [94,95,96,97]. However, its abundance can generally be affected by soil regimens (reduced in intensive cultures), landscape effects, or prey abundance, among others [79].
Notwithstanding, the real effect of predation on the damage reduction by the olf is still unknown. Thus, it becomes absolutely necessary to perform the study of the trophic webs and analyse the arthropods community according to their relative abundances and feeding preferences in the context of pest control [80,88,98]. In this line, post mortem studies analysing the presence of preys in the predator’s gut would provide a clearer profile of the trophic web, confirming the real role of each group in the biological control of each pest. For instance, carabids, ants, spiders, and earwigs have already been confirmed as B. oleae predators in molecular gut contain assays using individuals that were picked up during the fall and spring in olive cultures in Madrid [99,100].
As previously said, several arthropod groups can predate the different olive pest species. Chrysopidae, or green lacewings (Neuroptera), have been recognized as one of the most effective biological control agents of many orchard pests, including the olive moth [101]. Indeed, lacewings are currently cultivated for sale and release in different crops. Among others, Chrysoperla carnea, whose larvae are oophagous, plays an important role in the regulation of P. oleae populations [102]. Other Neuroptera species reported to predate on P. oleae eggs are Mallada flavifrons, Mallada picteti, Mallada prasina, Nineta vittata, and Rexa lordina [103]. In contrast to the voracious larvae, adults just feed upon vegetal substances such as pollen or nectar. This fact highlights, again, the importance of the surrounding landscape and surrounding vegetation to promote IPM [35,38,104]. Ants also have beneficial effects in biological control because they consume large numbers of pest insects [105]. For instance, Tapinoma complex, one of the most abundant groups in the olive agroecosystem, is a strong candidate for olive moth control in Spanish olive groves [106]. Indeed, although IPM is certainly important to maintain ant communities and promote their roles as pest control agents, the Tapinoma species seems to be little affected by the landscape heterogeneity [91]. Other species referenced as predators are Camponotus sp., Formica subrufa, or Crematogaster scutellaris [107].
Regarding the predation upon the described Xylella fastidiosa vectors, generalist predators that are present in ground covers play major roles in their biological control. Due to their abundance and ubiquity, spiders have been considered as potential enemies. Their contribution has been tested by post mortem gut content genetic assays in the southeast of Madrid (Spain), where 6.34% of them consumed the meadow spittlebug (Philaenus spumarius), the prey [108]. Also, through molecular protocols, the species Oxyopes sp was confirmed as a predator of this vector [109], whereas the roles of Araniela cucurbitina and Synema globosum were also tested using functional response assays in two groves with herbaceus strata in Portugal [110].
4.2. Parasitism
At the end of the last century, the California Department of Food and Agriculture (CDFA) focused on determining the efficacy of different parasitoids in order to eradicate B. oleae populations in the USA [111]. Several species of the Psyttalia genus were identified as olf parasitoids. For instance, P. lonsburyi is considered to be specific to B. oleae, but its effectiveness against this pest is compromised due to its fecundity problems and its difficulty of being reared under laboratory conditions [112]. The P. concolor or P. humilis species are also good candidates because they have already shown their potentials in biological control in the Mediterranean fruit fly, Ceratitis capitata, but generalist species would compromise the efficacy of the parasitism [113]. The main problem of this genus is that most of the species have small ovipositors compared to the fruit size in cultivated varieties. This fact decreases the probability of parasitizing the olf larvae [114].
Regarding parasitism upon the olive moth, efforts to determine the main candidates to perform this ecosystem service are focused on the species that are present during the most sensitive generations, phyllopagous and antophagous. For instance, the braconid Chelonus elaeaphilus has been pointed out as an efficient parasitoid with values of parasitism of up to 80% [107]. A relevant role in moth control is also played by the specialist parasitoid Ageniaspis fuscicollis or the generalist Elasmus flabellatus [115,116].
Despite the enormous impact caused by Xylella fastidiosa and its vectors, very little is known about the biological control agents of aphroporids. Few studies have determined the parasitism in these cercopids. For instance, Verralia aucta is a parasite of the adults but does not have an immediate effect on Xylella transmission given that it kills the vector after several weeks [117,118]. Recently, the species Ooctonus vulgatus has been proposed as a good candidate to its control due to the overlapping distribution of the parasitoid and its detection via Polymerase Chain Reaction (PCR) in different aphrophorid samples [119].
4.3. Pathogens
Bacteria, fungi, algae, protozoa, or viruses, naturally occurring or genetically engineered, are pathogens of pest crops, and hence, are considered microbial pesticides [120]. The main advantages of this type of pesticide are their safety and specificity. Due to their toxic mode of action that is specific to a single group or species of insects, they have non-toxicity and non-pathogenic natures towards humans, wildlife, and non-target organisms, particularly those that are unrelated to the target pest or other beneficial organisms [121,122,123,124]. Of course, there are limitations as well. New formulations and storage methods are needed for microbial pesticides. And because of their specificity, some microbial insecticides are not widely available or are relatively expensive [120,125,126]. Bacillus thuringiensis is a naturally occurring soil bacterium and the most widely used microbial biopesticide in IPM programs [127,128]. The bacteria and its toxins have been used for decades to control various insect pests such as caterpillars, mosquitoes, black flies, or beetles [121,127,129,130], although further research is needed to control B. oleae or P. oleae, as they are approved for use in olive groves, for example, in Spain [6,131,132,133]. During sporulation, B. thuringiensis produces an endotoxin called Cry protein, which, once ingested, causes an osmotic imbalance within the cell, resulting in the loss of the integrity of the midgut epithelium and thus killing the insect [134]. However, these toxins do not prevent insects from attacking the roots or internal parts of the plant when used as sprays [135]. These limitations have led to an interest in the development of novel biotechnological solutions to overcome these problems, as will be shown below.
Symbiosis disruption in olive pests refers to the interference or alteration of the mutualistic relationship between the insect and its endosymbionts [136,137]. Endosymbionts are microorganisms that live inside the body of the olive fruit fly and play crucial roles in its development, nutrition, and other physiological processes [138]. B. oleae has been studied regarding its endosymbionts for over a century [139] and it has been identified as bacteria of the genera Lactobacillus, Micrococcus, Pseudomonas, Streptococcus, Citrobacter, Proteus, Providencia, Enterobacter, Hafnia, Klebsiella, Serratia, Pantoea, and Xanthomonas through traditional microbiological approaches [140,141,142,143]. Molecular analyses have also shown the presence of Acetobacter tropicalis [144], Pseudomonas putida, Asaia sp. [145], Enterobacter sp. [146], or Tatumella sp. [147], among others. However, the most notable endosymbiont associated with this pest is Candidatus Erwinia dacicola, a non-culturable bacterial species that represents nearly the entire symbiotic population associated with the olive fruit fly [148,149]. It is an endosymbiotic bacterium found in the olf and is essential for its development and survival. This bacterium produces nutrients and other compounds that are important for the growth of fly larvae, thus influencing their abilities to complete their life cycles [150,151].
Additionally, disrupting the symbiosis between B. oleae and its endosymbionts has the potential to impact the fly’s fitness, reproduction, and overall population dynamics. Symbiosis disruption can be achieved through various methods, but chemical treatments are, perhaps, the most studied ones, and Ca. Erwinia dacicola is the most evident target to implement this approach. In this respect, several compounds have been tested such as viridiol, propolis, or copper oxychloride [151,152,153,154]. The former is a secondary metabolite biosynthesized by beneficial fungi belonging to the genus Trichoderma (Hendrik) [155,156], while copper oxychloride is widely used in olive groves for disease control [157,158,159]. All of them have proven to cause a reduction in fitness due to the drastic reduction in the number of offspring after treatments, although with some drawbacks such as the lack of specificity and, in the specific case of copper, its contaminating effects on soil and water. On the contrary, viridiol also shows no direct negative effects on B. oleae, an interesting finding when considering the direct toxic effect that this compound may have on non-target arthropods [151]. The disruption of the symbiosis would therefore be a potential target for the development of new pest control strategies. But before this approach can become a reality, much more research is needed both “in silico” and in the field.
5. Biopesticides and Inert Materials in IPM
As already discussed above, current legislation and recommendations on IPM emphasises the reduction in chemical products, promoting natural products that are used in organic agriculture and that are useful in insect control.
The first forms of control in olive groves included the anticipation of the harvest and the use of mixtures based on copper, cobalt, potassium/sodium arsenite, or tar [160]. In fact, some of these, although valuable [161], are not approved under the CE Nº1107/2009 regulation or are candidates for replacement in some European countries, such as Spain [10].
Among the approved substances is spinosad. It is a molecule with a potent larvicidal effect discovered in the 1980s that has gained popularity in the field of IPM due to its effectiveness against a wide range of insect pests, including caterpillars, leaf miners, thrips, fruit flies, spider mites, and certain beetles. Spinosad, produced by the bacterium Saccharopolyspora spinose, has a similar efficacy to pyrethroid insecticides and is superior to organophosphates [162]. It contains two active ingredients, spinosyn A and spinosyn D, which target the nervous systems of insects. Spinosad acts on nicotinic acetylcholine receptors in insect nerve cells, leading to the overstimulation and paralysis of the insects, ultimately causing their deaths [163,164]. The use of spinosad (a commercial mixture of spinosins) provides enormous benefits compared to other traditional chemicals. It is also worth noting that spinosad has a relatively short residual activity, i.e., it degrades relatively quickly in the environment. This property reduces the risk of long-term environmental impact when used properly, and it allows for a flexible approach to IPM, including the timely release of beneficial organisms after spinosad application. However, mutations in detoxification enzyme genes that confer resistance to this product have already been described in some insect species such as Drosphila suzukii, Zeugodacus cucurbitae, Ceratitis capitata, Musca domestica, Tribolim castaneum, or Plutella xylostella, among many others [165,166,167,168,169,170], maybe as a consequence of treating consecutive insect generations with the same mode of action that therefore result in the evolution of insecticide resistance [171]. Despite the fact that spinosad’s unique mode of action makes it less prone to insect resistance compared to some other classes of insecticides, it is still essential to practise responsible use and to avoid overreliance on any single pest control method to prolong its effectiveness. Until recently, one of the most promising key advantages of spinosad in IPM is its selectivity and low toxicity to beneficial insects and non-target organisms, such as bees, butterflies, and predators like ladybugs and lacewings. However, spinosad has an undesirable effect on populations of natural enemies of the olive fly, mainly on predatory arthropods inhabiting the tree canopy but also pollinators such as Apis mellifera or natural enemies of some other pests [172,173,174,175,176]. Nevertheless, sustainable olive grove management holds the potential to alleviate this adverse impact, as it distinctly enhances various soil quality parameters, encompassing the abundance and diversity of microbial and pedofaunal communities [160,177,178].
Next, particle film technologies refer to aqueous formulations of chemically inert clay or mineral particles specifically designed for coating purposes. These clays are widely used to reduce damage from insects, diseases, and solar radiation [179]. Kaolin is an aluminosilicate mineral that disperses readily in water and is chemically inert over a wide pH range [180]. It is often applied to plants as a thin film that does not interfere with plant respiration, and once the water evaporates, it forms a protective barrier that acts as a physical barrier to pests [181]. When insects come into contact with the kaolin-coated surfaces, they find it difficult to move and feed, which can help to reduce the populations of a wide range of insect pests in a wide variety of crops, including olive groves [160,161,182,183,184,185,186,187]. One of the main advantages of using kaolin in IPM is that it does not leave harmful residues on the products and even mitigates the deleterious effect of drought on some plants [188,189]. However, it contains aluminium, which has raised concerns about how it ends up in the food chain and how it affects human health [190,191], and even kaolin is generally considered safe for beneficial insects, pollinators, and other non-target organisms [192]; this should be taken with caution [161,193]. Additionally, kaolin’s effectiveness may vary depending on the pest species and the environmental conditions [160,192].
Trials on the efficacy of spinosad-based clay baits to control the olive fruit fly showed that spinosad-based baits were less effective than kaolin sprays in controlling B. oleae infestations [194]. Similar results were found when comparing copper and kaolin treatments, as copper treatments provided some protection that was insufficient at high infestation levels [167]. In addition, spinosad has been shown to have a significant detrimental effect on natural enemies in the medium term [172]. On the contrary, spinosad did not have a significant effect on either the feeding behaviour or the survival rate of P. spumarius, and nor did kaolin [195]. Laboratory tests with kaolin and copped-based products and some natural enemies of the olive fruit fly and olive moth have shown that, despite some negative effects, the negative impact on natural enemies is less than that caused by products that are commonly used in olive crops to control these pests [196,197]. However, field trials found a lower arthropod diversity and a significant change in the natural enemy community in the kaolin-sprayed plots [167]. In an attempt to avoid these drawbacks, the combined use of spinosad and kaolin was tested and proven to be an effective strategy to control this pest in organic olive groves. The combined treatment reduces the total and damaging infestation, but not the active infestation, without adversely affecting the natural agonists of the olive fruit fly [160].
To continue with a group comprising a diverse range of compounds, botanical insecticides are formulated from plant materials with minimal processing or modifications. Among the products that have been used for the longest periods of time are natural pyrethrins, which are among the organic alternatives to conventional synthetic insecticides. They are natural insecticides derived from the flowers of certain species of chrysanthemum, Dalmatian pyrethrum (Tanacetum cinerariifolium) [198]. Pyrethrins have certain advantages as IPM tools such as their low toxicity to mammals, birds, and beneficial insects, such as predators and pollinators [198,199,200,201,202,203], and their short environmental persistence as they break down quickly in sunlight and air, leaving no residues [203,204]. However, pyrethrins also have some limitations and challenges such as reductions in longevity and fecundity in certain parasitoids [205], a repellent effect in some insects [206], and the emergence of resistance [207,208,209,210]. Depending on the active ingredients and target insects, botanical insecticides work in a variety of ways. Some of the most common modes of action are contact toxicity or their uses as antifeedants or as repellents. The former kills the insect via direct contact, either by disrupting its nervous system, cell membranes, or metabolic processes. For example, pyrethrum, extracted from the flowers of Tanacetum cinerariaefolium, is a contact toxin that affects the sodium channels of insect nerve cells [210]. As antifeedants, they reduce or prevent the feeding behaviour of the insect, either by repelling it or by making the plant unpalatable or toxic. For example, neem, extracted from the seeds of Azadirachta indica, contains azadirachtin, which is an antifeedant that interferes with an insect’s hormonal system and disrupts its growth and reproduction [211]. Finally, repellents keep the insect away from the plant, either by emitting an unpleasant odour or by masking the plant’s attractants. For example, garlic, obtained from the bulbs of Allium sativum, is a repellent that produces sulphur compounds that deter many insects [212].
Essential oils can be considered botanical insecticides due to their natural origins and abilities to control insect pests. Essential oils can therefore also play roles in IPM as they often have natural insecticidal, antifungal, and antimicrobial properties [213,214,215,216]. They can be used as natural insecticides given that certain ones contain compounds that can repel or kill pests. For example, oils from celery, eucalyptus, neem, oregano, peppermint, pepper, and lavender have insecticidal properties [217,218,219,220,221,222,223] and can be used to control pests like aphids, beetles, mites, and mosquitoes [218,219,223,224,225]. Indeed, essential oils’ insecticidal properties have been mostly investigated against tephritid species [226,227,228] including B. oleae [194,229,230]. Although citrus, piperaceae, thyme, peppermint, clove, and eucalyptus are essential oils that have been used for integrated pest control in lepidopteran pests [218,231,232], research is needed on Prays oleae. Studies on P. spumarius, the main vector of X. fastidiosa in Europe, show that essential oils can be effective as either repellents or traps. However, the results are highly dependent on the oil used, its concentration, and the insect stage. Importantly, these are laboratory tests that need to be confirmed by field trials [233,234,235,236].
Essential oils such as eucalyptus, neem, or peppermint, to name just a few examples of those mentioned above, also have some drawbacks that limit their widespread applications. They can vary in chemical composition and bioactivity depending on the plant source, extraction method, environmental factors, and storage conditions [231,237]. Therefore, their effects on pests may not be stable or predictable in different situations. In addition, studies on essential oils have been used to be conducted in controlled laboratory or greenhouse settings, which may not accurately reflect real field scenarios where pests are exposed to a variety of biotic and abiotic influences [231,237,238,239,240]. They are also often expensive to produce and require large amounts of plant material. Moreover, they may need special formulations or delivery systems to enhance their stability, solubility, and penetration. These factors may increase the costs of using essential oils for IPM compared to synthetic pesticides [238,241,242]. In a recent work where insecticides, biopesticides, and clay were evaluated for the management of fruit flies, specifically Bactrocera spp. infesting bottle gourd, among the biopesticides, neem oil appeared to be superior to others in its class, but was less effective than, for example, spinosad treatment [243]. As many other compounds, essential oils are subject to strict regulations; they need to undergo rigorous testing and registration processes before they can be marketed as pesticides. This may take several years and require substantial financial resources, which may discourage the development and commercialization of essential oils for IPM [231,237].
In sum, much more research needs to be conducted on olive crops with these compounds, especially considering the upcoming more extreme environmental conditions and their costs compared to the synthetic insecticides that growers are already used to employing and know what to expect.
6. Genetics and Biotechnology in the Service of IPM
The development and implementation of IPM strategies has been greatly enhanced by genetics and biotechnology in order to apply the ever-increasing array of molecular genetic methods. Advances in genetics and biotechnology may lead to improved pesticide formulations and delivery systems, enabling more precise and efficient application as well as the efficacy and safety of biological pesticides [244,245,246]. This will minimise the impact on non-target organisms and the environment.
Biotechnology is supporting the production of biopesticides derived from beneficial microorganisms (such as bacteria, fungi, and viruses) that can target pests. As already discussed, the use of B. thuringiensis in pest control has some drawbacks. To overcome these problems, some of the genetics and biotechnological solutions developed have consisted of improving its stability, specificity, and delivery, as recently reviewed in Azizoglu et al. [134]. According to European and US legislation, B. thuringiensis can be applied as a spray or as a plant-incorporated protectant or PIP. The spray formulation must be approved by the European Commission and Member States and must comply with the requirements of the European Commission’s Directorate-General for Health and Food Safety for the placing of plant protection products on the market [247] or by the Environmental Protection Agency. Also, it must comply with the requirements of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) [248]. The spray formulation must also respect the maximum residue levels (MRLs) established for B. thuringiensis on the maximum residue levels of pesticides in or on foods and feeds of plant and animal origin [249] or those established in the Code of Federal Regulations [250]. As a PIP, B. thuringiensis can be incorporated into the plant genome via genetic engineering so that the plant produces the B. thuringiensis toxin in its tissues and protects itself from pest attack. The PIP must be authorised by the European Commission and the Member States and must comply with the requirements of the current directive [251] on the deliberate release of genetically modified organisms into the environment. In the USA, the PIP must be authorised by the EPA and must comply with the requirements of the Toxic Substances Control Act and FIFRA [252]. The PIP must also respect the MRLs established for B. thuringiensis in European and USA regulation, as mentioned earlier. Research on spraying applications continues today, finding new solutions and improving existing ones [253,254,255,256,257,258,259,260]. There is also an increasing amount of research on the genetic variability of Bacillus thuringiensis and others in the genus for the improvement of PIPs [260,261,262,263,264].
But the use of plant-incorporated protectants (PIPs) is not the only genetic approach that can boost IPM. Genetic modification can enhance the effectiveness of natural enemies used in biological control. This goal can be achieved by using conventional breeding or genetic engineering techniques. Conventional breeding involves crossing different strains or species of beneficial organisms, such as predators, parasitoids, or pathogens, to produce hybrids with improved traits, such as higher virulence, specificity, stability, or dispersal [265]. Genetic engineering involves the introduction of foreign genes or the modification of existing genes in beneficial organisms to confer new or enhanced traits, such as the resistance to pesticides, environmental stress, or host defence [76]. The genetic modification of beneficial organisms can offer some advantages over conventional biological control, such as increasing the efficacy, specificity, and persistence of these organisms, reducing the need for mass production and release, and facilitating their integration with other IPM tactics. However, the genetic modification of beneficial organisms also poses some challenges and risks, such as ethical, social, environmental, and regulatory issues. Even so, there are some genetically modified beneficial organisms that have been developed or tested for biological control. A strain of the entomopathogenic fungus Beauveria bassiana has been modified to increase its virulence and stability against diverse insects [266,267,268,269]. A transgenic strain of the entomopathogenic bacterium Bacillus thuringiensis that produces a scorpion toxin that is highly specific and lethal to the cotton bollworm has also been tested [267,270,271]. These are just two examples of the latest benefits in the area, of which there are many others [272].
The insect pests themselves may also be susceptible to genetic modification. The sterile insect technique (SIT) is a pest control method where large numbers of sterile insects are released into a target population to suppress reproduction [273]. In this respect, genetics and biotechnology can improve the mass rearing and sterilization processes, making SIT more efficient and cost-effective. The four main strategic options in using the SIT—suppression, containment, prevention, and eradication—with examples of each option are described in detail in the study by Dyck, Hendrichs, and Robinson [274]. Another approach that involves genetic engineering and pests is the use of RNA interference (RNAi). RNAi is a method that silences specific genes in pests by delivering double-stranded RNA molecules that trigger a natural mechanism of gene regulation [275]. RNAi technology can be used to silence specific genes in pests, causing them to become less viable or develop abnormally [276]. Gene drives consist of genetic elements that are efficiently transmitted among sexually reproducing individuals. They inherit genetic traits that are passed to future generations with a higher probability than with Mendelian inheritance, even if these elements reduce the fitness of such individuals. Therefore, this is a method that involves spreading a desired genetic trait through a population quickly [277]. There are several approaches to achieving this goal, all with advantages and disadvantages [278,279,280], but despite all their potentials, these products are unlikely to have an easy route to market. The applicability of these strategies to new target pests, their efficacy in the natural environment, and their potential safety and acceptability as control methods are surrounded by considerable controversy and uncertainty. International agreements before the release of approved insect strains are necessary [281].
Some of these measures are well established. They have been used for years or decades to control some of the pests that plague olive groves. For example, the use of B. thuringiensis has been researched and used for the control of major olive pests for at least 30 years [282]. Indeed, it is currently included in the community list of active substances approved for use in olive groves in EU countries such as Spain. There are different substances derived from 11 species of Bacillus that were approved for use as fungicides, insecticides, and/or bactericides. Specifically, there are eight strains of three subspecies of B. thuringiensis that were approved as insecticides [10].
Particularly in olive grove IPM, biotechnology and genetic engineering also play important roles. Although genetic modifications to the tree itself may take time to prove to be effective due to the nature of the plant, several genetic markers have been tested to obtain modifications against, for example, drought or salinity stress [282,283,284]. Several genetic and serological tests for the specific detection have been developed for main olive pathogens, highlighting those for X. fastidiosa [285,286] that produce vascular infection and may have an asymptomatic infection phase. But it is the genetic modification of the olive pests themselves that is without a doubt the most studied and the most widely used method. As mentioned above, SIT is being used to control important pests, including olive pests [287,288,289]. Other genetic markers, such as olfactory receptors, have also been studied to make genetically modified organisms more reluctant to reproduce. When released, they interfere with the mating behaviours and reproductive potentials of the flies, helping to control the pests [290]. Likewise, in 2012, a British company presented an initiative to Spanish MAPA and the Autonomous Government of Catalonia to test, in a controlled way, in 48 olive trees in Tarragona, a genetically modified strain of Bactrocera oleae whose females died at the larvae and early pupal stages. That could help to fight against this olive pest. In 2013, the company withdrew the application, overwhelmed by political and social obstacles. Several scientists, most of them from the company, demonstrated that the modified strain was effective in combating the pest [291]. As said, gene drive has several potential opportunities for pest control but also several uncertainties surrounding its employment in agricultural crops [291,292].
The knowledge of the nature of the genetic variation in olive pests at the intra- and inter- population levels is relevant for the design of IPMs and their success. The advance and cheapening of genetic techniques are providing us with new and more sensitive tools for genetic analysis, showing relevant data about the unequivocal species identifications, genetic diversities, structures, and gene flows of these damaging pests.
The use of microsatellite markers (very short DNA sequences—two to seven nucleotides—repeated many times, found in thousands of the location in the whole genome of an organism) has allowed researchers to determine the geographical origin, relatedness, and reproduction of B. oleae as well as to assess the impact of the sterile insect technique (SIT) on wild populations [293,294].
The resistance to pesticides is another important area where genetics can provide valuable information. Agricultural pest control relies mostly on synthetic insecticides, such as organophosphates (OPs) and pyrethroids (PYs), but the effectiveness of many pesticides is recurrently compromised by the evolution of resistances that have become major concerns. The intensive use of OPs to control the olive fruit fly led to an increase in resistance in field populations. Hence, a dramatic increase in the resistance to PYs such as alpha-cypermethrin has been observed in B. oleae populations in Greece, especially in Crete [55,295]. Spanish olive fruit fly populations also have high frequencies of alleles conferring resistance to organophosphate insecticides, as determined by studying mutations at the ace gene [56]. All of this information helps farmers to make better decisions about when to carry out insecticide interventions, as well as the most appropriate ones. This information is not only used to programme chemical insecticide interventions but is also used for compounds in IPM. For example, a resistance to spinosad has already been detected, although it remains low in most populations [296].
The analysis via PCR and the sequencing of a fragment of the mitochondrial gene of cytochrome oxidase subunit I (COI) have addressed the genetic diversity, population structure, and gene flow of B. oleae in different regions of the Mediterranean. The data revealed that olf populations, in general, hold high to moderate levels of genetic diversity and have been long established in the Mediterranean Basin with two main genetic groups. Gene flow seems to be the main process in shaping this genetic structure as well as the fly’s colonisation routes that have paralleled those of the olive tree. This genetic marker was sensitive to identify the source of origin of new infestations, making global collaboration easier [32,297,298,299,300,301,302,303].
The COI mitochondrial gene is also a useful and reliable taxonomic tool since it is the universal barcode for animal species identification. This trait makes it possible to identify olive pests at early stages, which is essential in the prompt detection and control of pests. The PCR analysis and sequencing of a fragment of COI lead to a reliable identification of P. spumarius at any life stage [108,109].
Ecosystem services are ecosystem resources or processes that benefit natural pest control. Generalist predators could provide a way of managing olive pests naturally. In this context, the use of genetic methods to detect the target DNA in environmental samples facilitates the monitoring and evaluation of biological control strategies. Post-mortem genetic analyses have been developed to detect the DNA of pests in soil arthropod fauna generalist predators’ guts. The PCR technique is employed to detect minute amounts of degraded prey DNA inside the digestive tract of the predator. These data would help to confirm whether these main candidates found in the olive agroecosystem predate the pest species. In this regard, several studies have been carried out not only in the laboratory but also in the field, helping to elucidate candidate species for the predation of B. oleae and X. fastidiosa vectors [84,88,92,93,100,108,109,304]. This research leads to better knowledge of the trophic chain of the olive grove and allows us to know which are the natural enemies that prey most on the pest and which should therefore be strengthened.
In short, biotechnology and genetics can enable the development of tools to assist in the rapid and accurate identification and genetic characterization of pest species, pathogens, and strains. Incorporating these advances and techniques into IPM programmes can provide many benefits and innovative tools to raise the precision, effectiveness, and sustainability of pest management practices.
7. Conclusions and Future Directions
With an ever-increasing world population and impending, if not already present, environmental challenges such as ecosystem degradation and climate change, it is imperative to improve the sustainability of crop production and protection. The Mediterranean Basin accounts for 95% of the world’s olive groves and the EU produces about 67% of the world’s olive oil. Approximately 4 million hectares, mainly in the Mediterranean countries of the EU, are devoted to olive cultivation, combining traditional, intensive, and super-intensive groves. This region is suffering from strong warming and water stress, which may lead to severe environmental conditions. These conditions are likely to modify the latitudinal and longitudinal ranges of olive pests and their attacks in ways that are still unknown given the complexities of the ecosystem interactions along the diversity of olive crops. Although the need to develop and implement more effective strategies for the control of olive pests and pathogens has always been urgent, the necessity of this challenge has increased sharply in recent years due to the globalisation of trade, the growth of the human population, the consequent increase in the demand for food, and, more recently, due to the social awakening in terms of health and biodiversity following the relentless COVID-19 pandemic and the increasingly extreme environmental conditions we are experiencing.
Although the use of synthetic pesticides has traditionally been, and still is, the most effective method, their misuse and prolonged use has led to serious environmental problems. In this context, the management of olive pests and diseases must be efficient, responsible, and environmentally friendly. The use of pesticides is now more restricted, with national and international regulatory frameworks in place to achieve the sustainable use of pesticides. One of the strategies to reduce the dependence on agrochemicals is to strengthen the wider implementation of IPM practices. It is important to emphasise that the combination of many IPM practices often has a greater effect than any single one. Some strategies used to control olive pest populations are insufficient or may also have undesirable effects, such as mass trapping or kaolin (also used against X. fastidiosa vectors (MAPA resolution 28 February 2018)). Therefore, research must focus on plant protection, soil health and conservation practices, irrigation and fertilisation, and the provision of ecosystem services with the enhancement of biodiversity to promote natural pest control via natural enemies. To achieve these objectives, it is essential to involve, guide, and financially support farmers in the knowledge and application of these good practices in their olive groves and the potential benefits they bring in terms of pest and disease control and plant health. Finally, the use and improvement of genetic and biotechnological tools, together with the development of new technologies, are essential to increase the success of IPM and the sustainability of this key Mediterranean crop.
Farmers, advisers, the olive industry, local and national governments, non-governmental institutions, researchers, and civil society together have the task of raising environmental awareness and recognizing the shared global responsibility to protect the health of people, ecosystems, and the planet, as embodied in the One Health approach.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app132112078/s1, Supplementary Material S1: A summary of potential biological control organisms, pathogens and OEs.
Author Contributions
Conceptualization E.L.; Methodology, C.C., E.L. and B.M.; Investigation, C.C., E.L. and B.M.; Resources, C.C., E.L. and B.M.; Data Curation, C.C., E.L. and B.M.; Writing—Original Draft Preparation, C.C., E.L. and B.M.; Writing—Review and Editing, C.C., E.L. and B.M.; Supervision, C.C., E.L. and B.M.; Project Administration, C.C.; Funding Acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the editor of the Special Issue and the reviewers for their helpful comments during the preparation of this review. This review article contains information from many published sources, and we would like to thank all the authors of the references used in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaniewsky, D.; Van Campo, E.; Boiy, T.; Terral, J.F.; Khadari, B.; Besnard, G. Primary domestication and early uses of emblematic olive tree: Paleobotanical, historical and molecular evidence from the Middle East. Biol. Rev. 2012, 87, 855–899. [Google Scholar] [CrossRef] [PubMed]
- Loukas, M.; Kimbras, C.B. History of olive cultivars based on their genetic distances. J. Hortic. Sci. 1983, 58, 121–127. [Google Scholar] [CrossRef]
- Besnard, G.; Terral, J.F.; Cornille, A. On the origins and domestication of the olive: A review and perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef]
- Namdar, D.; Amrani, A.; Getzov, N.; Milevski, I. Olive oil storage during the fifth and sixth millennia BC at Ein Zippori, Northern Israel. Isr. J. Plant Sci. 2015, 62, 65–74. [Google Scholar] [CrossRef]
- Liphschitz, N.; Gophna, R.; Hartman, M.; Biger, G. The beginning of olive (Olea europaea) cultivation in the Old World: A reassessment. J. Archaeol. Sci. 1991, 18, 441–453. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Shahidi, F. Olives and Olive Oil as Functional Foods; John Wiley & Sons: Chichester, UK, 2017. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2021, 11, 56. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. Statistics. Available online: https://www.fao.org/statistics/es/ (accessed on 18 August 2023).
- IOC, International Olive Council. Available online: https://www.internationaloliveoil.org/ (accessed on 18 August 2023).
- MAPA, Ministerio de Agricultura, Pesca y Alimentación. Available online: https://www.mapa.gob.es/es/ (accessed on 18 August 2023).
- UNESCO, United Nations Educational, Scientific and Cultural Organization. World Heritage Convention. Available online: https://whc.unesco.org/ (accessed on 18 August 2023).
- Campón-Cerro, A.M.; Folgado-Fernández, J.A.; Hernández-Mogollón, J.M. Rural Destination Development Based on Olive Oil Tourism: The Impact of Residents’ Community Attachment and Quality of Life on Their Support for Tourism Development. Sustainability 2017, 9, 1624. [Google Scholar] [CrossRef]
- Rodríguez Sousa, A.A.; Parra-López, C.; Sayadi-Gmada, S.; Barandica, J.M.; Rescia, A.J. A multifunctional assessment of integrated and ecological farming in olive agroecosystems in southwestern Spain using the Analytic Hierarchy Process. Ecol. Econ. 2020, 173, 106658. [Google Scholar] [CrossRef]
- Picornell, A.; Abreu, I.; Ribeiro, H. Trends and future projections of Olea flowering in the western Mediterranean: The example of the Alentejo region (Portugal). Agric. Meteorol. 2023, 339, 109559. [Google Scholar] [CrossRef]
- Victoriano, M.; Oliveira, L.; Oliveira, H.P. Automated Detection and Identification of Olive Fruit Fly Using YOLOv7 Algorithm. In Iberian Conference on Pattern Recognition and Image Analysis; Springer: Cham, Switzerland, 2023; pp. 211–222. [Google Scholar]
- IPPC Secretariat. Scientific Review of the Impact of Climate Change on Plant Pests—A Global Challenge to Prevent and Mitigate Plant Pest Risks in Agriculture, Forestry and Ecosystems; FAO on behalf of the IPPC Secretariat: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. IPM Practices. Available online: www.fao.org/agriculture/crops/thematic-sitemap/theme/compendium/scpi-practices/integrated-pest-management/en/ (accessed on 26 October 2023).
- European Commission. Directorate-General for Agriculture and Rural Development, Farmer’s Toolbox for Integrated Pest Management: Final Report, Publications Office of the European Union. Available online: https://data.europa.eu/doi/10.2762/457165 (accessed on 18 August 2023).
- EPPO, European and Mediterranean Plant Protection Organization. Available online: https://gd.eppo.int/taxon/DACUOL/distribution (accessed on 18 August 2023).
- Caselli, A.; Petacchi, R. Climate change and major pests of Mediterranean olive orchards: Are we ready to face the global heating? Insects 2021, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Haniotakis, G.E. Olive pest control: Present status and prospects. IOBC WPRS Bull. 2005, 28, 1. [Google Scholar]
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Ann. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; De Meyer, M.; Stonehouse, J.M. A review of native and introduced fruit flies (Diptera, Tephritidae) in the Indian Ocean islands of Mauritius, Reunion, and Seychelles. In Proceedings of the Indian Ocean Commission, Regional Fruit Fly Symposium, Flic en Flac, Mauritius, 5–9 June 2000; Price, N.S., Seewooruthun, S.I., Eds.; Indian Ocean Commission/European Union: Flic en Flac, Mauritius, 2000; pp. 15–21. [Google Scholar]
- Vargas, R.I.; Piñero, J.C.; Leblanc, L. An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects 2015, 6, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Duyck, P.F.; David, P.; Quilici, S. A review of relationships between interspecific competition and invasions in fruit flies (Diptera: Tephritidae). Ecol. Entomol. 2004, 29, 511–520. [Google Scholar] [CrossRef]
- Aluja, M.; Norrbom, A. (Eds.) Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Rice, R.E. Bionomics of the olive fruit fly Bactrocera (Dacus) oleae. Plant Prot. Q. 2000, 10, 1–5. [Google Scholar]
- Ruiz Castro, A. Fauna entomológica del olivo en España (SI). In Estudio Sistemático—Biológico de las Especies de Mayor Importancia Económica; Instituto Español de Entomología; CSIC: Madrid, Spain, 1941. [Google Scholar]
- Trombik, J.; Ward, S.F.; Norrbom, A.L.; Liebhold, A.M. Global drivers of historical true fruit fly (Diptera: Tephritidae) invasions. J. Pest. Sci. 2023, 96, 345–357. [Google Scholar] [CrossRef]
- Collier, T.; Van Steenwyk, R. Prospects for integrated control of olive fruit fly are promising in California. Calif. Agric. 2003, 1, 28–32. [Google Scholar] [CrossRef]
- Marchini, D.; Petacchi, R.; Marchi, S. Bactrocera oleae reproductive biology: New evidence on wintering wild populations in olive groves of Tuscany (Italy). Bull. Insectol. 2017, 70, 121–128. [Google Scholar]
- Segura, M.D. Estudio Poblacional y Evolutivo de la Especie Bactrocera oleae Mediante el Uso de Marcadores Moleculares. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2002. [Google Scholar]
- Ramos, P.; Campos, M.; Ramos, J.M. Long-term study on the evaluation of yield and economic losses caused by Prays oleae Bern. in the olive crop of Granada (southern Spain). Crop Prot. 1998, 17, 645–647. [Google Scholar] [CrossRef]
- Alves, J.F.; Mendes, S.; Alves da Silva, A.; Sousa, J.P.; Paredes, D. Land-use effect on olive groves pest Prays oleae and on its potential biocontrol agent Chrysoperla carnea. Insects 2021, 12, 46. [Google Scholar] [CrossRef]
- Armendáriz, I.; De la Iglesia, L.; Santiago, Y.; Campillo, G.; Alberte, C.; Miranda, L.; Juárez, S.; Pérez-Sanz, A. Ciclo del prays del olivo (Prays oleae, Bern.) en Arribes del Duero. Bol. San. Veg. Plagas. 2007, 33, 443–445. [Google Scholar]
- Bjeliš, M.; Radunić, D.; Maček, J.L. Control of olive moth—Prays oleae Bernhard (Lepidoptera, Hyponomeutidae) flower generation by insecticide cover sprays. Zb. Pred. Ref. 2009, 9, 403–409. [Google Scholar]
- Morris, T.I.; Symondson, W.O.C.; Kidd, N.A.C.; Campos, M. The effect of different ant species on the olive moth, Prays oleae (Bern.), in Spanish olive orchard. J. Appl. Entomol. 2002, 126, 224–230. [Google Scholar] [CrossRef]
- Villa, M.; Santos, S.A.P.; Sousa, J.P.; Ferreira, A.; Martins da Silva, P.; Patanita, I.; Ortega, M.; Pascual, S.; Pereira, J.A. Landscape composition and configuration affect the abundance of the olive moth (Prays oleae, Bernard) in olive groves. Agric. Ecosyst. Environ. 2020, 294, 106854. [Google Scholar] [CrossRef]
- Drosopoulos, S.; Remane, R. Biogeographic studies on the spittlebug Philaenus signatus Melichar, 1896 species group (Hemiptera: Aphrophoridae) with the description of two new allopatric species. In Annales de la Société Entomologique de France; Société Entomologique de France: Paris, France, 2000; Volume 36, pp. 269–277. [Google Scholar]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Available online: https://www.efsa.europa.eu/en/topics/topic/xylella-fastidiosa (accessed on 18 August 2023).
- Cornara, D.; Sicard, A.; Zeilinger, A.R.; Porcelli, F.; Purcell, A.H.; Almeida, R.P.P. Transmission of Xylella fastidiosa to Grapevine by the Meadow Spittlebug. Phytopathology 2016, 106, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- Almeida, R.P.P. Xylella fastidiosa vector transmission biology. In Vector-Mediated Transmission of Plant Pathogens; Brown, J.K., Ed.; American Phytopathological Society Press: St. Paul, MN, USA, 2016; pp. 165–174. [Google Scholar]
- Weaver, C.R.; King, D.R. Meadow spittlebug, Philaenus leucophthalmus (L.). Res. Bull. Ohio Agric. Exp. Stn. 1954, 741, 1–99. [Google Scholar]
- Halkka, A.; Halkka, L.; Halkka, O.; Roukka, K.; Pokki, J. Lagged effects of North Atlantic Oscillation on spittlebug Philaenus spumarius (Homoptera) abundance and survival. Glob. Chang. Biol. 2006, 12, 2250–2262. [Google Scholar] [CrossRef]
- Picciotti, U.; Lahbib, N.; Sefa, V.; Porcelli, F.; Garganese, F. Aphrophoridae Role in Xylella fastidiosa subsp. pauca ST53 Invasion in Southern Italy. Pathogens 2021, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Acquasanta, F.; Bacci, L.; Baser, N.; Carmignano, P.M.; Cavalieri, V.; Cioffi, M.; Convertini, S.; D’Accolti, A.; Dal Maso, E.; Diana, F.; et al. Tradizione e Innovazione nel Controllo del Philaenus spumarius Linnaeus, 1758 (Hemiptera Aphrophoridae). In Proceedings of the Giornate Fitopatologiche; Atti Giornate Fitopatologiche I: Chianciano Terme, Italy, 2018; pp. 181–190. [Google Scholar]
- Dáder, B.; Viñuela, E.; Moreno, A.; Plaza, M.; Garzo, E.; Del Estal, P.; Fereres, A. Sulfoxaflor and natural Pyrethrin with Piperonyl Butoxide are effective alternatives to Neonicotinoids against juveniles of Philaenus spumarius, the European vector of Xylella fastidiosa. Insects 2019, 10, 225. [Google Scholar] [CrossRef]
- Commission Implementing Decision (EU) 2015/789 of 18 May 2015 as Regards Measures to Prevent the Introduction into and the Spread within the Union of Xylella fastidiosa (Wells et al.) (Notified under Document C(2015) 3415). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015D0789 (accessed on 17 October 2023).
- EIP-AGRI Focus Group. Pests and Diseases of the Olive Tree. 2020. Available online: https://ec.europa.eu/eip/agriculture/sites/default/files/fg33_mp_biodiversitypestmanagement_2019_en.pdf (accessed on 18 October 2023).
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Phys. 2014, 121, 122–128. [Google Scholar] [CrossRef]
- Dogaç, E.; Kandemir, I.; Taskin, V. Geographical distribution and frequencies of organophosphate-resistant ace alleles and morphometric variations in olive fruit fly populations. Pest. Manag. 2014, 71, 1529–1539. [Google Scholar] [CrossRef]
- Pereira-Castro, I.; Van Asch, B.; Trinidade-Rei, F.; Teixeira Da Costa, L. Bactrocera oleae (Diptera: Tephritidae) organophosphate resistance alleles in Iberia: Recent expansion and variable frequencies. Eur. J. Entomol. 2015, 112, 20–26. [Google Scholar]
- Kampouraki, A.; Stavrakaki, M.; Karataraki, A.; Katsikogiannis, G.; Pitika, E.; Varikou, K.; Vlachaki, A.; Chrysargyris, A.; Malandraki, E.; Sidiropoulos, N.; et al. Recent evolution and operational impact of insecticide resistance in olive fruit fly Bactrocera oleae populations from Greece. J. Pest. Sci. 2018, 91, 1429–1439. [Google Scholar] [CrossRef]
- Lantero, E.; Matallanas, B.; Pascual, S.; Ochando, M.D.; Callejas, C. Phylogeography of organophosphate resistant ace alleles in Spanish olive fruit fly populations: A Mediterranean perspective in global change context. Insects 2020, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Porras, A.; Marti, J.; Tudela, A.; Rodríguez-González, Á.; Sambado, P. Mating Disruption of the Olive Moth Prays oleae (Bernard) in Olive Groves Using Aerosol Dispensers. Insects 2021, 12, 1113. [Google Scholar] [CrossRef] [PubMed]
- Budzinski, H.; Couderchet, M. Environmental and human health issues related to pesticides: From usage and environmental fate to impact. Environ. Sci. Pollut. R. 2018, 25, 14277–14279. [Google Scholar] [CrossRef] [PubMed]
- Brühl, C.A.; Zaller, J.G. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front. Env. Sci. 2019, 7, 177. [Google Scholar] [CrossRef]
- European Directive 2009/128/CE 21 of the European Parliament and of the Council. A Framework to Achieve a Sustainable Use of Pesticides by Reducing the Risks and Impacts of Pesticide Use on Human Health and the Environment and Promoting the Use of Integrated Pest Management and of Alternative Approaches or Techniques Such as Non-Chemical Alternatives to Pesticides; Bulletin 309; October 2009; pp. 71–83. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32009L0128 (accessed on 17 October 2023).
- MAPA, Ministerio de Agricultura Pesca y Alimentación de España. Registro de Productos Fitosanitarios. Available online: https://www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/productos-fitosanitarios/registro-productos/ (accessed on 16 October 2023).
- BOE-A-2012-11605, Real Decreto 1311/2012, de 14 de Septiembre de 2012 BOE 223. Marco de Actuación para Conseguir un Uso Sostenible de los Productos Fitosanitarios. Available online: https://www.boe.es/diario_boe/txt.php?id=BOE-A-2012-11605 (accessed on 17 October 2023).
- Selim, M. A review of advantages, disadvantages and challenges of crop rotations. Egypt. J. Agron. 2019, 41, 1–10. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations Conservation Agriculture: Case Studies in Latin America and Africa; FAO Soils Bulletin; FAO: Rome, Italy, 2002; Volume 78. [Google Scholar]
- Gordon, P.; Lampinen, B.; Milliron, L.; Duncan, R.; Lightle, D.; Connell, J.; Brar, R.; Reyes, C.; Vasquez-Mendoza, J. Evaluation of almond cultivars from an integrated pest management perspective. In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Innovative Perennial Crops Management, Angers, France, 14 August 2022; Volume 1366, pp. 435–442. [Google Scholar]
- Dolatabadian, A.; Cornelsen, J.; Huang, S.; Zou, Z.; Fernando, W.D. Sustainability on the farm: Breeding for resistance and management of major canola diseases in Canada contributing towards an IPM approach. Can. J. Plant Pathol. 2022, 44, 157–190. [Google Scholar]
- Darwish, A.; Attia, M.M.; Khozimy, A.M. Effect of some integrated pest management elements on the population density of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) on tomato plants (Solanum lycopersicum L.). Alex. Sci. Exch. 2021, 42, 57–68. [Google Scholar]
- Abbasi, N.; Ghassemi-Kahrizeh, A.; Hosseinzadeh, A. The effect of cultivar and planting date on density and damage of chickpea leaf-miner (Liriomyza congesta Becker) in the Oshnavia region, West Azarbaijan province, Iran. Iran. J. Pulses Res. 2021, 12, 165–182. [Google Scholar]
- Uyi, O.; Reay-Jones, F.P.; Ni, X.; Buntin, D.; Jacobson, A.; Punnuri, S.; Toews, M.D. Impact of Planting Date and Insecticide Application Methods on Melanaphis sorghi (Hemiptera: Aphididae) Infestation and Forage Type Sorghum Yield. Insects 2022, 13, 1038. [Google Scholar]
- Han, P.; Desneux, N.; Becker, C.; Larbat, R.; Le Bot, J.; Adamowicz, S.; Zhang, J.; Lavoir, A.-V. Bottom-up effects of irrigation, fertilization and plant resistance on Tuta absoluta: Implications for Integrated Pest Management. J. Pest. Sci. 2019, 92, 1359–1370. [Google Scholar]
- Devkota, K.P.; Pasuquin, E.; Elmido-Mabilangan, A.; Dikitanan, R.; Singleton, G.R.; Stuart, A.M.; Vithoonjit, D.; Vidiyangkura, L.; Pustika, A.B.; Afriani, R.; et al. Economic and environmental indicators of sustainable rice cultivation: A comparison across intensive irrigated rice cropping systems in six Asian countries. Ecol. Indic. 2019, 105, 199–214. [Google Scholar]
- Liu, L.; Zheng, X.; Wei, X.; Kai, Z.; Xu, Y. Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication. Sci. Rep. 2021, 11, 23015. [Google Scholar] [CrossRef]
- Desneux, N.; Han, P.; Mansour, R.; Arnó, J.; Brévault, T.; Campos, M.R.; Chailleux, A.; Guedes, R.N.C.; Karimi, J.; Konan, K.A.J.; et al. Integrated pest management of Tuta absoluta: Practical implementations across different world regions. J. Pest. Sci. 2022, 95, 17–39. [Google Scholar]
- MAPA, Ministerio de Agricultura Pesca y Alimentación de España. Guidelines for IPM. Available online: www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/productos-fitosanitarios/guias-gestion-plagas (accessed on 18 August 2023).
- MAPA, Ministerio de Agricultura, Pesca y Alimentación. Olive Crops Guidelines. Available online: www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/productos-fitosanitarios/guias-gestion-plagas/olivar/default.aspx (accessed on 18 August 2023).
- Deguine, J.P.; Aubertot, J.N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.G.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- MAPA, Ministerio de Agricultura, Pesca y Alimentación. Informe de Resultados de Aplicación del PAN 2021. Available online: https://www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/informepan2021_tcm30-620872.pdf (accessed on 18 August 2023).
- European Commission. IPM Toolbox for Farmers. Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/cmef/sustainability/ipm-toolbox-farmers_en (accessed on 18 August 2023).
- Cloyd, R.A. How Effective Is Conservation Biological Control in Regulating Insect Pest Populations in Organic Crop Production Systems? Insects 2020, 11, 744. [Google Scholar] [CrossRef]
- Picchi, M.S.; Bocci, G.; Petacchi, R.; Entling, M.H. Effects of local and landscape factors on spiders and olive fruit flies. Agric. Ecosyst. Environ. 2016, 222, 138–147. [Google Scholar] [CrossRef]
- Carpio, A.J.; Castro, J.; Tortosa, F.S. Arthropod biodiversity in olive groves under two soil management systems: Presence versus absence of herbaceous cover crop. Agric. For. Entomol. 2019, 21, 58–68. [Google Scholar] [CrossRef]
- Paredes, D.; Rosenheim, J.A.; Karp, D.S. The causes and consequences of pest population variability in agricultural landscapes. agriRxiv 2022, 32, 5. [Google Scholar]
- Boccaccio, L.; Petacchi, R. Landscape effects on the complex of Bactrocera oleae parasitoids and implications for conservation biological control. BioControl 2009, 54, 607–616. [Google Scholar] [CrossRef]
- Lantero, E.; Ortega, M.; Sánchez-Ramos, I.; Núñez, M.G.; Fernández, C.E.; Rescia, A.J.; Matallanas, B.; Callejas, C.; Pascual, S. Effect of local and landscape factors on abundance of ground beetles and assessment of their role as biocontrol agents in the olive growing area of southeastern Madrid, Spain. BioControl 2019, 64, 685–696. [Google Scholar]
- Ortega, M.; Moreno, N.; Fernández, C.E.; Pascual, S. Olive landscape affects Bactrocera oleae abundance, movement and infestation. Agronomy 2021, 12, 4. [Google Scholar] [CrossRef]
- Ortega, M.; Pascual, S. Spatio-temporal analysis of the relationship between landscape structure and the olive fruit fly Bactrocera oleae (Diptera: Tephritidae). Agric. For. Entomol. 2014, 16, 14–23. [Google Scholar] [CrossRef]
- Jaworski, E.T.; Rusch, A.; Lavoir, A.V.; Wang, S.; Desneux, N. Crop diversification to promote arthropod pest management: A review. Agric. Commun. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Dinis, A.M.; Pereira, J.A.; Pimenta, M.C.; Oliveira, J.; Benhadi-Marín, J.; Santos, S.A.P. Suppression of Bactrocera oleae (Diptera: Tephritidae) pupae by soil arthropods in the olive grove. J. Appl. Entomol. 2016, 140, 677–687. [Google Scholar] [CrossRef]
- Picchi, M.S.; Marchi, S.; Albertini, A.; Petacchi, R. Organic management of olive orchards increases the predation rate of overwintering pupae of Bactrocera oleae (Diptera: Tephritidae). BioControl 2017, 108, 9–15. [Google Scholar] [CrossRef]
- Ortega, M.; Sanchez-Ramos, I.; González-Nuñez, M.; Pascual, S. Time course study of Bactrocera oleae (Diptera: Tephritidae) pupae predation in soil: The effect of landscape structure and soil condition. Agric. For. Entomol. 2018, 20, 201–207. [Google Scholar] [CrossRef]
- Martínez-Núñez, P.J.; Rey, T.; Salido, A.J.; Manzaneda, F.M.; Camacho, J.I. Ant community potential for pest control in olive groves: Management and landscape effects. Agric. Ecosyst. Environ. 2021, 305, 107185. [Google Scholar] [CrossRef]
- Albertini, A.; Pizzolotto, R.; Petacchi, R. Carabid patterns in olive orchards and woody semi-natural habitats: First implications for conservation biological control against Bactrocera oleae. BioControl 2017, 62, 71–83. [Google Scholar] [CrossRef]
- Albertini, A.; Marchi, S.; Ratti, C.; Burgio, G.; Petacchi, R.; Magagnoli, S. Bactrocera oleae pupae predation by Ocypus olens detected by molecular gut content analysis. BioControl 2018, 63, 227–239. [Google Scholar] [CrossRef]
- Benhadi-Marín, J.; Pereira, J.A.; Barrientos, J.A.; Sousa, J.P.; Santos, S.A.P. Stones on the ground in olive groves promote the presence of spiders (Araneae). Eur. J. Entomol. 2018, 115, 372–379. [Google Scholar] [CrossRef]
- Benhadi-Marín, J.; Pereira, J.A.; Sousa, J.P.; Santos, S.A.P. Distribution of the spider community in the olive grove agroecosystem (Portugal): Potential bioindicators. Agric. For. Entomol. 2020, 22, 10–19. [Google Scholar] [CrossRef]
- Picchi, M.S. Spiders (Araneae) of olive groves and adjacent semi-natural habitats from central Italy. Arachnol. Mitt. 2020, 60, 1–11. [Google Scholar] [CrossRef]
- Picchi, M.S.; Bocci, G.; Petacchi, R.; Entling, M.H. Taxonomic and functional differentiation of spiders in habitats in a traditional olive producing landscape in Italy. Eur. J. Entomol. 2020, 117, 18–26. [Google Scholar] [CrossRef]
- Gkisakis, V.; Volakakis, N.; Kollaros, D.; Bàrberi, P.; Kabourakis, E.M. Soil arthropod community in the olive agroecosystem: Determined by environment and farming practices in different management systems and agroecological zones. Agric. Ecosyst. Env. 2016, 218, 178–189. [Google Scholar] [CrossRef]
- Lantero, E. Estudio Genético de la Plaga del Olivo Bactrocera oleae (Rossi 1790) y su Aplicación al Control Biológico. Ph.D. Thesis, Universidad Complutense of Madrid, Alcala de Henares, Spain, 2018. [Google Scholar]
- Lantero, E.; Pascual, S.; Ortega, M.; Rescia, A.; González-Núñez, M.; Sánchez-Ramos, I.; Pérez, S.; Matallanas, B.; Callejas, C. Post mortem gut content analysis for determining the contribution of different soil predators to control Bactrocera oleae. Bull. IOBC-WPRS 2019, 141, 161–167. [Google Scholar]
- Porcel, M.; Ruano, F.; Cotes, B.; Peña, A.; Campos, M. Agricultural management systems affect the green lacewing community (Neuroptera: Chrysopidae) in olive orchards in Southern Spain. Environ. Entomol. 2013, 42, 97–106. [Google Scholar] [CrossRef]
- Pappas, M.L.; Broufas, G.D.; Koveos, D.S. Chrysopid predators and their role in biological control. J. Entomol. 2011, 8, 301–326. [Google Scholar] [CrossRef]
- Bento, A.; Lopes, J.; Torres, L.; Passos-Carvalho, P. Biological control of Prays oleae (Bern.) by chrysopids in Trás-os-Montes region (Northeastern Portugal). In Proceedings of the III International Symposium on Olive Growing, Crete, Greece, 22–26 September 1997; International Society for Horticultural Science: Leuven, Beligum, 1999; Volume 474, pp. 535–540. [Google Scholar]
- Pascual, S.; Ortega, M.; Villa, M. Prays oleae (Bernard), its potential predators and biocontrol depend on the structure of the surrounding landscape. Biol. Control. 2022, 176, 105092. [Google Scholar] [CrossRef]
- Choae, B.; Drummond, F.A. Ants as biological control agents in agricultural cropping systems. Terr. Arthropod Rev. 2011, 4, 157–180. [Google Scholar]
- Álvarez, H.A.; García-García, A.; Sandoval, P.; Martín-Blázquez, R.; Seifert, B.; Tinaut, A.; Ruano, F. Elucidating the trophic role of Tapinoma ibericum (Hymenoptera: Formicidae) as a potential predator of olive pests. J. Appl. Entomol. 2023, 147, 667–675. [Google Scholar] [CrossRef]
- EIP-AGRI Focus Group. Pest and Diseases of Olive Trees, Biodiversity and Pest Management. Available online: https://ec.europa.eu/eip/agriculture/sites/default/files/eip-agri_fg_pests_diseases_olive_tree_final_report_2020_en.pdf (accessed on 18 August 2023).
- Lantero, E.; Matallanas, B.; Pascual, S.; Callejas, C. PCR Species-Specific Primers for Molecular Gut Content Analysis to Determine the Contribution of Generalist Predators to the Biological Control of the Vector of Xylella fastidiosa. Sustainability 2018, 10, 2207. [Google Scholar] [CrossRef]
- Rodrigues, I.; Ramos, V.; Benhadi-Marín, J.; Moreno, A.; Fereres, A.; Pereira, J.A.; Baptista, P. A novel molecular diagnostic method for the gut content analysis of Philaenus DNA. Sci. Rep. 2022, 12, 492. [Google Scholar] [CrossRef]
- Benhadi-Marín, J.; Villa, M.; Pereira, L.F.; Rodrigues, I.; Morente, M.; Baptista, P.; Pereira, J.A. A Guild-Based Protocol to Target Potential Natural Enemies of Philaenus spumarius (Hemiptera: Aphrophoridae), a Vector of Xylella fastidiosa (Xanthomonadaceae): A Case Study with Spiders in the Olive Grove. Insects 2020, 11, 100. [Google Scholar] [CrossRef]
- Daane, K.; Johnson, M.; Pickett, C.; Sime, K.; Wang, X.G.; Nadel, H.; Andrews, J.; Hoelmer, K. Biological controls investigated to aid management of olive fruit fly in California. Calif. Agric. 2011, 65, 21–28. [Google Scholar] [CrossRef]
- Daane, K.M.; Sime, K.; Wang, X.G.; Nadel, H.; Johnson, M.; Walton, V.; Kirk, A.; Pickett, C. Psyttalia lonsburyi (Hymenoptera: Braconidae), potential biological control agent for the olive fruit fly in California. Biol. Control 2008, 44, 79–89. [Google Scholar] [CrossRef]
- Yokoyama, V.Y.; Cáceres, C.E.; Kuenen, L.P.S.; Wang, X.G.; Rendón, P.A.; Johnson, M.W.; Daane, K.M. Field performance and fitness of an olive fruit fly parasitoid, Psyttalia humilis (Hymenoptera: Braconidae), mass reared on irradiated Medfly. Biol. Control 2010, 54, 90–99. [Google Scholar] [CrossRef]
- Wang, X.G.; Johnson, M.; Daane, K.; Yokoyama, V. Larger olive fruit size reduces the efficiency of Psyttalia concolor as a parasitoid of the olive fruit fly. Biol. Control 2009, 49, 45–51. [Google Scholar] [CrossRef]
- Villa, M.; Santos, S.A.P.; Mexia, A.; Bento, A.; Pereira, J.A. Ground cover management affects parasitism of Prays oleae (Bernard). Biol. Control 2016, 96, 72–77. [Google Scholar] [CrossRef]
- Pappalardo, S.; Villa, M.; Santos, S.A.P.; Benhadi-Marín, J.; Pereira, J.A.; Venturino, E. A tritrophic interaction model for an olive tree pest, the olive moth—Prays oleae (Bernard). Ecol. Model. 2021, 462, 109776. [Google Scholar] [CrossRef]
- Whittaker, J.B. Density Regulation in a Population of Philaenus spumarius (L.) (Homoptera: Cercopidae). J. Anim. Ecol. 1973, 42, 163–172. [Google Scholar] [CrossRef]
- Molinatto, G.; Demichelis, S.; Bodino, N.; Giorgini, M.; Mori, N.; Bosco, D. Biology and Prevalence in Northern Italy of Verrallia aucta (Diptera, Pipunculidae), a Parasitoid of Philaenus spumarius (Hemiptera, Aphrophoridae), the Main Vector of Xylella fastidiosa in Europe. Insects 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, X.; Chartois, M.; Genson, G.; Rossi, J.; Cruaud, A.; Rasplus, J. Ooctonus vulgatus (Hymenoptera, Mymaridae), a potential biocontrol agent to reduce populations of Philaenus spumarius (Hemiptera, Aphrophoridae) the main vector of Xylella fastidiosa in Europe. PeerJ 2020, 8, e8591. [Google Scholar] [CrossRef]
- Vongati, M.; Mohanty, S.; Das, K. Role of Microbial Pesticides in IPM. Just Agric. 2022, 2, 12. [Google Scholar]
- Ortiz, A.; Sansinenea, E. Bacillus thuringiensis based biopesticides for integrated crop management. In Biopesticides; Rakshit, M., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L.F., Parihar, M., Singh, A.K., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Arora, N.; Agrawal, N.; Yerramilli, V.; Bhatnagar, R.K. Biology and Applications of Bacillus thuringiensis. In Integrated Pest Management. General Concepts in Integrated Pest and Disease Management; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 1, pp. 227–244. [Google Scholar] [CrossRef]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as promising alternatives to chemical pesticides: A review of their current and future status. Online J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Jhala, J.; Baloda, A.S.; Rajput, V.S. Role of bio-pesticides in recent trends of insect pest management: A review. J. Pharmacogn. Phytochem. 2020, 9, 2237–2240. [Google Scholar]
- Essiedu, J.A.; Adepoju, F.O.; Ivantsova, M.N. Benefits and limitations in using biopesticides: A review. In Proceedings of the AIP Conference Proceedings, Ekaterinburg, Russia, 18–22 May 2020; AIP Publishing: Melville, NY, USA, 2020; Volume 2313, p. 1. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Botanical pesticides for eco-friendly pest management: Drawbacks and limitations. In Pesticides in Crop Production: Physiological and Biochemical Action; Srivastava, P.K., Singh, V.P., Singh, A., Tripathi, D.K., Singh, S., Prasad, S.M., Chauhan, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 181–193. [Google Scholar] [CrossRef]
- Fernández-Chapa, D.; Ramírez-Villalobos, J.; Galán-Wong, L. Toxic potential of Bacillus thuringiensis: An overview. In Protecting Rice Grains in the Post-Genomic Era; Jia, Y., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Recent advancements for microorganisms and their natural compounds useful in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Unzué Pozas, A. Multifunctional Analysis of New Bacillus thuringiensis Strains and Their Potential as Pest Control Agents. Ph.D. Thesis, Universidad de Navarra, Pamplona, Spain, 2023. [Google Scholar]
- Sanchis, V. From microbial sprays to insect-resistant transgenic plants: History of the biospesticide Bacillus thuringiensis. A Review. Agron. Sustain. Dev. 2011, 31, 217–231. [Google Scholar] [CrossRef]
- Cárdenas, A.R.T.; Rebull, J. Control de” Prays oleae” con” Bacillus thuringiensis”, y efecto sobre la comunidad entomológica. Phytoma España Rev. Prof. Sanid. Veg. 2022, 343, 132–135. [Google Scholar]
- Godena, S.; Rojnić, I.D.; Žanić, K.; Dean, B.A.N. Monitoring and population characteristics of Prays oleae (Lepidoptera: Yponomeutidae) on different insecticidal treatments. Rev. Soc. Entomol. 2019, 78, 65–74. [Google Scholar] [CrossRef]
- Blibech, I.; Ksantini, M.; Shete, M. Insecticidal Activity of an Indian Botanical Insecticide ULTRA ACT® against the Olive Pest Bactrocera oleae (Diptera: Tephritidae) in Tunisia. Adv. Chem. Eng. 2020, 10, 69. [Google Scholar] [CrossRef]
- Azizoglu, U.; Jouzani, G.S.; Sansinenea, E.; Sanchis-Borja, V. Biotechnological advances in Bacillus thuringiensis and its toxins: Recent updates. Rev. Env. Sci. Biotechnol. 2023, 22, 319–348. [Google Scholar] [CrossRef]
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Darby, A.C. Symbiosis research as a novel strategy for insect pest control. In Biorational Control of Arthropod Pests: Application and Resistance Management; Ishaaya, I., Horowitz, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 207–231. [Google Scholar]
- Klepzig, K.D.; Adams, A.S.; Handelsman, J.; Raffa, K.F. Symbioses: A key driver of insect physiological processes, ecological interactions, evolutionary diversification, and impacts on humans. Environ. Entomol. 2009, 38, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.K.; Douglas, A.E. Hype or opportunity? Using microbial symbionts in novel strategies for insect pest control. J. Insect. Physiol. 2017, 103, 10–17. [Google Scholar] [CrossRef]
- Petri, L. Ricerche sopra i batteri intestinali della mosca olearia. In Memorie della Regia Stazione di Patologia Vegetale di Roma; Tipografia nazionale di G. Bertero e c.: Roma, Italy, 1909. [Google Scholar]
- Yamvrias, C.; Panagopiulos, C.G.; Psallidas, P.G. Preliminary study of the internal bacterial flora of the olive fruit fly (Dacus oleae Gmelin). Ann. L’institut Phytopathol. Benaki 1970, 9, 201–206. [Google Scholar]
- Daser, U.; Brandl, R. Microbial gut floras of eight species of tephritids. Biol. J. Linn. Soc. 1992, 45, 155–165. [Google Scholar] [CrossRef]
- Konstantopoulou, M.A.; Raptopoulos, D.G.; Stavrakis, N.G.; Mazomenos, B.E. Microflora species and their volatile compounds affecting development of an alcohol dehydrogenase homozygous strain (Adh-I) of Bactrocera (Dacus) oleae (Diptera: Tephritidae). J. Econ. Entomol. 2009, 98, 1943–1949. [Google Scholar] [CrossRef]
- Rempoulakis, P.; Sela, S.; Nemny-Lavy, E.; Pinto, R.; Birke, A.; Nestel, D. Microbial composition affects the performance of an artificial Tephritid larval diet. Bull. Entomol. Res. 2018, 108, 434–441. [Google Scholar] [CrossRef]
- Kounatidis, I.; Crotti, E.; Sapountzis, P.; Sacchi, L.; Rizzi, A.; Chouaia, B.; Bandi, C.; Alma, A.; Daffonchio, D.; Mavragani-Tsipidou, P.; et al. Acetobacter tropicalis is a major symbiont of the olive fruit fly (Bactrocera oleae). Appl. Environ. Microbiol. 2009, 75, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, P.; Granchietti, A.; Landini, S.; Viti, C.; Giovannetti, L.; Belcari, A. Relationships between the olive fly and bacteria. J. Appl. Entomol. 2008, 132, 682–689. [Google Scholar] [CrossRef]
- Estes, A.M.; Hearn, D.J.; Bronstein, J.L.; Pierson, E.A. The olive fly endosymbiont, “Candidatus Erwinia dacicola,” switches from an intracellular existence to an extracellular existence during host insect development. Appl. Environ. Microbiol. 2009, 75, 7097–7106. [Google Scholar] [PubMed]
- Blow, F.; Gioti, A.; Goodhead, I.B.; Kalyva, M.; Kampouraki, A.; Vontas, J.; Darby, A.C. Functional genomics of a symbiotic community: Shared traits in the olive fruit fly gut microbiota. Genome Biol. Evol. 2019, 12, 3778–3791. [Google Scholar]
- Capuzzo, C.; Firrao, G.; Mazzon, L.; Squartini, A.; Girolami, V. ‘Candidatus Erwinia dacicola’, a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin). Int. J. Syst. Evol. Microbiol. 2005, 55, 1641–1647. [Google Scholar] [PubMed]
- Savio, C.; Mazzon, L.; Martinez-Sanudo, I.; Simonato, M.; Squartini, A.; Girolami, V. Evidence of two lineages of the symbiont ‘Candidatus Erwinia dacicola’ in Italian populations of Bactrocera oleae (Rossi) based on 16S rRNA gene sequences. Int. J. Syst. Evol. Microbiol. 2012, 62, 179–187. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive fly larvae to overcome host defences. Roy. Soc. Open Sci. 2015, 2, 150–170. [Google Scholar]
- Sinno, M.; Bézier, A.; Vinale, F.; Giron, D.; Laudonia, S.; Garonna, A.P.; Pennacchio, F. Symbiosis disruption in the olive fruit fly, Bactrocera oleae (Rossi), as a potential tool for sustainable control. Pest. Manag. Sci. 2020, 76, 3199–3207. [Google Scholar] [CrossRef]
- Belcari, A.; Sacchetti, P.; Rosi, M.C.; Del Pianta, R. The use of copper products to control the olive fly (Bactrocera oleae) in central Italy. IOBC WPRS Bull. 2005, 28, 45. [Google Scholar]
- Caleca, V.; Lo Verde, G.; Lo Verde, V.; Piccionello, M.P.; Rizzo, R. Control of Bactrocera oleae and Ceratitis capitata in organic orchards: Use of clays and copper products. Acta Hortic. 2010, 873, 227–234. [Google Scholar] [CrossRef]
- Nobre, T. Symbiosis in sustainable agriculture: Can olive fruit fly bacterial microbiome be useful in pest management? Microorganisms 2019, 7, 238. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Ruocco, M.; Woo, S.L.; Lorito, M. Trichoderma secondary metabolites that affect plant metabolism. Nat. Prod. Commun. 2012, 7, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Keswani, C.; Singh, H.B.; Hermosa, R.; García-Estrada, C.; Caradus, J.; He, Y.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Vinale, F.; et al. Antimicrobial secondary metabolites from agriculturally important fungi as next biocontrol agent. Appl. Microbiol. Biotechnol. 2019, 103, 9287–9303. [Google Scholar]
- Bigiotti, G.; Pastorelli, R.; Belcari, A.; Sacchetti, P. Symbiosis interruption in the olive fly: Effect of copper and propolis on Candidatus Erwinia dacicola. J. Appl. Entomol. 2019, 143, 357–364. [Google Scholar] [CrossRef]
- Tsolakis, H.; Ragusa, E.; Tarantino, P. Control of Bactrocera oleae by low environmental impact methods: NPC methodology to evaluate the efficacy of lure-and-kill method and copper hydroxide treatments. Bull. Insectol. 2011, 64, 1–8. [Google Scholar]
- Gonçalves, F.; Torres, L. Effect of copper oxychloride on the olive infestation by Bactrocera oleae in northeastern Portugal. Acta Hortic. 2012, 949, 333–340. [Google Scholar] [CrossRef]
- Vizzarri, V.; Lombardo, L.; Novellis, C.; Rizzo, P.; Pellegrino, M.; Cruceli, G.; Godino, G.; Zaffina, F.; Ienco, A. Testing the Single and Combined Effect of Kaolin and Spinosad against Bactrocera oleae and Its Natural Antagonist Insects in an Organic Olive Grove. Life 2023, 13, 607. [Google Scholar] [CrossRef]
- González-Núñez, M.; Pascual, S.; Cobo, A.; Seris, E.; Cobos, G.; Fernández, C.E.; Sánchez-Ramos, I. Copper and kaolin sprays as tools for controlling the olive fruit fly. Entomol. Gen. 2021, 41, 97–110. [Google Scholar] [CrossRef]
- Sparks, T.; Crouse, G.; Durst, G. Natural products as insecticides: The biology, biochemistry and quantitative structure activity relationships of spinosyns and spinosoids. Pest. Manag. Sci. 2001, 57, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.S.V.; Silva, C.E.; Oliveira, C.M.; de Morais, C.R.; Limongi, J.E.; Pereira, B.B. Evaluation of toxicity and environmental safety in use of spinosad to rationalize control strategies against Aedes aegypti. Chemosphere 2019, 226, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Chio, E.H.; Li, Q.X. Pesticide Research and Development: General Discussion and Spinosad Case. J. Agric. Food Chem. 2022, 70, 8913–8919. [Google Scholar] [CrossRef] [PubMed]
- Sagri, E.; Reczko, M.; Gregoriou, M.E.; Tsoumani, K.T.; Zygouridis, N.E.; Salpea, K.D.; Zalom, F.G.; Ragoussis, J.; Mathiopoulos, K.D. Olive fly transcriptomics analysis implicates energy metabolism genes in spinosad resistance. BMC Genom. 2014, 15, 714. [Google Scholar] [CrossRef] [PubMed]
- Gress, B.E.; Zalom, F.G. Identification and risk assessment of spinosad resistance in a California population of Drosophila suzukii. Pest. Manag. Sci. 2019, 75, 1270–1276. [Google Scholar] [CrossRef]
- Guillem-Amat, A.; Sánchez, L.; López-Errasquín, E.; Ureña, E.; Hernández-Crespo, P.; Ortego, F. Field detection and predicted evolution of spinosad resistance in Ceratitis capitata. Pest. Manag. Sci. 2020, 76, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, M.; Ma, Z.; You, C.; Gao, X.; Shi, X. Esterase-mediated spinosad resistance in house flies Musca domestica (Diptera: Muscidae). Ecotoxicology 2020, 29, 35–44. [Google Scholar] [CrossRef]
- Hsu, J.C.; Chou, M.Y.; Mau, R.F.; Maeda, C.; Shikano, I.; Manoukis, N.C.; Vargas, R.I. Spinosad resistance in field populations of melon fly, Zeugodacus cucurbitae (Coquillett), in Hawaii. Pest. Manag. Sci. 2021, 77, 5439–5444. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ramasamy, G.G.; Pathak, J.; Nayyar, N.; Muthugounder, M.; Maria, P.; Rai, A.; Thiruvengadam, V. Deciphering the Molecular Mechanisms of Insecticide Resistance from the Transcriptome Data of Field Evolved Spinosad Resistant and Susceptible Populations of Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2022, 115, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, R.; van den Berg, J.; van Rensburg, P.J.; du Plessis, H. Residual activity of spinosad applied as a soil drench to tomato seedlings for control of Tuta absoluta. Pest. Manag. Sci. 2023, 79, 1860–1867. [Google Scholar] [CrossRef]
- Pascual, S.; Cobos, G.; Seris, E.; Sánchez-Ramos, I.; González-Núñez, M. Spinosad bait sprays against the olive fruit fly (Bactrocera oleae (Rossi)): Effect on the canopy non-target arthropod fauna. Int. J. Pest. Manag. 2014, 60, 258–268. [Google Scholar] [CrossRef]
- Martelli, F.; Hernandes, N.H.; Zuo, Z.; Wang, J.; Wong, C.O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Robin, C.; Venkatachalam, K.; et al. Low doses of the organic insecticide spinosad trigger lysosomal defects, elevated ROS, lipid dysregulation, and neurodegeneration in flies. Elife 2022, 11, e73812. [Google Scholar] [CrossRef]
- Araújo, R.D.S.; Lopes, M.P.; Viana, T.A.; Bastos, D.S.S.; Machado-Neves, M.; Botina, L.L.; Martins, G.F. Bioinsecticide spinosad poses multiple harmful effects on foragers of Apis mellifera. Environ. Sci. Pollut. Res. 2023, 30, 66923–66935. [Google Scholar] [CrossRef]
- Perrin, M.; Borowiec, N.; Thaon, M.; Siegwart, M.; Delattre, T.; Moiroux, J. Differential influence of temperature on the toxicity of three insecticides against the codling moth Cydia pomonella (L.) and two natural enemies. J. Pest. Sci. 2023, 76, 1410–1415. [Google Scholar] [CrossRef]
- Anjum, F.; Wright, D.J. Foliar Residual Toxicity of Insecticides to Brassica Pests and Their Natural Enemies. J. Econ. Entom. 2023, 116, 153–159. [Google Scholar] [CrossRef]
- Calabrese, G.; Perrino, E.V.; Ladisa, G.; Aly, A.; Tesfmichael Solomon, M.; Mazdaric, S.; Benedetti, A.; Ceglie, F.G. Short-term effects of different soil management practices on biodiversity and soil quality of Mediterranean ancient olive orchards. Org. Agric. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- Lombardo, L.; Palese, A.M.; Grasso, F.; Duffy, D.H., III; BriccoliBati, C.; Xiloyannis, C. Mechanical Tillage Diversely Affects Glomalin Content, Water Stable Aggregates and AM Fungal Community in the Soil Profiles of Two Differently Managed Olive Orchards. Biomolecules 2019, 9, 639. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.V.R.; Datta, S.C. Particle films and their applications in horticultural crops. Appl. Clay Sci. 2015, 116–117, 54–68. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J. Particle films: A new technology for agriculture. Hort. Rev. 2005, 31, 1–44. [Google Scholar]
- Salerno, G.; Rebora, M.; Kovalev, A.; Gorb, E.; Gorb, S. Kaolin nano-powder effect on insect attachment ability. J. Pest. Sci. 2020, 93, 315–327. [Google Scholar] [CrossRef]
- Marcotegui, A.; Sánchez-Ramos, I.; Pascual, S.; Fernández, C.E.; Cobos, G.; Armendáriz, I.; Cobo, A.; González-Núñez, M. Kaolin and potassium soap with thyme essential oil to control Monosteira unicostata and other phytophagous arthropods of almond trees in organic orchards. J. Pest. Sci. 2015, 88, 753–765. [Google Scholar] [CrossRef]
- Faghih, S.; Zamani, Z.; Fatahi, R.; Liaghat, A. Effects of deficit irrigation and kaolin application on vegetative growth and fruit traits of two early ripening apple cultivars. Biol. Res. 2019, 52, 43. [Google Scholar] [CrossRef]
- Arbabi, M.; Shokat, G.A.A.; Khiavi, H.K.; Imami, M.S.; Kamali, H.; Farazmand, H. Evaluation of kaolin in control of Panonychus ulmi in apple orchards of Iran. Indian. J. Plant Prot. 2020, 34, 47–53. [Google Scholar]
- Singh, R.K.; Afonso, J.; Nogueira, M.; Oliveira, A.A.; Cosme, F.; Falco, V. Silicates of potassium and aluminium (kaolin); comparative foliar mitigation treatments and biochemical insight on grape berry quality in Vitis vinifera L. (cv. Touriga National and Touriga Franca). Biology 2020, 9, 58. [Google Scholar] [CrossRef]
- Cahenzli, F.; Boutry, C. Autumn Kaolin Treatments and Early Spring Oil Treatments against Myzus cerasi in Sweet Cherries. In Proceedings of the 20th International Conference on Organic Fruit-Growing, Hohenheim, Germany, 21–23 February 2022; pp. 175–179. Available online: https://www.ecofruit.net/wp-content/uploads/2022/02/175_179_BP_12_ShortContribution_CahenzliBoutry_AutumnKaolinTreatmentsSweetcherries_formatiert.pdf (accessed on 17 October 2023).
- Nottingham, L.B.; Orpet, R.J.; Beers, E.H. Integrated pest management programs for pear psylla, Cacopsylla pyricola (Förster) (Hemiptera: Psyllidae), using kaolin clay and reflective plastic mulch. J. Econ. Entomol. 2022, 115, 1607–1619. [Google Scholar] [CrossRef]
- Linder, C.; Rösti, J.; Lorenzini, F.; Deneulin, P.; Badertscher, R.; Kehrli, P. Efficacy of kaolin treatments against Drosophila suzukii and their impact on the composition and taste of processed wines. Vitis 2020, 59, 49–52. [Google Scholar] [CrossRef]
- Azizifar, S.; Abdossi, V.; Gholami, R.; Ghavami, M.; Torkashvand, A.M. Effect of salicylic acid and kaolin on yield, physiological traits, and fatty acid composition in olive cultivars under regulated deficit irrigation. Acta Sci. Pol-Hortoru 2022, 21, 131–140. [Google Scholar] [CrossRef]
- Dey, M.; Singh, R.K. Neurotoxic effects of aluminium exposure as a potential risk factor for Alzheimer’s disease. Pharmacol. Rep. 2022, 74, 439–450. [Google Scholar] [CrossRef]
- Stahl, T.; FalkLK, S.; RohrbeCK, A.; Georgii, S.; Herzog, C.; Wiegand, A.; Hotz, S.; Boschek, B.; Zorn, H.; Brunn, H. Migration of aluminium from food contact materials to food—A health risk for consumers? Part I of III: Exposure to aluminium, release of aluminium, tolerable weekly intake (TWI), toxicological effects of aluminium, study design, and methods. Environ. Sci. Eur. 2017, 29, 19. [Google Scholar] [CrossRef]
- Torres, E.; Mancebo, J.; Lara, A.; Maurer, W.; Geraldo, Y.; Pilar, M.; Torres-Quezada, I.; Caro, J.C.; Lopez, L. Early Results of Kaolin Clay Applications in the Dominican Republic. EC Agric. 2021, 7, 8–16. [Google Scholar]
- Moarefi, M.; Seddigh, S.; Hamrahi, A. Effects of processed kaolin on Aphis fabae and Hippodamia variegata on broad bean: A lab and field case study. J. Crop Prot. 2022, 11, 211–227. [Google Scholar]
- Rizzo, R.; Pistillo, M.; Germinara, G.S.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Petrelli, R.; Spinozzi, E.; Cappellacci, L.; Zeni, V.; et al. Bioactivity of Carlina acaulis Essential Oil and Its Main Component towards the Olive Fruit Fly, Bactrocera oleae: Ingestion Toxicity, Electrophysiological and Behavioral Insights. Insects 2021, 12, 880. [Google Scholar] [CrossRef]
- Blasco, C.L. Dispersal Behaviour, Ecology and Control of Philaenus spumarius: The Main Vector of Xylella fastidiosa in Europe. Ph.D. Dissertation, Universidad Politécnica de Madrid, Madrid, Spain, 2022. [Google Scholar]
- Bengochea, P.; Amor, F.; Saelices, R.; Hernando, S.; Budia, F.; Adán, A.; Medina, P. Kaolin and copper-based products applications: Ecotoxicology on four natural enemies. Chemosphere 2013, 91, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Bengochea, P.; Budia, F.; Viñuela, E.; Medina, P. Are kaolin and copper treatments safe to the olive fruit fly parasitoid Psyttalia concolor? J. Pest. Sci. 2014, 87, 351–359. [Google Scholar] [CrossRef]
- Gómez-Guzmán, J.A.; Sainz-Pérez, M.; González-Ruiz, R. Monitoring and inference of behavioral resistance in beneficial insects to insecticides in two pest control systems: IPM and organic. Agronomy 2022, 12, 538. [Google Scholar] [CrossRef]
- Pecenka, J.R.; Ingwell, L.L.; Foster, R.E.; Krupke, C.H.; Kaplan, I. IPM reduces insecticide applications by 95% while maintaining or enhancing crop yields through wild pollinator conservation. Proc. Natl. Acad. Sci. USA 2021, 118, e2108429118. [Google Scholar] [CrossRef] [PubMed]
- Lafargue, G.L.; Medina, J.M.A.; Acosta, A.L.; Llanes, Y.M. Piretrinas y Piretroides. Anu. Cienc. UNAH 2018, 16, 4–13. [Google Scholar]
- Alfaro-Tapia, A.; Alvarez-Baca, J.K.; Fuentes-Contreras, E.; Figueroa, C.C. Biological control may fail on pests applied with high doses of insecticides: Effects of sub-lethal concentrations of a pyrethroid on the host-searching behaviour of the aphid parasitoid Aphidius colemani (Hymenoptera, Braconidae) on aphid pests. Agriculture 2021, 11, 539. [Google Scholar] [CrossRef]
- Ravula, A.R.; Yenugu, S. Pyrethroid based pesticides–chemical and biological aspects. Crit. Rev. Toxicol. 2021, 51, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Dávila, O.L.; Sánchez-Martínez, G.; Rico-Martínez, R. Toxicity tests, bioaccumulation and residuality of pyrethroid insecticides commonly used to control conifer bark beetles in Mexico. Ecotoxicology 2022, 31, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Lybrand, D.B.; Xu, H.; Last, R.L.; Pichersky, E. How plants synthesize Pyrethrins: Safe and biodegradable insecticides. Trends. Plant. Sci. 2020, 25, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.V.; Holle, S.G.; Hutchison, W.D.; Koch, R.L. Lethal and sublethal effects of conventional and organic insecticides on the parasitoid Trissolcus japonicus, a biological control agent for Halyomorpha halys. Front. Insect. Sci. 2021, 1, 5. [Google Scholar] [CrossRef]
- Yan, R.; Zhou, Q.; Xu, Z.; Wu, Y.; Zhu, G.; Wang, M.; Guo, Y.; Dong, K.; Chen, M. Pyrethrins elicit olfactory response and spatial repellency in Aedes albopictus. Pest. Manag. Sci. 2021, 77, 3706–3712. [Google Scholar] [CrossRef]
- Dara, S.K. Five shades of gray mold control in strawberry: Evaluating chemical, organic oil, botanical, bacterial, and fungal active ingredients. EJ Entomol. Biol. 2019. [Google Scholar]
- Ciceoi, R.; IordĂChescu, M.; UdriȘTe, A.A.; BĂDulescu, L.A. Insights on solanaceous resistance against tomato leafminer (Tuta absoluta), with emphasis on chemical compounds useful in integrated pest management. Not. Bot. Horti Agrobo 2021, 49, 12543. [Google Scholar] [CrossRef]
- Balanza, V.; Mendoza, J.E.; Cifuentes, D.; Bielza, P. Selection for resistance to pyrethroids in the predator Orius laevigatus. Pest. Manag. Sci. 2021, 77, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Dougoud, J.; Toepfer, S.; Bateman, M.; Jenner, W.H. Efficacy of homemade botanical insecticides based on traditional knowledge. A review. Agron. Sustain. Dev. 2019, 39, 1–22. [Google Scholar] [CrossRef]
- Fita, T.; Getu, E.; Wakgari, M.; Woldetsadike, K. Potency of Neem, Azadirachta indica L. (A. juss) Leaf Aqueous Extract Insecticide against White Mango Scale, Aulacuspis tubercularis Newstead (Homoptera: Diaspididae) Infesting Mango (Mangifera indica L.). In Insecticides—Advances in Insect Control and Sustainable Pest Management; Habib, A., Adnan, N.S., Muhammad, B.T., Sajid, F., Basharat, A., Eds.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Show, N. Determining the Efficacy of Syringa (Melia azedarach L.) Extracts and Garlic (Allium sativum L.) Extracts in the Control of Tobacco Spider Mites (Tetranychus evansi) in Tomatoes (Lycopersicum esculentum) under Field Conditions in Chiredzi District, in Zimbabwe. Ph.D. Dissertation, University of South Africa, Pretoria, South Africa, 2023. [Google Scholar]
- Miresmailli, S.; Isman, M.B. Botanical insecticides inspired by plant herbivore chemical interactions. Trends Plant Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Abd Rashed, A.; Rathi, D.-N.G.; Ahmad Nasir, N.A.H.; Abd Rahman, A.Z. Antifungal Properties of Essential Oils and Their Compounds for Application in Skin Fungal Infections: Conventional and Nonconventional Approaches. Molecules 2021, 26, 1093. [Google Scholar] [CrossRef] [PubMed]
- Visakh, N.U.; Pathrose, B.; Narayanankutty, A.; Alfarhan, A.; Ramesh, V. Utilization of pomelo (Citrus maxima) peel waste into bioactive essential oils: Chemical composition and insecticidal properties. Insects 2022, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Lalruatsangi, K. Botanicals as selective pesticides for the integrated pest management in vegetables: A review. Agric. Rev. 2022, 43, 239–242. [Google Scholar] [CrossRef]
- Srivastava, R.P. Neem and pest Management. In International Book; Distributing Company: Lucknow, India, 2001; 620p. [Google Scholar]
- Amin, A.R.H.; Bayoumi, A.E.D.; Dimetry, N.Z.; Youssef, D.A. Efficiency of Nano-formulations of neem and peppermint oils on the bionomics and enzymatic activities of Agrotis ipsilon larvae (Lepidoptera: Noctuidae). J. Nat. Resour. Ecol. Manag. 2019, 4, 102–111. [Google Scholar]
- Spinozzi, E.; Maggi, F.; Bonacucina, G.; Pavela, R.; Boukouvala, M.C.; Kavallieratos, N.G.; Canale, A.; Romano, D.; Desneux, N.; Wilke, A.B.B.; et al. Apiaceae essential oils and their constituents as insecticides against mosquitoes—A review. Ind. Crop. Prod. 2021, 171, 113892. [Google Scholar] [CrossRef]
- Peace, K.; Bakaloudis, D.E.; Callaghan, A.; Holloway, G.J. Essential oils: A potential addition to integrated pest management strategies against adult varied carpet beetle, Anthrenus verbasci, in natural science collections. Bull. Insectol. 2022, 75, 247–252. [Google Scholar]
- Plata-Rueda, A.; Santos, M.H.D.; Serrão, J.E.; Martínez, L.C. Chemical composition and insecticidal properties of Origanum vulgare (Lamiaceae) essential oil against the stored product beetle, Sitophilus granarius. Agronomy 2022, 12, 2204. [Google Scholar] [CrossRef]
- Merlimau, M. Essential oil from Piperaceae as a potential for biopesticide agents: A review. Food Res. 2020, 4, 1–10. [Google Scholar]
- Danna, C.; Malaspina, P.; Cornara, L.; Smeriglio, A.; Trombetta, D.; De Feo, V.; Vanin, S. Eucalyptus essential oils in pest control: A review of chemical composition and applications against insects and mites. Crop Prot. 2023; 106319, in press. [Google Scholar] [CrossRef]
- Casas, J.L.; Sagarduy-Cabrera, A.; López Santos-Olmo, M.; Marcos-García, M.Á. Essential Oils from Selected Mediterranean Aromatic Plants—Characterization and Biological Activity as Aphid Biopesticides. Life 2023, 13, 1621. [Google Scholar] [CrossRef] [PubMed]
- Werrie, P.Y. Essential Oils as Potential Botanical Insecticide against Rosy Apple Aphid (Dysaphis plantaginea P.) by Trunk Injection. Ph.D. Dissertation, University of Liège—Gembloux Agro-Bio Tech, Gembloux, Belgium, 2022. [Google Scholar]
- El-Minshawy, A.M.; Abdelgaleil, S.A.; Gadelhak, G.G.; AL-Eryan, M.A.; Rabab, R.A. Effects of monoterpenes on mortality, growth, fecundity, and ovarian development of Bactrocera zonata (Saunders) (Diptera: Tephritidae). Environ. Sci. Pollut. R. 2018, 25, 15671–15679. [Google Scholar] [CrossRef] [PubMed]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Rizzo, R.; Lo Verde, G.; Lucchi, A.; Canale, A. Toxics or lures? Biological and behavioural effects of plant essential oils on tephritidae fruit flies. Molecules 2021, 26, 5898. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, S.; Lu, Y. Toxicity of Some Essential Oils Constituents against Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Insects 2022, 13, 954. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Caruso, T.; Caleca, V. Control of Bactrocera oleae (Rossi) in organic olive orchards: Use of clays and spinosad-based bait. In Proceedings of the 8th Meeting, Florence, Italy, 9–12 October 2023; Dionyssios, P., Patrizia, S., Antonio, B., Marzia, C.R., Eds.; IOBC/WPRS Bulletins: Florence, Italy, 2019; Volume 141, pp. 67–70. [Google Scholar]
- Giunti, G.; Laudani, F.; Lo Presti, E.; Bacchi, M.; Palmeri, V.; Campolo, O. Contact toxicity and ovideterrent activity of three essential oil-based nano-emulsions against the olive fruit fly Bactrocera oleae. Horticulturae 2022, 8, 240. [Google Scholar] [CrossRef]
- Durán Aguirre, C.E.; Pratissoli, D.; Carvalho, J.R.D.; Pacheco Damascena, A.; Araujo Junior, L.M.D.; Bolsoni Zago, H. Actividad insecticida de aceites esenciales sobre Helicoverpa armígera (Hübner) (Lepidoptera: Noctuidae). Idesia 2020, 38, 59–64. [Google Scholar] [CrossRef]
- Ramadan, E.A. The efficiency of some plant extracts against Agrotis ipsilon (Lepidoptera: Noctuidae) regarding to their activity on vital biochemical parameters. GSC Biol. Pharm. Sci. 2020, 12, 240–248. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Di Carolo, M.; Fumarola, G.; Tauro, D.; Altamura, G.; Cavalieri, V. Evaluation of insecticides for the control of juveniles of Philaenus spumarius L.; 2015–2017. Arthropod Manag. Tests 2018, 43, tsy073. [Google Scholar] [CrossRef]
- Domenico, C.D.; Ganassi, S.; Delfine, S.; Pistillo, M.; Germinara, G.S.; Cristofaro, A.D. Biological activities of some essential oils towards Philaenus spumarius adults. In Proceedings of the IOBC/WPRS Working Group. “Integrated Protection of Olive Crops”; IOBC/WPRS Bulletins: Florence, Italy, 2019; Volume 141, pp. 88–90. [Google Scholar]
- Ganassi, S.; Cascone, P.; Domenico, C.D.; Pistillo, M.; Formisano, G.; Giorgini, M.; Grazioso, P.; Germinara, G.S.; de Cristofaro, A.; Guerrieri, E. Electrophysiological and behavioural response of Philaenus spumarius to essential oils and aromatic plants. Sci. Rep. 2020, 10, 3114. [Google Scholar] [CrossRef]
- Anastasaki, E.; Psoma, A.; Partsinevelos, G.; Papachristos, D.; Milonas, P. Electrophysiological responses of Philaenus spumarius and Neophilaenus campestris females to plant volatiles. Phytochemistry 2021, 189, 112848. [Google Scholar] [CrossRef]
- Greff, B.; Sáhó, A.; Lakatos, E.; Varga, L. Biocontrol Activity of Aromatic and Medicinal Plants and Their Bioactive Components against Soil-Borne Pathogens. Plants 2023, 12, 706. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Maggi, M.D.; Ruffnengo, S.R.; Gende, L.B.; Sarlo, E.G.; Eguaras, M.J.; Bailac, P.N.; Ponzi, M.I. Laboratory evaluations of Syzygium aromaticum (L.) Merr. et Perry essential oil against Varroa Destr. J. Essent. Oil Res. 2010, 22, 119–122. [Google Scholar] [CrossRef]
- Habashy, M.G.; Abou El Atta, D.A.; Saleh, F.M. Efficacy of selected plant-derived oils against Tetranychid Mite (Tetranychus urticae) (Acari: Tetranychidae), in laboratory and semi field conditions. Int. J. Zool. Stud. 2023, 8, 47–52. [Google Scholar]
- Ziaee, M.; Hamzavi, F. A review of plant essential oils as a component of integrated pest management in stored products protection. In Proceedings of the International Conference on Green Agro-Industry (ICGAI), Yogyakarta, Indonesia, 11–14 November 2013; p. 394. [Google Scholar]
- Lu, J.; Wu, S. Bioactivity of essential oil from Ailanthus altissima bark against 4 major stored-grain insects. Afr. J. Microbiol. Res. 2010, 4, 154–157. [Google Scholar]
- Abrol, D.; Gupta, D.; Sharma, I. Evaluation of insecticides, biopesticides and clay for the management of fruit fly, Bactrocera spp. infesting bottle gourd. J. Entomol. Zool. Stud. 2019, 7, 311–314. [Google Scholar]
- Nair, P.P. A Unique Perspective in Precision of Nano-biotechnology for Sustainable Agricultural Fields. In Bio-Manufactured Nanomaterials: Perspectives and Promotion; Springer International Publishing: Cham, Switzerland, 2021; pp. 299–320. [Google Scholar]
- Kumar, B.A.; Niralwad, K.S.; Roghini, R.; Geethalakshmi, V. Sustainable solutions: Biotechnology’s role in environmental conservation. Eur. Chem. Bull. 2023, 12, 287–294. [Google Scholar]
- Das, S.; Ray, M.K.; Panday, D.; Mishra, P.K. Role of biotechnology in creating sustainable agriculture. PLOS Sustain. Transform. 2023, 2, e0000069. [Google Scholar] [CrossRef]
- Regulation (EC) No 1107/2009. Health and Food Safety Directorate General. European Commission. Standing Committee on Plants, Animals, Food and Feed Section Phytopharmaceuticals—Pesticide Residues 13–14 February 2023. Available online: https://food.ec.europa.eu/system/files/2023-03/sc_phyto_20230213_ppr_sum_1.pdf (accessed on 20 August 2023).
- EPA Pesticides. United States Environmental Protection Agency. Are Bt Crops Safe? Available online: https://www.epa.gov/sites/default/files/2015-08/documents/are_bt_crops_safe.pdf (accessed on 20 August 2023).
- Regulation (EC) No 396/2005. Health and Food Safety Directorate General. European Commission. Standing Committee on Plants, Animals, Food and Feed Section Phytopharmaceuticals—Pesticide Residues 22–23 November 2021. Available online: https://food.ec.europa.eu/system/files/2021-12/sc_phyto_20211122_ppr_sum_0.pdf (accessed on 20 August 2023).
- EPA—Fact Sheet for Bacillus thuringiensis. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-006400_24-Mar-98.pdf (accessed on 20 August 2023).
- Directive 2001/18/EC. Document 32023R1004. Commission Implementing Regulation (EU) 2023/1004 of 23 May 2023 Renewing the Approval of the Active Substance Bacillus thuringiensis subsp. Kurstaki SA-11 in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) No 540/2011 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R1004 (accessed on 20 August 2023).
- EPA. PIP Regulation. Overview of Plant Incorporated Protectants. Available online: https://www.epa.gov/regulation-biotechnology-under-tsca-and-fifra/overview-plant-incorporated-protectants#regulation (accessed on 20 August 2023).
- Eski, A.; Demirbağ, Z.; Demir, İ. Microencapsulation of an indigenous isolate of Bacillus thuringiensis by spray drying. J. Microencapsul. 2019, 36, 1–9. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Fraceto, L.F.; Bravo, A.; Polanczyk, R.A. Encapsulation strategies for Bacillus thuringiensis: From now to the future. J. Agric. Food Chem. 2021, 69, 4564–4577. [Google Scholar] [CrossRef]
- Poulin, B.; Lefebvre, G.; Hilaire, S.; Després, L. Long-term persistence and recycling of Bacillus thuringiensis israelensis spores in wetlands sprayed for mosquito control. Ecotox. Environ. Safe 2022, 243, 114004. [Google Scholar] [CrossRef] [PubMed]
- Pola, S.; Kesharwani, A.K.; Singh, J.; Singh, D.; Kalia, V.K. Endophytic ability of indigenous Bacillus thuringiensis strain VKK-BB2: New horizons for the development of novel insect pest-resistant crops. Egypt. J. Biol. Pest. Co. 2022, 32, 8. [Google Scholar] [CrossRef]
- Kumar, M.; Mehta, C.R.; Bhargav, V.K.; Tripathi, M.K.; Agrawal, K.N.; Babu, V.B. Effect of Spray Operating Parameters on Viability of Bacillus thuringiensis Based Biopesticide under Laboratory Condition. Agric. Res. 2023, 12, 189–196. [Google Scholar] [CrossRef]
- Kumar, M.; Mehta, C.R.; Agrawal, K.N.; Tripathi, M.K. Optimization of operating parameters for spraying microbial (Bacillus thuringiensis and Beauveria bassiana) based bio-pesticide solutions for foliar application. Int. J. Pest. Manag. 2023, 1–13. [Google Scholar] [CrossRef]
- Fuentealba, A.; Pelletier-Beaulieu, É.; Dupont, A.; Hébert, C.; Berthiaume, R.; Bauce, É. Optimizing Bacillus thuringiensis (Btk) Aerial Spray Prescriptions in Mixed Balsam Fir-White Spruce Stands against the Eastern Spruce Budworm. Forests 2023, 14, 1289. [Google Scholar] [CrossRef]
- Cherif, A.; Ettoumi, B.; Raddadi, N.; Daffonchio, D.; Boudabous, A. Genomic diversity and relationship of Bacillus thuringiensis and Bacillus cereus by multi-REP-PCR fingerprinting. Can. J. Microbiol. 2007, 53, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Punina, N.V.; Zotov, V.S.; Parkhomenko, A.L.; Parkhomenko, T.U.; Topunov, A.F. Genetic diversity of Bacillus thuringiensis from different geo-ecological regions of Ukraine by analyzing the 16S rRNA and gyrB genes and by AP-PCR and saAFLP. Acta Nat. 2013, 5, 90–100. [Google Scholar] [CrossRef]
- Subbanna, A.R.N.S.; Khan, M.S.; Srivastava, R.M.; Mishra, P.K.; Babu, B.K.; Venkateswarlu, V. Interspecies diversity of Bacillus thuringiensis isolates native from North Western Indian Himalayas. J. Environ. Biol. 2018, 39, 306–313. [Google Scholar] [CrossRef]
- Machado, D.H.B.; Livramento, K.G.D.; Máximo, W.P.F.; Negri, B.F.; Paiva, L.V.; Valicente, F.H. Molecular characterization of Bacillus thuringiensis strains to control Spodoptera eridania (Cramer) (Lepidoptera: Noctuidae) population. Rev. Bras. Entomol. 2020, 64, e201947. [Google Scholar] [CrossRef]
- Baranek, J.; Pluskota, M.; Rusin, M.; Konecka, E.; Kaznowski, A.; Wiland-Szymańska, J. Insecticidal activity of Bacillus thuringiensis strains isolated from tropical greenhouses towards Cydia pomonella and Spodoptera exigua larvae. BioControl 2023, 68, 39–48. [Google Scholar] [CrossRef]
- Orr, D. Biological Control and Integrated Pest Management. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 207–239. [Google Scholar] [CrossRef]
- Deng, S.Q.; Zou, W.H.; Li, D.L.; Chen, J.T.; Huang, Q.; Zhou, L.J.; Tian, X.X.; Chen, Y.J.; Peng, H.J. Expression of Bacillus thuringiensis toxin Cyt2Ba in the entomopathogenic fungus Beauveria bassiana increases its virulence towards Aedes mosquitoes. PLoS Negl. Trop. Dis. 2019, 13, e0007590. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef]
- Zeng, S.; Lin, Z.; Yu, X.; Zhang, J.; Zou, Z. Expressing Parasitoid Venom Protein VRF1 in an Entomopathogen Beauveria bassiana Enhances Virulence toward Cotton Bollworm Helicoverpa armigera. Appl. Environ. Microbiol. 2023, 89, e00705-23. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.Q.; Chen, J.T.; Li, W.W.; Chen, M.; Peng, H.J. Application of the scorpion neurotoxin AaIT against insect pests. Int. J. Mol. Sci. 2019, 20, 3467. [Google Scholar] [CrossRef]
- Yadav, A.; Reddy, D.C.; Chaudhary, M.; Chand, A.; Batham, P. Role of Genetically Engineering in Insect Pest Management. In Recent Trends in Insect Pest Management; Raju, S.V.S., Ed.; AkiNik Publications: Delhi, India, 2020; Volume 2, pp. 131–143. [Google Scholar]
- Hoy, M.A. Transgenic arthropods for pest management programs: Risks and realities. Exp. Appl. Acarol. 2000, 24, 463–495. [Google Scholar] [CrossRef] [PubMed]
- Hendrichs, J.; Robinson, A. Sterile insect technique. In Encyclopedia of Insects; Academic Press: Cambridge, MA, USA, 2009; pp. 953–957. [Google Scholar]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Taylor & Francis: Abingdon, UK, 2021; p. 1216. [Google Scholar]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Mateos Fernández, R.; Petek, M.; Gerasymenko, I.; Juteršek, M.; Baebler, Š.; Kallam, K.; Moreno Giménez, E.; Gondolf, J.; Nordmann, A.; Gruden, K.; et al. Insect pest management in the age of synthetic biology. Plant Biotechnol. J. 2022, 20, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ren, B.; Zeng, B.; Shen, J. Improving RNAi efficiency for pest control in crop species. BioTechniques 2020, 68, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, O.S.; Riaz, S.; Toufiq, N.; Yousaf, I.; Bhatti, M.U.; Batcho, A.; Olajide, A.A.; Nasir, I.A.; Tabassum, B. Advances in exogenous RNA delivery techniques for RNAi-mediated pest control. Mol. Biol. Rep. 2020, 47, 6309–6319. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Tang, X. RNAi-based pest control: Production, application and the fate of dsRNA. Front. Bioeng. Biotechnol. 2022, 10, 1080576. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Sweet, J.; Dzhambazova, T.; Urru, I.; Smagghe, G.; Kostov, K.; Arpaia, S. Implementation of RNAi-based arthropod pest control: Environmental risks, potential for resistance and regulatory considerations. J. Pest. Sci. 2022, 95, 1–15. [Google Scholar] [CrossRef]
- Baltzegar, J.; Cavin Barnes, J.; Elsensohn, J.E.; Gutzmann, N.; Jones, M.S.; King, S.; Sudweeks, J. Anticipating complexity in the deployment of gene drive insects in agriculture. J. Responsible Innov. 2018, 5, S81–S97. [Google Scholar] [CrossRef]
- Delrio, G. Integrated control in olive groves. In Biological Control and Integrated Crop Protection: Towards Environmentally Safer Agriculture: Proceedings of an International Conference, Veldhoven, The Netherlands, 8–13 September 1991; IOBC-WPRS Bulletins: Florence, Italy, 1992; pp. 67–76. [Google Scholar]
- Behelgardy, M.F.; Motamed, N.; Jazii, F.R. Expression of the P5CS gene in transgenic versus nontransgenic olive (Olea europaea) under salinity stress. World Appl. Sci. J. 2012, 18, 580–583. [Google Scholar]
- Chiappetta, A.; Muto, A.; Bruno, L.; Woloszynska, M.; Lijsebettens, M.V.; Bitonti, M.B. A dehydrin gene isolated from feral olive enhances drought tolerance in Arabidopsis transgenic plants. Front. Plant Sci. 2015, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Celletti, S.; Cristofori, V.; Astolfi, S.; Ruggiero, B.; Rugini, E. Olive (Olea europaea L.) plants transgenic for tobacco osmotin gene are less sensitive to in vitro-induced drought stress. Acta Physiol. Plant 2017, 39, 229. [Google Scholar]
- Loconsole, G.; Oriana, P.; Boscia, D.; Altamura, G.; Djelouah, K.; Elbeaino, T.; Frasheri, D.; Lorusso, D.; Palmisano, F.; Pollastro, P.; et al. Detection of Xylella fastidiosa in olive trees by molecular and serological methods. J. Plant Pathol. 2014, 96, 7–14. [Google Scholar]
- D’onghia, A.M.; Santoro, F.; Minutillo, S.A.; Frasheri, D.; Gallo, M.; Gualano, S.; Giuseppe, C.; Valentini, F. Optimisation of sampling and testing for asymptomatic olive trees infected by Xylella fastidiosa in Apulia region, Italy. Phytopathol. Mediterr. 2022, 61, 439–449. [Google Scholar] [CrossRef]
- Ant, T.; Koukidou, M.; Rempoulakis, P.; Gong, H.F.; Economopoulos, A.; Vontas, J.; Alphey, L. Control of the olive fruit fly using genetics-enhanced sterile insect technique. BMC Biol. 2012, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.M.; Nestel, D.; Belcari, A.; Jessup, A.; Rempoulakis, P.; Economopoulos, A.P. A basis for the renewal of sterile insect technique for the olive fly, Bactrocera oleae (Rossi). J. Appl. Entomol. 2012, 136, 1–16. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Abd-Alla, A.M.M.; Bourtzis, K.; Bouyer, J.; Caceres, C.; de Beer, C.; Oliveira Carvalho, D.; Maiga, H.; Mamai, W.; Nikolouli, K.; et al. The insect pest control laboratory of the joint FAO/IAEA programme: Ten years (2010–2020) of research and development, achievements and challenges in support of the sterile insect technique. Insects 2021, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Tsoumani, K.T.; Belavilas-Trovas, A.; Gregoriou, M.E.; Mathiopoulos, K.D. Anosmic flies: What Orco silencing does to olive fruit flies. BMC Genet. 2020, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Legros, M.; Marshall, J.M.; Macfadyen, S.; Hayes, K.R.; Sheppard, A.; Barrett, L.G. Gene drive strategies of pest control in agricultural systems: Challenges and opportunities. Evol. Appl. 2021, 14, 2162–2178. [Google Scholar] [CrossRef] [PubMed]
- Then, C.; Kawall, K.; Valenzuela, N. Spatiotemporal controllability and environmental risk assessment of genetically engineered gene drive organisms from the perspective of European Union genetically modified organism regulation. Integr. Environ. Assess. Manag. 2020, 16, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Augustinos, A.A.; Stratikopoulos, E.E.; Drosopoulos, E.; Kakani, E.G.; Mavragani-Tsipidou, P. Isolation and Characterization of microsatellite markers from the olive fly, Bactrocera oleae, and their cross-amplification in the Tephritidae family. BMC Genom. 2008, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Zygouridis, N.E.; Augustinos, A.A.; Zalom, F.G.; Mathiopoulos, K.D. Analysis of olive fly invasion in California based on microsatellite markers. Hered 2009, 102, 402–412. [Google Scholar] [CrossRef]
- Skouras, P.J.; Margaritopoulos, J.T.; Seraphides, N.A.; Ioannides, I.M.; Kakani, E.G.; Mathiopoulos, K.D.; Tsitsipis, J.A. Organophosphate resistance in olive fruit fly, Bactrocera oleae, populations in Greece and Cyprus. Pest. Manag. Sci. 2007, 63, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, N.; Kampouraki, A.; Tseliou, V.; Wybouw, N.; Dermauw, W.; Roditakis, E.; Nauen, R.; Van Leeuwen, T.; Vontas, J. Molecular characterization of pyrethroid resistance in the olive fruit fly Bactrocera oleae. Pestic. Biochem. Phys. 2018, 148, 1–7. [Google Scholar] [CrossRef]
- Nardi, F.; Carapelli, A.; Dallai, R.; Roderick, G.K.; Frati, F. Population structure and colonization history of the olive fly, Bactrocera oleae (Diptera, Tephritidae). Mol. Ecol. 2005, 14, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Matallanas, B.; Lantero, E.; M’Saad, M.; Callejas, C.; Ochando, M.D. Genetic polymorphism at the cytochrome oxidase I gene in Mediterranean populations of Batrocera oleae (Diptera: Tephritidae). J. Appl. Entomol. 2013, 137, 624–630. [Google Scholar] [CrossRef]
- Van Asch, B.; Pereira—Castro, I.; Rei, F.; Teixeira da Costa, L. Mitochondrial haplotypes revealed olive fly (Bactrocera oleae) population substructure in the Mediterranean. Genet 2012, 140, 181–187. [Google Scholar] [CrossRef][Green Version]
- Dogaç, E.; Kandemir, I.; Taskin, V. The genetic polymorphisms and colonization process of olive fly populations in Turkey. PLoS ONE 2013, 8, e56067. [Google Scholar] [CrossRef]
- Van Asch, B.; Pereira—Castro, I.; Rei, F.; Teixeira da Costa, L. Marked genetic differentiation between western Iberian and Italic populations of olive fly: Southern France as intermediate area. PLoS ONE 2015, 10, e0126702. [Google Scholar] [CrossRef] [PubMed]
- Eti, C.N.; Dogac, E.; Gocmen Taskin, B.; Gokdere, G.; Taskin, V. Population structure and patterns of geographic differentiation of Bactrocera oleae (Diptera: Tephritidae) in Eastern Mediterranean Basin. Mitochondrial DNA Part A 2018, 29, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Lantero, E.; Matallanas, B.; Ochando, M.D.; Callejas, C. Vast Gene Flow among the Spanish Populations of the Pest Bactrocera oleae (Diptera, Tephritidae), Phylogeography of a Metapopulation to Be Controlled and Its Mediterranean Genetic Context. Insects 2022, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Rejili, M.; Fernandes, T.; Dinis, A.M.; Pereira, J.A.; Baptista, P.; Santos, S.A.P.; Lino-Neto, T. A PCR-based diagnostic assay for detecting DNA of the olive fruit fly, Bactrocera oleae, in the gut of soil-living arthropods. Bull. Entomol. Res. 2016, 106, 695–699. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).