Particle Formation Mechanism of TiCl4 Hydrolysis to Prepare Nano TiO2
Abstract
:1. Introduction
2. TiCl4 Hydrolysis Process and Mathematical Model
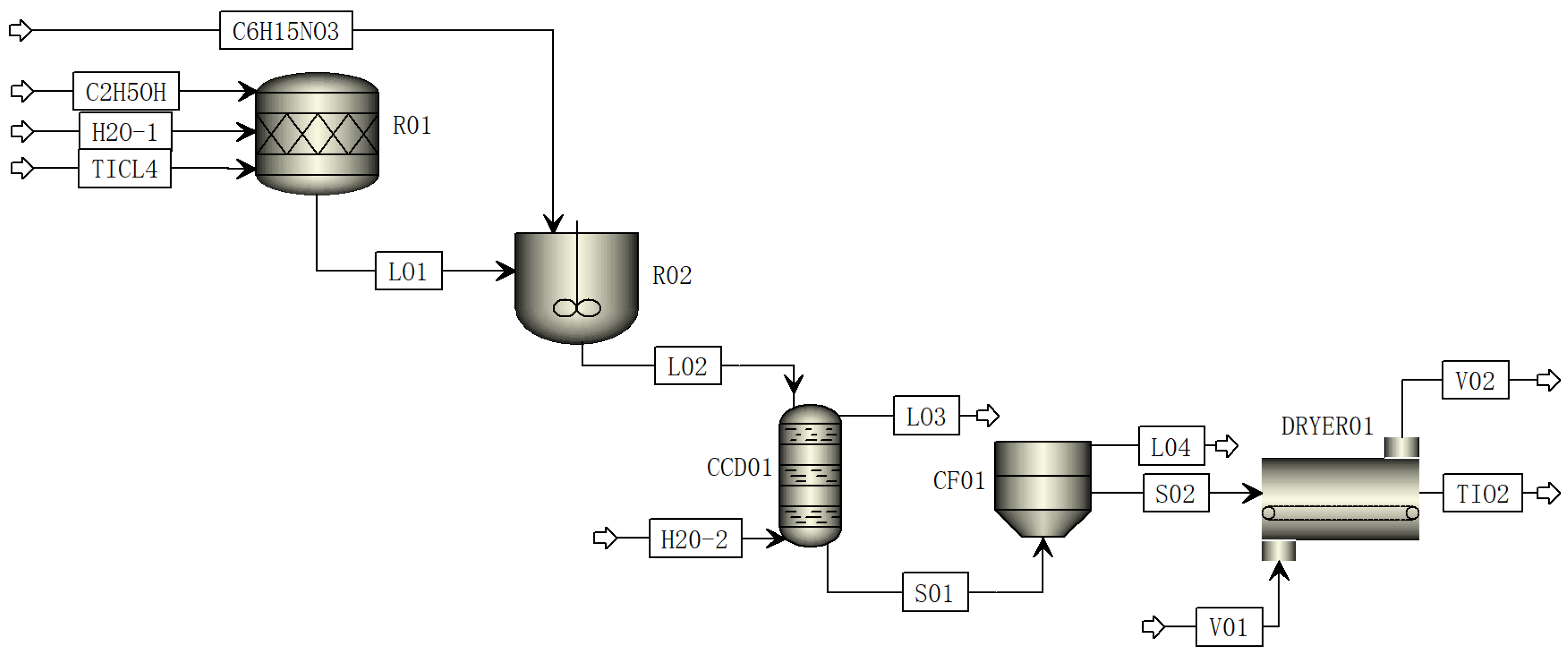
2.1. Process Flow of TiCl4 Hydrolysis to Prepare TiO2
2.2. Mathematical Model
2.2.1. POWERLAW Dynamics
2.2.2. Nucleation Dynamics
2.2.3. Growth Kinetics
2.2.4. PSD Calculation
3. Experimental Methods
4. Results and Discussion
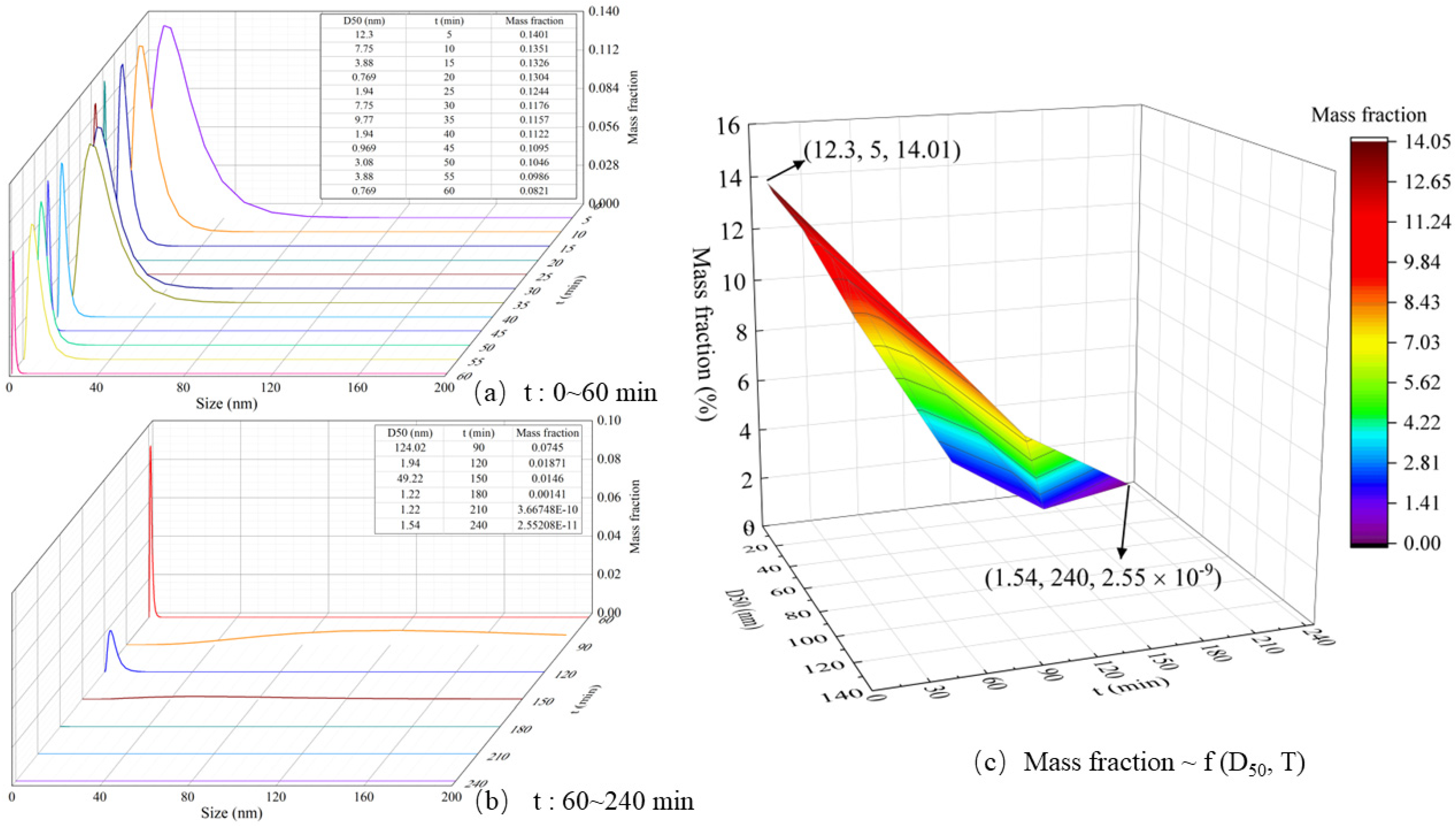
4.1. Particle Formation Process
4.2. Effect of Reaction Temperature on Particle Formation Behavior
4.3. Effect of Water Addition on Particle Formation Behavior
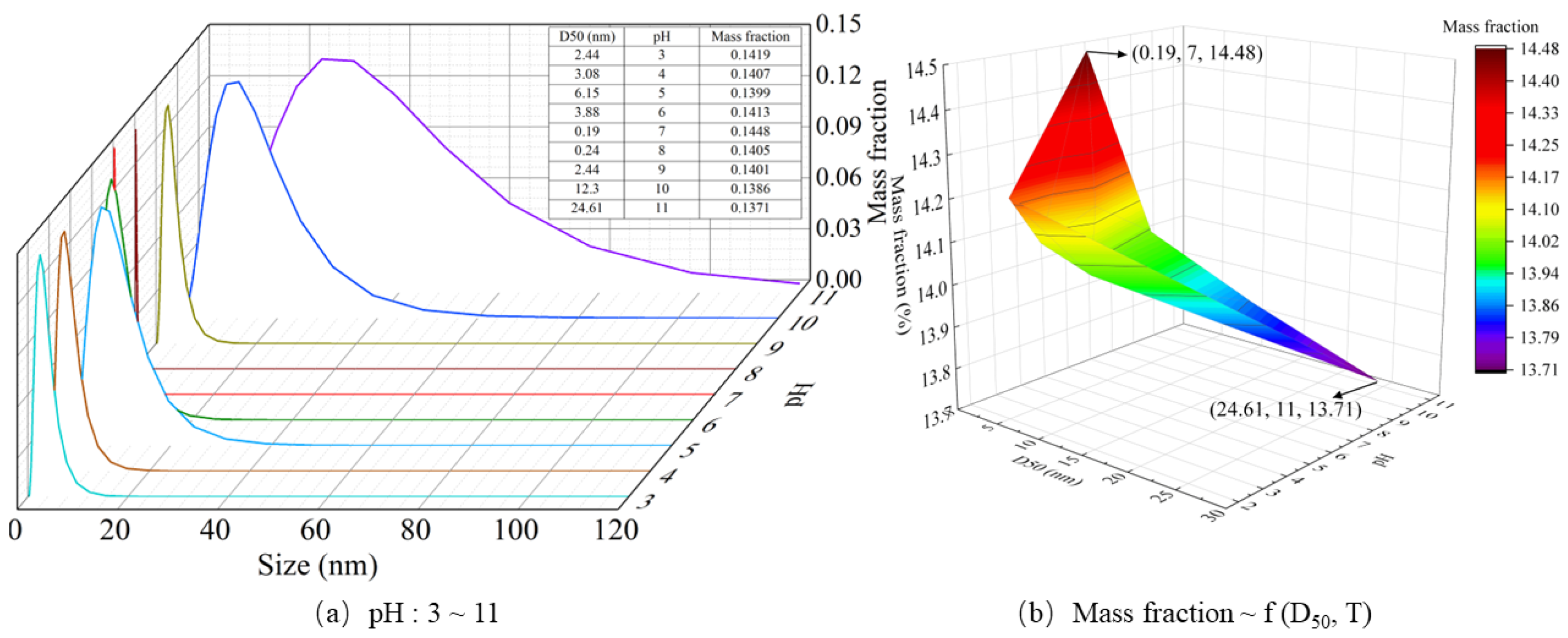
4.4. Effect of pH on Particle Formation Behavior
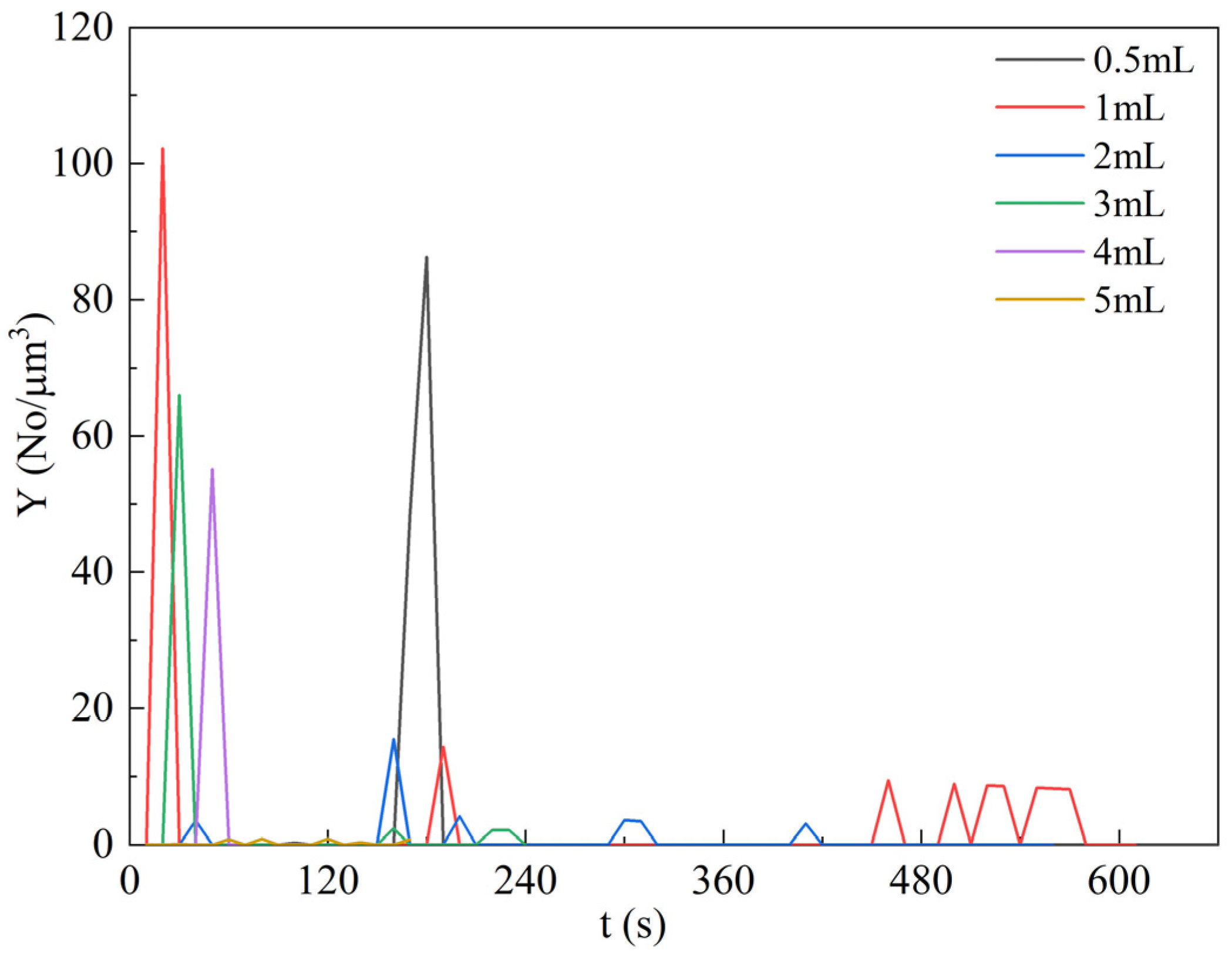
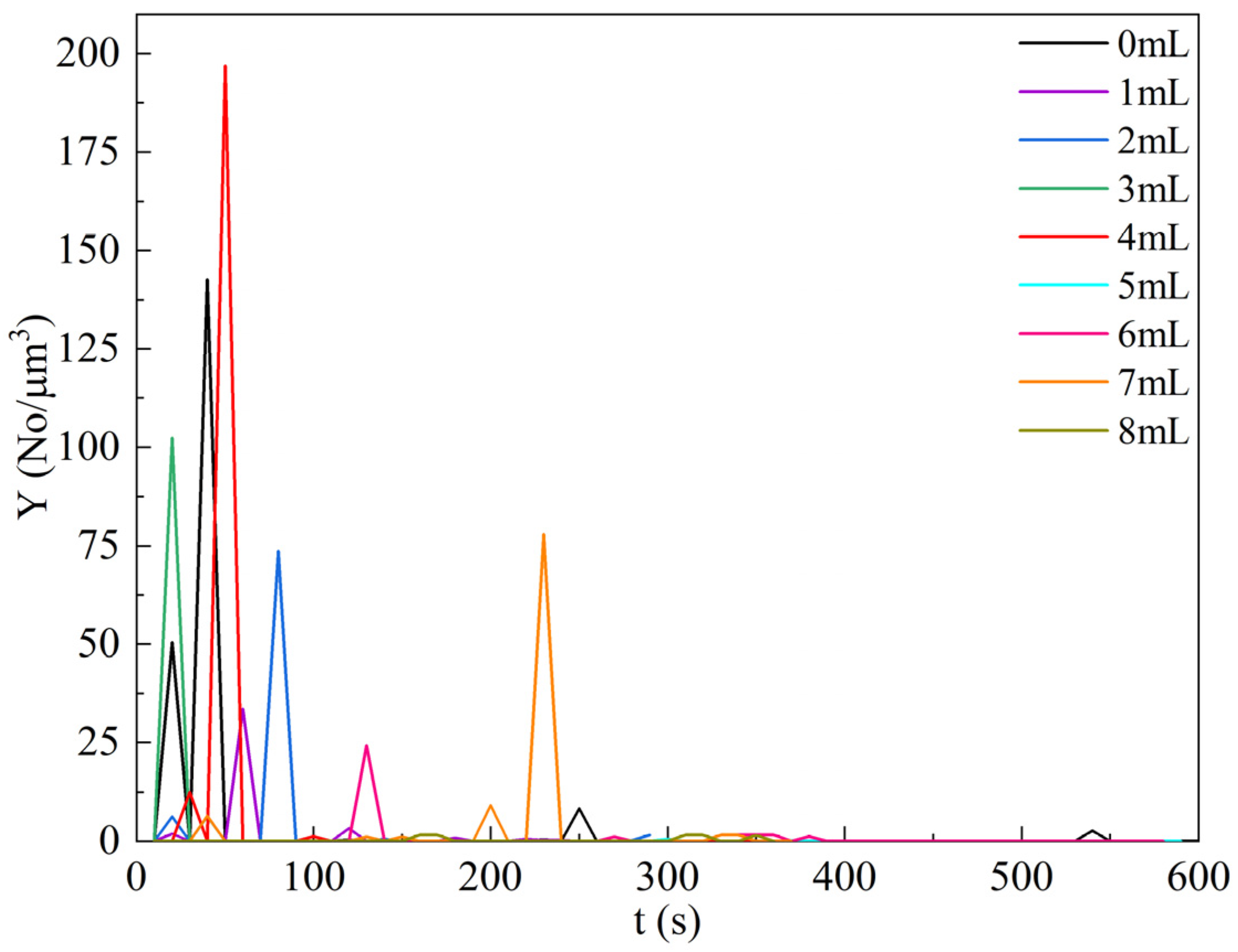
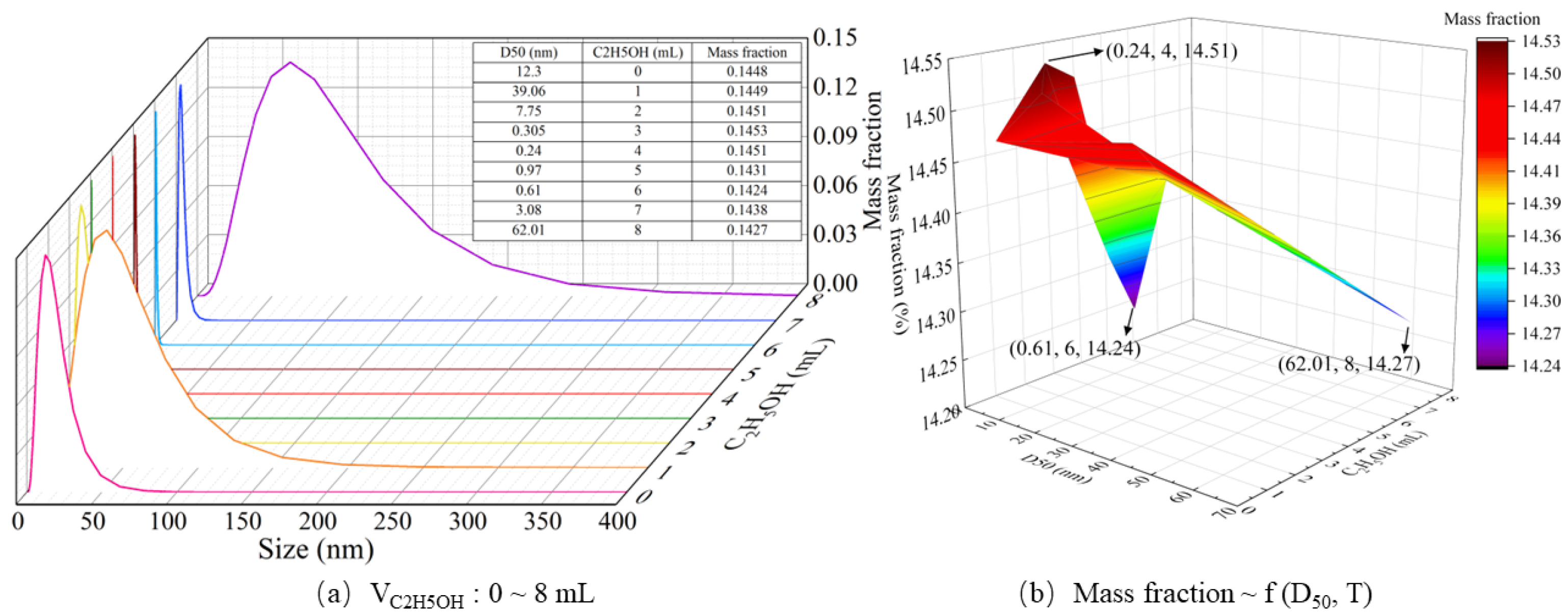
4.5. Effect of Ethanol Amount on Particle Formation Behavior
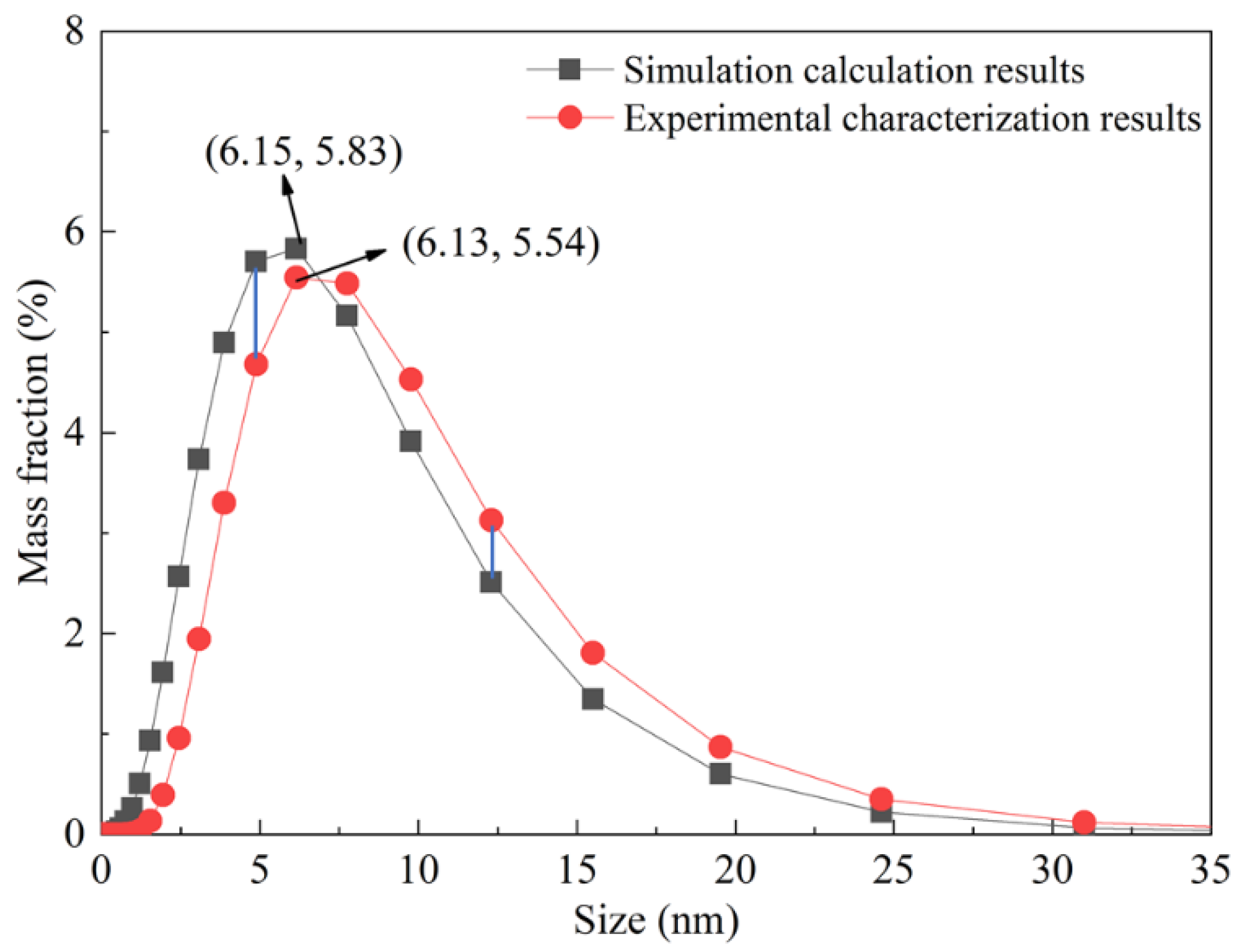
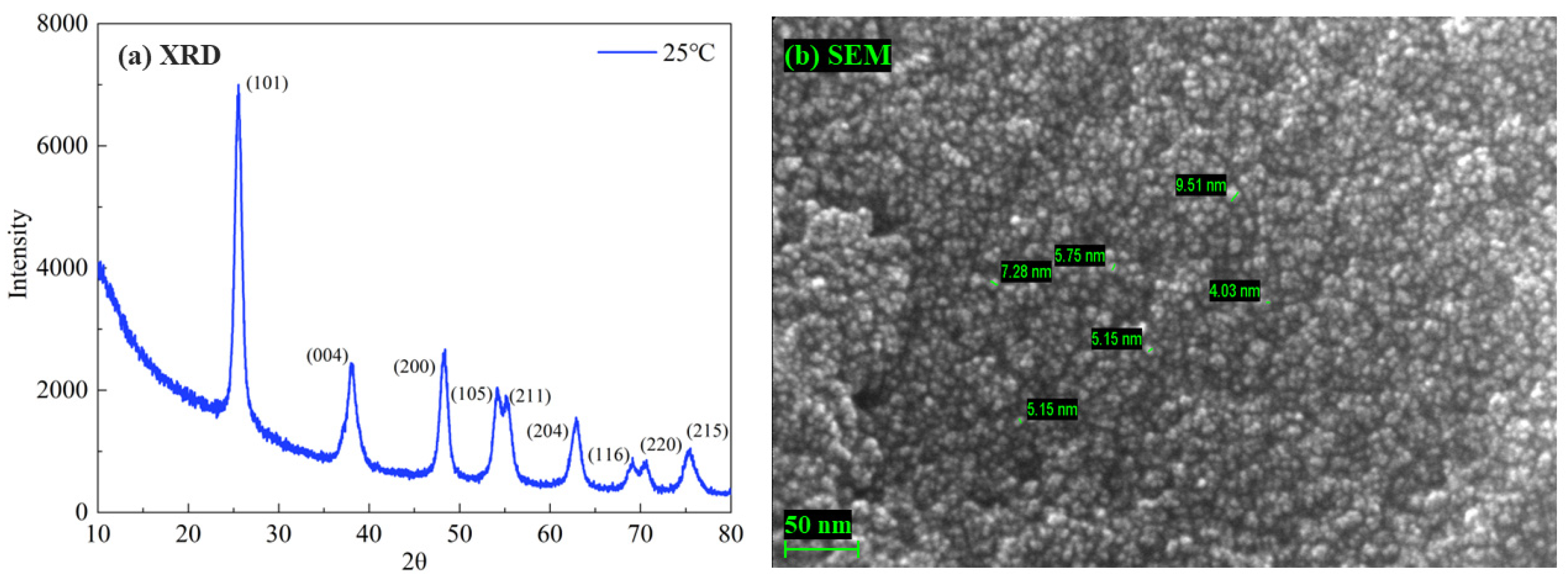
4.6. Experimental Verification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L. TiO2 Research on the Regulation Mechanism and Performance of 2 Morphology and Crystal Structure; Chongqing University: Chongqing, China, 2021. [Google Scholar]
- Zhao, X.; Xu, W.; Huang, B.; Ma, J.; Chen, H.; Hao, X.; Ge, B.; Wang, C. Effect of TiH2 intermediate on the structure and properties of TiO2 coating induced on titanium by induction heating and its multifunctional mechanism. Appl. Surf. Sci. 2021, 536, 147968. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, J.; Luo, W.; Zhang, K.; Li, X.; Liu, Y. Titanium Dioxide Nanocomposites and Their Preparation Methods and Applications, Hunan Province. CN 109847662B, 29 October 2021. [Google Scholar]
- Song, J.; Qin, J.; Qu, J.; Song, Z.; Zhang, W.; Xue, X.; Shi, Y.; Zhang, T.; Ji, W.; Zhang, R.; et al. The effects of particle size distribution on the optical properties of titanium dioxide rutile pigments and their applications in cool non-white coatings. Sol. Energy Mater. Sol. Cells 2014, 130, 42–50. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. Effects of Water-to-Methanol Ratio on the Structural, Optical and Photocatalytic Properties of Titanium Dioxide Thin Films Prepared by Mist Chemical Vapor Deposition. Catal. Today 2019, 358, 172–176. [Google Scholar] [CrossRef]
- Horvat, P.; Škapin, A.S.; Štangar, U.L.; Korošec, R.C. Thermal techniques as a tool for the direction of the preparation of photocatalytically efficient titania thin films and powders. J. Therm. Anal. Calorim. 2020, 146, 1121–1131. [Google Scholar] [CrossRef]
- Kompa, A.; Mahesha, M.G.; Kekuda, D.; Rao, K.M. Spectroscopic investigation of defects in spin coated titania based thin films for photocatalytic applications. J. Solid-State Chem. 2021, 303, 122488. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, S.; Yang, Y.; Luo, R.; Xia, Y.; Wang, X.; Liu, Q.; Hu, B. Phase transition of titanium dioxide based on quantum dynamics. Phys. Chem. Chem. Phys. 2023, 25, 23024–23032. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, L.; Ai, C.-Z.; Wang, Z.; Lin, S.-W. Elastic properties of anatase titanium dioxide nanotubes: A molecular dynamics study. Chin. Phys. B 2019, 28, 103102. [Google Scholar] [CrossRef]
- Aljaerani, H.A.; Samykano, M.; Pandey, A.K.; Said, Z.; Sudhakar, K.; Saidur, R. Effect of TiO2 nanoparticles on the thermal energy storage of HITEC salt for concentrated solar power applications. J. Energy Storage 2023, 72, 108449. [Google Scholar] [CrossRef]
- Wang, M.-C.; Wu, B.-H.; Liu, S.-E.; Li, Y.-C.; Lin, S.-K.; Tsai, T.-M.; Chang, T.-C. A solid-state semiconductor battery with silica-coated TiO2 core–shell energy storage nanoparticles for rechargeable device application. AIP Adv. 2023, 13, 095011. [Google Scholar] [CrossRef]
- Yang, M.; Ma, R.; Chen, L.; Zhang, C.; Cao, R.; Wen, Q. Preparation and photo-catalytic performance of β2-SiW11/PANI/TiO2 photo-catalyst. J. Coord. Chem. 2020, 73, 3420–3430. [Google Scholar] [CrossRef]
- El-Ola, S.M.A.; Kotb, R.M. Functional versatility of hybrid composite finishing of chitosan-titania NPs-organic UV-absorber for polyacrylonitrile fabric. J. Eng. Fibers Fabr. 2021, 16, 15589250211064845. [Google Scholar] [CrossRef]
- ASPEN PLUS V11 User’s Manual; Aspen Technology Inc.: Bedford, MA, USA, 2023.
- Hachhach, M.; Akram, H.; Hanafi, M.; Achak, O.; Chafik, T. Simulation and Sensitivity Analysis of Molybdenum Disulfide Nanoparticle Production Using Aspen Plus. Int. J. Chem. Eng. 2019, 2019, 3953862. [Google Scholar] [CrossRef]
- Dehury, P.; Mahanta, U.; Singh, R.; Banerjee, T. Potential of deep eutectic solvent based nanofluids as a new generation heat transfer media. J. Mol. Liq. 2023, 379, 121700. [Google Scholar] [CrossRef]
- ASPEN PLUS V11 Modeling Solid Operations in Aspen Plus; Aspen Technology Inc.: Bedford, MA, USA, 2023.
- Schefflan, R. Introduction to Aspen Plus. In Teach Yourself the Basics of Aspen Plus™; Schefflan, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Mangum, J.S.; Garten, L.M.; Ginley, D.S.; Gorman, B.P. Utilizing TiO2 amorphous precursors for polymorph selection: An in-situ TEM study of phase formation and kinetics. J. Am. Ceram. Soc. 2019, 103, 2899–2907. [Google Scholar] [CrossRef]
- Fang, X. TiCl Preparation of Nano TiO2 by Liquid Phase Hydrolysis Research on 2 Powders; South China University of Technology: Guangzhou, China, 2002. [Google Scholar]
- Wei, H.-y. Computer-aided design and scale-up of crystallization processes: Integrating approaches and case studies. Chem. Eng. Res. Des. 2010, 88, 1377–1380. [Google Scholar] [CrossRef]
- Vlasov, V.A. Activated complex theory of nucleation. Eur. Phys. J. E 2019, 42, 36. [Google Scholar] [CrossRef]
- Rojas Molina, R.; Liese, S.; Alimohamadi, H.; Rangamani, P.; Carlson, A. Diffuso-kinetic membrane budding dynamics. Soft Matter 2020, 16, 10889. [Google Scholar] [CrossRef]
- Hao, B.; Huang, J. Research Review on Crystal Growth Mechanism. J. Beijing Inst. Pet. Chem. Technol. 2006, 58–64. [Google Scholar]
- Fu, M.-Q.; Bi, Q.-L.; Lü, Y.-J. Kinetics of Spherical Interface in Crystal Growth. Chin. Phys. Lett. 2017, 34, 048102. [Google Scholar] [CrossRef]
- Fytopoulos, A.A.; Kavousanakis, M.E.; Van Gerven, T.; Boudouvis, A.G.; Stefanidis, G.D.; Xiouras, C. Crystal Growth, Dissolution, and Agglomeration Kinetics of Sodium Chlorate. Ind. Eng. Chem. Res. 2021, 60, 7367–7384. [Google Scholar] [CrossRef]
- Vander Voort, G.; Pakhomova, O.; Kazakov, A. Evaluation of Normal Versus Non-Normal Grain Size Distributions. Mater. Perform. Charact. 2016, 5, 521–534. [Google Scholar] [CrossRef]
- Hu, Z. Based on Different Crystal Forms of TiO2 Research on the Structure and Performance of Quantum Dot Sensitized Solar Cells with 2 Photoanodes; Changchun University of Technology: Changchun, China, 2022. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, Q.; Yu, S.; Luo, W. Particle Formation Mechanism of TiCl4 Hydrolysis to Prepare Nano TiO2. Appl. Sci. 2023, 13, 12213. https://doi.org/10.3390/app132212213
Le Q, Yu S, Luo W. Particle Formation Mechanism of TiCl4 Hydrolysis to Prepare Nano TiO2. Applied Sciences. 2023; 13(22):12213. https://doi.org/10.3390/app132212213
Chicago/Turabian StyleLe, Qianjun, Shengfei Yu, and Wusheng Luo. 2023. "Particle Formation Mechanism of TiCl4 Hydrolysis to Prepare Nano TiO2" Applied Sciences 13, no. 22: 12213. https://doi.org/10.3390/app132212213



