Oral Cavity Mucocele and Different Surgical Treatment Strategies: Is Laser Excision Effective? A Scoping Review
Abstract
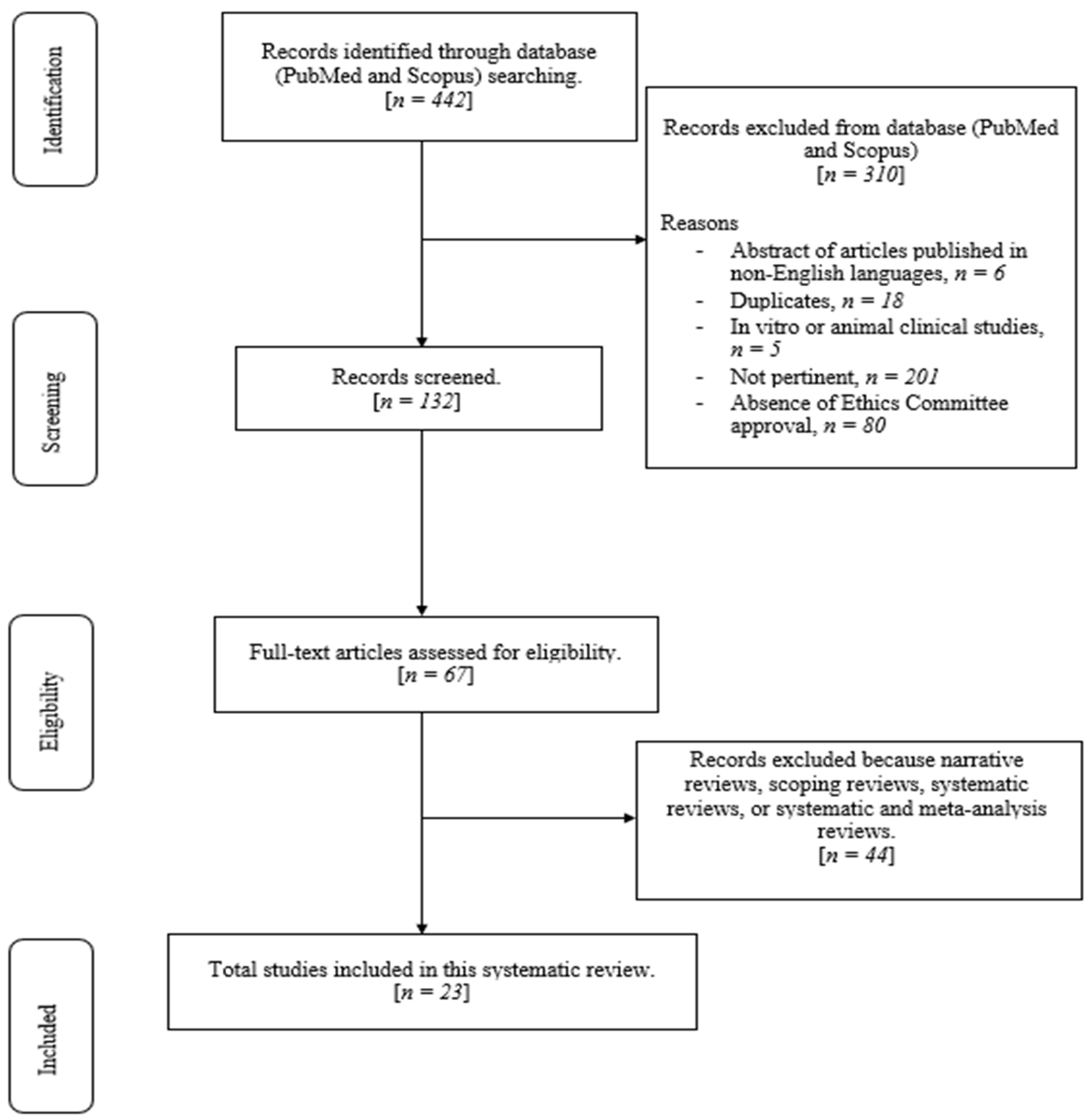
:1. Introduction
2. Materials and Methods
2.1. Focused Questions
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Research
2.5. Quality Assessment of Included Studies
3. Results
Risk of Bias
4. Discussion
4.1. Laser-Assisted Surgery in Stomatology
4.2. Literature Review Results
4.3. Laser-Assisted Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adachi, P.; Soubhia, A.M.; Horikawa, F.K.; Shinohara, E.H. Mucocele of the glands of Blandin-Nuhn—clinical, pathological, and therapeutical aspects. Oral Maxillofac. Surg. 2011, 15, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Agoob Alfergany, M.; Alaijah, F. Overview of the Clinical Benefits Using the Different Diode Laser Wavelengths in Treatment of the Mucocele: Clinical Cases Report Review. Photobiomodulation Photomed. Laser Surg. 2020, 38, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Byun, J.S.; Choi, J.K.; Jung, J.K. Identification of predictive variables for the recurrence of oral mucocele. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e231–e235. [Google Scholar] [CrossRef] [PubMed]
- Shapira, M.; Akrish, S. Mucoceles of the oral cavity in neonates and infants—Report of a case and literature review. Pediatr. Dermatol. 2014, 31, e55–e58. [Google Scholar] [CrossRef] [PubMed]
- Valerio, R.A.; Mussolino dei Qeiroz, A.; Coutinho Romulado, P.; Brentegani, L.R.; de Paula Silva, F. Mucocele and fibroma: Treatment and Clinical Features for Differential Diagnosis. Braz. Dent. J. 2013, 24, 537–541. [Google Scholar] [CrossRef]
- Mínguez-Martinez, I.; Bonet-Coloma, C.; Ata-Ali-Mahmud, J.; Carrillo-García, C.; Peñarrocha-Diago, M.; Peñarrocha-Diago, M. Clinical characteristics, treatment, and evolution of 89 mucoceles in children. J. Oral Maxillofac. Surg. 2010, 68, 2468–2471. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Implement. 2021, 19, 3–10. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. 2020. Available online: https://synthesismanual.jbi.global (accessed on 24 April 2023).
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. Joanna Briggs Inst. Rev. Manual. Joanna Briggs Inst. 2020, 5, 217–269. [Google Scholar]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Aulakh, K.K.; Brar, R.S.; Azad, A.; Sharma, S.; Anand, A.; Jyoti, B. Cryotherapy for Treatment of Mouth Mucocele. Niger. J. Surg. 2016, 22, 130–133. [Google Scholar] [CrossRef]
- Besbes, A.; Elelmi, Y.; Khanfir, F.; Belgacem, R.; Ghedira, H. Recurrent Oral Mucocele Management with Diode Laser. Case Rep. Dent. 2020, 3, 8855759. [Google Scholar] [CrossRef] [PubMed]
- De Falco, D.; Di Venere, D.; Maiorano, E. Diode Laser Excision of Blandin-Nuhn Mucocele. Cureus 2020, 28, e7441. [Google Scholar] [CrossRef]
- Essaket, S.; Hakkou, F.; Chbicheb, S. Mucocèle de la muqueuse buccale [Mucocele of the oral mucous membrane]. Pan. Afr. Med. J. 2020, 29, 140. [Google Scholar]
- Gaikwad, T.V.; Maini, A.P.; Das, S.; Lokhande, S.; Patil, S.K.; Sarma, A. Nonsurgical Management of Oral Mucocele Occurring on a Rare Site. Contemp. Clin. Dent. 2022, 13, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Tripathi, A.; Chowdhry, S.; Sharma, A.; Biswas, G. Cryosurgery: Painless and fearless management of mucocele in young patient. J. Clin. Diagn. Res. 2014, 8, ZD04–ZD06. [Google Scholar] [CrossRef] [PubMed]
- Mouravas, V.; Sfoungaris, D.; Papageorgiou, I.; Kepertis, C.; Spyridakis, I. Mucoceles of the lesser salivary glands in neonates demonstrate a particular clinicopathological pattern and mandate urgent management. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 238–241. [Google Scholar] [CrossRef]
- Nagar, S.R.; Fernandes, G.; Sinha, A.; Rajpari, K.N. Mucocele of the tongue: A case report and review of literature. J. Oral Maxillofac. Pathol. 2021, 25 (Suppl. S1), S37–S41. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, M.H.; Choi, I. Oral mucocele treated with acupuncture: A case report. Acupunct. Med. 2021, 39, 400–402. [Google Scholar] [CrossRef]
- Vitale, M.C.; Sfondrini, M.F.; Croci, G.A.; Paulli, M.; Carbone, L.; Gandini, P.; Scribante, A. Diode Laser-Assisted Surgical Therapy for Early Treatment of Oral Mucocele in a Newborn Patient: Case Report and Procedures Checklist. Case Rep. Dent. 2018, 24, 3048429. [Google Scholar] [CrossRef]
- Amaral, M.B.; Freitas, I.Z.; Pretel, H.; Abreu, M.H.; Mesquita, R.A. Low level laser effect after micro-marsupialization technique in treating ranulas and mucoceles: A case series report. Lasers Med. Sci. 2012, 27, 1251–1255. [Google Scholar] [CrossRef]
- Feng, H.; Wang, S.; Liu, Y.; Liao, X.; Tang, Y.; Liang, X. Microwave Ablation: A Novel Treatment for the Mucoceles of Anterior Lingual Salivary Glands. J. Oral Maxillofac. Surg. 2017, 75, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Giraddi, G.B.; Saifi, A.M. Micro-marsupialization versus surgical excision for the treatment of mucoceles. Ann. Maxillofac. Surg. 2016, 6, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Graillon, N.; Mage, C.; Le Roux, M.K.; Scemama, U.; Chossegros, C.; Foletti, J.M. Mucoceles of the anterior ventral surface of the tongue and the glands of Blandin-Nuhn: 5 cases. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Wang, T.; Liu, S.; Chen, A. Preliminary experience with promethazine hydrochloride injection in the sclerotherapy of oral mucocele. Int. J. Oral Maxillofac. Surg. 2021, 50, 516–521. [Google Scholar] [CrossRef]
- Farah, C.S.; Koelmeyer, N.; Kaney, A.; Simanovic, B. Nitrous oxide cryotherapy for the management of benign lesions of the oral cavity. J. Oral Pathol. Med. 2019, 48, 611–618. [Google Scholar] [CrossRef]
- Mori, K.; Uchino, K.; Komukai, S.; Aijima, R.; Shimohira, D.; Danjo, A.; Yamashita, Y. Clinical evaluation of steroid ointment for the treatment of mucoceles. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 307–311. [Google Scholar] [CrossRef]
- Ohta, N.; Fukase, S.; Suzuki, Y.; Aoyagi, M. Treatment of salivary mucocele of the lower lip by OK-432. Auris Nasus Larynx 2011, 38, 240–243. [Google Scholar] [CrossRef]
- Piazzetta, C.M.; Torres-Pereira, C.; Amenábar, J.M. Micro-marsupialization as an alternative treatment for mucocele in pediatric dentistry. Int. J. Paediatr. Dent. 2012, 22, 318–323. [Google Scholar] [CrossRef]
- Romeo, U.; Palaia, G.; Tenore, G.; Del Vecchio, A.; Nammour, S. Excision of oral mucocele by different wavelength lasers. Indian J. Dent. Res. 2013, 24, 211–215. [Google Scholar] [CrossRef]
- Sharma, B.B.; Lamey, P.J. Multiple oral mucoceles treated with evening primrose oil: A report of two cases. Br. J. Oral Maxillofac. Surg. 2022, 60, 365–367. [Google Scholar] [CrossRef]
- Sinha, R.; Sarkar, S.; Khaitan, T.; Kabiraj, A.; Maji, A. Nonsurgical Management of Oral Mucocele by Intralesional Corticosteroid Therapy. Int. J. Dent. 2016, 2016, 2896748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C. The Application of Absolute Ethanol in the Treatment of Mucocele of the Glands of Blandin-Nuhn. J. Craniofac. Surg. 2016, 27, e641–e642. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.L.; Dean, D.R.; Hull, K.; Hu, S.J.; Sim, Y.F.; Nadeau, C.; Gonçalves, S.; Lodi, G.; Hodgson, T.A. World Workshop on Oral Medicine VII: Relative frequency of oral mucosal lesions in children, a scoping review. Oral Dis. 2019, 25 (Suppl. S1), 193–203. [Google Scholar] [CrossRef]
- Bowers, E.M.R.; Schaitkin, B. Management of Mucoceles, Sialoceles, and Ranulas. Otolaryngol. Clin. N. Am. 2021, 54, 543–551. [Google Scholar] [CrossRef]
- Khandelwal, S.; Patil, S. Oral mucoceles—Review of the literature. Minerva Stomatol. 2012, 61, 91–99. [Google Scholar] [PubMed]
- Tandon, A.; Sircar, K.; Chowdhry, A.; Bablani, D. Salivary duct cyst on lower lip: A rare entity and literature review. J. Oral Maxillofac. Pathol. 2014, 18 (Suppl. S1), S151–S156. [Google Scholar] [CrossRef]
- Zahid, E.; Bhatti, O.; Zahid, M.A.; Stubbs, M. Overview of common oral lesions. Malays. Fam. Physician 2022, 1, 9–21. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Varghese, A.K.; Vasupradha, G.; Dinakaran, J. Mucocele: A diagnostic dilemma!! J. Pharm. Bioallied Sci. 2016, 8 (Suppl. S1), S168–S170. [Google Scholar] [CrossRef]
- Nallasivam, K.U.; Sudha, B.R. Oral mucocele: Review of literature and a case report. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. S2), S731–S733. [Google Scholar] [CrossRef]
- More, C.B.; Bhavsar, K.; Varma, S.; Tailor, M. Oral mucocele: A clinical and histopathological study. J Oral Maxillofac Pathol. 2014, 18 (Suppl. S1), S72–S77. [Google Scholar] [CrossRef]
- Randall, D.A.; Wilson Westmark, N.L.; Neville, B.W. Common Oral Lesions. Am. Fam. Physician 2022, 1, 369–376. [Google Scholar]
- Rioboo-Crespo Mdel, R.; Planells-del Pozo, P.; Rioboo-García, R. Epidemiology of the most common oral mucosal diseases in children. Med. Oral Patol. Oral Cir. Bucal 2005, 10, 376–387. [Google Scholar] [PubMed]
- Owczarek-Drabińska, J.E.; Nowak, P.; Zimoląg-Dydak, M.; Radwan-Oczko, M. The Prevalence of Oral Mucosa Lesions in Pediatric Patients. Int. J. Environ. Res. Public Health 2022, 8, 11277. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, W.C.; Chi, A.C.; Neville, B.W. Common oral lesions: Part, I. Superficial mucosal lesions. Am. Fam. Physician 2007, 15, 501–507. [Google Scholar]
- Çayan, T.; Hasanoğlu Erbaşar, G.N.; Akca, G.; Kahraman, S. Comparative Evaluation of Diode Laser and Scalpel Surgery in the Treatment of Inflammatory Fibrous Hyperplasia: A Split-Mouth Study. Photobiomodul. Photomed. Laser Surg. 2019, 37, 91–98. [Google Scholar] [CrossRef]
- Monteiro, L.; Delgado, M.L.; Garcês, F.; Machado, M.; Ferreira, F.; Martins, M.; Salazar, F.; Pacheco, J.J. A histological evaluation of the surgical margins from human oral fibrous-epithelial lesions excised with CO2 laser, Diode laser, Er:YAG laser, Nd:YAG laser, electrosurgical scalpel and cold scalpel. Med. Oral Patol. Oral Cir. Bucal 2019, 1, e271–e280. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Suter, V.G.; Stauffer, E.; Buser, D. Der CO2-Laser in der Stomatologie. Teil 1 [The CO2 laser in stomatology. Part 1]. Schweiz. Monatsschr. Zahnmed. 2003, 113, 559–570. [Google Scholar]
- Ortega-Concepción, D.; Cano-Durán, J.A.; Peña-Cardelles, J.F.; Paredes-Rodríguez, V.M.; González-Serrano, J.; López-Quiles, J. The application of diode laser in the treatment of oral soft tissues lesions. A literature review. J. Clin. Exp. Dent. 2017, 1, e925–e928. [Google Scholar] [CrossRef]
- Tuncer, I.; Ozçakir-Tomruk, C.; Sencift, K.; Cöloğlu, S. Comparison of conventional surgery and CO2 laser on intraoral soft tissue pathologies and evaluation of the collateral thermal damage. Photomed. Laser Surg. 2010, 28, 75–79. [Google Scholar] [CrossRef]
- Suter, V.G.; Altermatt, H.J.; Dietrich, T.; Warnakulasuriya, S.; Bornstein, M.M. Pulsed versus continuous wave CO2 laser excisions of 100 oral fibrous hyperplasias: A randomized controlled clinical and histopathological study. Lasers Surg. Med. 2014, 46, 396–404. [Google Scholar] [CrossRef]
- Walinski, C.J.; Ou, K.L.; Liang, T.M.; Schafer, D.R. Guidelines for the Biopsy of Oral Mucosal Lesions Using a Laser. Compend. Contin. Educ. Dent. 2020, 41, 313–317. [Google Scholar] [PubMed]
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A randomized controlled clinical and histopathological trial comparing excisional biopsies of oral fibrous hyperplasias using CO2 and Er:YAG laser. Lasers Med. Sci. 2017, 32, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Horvat Aleksijević, L.; Prpić, J.; Muhvić Urek, M.; Pezelj-Ribarić, S.; Ivančić-Jokić, N.; Peršić Bukmir, R.; Aleksijević, M.; Glažar, I. Oral Mucosal Lesions in Childhood. Dent. J. 2022, 9, 214. [Google Scholar] [CrossRef]
| Author, Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Overall Appraisal |
|---|---|---|---|---|---|---|---|---|---|
| Aulakh et al., 2016 [11] |  |  |  |  |  |  |  |  |  |
| Besbes et al., 2020 [12] |  |  |  |  |  |  |  |  |  |
| De Falco et al., 2020 [13] |  |  |  |  |  |  |  |  |  |
| Essaket et al., 2020 [14] |  |  |  |  |  |  |  |  |  |
| Gaikwad et al., 2022 [15] |  |  |  |  |  |  |  |  |  |
| Garg et al., 2014 [16] |  |  |  |  |  |  |  |  |  |
| Mouravas et al., 2018 [17] |  |  |  |  |  |  |  |  |  |
| Nagar et al., 2021 [18] |  |  |  |  |  |  |  |  |  |
| Park et al., 2020 [19] |  |  |  |  |  |  |  |  |  |
| Vitale et al., 2018 [20] |  |  |  |  |  |  |  |  |  |
| Author, Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Overall Appraisal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amaral et al., 2012 [21] |  |  |  |  |  |  |  |  |  |  |  |
| Feng et al., 2017 [22] |  |  |  |  |  |  |  |  |  |  |  |
| Giraddi et al., 2016 [23] |  |  |  |  |  |  |  |  |  |  |  |
| Graillon et al., 2021 [24] |  |  |  |  |  |  |  |  |  |  |  |
| Huang et al., 2021 [25] |  |  |  |  |  |  |  |  |  |  |  |
| Farah et al., 2019 [26] |  |  |  |  |  |  |  |  |  |  |  |
| Mori et al., 2021 [27] |  |  |  |  |  |  |  |  |  |  |  |
| Ohta et al., 2010 [28] |  |  |  |  |  |  |  |  |  |  |  |
| Piazzetta et al., 2012 [29] |  |  |  |  |  |  |  |  |  |  |  |
| Romeo et al., 2013 [30] |  |  |  |  |  |  |  |  |  |  |  |
| Sharma et al., 2022 [31] |  |  |  |  |  |  |  |  |  |  |  |
| Sinha et al., 2016 [32] |  |  |  |  |  |  |  |  |  |  |  |
| Zhang et al., 2016 [33] |  |  |  |  |  |  |  |  |  |  |  |
| Author, Year | Type of Laser | Power Setting | Wavelength |
|---|---|---|---|
| Besbes et al., 2020 [12] | Diode laser | 2 W | Not clear |
| De Falco et al., 2020 [13] | Diode laser | 1.5 W | 808 nm |
| Vitale et al., 2018 [20] | Diode laser | 3 W | 810 nm |
| Romeo et al., 2013 [30] | Diode laser and Er,Cr:YSGG laser | Not clear | 808 nm for diode laser and 2780 nm for Er,Cr:YSGG laser |
| Author, Year | Type of Article | Number of Cases | Age | Sex | Position | Size | Intervention | Outcome | Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| Aulakh et al., 2016 [11] | Case report | 1 | 35 | F | Floor of the mouth near 3.6 | 0.5 cm | Cryosurgery | Lesion excision and cauterization | No recurrence, 3-month follow-up |
| Besbes et al., 2020 [12] | Case report | 1 | 10 | F | Lower lip | 0.05 cm | Diode laser excision (2 W) | Lesion excision and cauterization | No recurrence, 4-week follow-up |
| De Falco et al., 2020 [13] | Case report | 1 | 28 | F | Floor of the mouth, ventral tongue | - | Diode laser excision (1.5 W, 800 nm) | Lesion excision and cauterization | Not clear |
| Essaket et al., 2019 [14] | Case report | 1 | 43 | M | Lower lip | 2 cm × 1.5 cm | Scalpel surgery | Lesion excision, suture needed | No recurrence, 8-week follow-up |
| Gaikwad et al., 2022 [15] | Case report | 1 | 49 | M | Buccal mucosa | 4 mm | Sclerotherapy with sodium tetradecyl sulfate (STS) | Lesion removed without surgery | No recurrence, 6-month follow-up |
| Garg et al., 2014 [16] | Case report | 1 | 10 | M | Floor of the mouth | 0.5 cm | Cryosurgery | Lesion excision and cauterization | No recurrence, 6-months follow-up |
| Nagar et al., 2021 [17] | Case report | 1 | 11 | F | Lower tongue | 10 mm × 8 mm | Scalpel surgery | Lesion excision, not clear if suture needed | No recurrence, 1-year follow-up |
| Mouravas et al., 2018 [18] | Case report | 1 | 3 days old | F | Lateral surface of the tongue | 0.5 cm | Scalpel surgery on general anesthesia | Lesion excision, suture needed | No recurrence, 4-year follow-up |
| Park et al., 2021 [19] | Case report | 1 | 21 | M | Lower lip | - | Seven sessions of acupuncture, 15 min per session, once or twice a week | Lesion removed without surgery | No recurrence, 15-month follow-up |
| Vitale et al., 2018 [20] | Case report | 1 | 4 months | F | Lower lip | 10 mm × 6 mm | Diode laser excision (3 W, 810 nm) | Lesion excision and cauterization | No recurrence, 4-month follow-up |
| Amaral et al., 2012 [21] | Case series | 11 | Mean: 17.4 years—range: 5 to 31 years | 72.7% F—27.3% M | Floor of the mouth, lower lip, ventral surface of the tongue | Mean: 16.7 mm | Micro-marsupialization and PBMT with diode laser | Laser excision, suture needed | No recurrence, 11-month follow-up |
| Feng et al., 2017 [22] | Case series | 78 | 0–10 y: 53%, 11–20 y: 17% >21 y: 8% | 38.5% M—48% F | Anterion lingual mucosa | 68.2% < 0.5 cm only 7.7% > 1 cm | Microwave ablation | Lesion removed without surgery | No recurrence in 88.5% of patients, 11.5% needed a second treatment |
| Giraddi et al., 2017 [23] | Case series | 20 | Mean: 19.6 y in group 1—21.9 y in group 2 | 50% F—50% M | Lower lip, floor of the mouth, buccal mucosa | Mean: 0.98 cm in group 1—1.15 cm in group 2 | Micro-marsupialization on group 1—scalpel surgery in group 2 | Lesion excision, suture needed in both groups | Micro-marsupialization showed recurrence in 20% of patients—scalpel surgery showed recurrence in 10% of patients and fibrosis in lower lip in 10% of cases. |
| Graillon et al., 2021 [24] | Case series | 5 | Mean: 18.2 y—range: 7 to 39 years | 90% M—10% F | Ventral surface of the tongue | - | Marsupialization and scalpel excision | Lesion excision, suture needed | No recurrence, follow-up from 6 to 12 months. |
| Huang et al., 2021 [25] | Case series | 37 | Mean: 16 years | 37.8% M—62.2% F | Ventral tongue tip, lower lip, floor of the mouth | Mean: 5 mm | Sclerotherapy with promethazine hydrochloride (25 mg/mL) | Lesion removed without surgery | Thirty-three patients showed no recurrence, nine patients needed two sessions of treatment, three patients showed no response to the therapy |
| Farah et al., 2019 [26] | Case series | 12 | Mean: 30.9 years | 50% F—50% M | Lower lip, floor of the mouth | Mean: 7.8 mm | Nitrous oxide cryotherapy | Lesion removed without surgery and cauterization | 50% of the patients showed resolution with one application, 67% showed resolution after two visits, 83% showed resolution after three treatment visits, 100% resolution after the fifth treatment. |
| Mori et al., 2021 [27] | Case series | 91 | Mean: 13.0 years | 55% M—45% F | Lower lip, floor of the mouth, upper labial mucosa, tongue, anterior lingual gland, soft palate, buccal mucosa | Mean: >6 mm | Surgical removal for group 1 and steroid ointment application (dexamethasone 0.1%) for group 2 | Lesion removed | Response rate of the ointment group was significantly lower than the surgical group. |
| Ohta et al., 2012 [28] | Case series | 20 | Mean: 30.8 years | 16% F—84% M | Lower lip | Mean: 7.9 mm | Injection of OK-432 solution | Lesion removed without surgery | Sixteen patients showed resolution, four patients showed marked reduction in the lesion size. |
| Piazzetta et al. 2012 [29] | Case Series | 86 | 0–6 years: 15%—7–12 years: 51%—13–18 years: 33% | 45% M—54% F | Lower lip, tongue, buccal mucosa | Mean: 0.6–1 cm | Micro-marsupialization vs. scalpel excision | Lesion excision, suture needed | Patients showed recurrence in 15% of cases treated with micro-marsupialization and in 6% of cases treated with scalpel excision |
| Romeo et al., 2013 [30] | Case series | 3 | Mean: 13 years | 100% M | Lower lip | 0.5 cm | Laser excision: one case with an Er,Cr:YSGG laser at 2780 nm, one case with a diode laser at 808 nm, one case with a KTP laser at 532 nm. | Lesion removed and cauterization | No recurrence in all cases. |
| Sharma et al., 2021 [31] | Case series | 2 | Mean: 50 years | 100% F | Soft palate | - | Primrose oil application of 500 mg, four times a day for three months. | Lesion removed without surgery | No recurrence in all cases, three-month follow-up. |
| Sinha et al., 2016 [32] | Case series | 20 | Range: 10–30 years | - | Lower lip, buccal mucosa | - | Intralesional corticosteroid therapy, four injections once a week | Lesion removed without surgery | No recurrence, two cases showed only a reduction in size. |
| Zhang et al. [33] | Case series | 14 | Mean: 14.4 years | 50% F—50% M | Lingual mucosa | Mean: 0.9 cm × 0.6 cm | Intralesional absolute ethanol (from 0.1 to 0.5 mL according to the lesions’ size) injection. | Lesion removed without surgery. | No recurrence in 1-year follow-up. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scribante, A.; Pellegrini, M.; Pulicari, F.; De Martino, F.; Li Vigni, G.; Ghizzoni, M.; Spadari, F. Oral Cavity Mucocele and Different Surgical Treatment Strategies: Is Laser Excision Effective? A Scoping Review. Appl. Sci. 2023, 13, 12327. https://doi.org/10.3390/app132212327
Scribante A, Pellegrini M, Pulicari F, De Martino F, Li Vigni G, Ghizzoni M, Spadari F. Oral Cavity Mucocele and Different Surgical Treatment Strategies: Is Laser Excision Effective? A Scoping Review. Applied Sciences. 2023; 13(22):12327. https://doi.org/10.3390/app132212327
Chicago/Turabian StyleScribante, Andrea, Matteo Pellegrini, Federica Pulicari, Francesca De Martino, Giacomo Li Vigni, Martina Ghizzoni, and Francesco Spadari. 2023. "Oral Cavity Mucocele and Different Surgical Treatment Strategies: Is Laser Excision Effective? A Scoping Review" Applied Sciences 13, no. 22: 12327. https://doi.org/10.3390/app132212327
APA StyleScribante, A., Pellegrini, M., Pulicari, F., De Martino, F., Li Vigni, G., Ghizzoni, M., & Spadari, F. (2023). Oral Cavity Mucocele and Different Surgical Treatment Strategies: Is Laser Excision Effective? A Scoping Review. Applied Sciences, 13(22), 12327. https://doi.org/10.3390/app132212327









