Icarifil, a Natural Mixture Based on L-Citrulline and L-Carnitine as a Novel Multicomponent Nutraceutical to Modulate ROS and PDE5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Icarifil
2.2. Cell Proliferation Studies of Human Muscular Epithelium and Murine Penile Muscle Epithelium
2.3. Determination of Induced Human Muscular Epithelium Cell Turgor
2.4. Measurement of Phosphodiesterase Type 5 (PDE5) Protein and Transcript Levels
2.5. Measurement of Reactive Oxygen Species (ROS) Levels in Murine Penile Muscle Epithelium Cells
2.6. Statistic Analysis
3. Results and Discussion
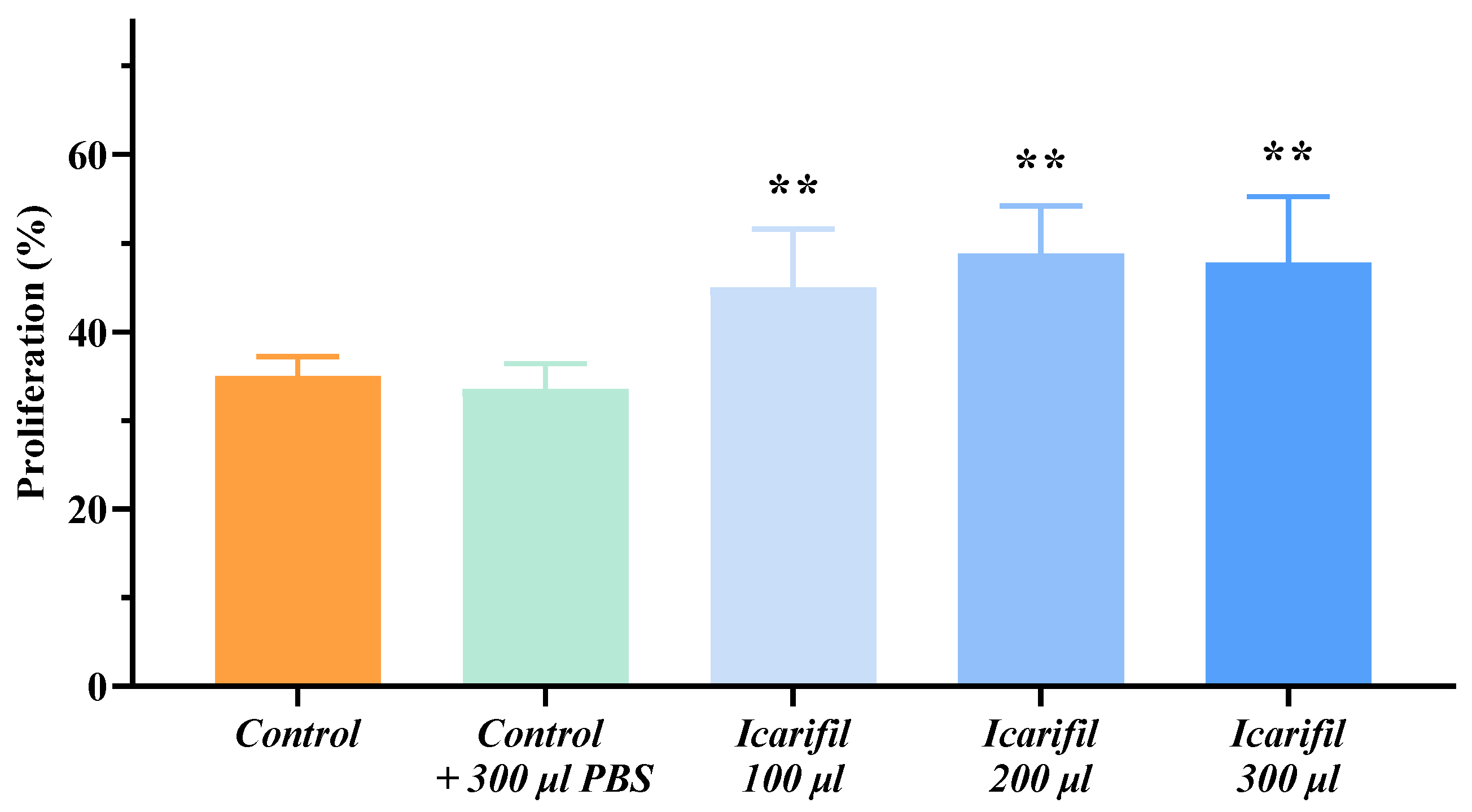
3.1. Cell Proliferation
3.2. Human Muscular Epithelium Cell Turgor
3.3. Reduction of Phosphodiesterase Type 5 (PDE5) Protein and Transcript Levels
3.4. Modulation of the Intracellular Level of ROS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institutes of Health. Consensus development conference statement. Int. J. Impot. Res. 1993, 5, 181–284. [Google Scholar]
- Wassersug, R.; Wibowo, E. Non-pharmacological and non-surgical strategies to promote sexual recovery for men with erectile dysfunction. Transl. Androl. Urol. 2017, 6, S776. [Google Scholar] [CrossRef]
- Kessler, A.; Sollie, S.; Challacombe, B.; Briggs, K.; Van Hemelrijck, M. The global prevalence of erectile dysfunction: A review. BJU Int. 2019, 124, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Angulo, J.; Chitaley, K.; Dai, Y.T.; Kim, N.N.; Paick, J.S.; Simonsen, U.; Uckert, S.; Wespes, E.; Andersson, K.E.; et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J. Sex Med. 2010, 7, 445–475. [Google Scholar] [CrossRef]
- Johannes, C.B.; Araujo, A.B.; Feldman, H.A.; Derby, C.A.; Kleinman, K.P.; McKinlay, J.B. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts male aging study. J. Urol. 2000, 163, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Shen, Y.C.; Weng, S.F.; Wang, J.J.; Tien, K.J. Increased Risk of Dementia in Patients With Erectile Dysfunction: A Population-Based, Propensity Score-Matched, Longitudinal Follow-Up Study. Medicine 2015, 94, e990. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, Y.H.; Qin, L.Q. Erectile dysfunction and risk of cardiovascular disease: Meta-analysis of prospective cohort studies. J. Am. Coll. Cardiol. 2011, 58, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Saenz de Tejada, I.; Goldstein, I.; Azadzoi, K.; Krane, R.J.; Cohen, R.A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N. Engl. J. Med. 1989, 320, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Krzastek, S.C.; Bopp, J.; Smith, R.P.; Kovac, J.R. Recent advances in the understanding and management of erectile dysfunction. F1000Research 2019, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rhim, H.C.; Kim, M.S.; Park, Y.-J.; Choi, W.S.; Park, H.K.; Kim, H.G.; Kim, A.; Paick, S.H. The potential role of arginine supplements on erectile dysfunction: A systemic review and meta-analysis. J. Sex. Med. 2019, 16, 223–234. [Google Scholar] [CrossRef]
- Aguayo, E.; Martínez-Sánchez, A.; Fernández-Lobato, B.; Alacid, F. L-Citrulline: A Non-Essential Amino Acid with Important Roles in Human Health. Appl. Sci. 2021, 11, 3293. [Google Scholar] [CrossRef]
- Cormio, L.; De Siati, M.; Lorusso, F.; Selvaggio, O.; Mirabella, L.; Sanguedolce, F.; Carrieri, G. Oral L-citrulline supplementation improves erection hardness in men with mild erectile dysfunction. Urology 2011, 77, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Gianfrilli, D.; Lauretta, R.; Di Dato, C.; Graziadio, C.; Pozza, C.; De Larichaudy, J.; Giannetta, E.; Isidori, A.M.; Lenzi, A. Propionyl-L-carnitine, L-arginine and niacin in sexual medicine: A nutraceutical approach to erectile dysfunction. Andrologia 2012, 44, 600–604. [Google Scholar] [CrossRef]
- Gentile, V.; Antonini, G.; Antonella Bertozzi, M.; Dinelli, N.; Rizzo, C.; Ashraf Virmani, M.; Koverech, A. Effect of propionyl-l-carnitine, l-arginine and nicotinic acid on the efficacy of vardenafil in the treatment of erectile dysfunction in diabetes. Curr. Med. Res. Opin. 2009, 25, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, V.; Piragine, E.; Gorica, E.; Citi, V.; Testai, L.; Pagnotta, E.; Matteo, R.; Pecchioni, N.; Montanaro, R.; Di Cesare Mannelli, L.; et al. Anti-Inflammatory Effect of the Natural H2S-Donor Erucin in Vascular Endothelium. Int. J. Mol. Sci. 2022, 23, 15593. [Google Scholar] [CrossRef]
- Jupiter, R.C.; Yoo, D.; Pankey, E.A.; Reddy, V.V.; Edward, J.A.; Polhemus, D.J.; Peak, T.C.; Katakam, P.; Kadowitz, P.J. Analysis of erectile responses to H2S donors in the anesthetized rat. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H835–H843. [Google Scholar] [CrossRef]
- Choi, Y.D.; Park, C.W.; Jang, J.; Kim, S.H.; Jeon, H.Y.; Kim, W.G.; Lee, S.J.; Chung, W.S. Effects of Korean ginseng berry extract on sexual function in men with erectile dysfunction: A multicenter, placebo-controlled, double-blind clinical study. Int. J. Impot. Res. 2013, 25, 45–50. [Google Scholar] [CrossRef]
- Leung, K.W.; Wong, A.S. Ginseng and male reproductive function. Spermatogenesis 2013, 3, e26391. [Google Scholar] [CrossRef]
- Ștefănescu, R.; Farczadi, L.; Huțanu, A.; Ősz, B.E.; Mărușteri, M.; Negroiu, A.; Vari, C.E. Tribulus terrestris Efficacy and Safety Concerns in Diabetes and Erectile Dysfunction, Assessed in an Experimental Model. Plants 2021, 10, 744. [Google Scholar] [CrossRef]
- Gauthaman, K.; Ganesan, A.P.; Prasad, R.N.V. Sexual Effects of Puncturevine (Tribulus terrestris) Extract (Protodioscin): An Evaluation Using a Rat Model. J. Altern. Complement. Med. 2003, 9, 257–265. [Google Scholar] [CrossRef]
- Szewczyk, K.; Zidorn, C. Ethnobotany, phytochemistry, and bioactivity of the genus Turnera (Passifloraceae) with a focus on damiana—Turnera diffusa. J. Ethnopharmacol. 2014, 152, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Q.; Shang, J.; Dong, W.; Wu, S.; Guo, X.; Xie, Z.; Chen, C. The role of taurine in male reproduction: Physiology, pathology and toxicology. Front. Endocrinol. 2023, 14, 1017886. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Li, M.; Wang, T.; Yang, J.; Rao, K.; Wang, S.; Yang, W.; Liu, J.; Ye, Z. Taurine Supplementation Improves Erectile Function in Rats with Streptozotocin-induced Type 1 Diabetes via Amelioration of Penile Fibrosis and Endothelial Dysfunction. J. Sex. Med. 2016, 13, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.M.; Senbel, A.M. Evaluation of vitamin E in the treatment of erectile dysfunction in aged rats. Life Sci. 2012, 90, 489–494. [Google Scholar] [CrossRef] [PubMed]
- El-Assmy, A. Erectile dysfunction in hemodialysis: A systematic review. World J. Nephrol. 2012, 1, 160–165. [Google Scholar] [CrossRef]
- Hempling, H.G.; Cicoria, A.D.; Dupre, A.M.; Thompson, S. State of water and electrolytes in mammalian cells during maturation and differentiation. J. Exp. Zool. 1981, 215, 259–276. [Google Scholar] [CrossRef]
- Aversa, A.; Fittipaldi, S.; Bimonte, V.M.; Wannenes, F.; Papa, V.; Francomano, D.; Greco, E.A.; Lenzi, A.; Migliaccio, S. Tadalafil modulates aromatase activity and androgen receptor expression in a human osteoblastic cell in vitro model. J. Endocrinol. Investig. 2016, 39, 199–205. [Google Scholar] [CrossRef]
- Ferrini, M.G.; Garcia, E.; Abraham, A.; Artaza, J.N.; Nguyen, S.; Rajfer, J. Effect of ginger, Paullinia cupana, muira puama and l- citrulline, singly or in combination, on modulation of the inducible nitric oxide-NO-cGMP pathway in rat penile smooth muscle cells. Nitric Oxide 2018, 76, 81–86. [Google Scholar] [CrossRef]
- Niu, Y.; Lin, G.; Pan, J.; Liu, J.; Xu, Y.; Cai, Q.; Wang, T.; Luan, Y.; Chen, Y.; Feng, Y.; et al. Deciphering the myth of icariin and synthetic derivatives in improving erectile function from a molecular biology perspective: A narrative review. Transl. Androl. Urol. 2022, 11, 1007–1022. [Google Scholar] [CrossRef]
- Shirai, M.; Hiramatsu, I.; Aoki, Y.; Shimoyama, H.; Mizuno, T.; Nozaki, T.; Fukuhara, S.; Iwasa, A.; Kageyama, S.; Tsujimura, A. Oral L-citrulline and transresveratrol supplementation improves erectile function in men with phosphodiesterase 5 inhibitors: A randomized, double-blind, placebo-controlled crossover pilot study. Sex. Med. 2018, 6, 291–296. [Google Scholar] [CrossRef]
- Su, L.; Yang, Z.-T.; Qu, H.; Luo, C.-L.; Yuan, G.-X.; Wu, J.; Jiao, Y.-Z. Effect of Antioxidants Supplementation on Erectile Dysfunction: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sex. Med. Rev. 2022, 10, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.; Ali, A. Effect of Pomegranate Juice and Fresh Leaves of Eruca vesicaria on Testosterone Hormone Level in Blood Serum of Male Rabbits. Syst. Rev. Pharm. 2020, 11, 1211–1214. [Google Scholar] [CrossRef]






| Sample | Amount (mg) |
|---|---|
| L-Citrulline | 1500 |
| L-Carnitine | 500 |
| Eruca vesicaria (Icariina 5%) | 200 |
| Panax ginseng (Ginsenosids 80%) | 150 |
| Tribulus terrestris | 100 |
| Turnera diffusa (damiana) | 100 |
| Taurine | 50 |
| Vitamin E | 50 |
| Zinc | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amante, C.; De Soricellis, C.; Luccheo, G.; Di Vernieri, A.; Luccheo, L.; Falcone, G.; Del Gaudio, P. Icarifil, a Natural Mixture Based on L-Citrulline and L-Carnitine as a Novel Multicomponent Nutraceutical to Modulate ROS and PDE5. Appl. Sci. 2023, 13, 12358. https://doi.org/10.3390/app132212358
Amante C, De Soricellis C, Luccheo G, Di Vernieri A, Luccheo L, Falcone G, Del Gaudio P. Icarifil, a Natural Mixture Based on L-Citrulline and L-Carnitine as a Novel Multicomponent Nutraceutical to Modulate ROS and PDE5. Applied Sciences. 2023; 13(22):12358. https://doi.org/10.3390/app132212358
Chicago/Turabian StyleAmante, Chiara, Chiara De Soricellis, Gianni Luccheo, Anna Di Vernieri, Luigi Luccheo, Giovanni Falcone, and Pasquale Del Gaudio. 2023. "Icarifil, a Natural Mixture Based on L-Citrulline and L-Carnitine as a Novel Multicomponent Nutraceutical to Modulate ROS and PDE5" Applied Sciences 13, no. 22: 12358. https://doi.org/10.3390/app132212358
APA StyleAmante, C., De Soricellis, C., Luccheo, G., Di Vernieri, A., Luccheo, L., Falcone, G., & Del Gaudio, P. (2023). Icarifil, a Natural Mixture Based on L-Citrulline and L-Carnitine as a Novel Multicomponent Nutraceutical to Modulate ROS and PDE5. Applied Sciences, 13(22), 12358. https://doi.org/10.3390/app132212358








