Assessment of the Fermentative Performance of Traditional Fresh Moroccan Sourdoughs and Their Freeze-Dried Forms Using Online Monitoring Device: Panigraph
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Lyophilization
2.3. Fermentation Monitoring System
2.4. CO2 and Ethanol Release
2.5. Total Titratable Acidity (TTA)
2.6. Preparation of Bread Dough
2.7. Dough Rising (cm)
2.8. Dough Yield (DY)
2.9. Dough Fermentation Temperature and Duration
2.10. Dough Moisture
2.11. Statistical Analysis of Data
3. Results
3.1. Sourdough Characterization
3.2. Assessment of Fermentation Capability
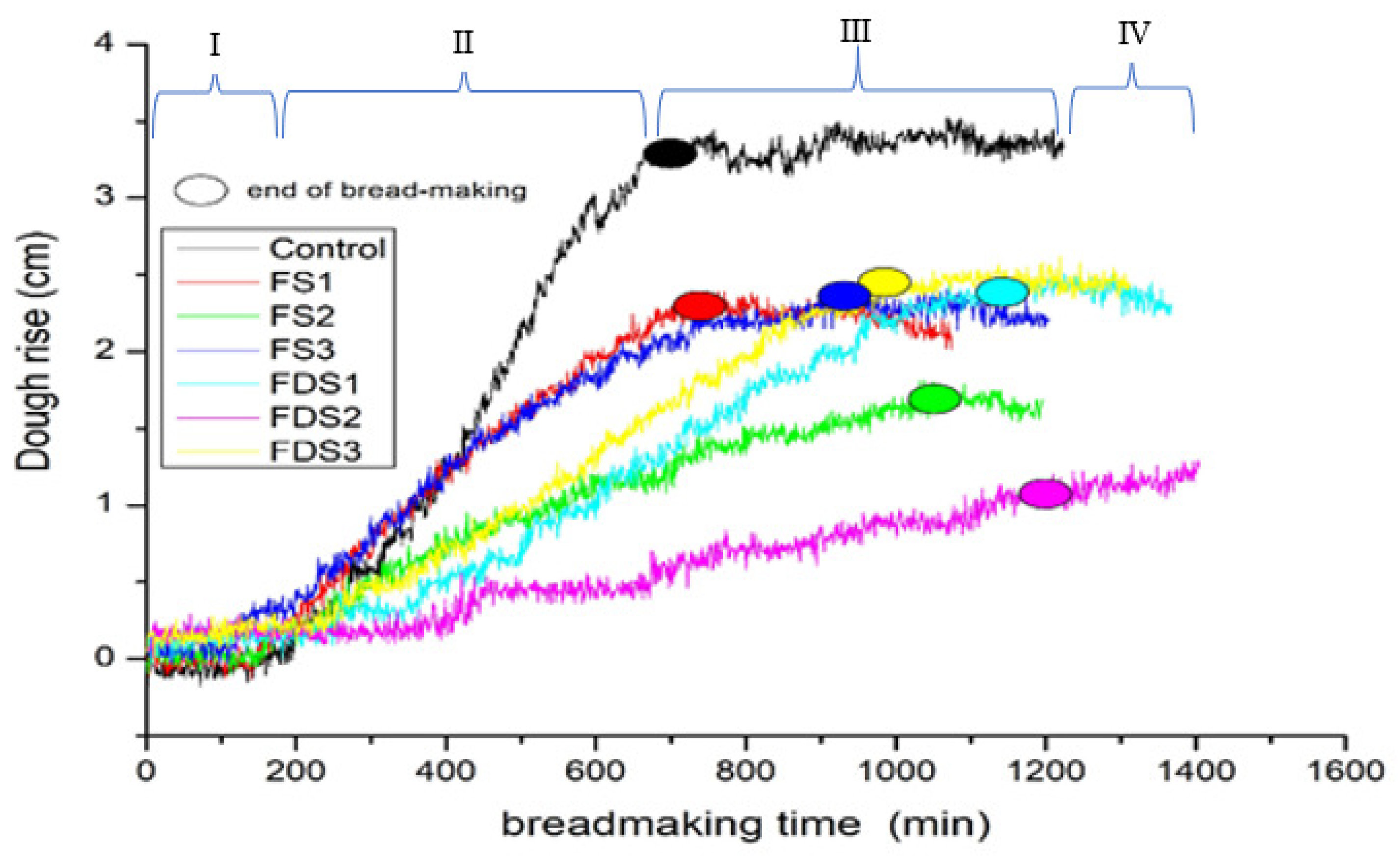
3.2.1. Dough Rise during Bread Making
3.2.2. Bread-Making Duration
3.2.3. Specific Volume
3.2.4. Loss of Mass
3.3. Biochemical Characterization of Dough
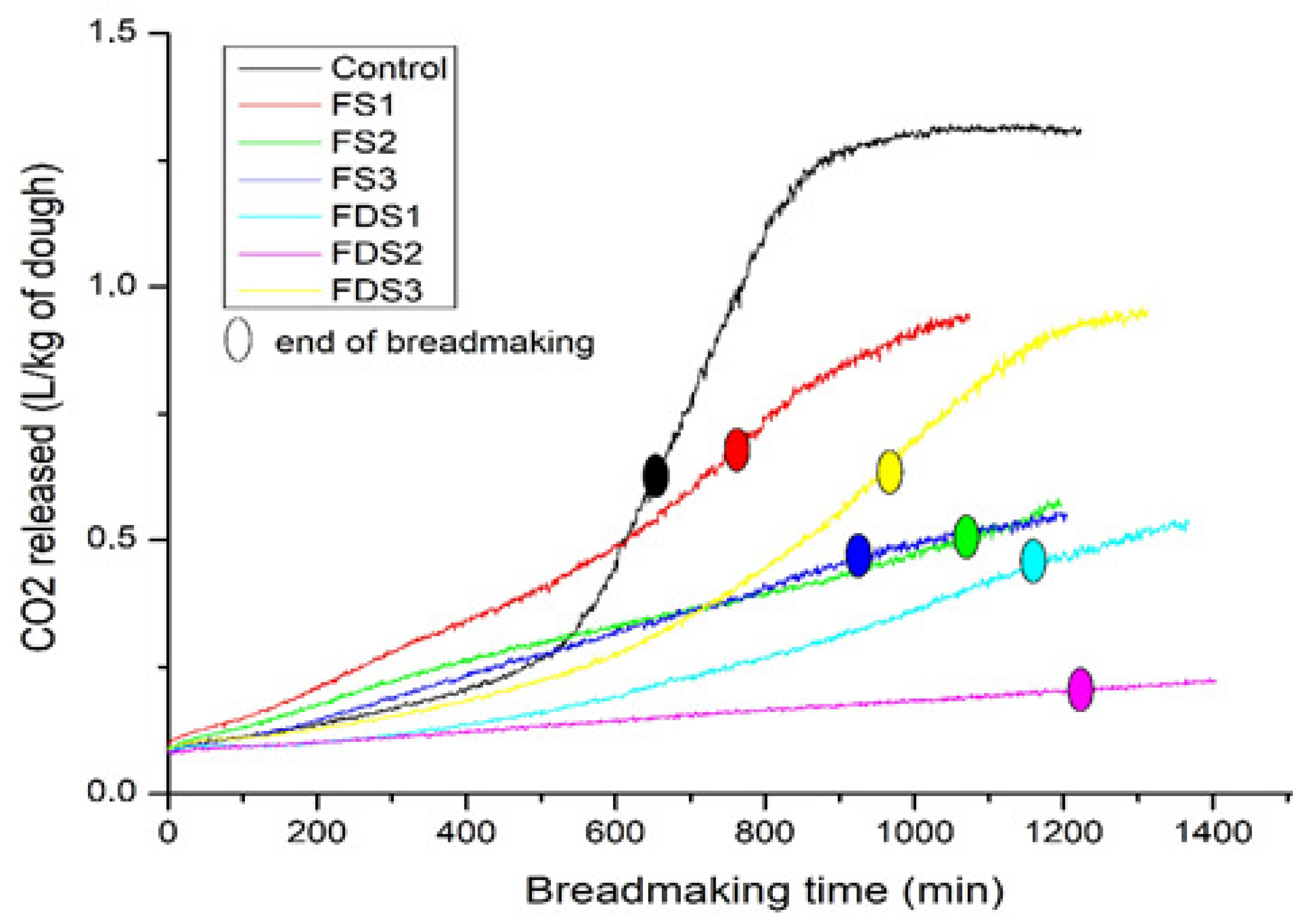
3.3.1. CO2 Release during Bread Making
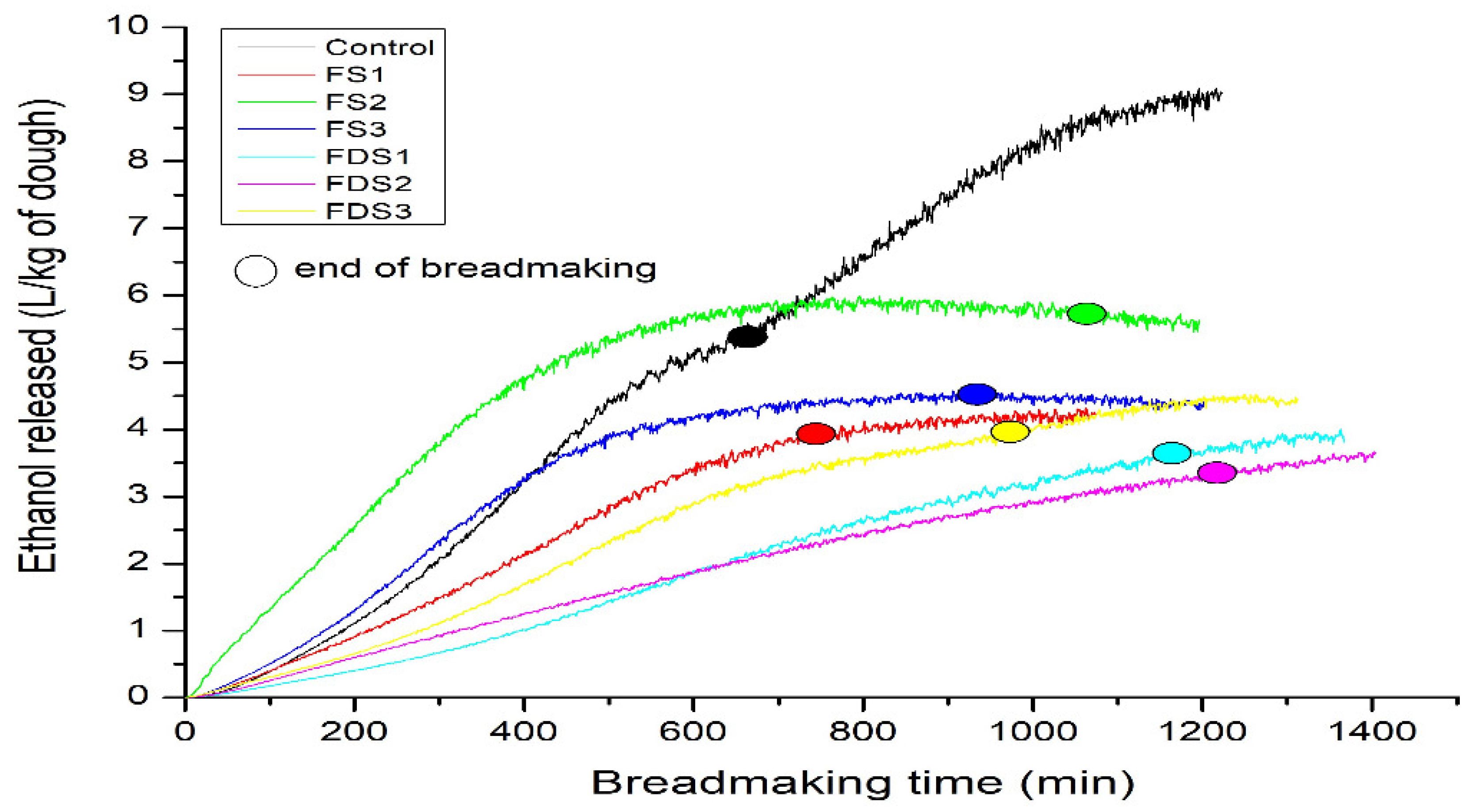
3.3.2. Ethanol Release during Bread Making
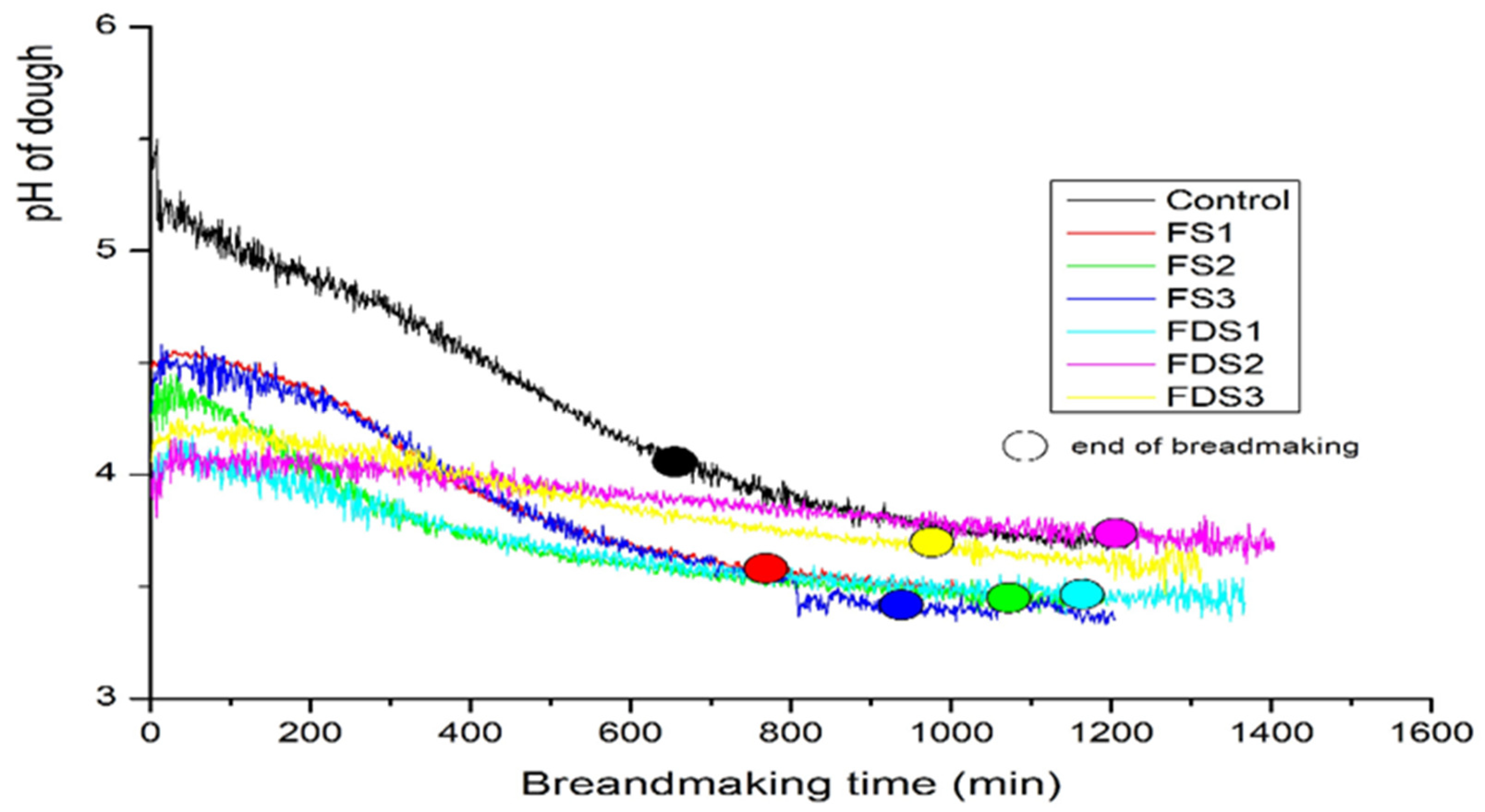
3.3.3. Variation in pH and TTA of Fermented Doughs
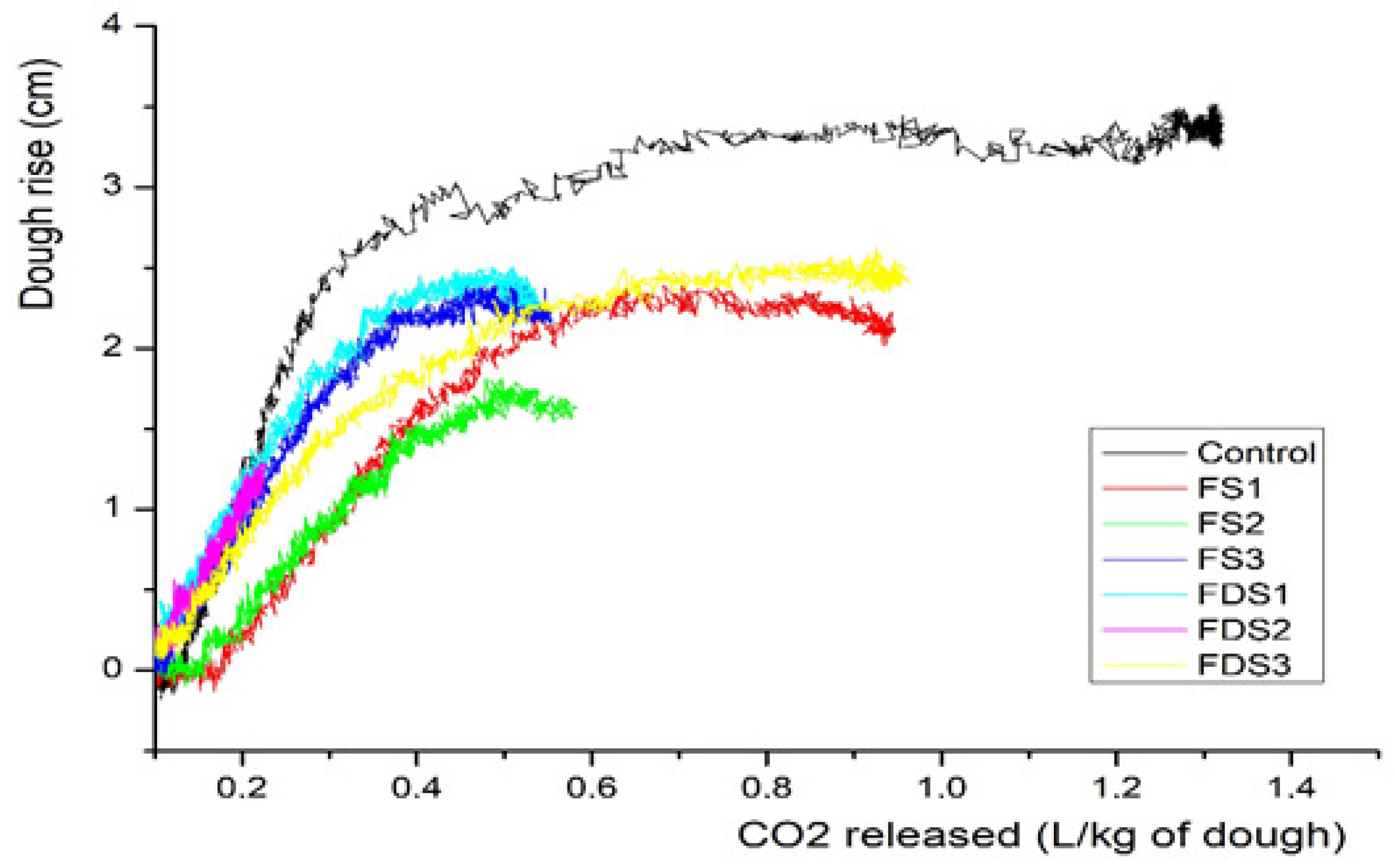
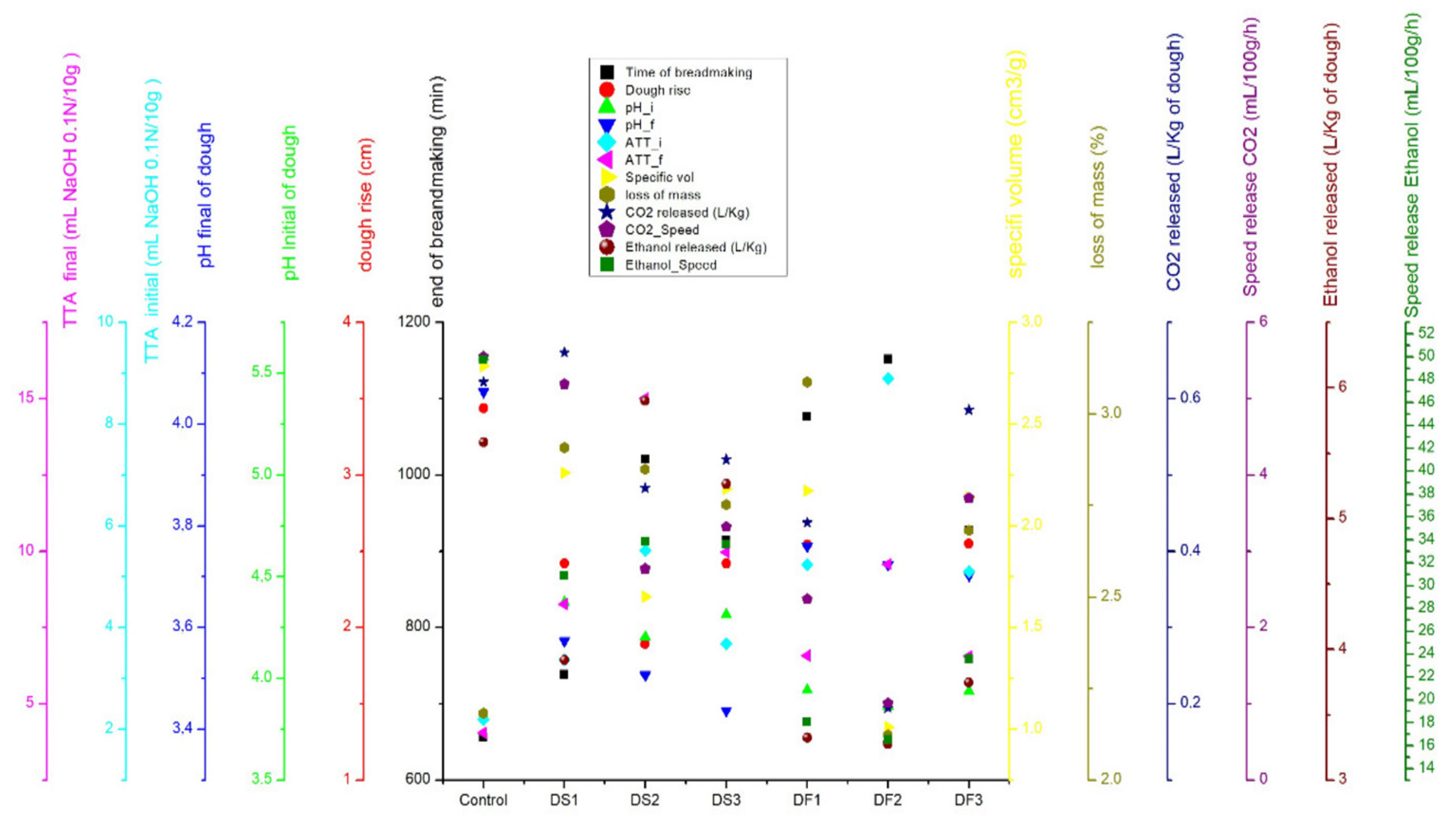
3.4. Correlations of Bread-Making Parameters
3.5. Individual Panigram of FS1 and FDS3 Sourdoughs
3.6. Vertical Panigram of All Doughs
4. Discussions
4.1. Sourdough Comparisons
4.2. Bread Fermentation Performance Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.-M.; Weckx, S. Microbial Ecology of Sourdough Fermentations: Diverse or Uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; De Angelis, M.; Di Cagno, R.; Gobbetti, M. Ecological Parameters Influencing Microbial Diversity and Stability of Traditional Sourdough. Int. J. Food Microbiol. 2014, 171, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Katina, K.; Rizzello, C.G. Bran Bioprocessing for Enhanced Functional Properties. Curr. Opin. Food Sci. 2015, 1, 50–55. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Maes, D.; De Vuyst, L. Sourdoughs as a Function of Their Species Diversity and Process Conditions, a Meta-Analysis. Trends Food Sci. Technol. 2017, 68, 152–159. [Google Scholar] [CrossRef]
- Comasio, A.; Verce, M.; Van Kerrebroeck, S.; De Vuyst, L. Diverse Microbial Composition of Sourdoughs From Different Origins. Front. Microbiol. 2020, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Minervini, F.; Pontonio, E.; Di Cagno, R.; De Angelis, M. Drivers for the Establishment and Composition of the Sourdough Lactic Acid Bacteria Biota. Int. J. Food Microbiol. 2016, 239, 3–18. [Google Scholar] [CrossRef]
- Pontonio, E.; Nionelli, L.; Curiel, J.A.; Sadeghi, A.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G. Iranian Wheat Flours from Rural and Industrial Mills: Exploitation of the Chemical and Technology Features, and Selection of Autochthonous Sourdough Starters for Making Breads. Food Microbiol. 2015, 47, 99–110. [Google Scholar] [CrossRef]
- Calvert, M.D.; Madden, A.A.; Nichols, L.M.; Haddad, N.M.; Lahne, J.; Dunn, R.R.; McKenney, E.A. A Review of Sourdough Starters: Ecology, Practices, and Sensory Quality with Applications for Baking and Recommendations for Future Research. PeerJ 2021, 9, e11389. [Google Scholar] [CrossRef]
- Tafti, A.G.; Peighambardoust, S.H.; Hesari, J.; Bahrami, A.; Bonab, E.S. Physico-Chemical and Functional Properties of Spray-Dried Sourdough in Breadmaking. Food Sci. Technol. Int. 2013, 19, 271–278. [Google Scholar] [CrossRef]
- Komlenić, D.K.; Ugarčić-Hardi, Ž.; Jukić, M.; Planinić, M.; Bucić-Kojić, A.; Strelec, I. Wheat Dough Rheology and Bread Quality Effected by Lactobacillus Brevis Preferment, Dry Sourdough and Lactic Acid Addition: Properties of Acidified Bread. Int. J. Food Sci. Technol. 2010, 45, 1417–1425. [Google Scholar] [CrossRef]
- Caglar, N.; Ermis, E.; Durak, M.Z. Spray-Dried and Freeze-Dried Sourdough Powders: Properties and Evaluation of Their Use in Breadmaking. J. Food Eng. 2021, 292, 110355. [Google Scholar] [CrossRef]
- Novotni, D.; Gänzle, M.; Rocha, J.M. Composition and Activity of Microbiota in Sourdough and Their Effect on Bread Quality and Safety. In Trends in Wheat and Bread Making; Elsevier: Amsterdam, The Netherlands, 2021; pp. 129–172. [Google Scholar]
- Raffak, A.; Chafai, Y.; Hamouda, A.; Mounir, M. Suivi en temps réel de la fermentation panaire de quatre levains liquides à base des farines du blé tendre, du blé dur complet, du baobab et du Millet. REMAV 2023, 11, 259–268. [Google Scholar] [CrossRef]
- Zhang, L.; Lucas, T.; Doursat, C.; Flick, D.; Wagner, M. Effects of Crust Constraints on Bread Expansion and CO2 Release. J. Food Eng. 2007, 80, 1302–1311. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Coda, R.; Wang, Y.; Verni, M.; Kajala, I.; Katina, K.; Laitila, A. Characterization of Indigenous Pediococcus Pentosaceus, Leuconostoc Kimchii, Weissella Cibaria and Weissella Confusa for Faba Bean Bioprocessing. Int. J. Food Microbiol. 2019, 302, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.M.; Gupta, R.K. Bread (Composite Flour) Formulation and Study of Its Nutritive, Phytochemical and Functional Properties. J. Pharmacogn. Phytochem. 2015, 4, 254–268. [Google Scholar]
- Chavan, R.S.; Chavan, S.R. Sourdough Technology—A Traditional Way for Wholesome Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 169–182. [Google Scholar] [CrossRef]
- Vrancken, G.; Rimaux, T.; Weckx, S.; Leroy, F.; De Vuyst, L. Influence of Temperature and Backslopping Time on the Microbiota of a Type I Propagated Laboratory Wheat Sourdough Fermentation. Appl. Environ. Microbiol. 2011, 77, 2716–2726. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of Sourdough Fermentation on Stabilisation, and Chemical and Nutritional Characteristics of Wheat Germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, Protein and Mineral Fortification of Wheat Bread through Milled and Fermented Brewer’s Spent Grain Enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Roussel, P.; Onno, B.; Michel, E.; Sicard, D. La Panification Au Levain Naturel; Éditions Quae: Versailles, France, 2020. [Google Scholar]
- Różyło, R.; Rudy, S.; Krzykowski, A.; Dziki, D.; Siastała, M.; Polak, R. Gluten-Free Bread Prepared with Fresh and Freeze-Dried Rice Sourdough-Texture and Sensory Evaluation: GF Bread with Freeze-Dried Rice Sourdough. J. Texture Stud. 2016, 47, 443–453. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Boscaino, F.; Nazzaro, F.; Fratianni, F.; Aponte, M. Lactic Acid Bacteria Biota and Aroma Profile of Italian Traditional Sourdoughs From the Irpinian Area in Italy. Front. Microbiol. 2019, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Corsetti, A.; Rossi, J. Interaction between Lactic Acid Bacteria and Yeasts in Sour-Dough Using a Rheofermentometer. World J. Microbiol. Biotechnol. 1995, 11, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A. Der EinflussorganiscerSauren Auf Die Vergarungverschiedener Z. Getr. Mehl. Brot. 1972, 26, 129–133. [Google Scholar]
- Casal, M.; Cardoso, H.; Leão, C. Effects of Ethanol and Other Alkanols on Transport of Acetic Acid in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1998, 64, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Meuser, F.; Busch, K.-G. Dough Preparation for Crispbread Production by Applying a High-Pressure Homogeniser. In Proceedings of the Vtt Symposium (Valtion Teknillinen Tutkimuskeskus), Espoo, Finland, 28 May–1 June 1995; Volume 161, pp. 169–187. [Google Scholar]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of Acetic Acid and Lactic Acid on the Growth of Saccharomyces Cerevisiae in a Minimal Medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, R.F.; Machado, A.A.R.; Pasqualin Cavalheiro, C.; Bartholomei Santos, M.L.; Nabeshima, E.H.; Copetti, M.V.; Fries, L.L.M. Trehalose as a Cryoprotectant in Freeze-Dried Wheat Sourdough Production. LWT 2018, 89, 510–517. [Google Scholar] [CrossRef]
- Stephan, D.; Da Silva, A.-P.M.; Bisutti, I.L. Optimization of a Freeze-Drying Process for the Biocontrol Agent Pseudomonas Spp. and Its Influence on Viability, Storability and Efficacy. Biol. Control 2016, 94, 74–81. [Google Scholar] [CrossRef]
- Lund, B.; Hansen, A.; Lewis, M.J. The Influence of Dough Yield on Acidification and Production of Volatiles in Sourdoughs. Lebensm. Wiss. Technol. 1989, 22, 150–153. [Google Scholar]
- Reed, G. Yeast Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Castro-Alba, V.; Lazarte, C.E.; Perez-Rea, D.; Carlsson, N.; Almgren, A.; Bergenståhl, B.; Granfeldt, Y. Fermentation of Pseudocereals Quinoa, Canihua, and Amaranth to Improve Mineral Accessibility through Degradation of Phytate. J. Sci. Food Agric. 2019, 99, 5239–5248. [Google Scholar] [CrossRef]
- Chiron, H.; Onno, B.; Dewalque, M. Radioscopie d’un Pain Au Levain. In Proceedings of the Journées Techniques de l’AEMIC (60. Journées Techniques des Industries Céréalières), Reims, France, 13–15 October 2009. [Google Scholar]
- Arendt, E.K.; Ryan, L.A.M.; Dal Bello, F. Impact of Sourdough on the Texture of Bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in Foods and Significance for Humans: Food Sources, Intake, Processing, Bioavailability, Protective Role and Analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef]






| Fresh Sourdough Code | Housewives’ Regions | Water | Soft Wheat Flour | Dried Grapes | Durum Wheat Flour Complete | Fermented Milk (Lben) |
|---|---|---|---|---|---|---|
| FS1 | Rabat | 55.00 | 43.50 | 1.50 | - | - |
| FS2 | Guelmim | 26.00 | - | 1.00 | 47.00 | 26.00 |
| FS3 | Tanger | 50.00 | 49.00 | 0.93 | - | - |
| Dough Code | Sourdough Code | Water | Soft Wheat Flour | Table Salt | Baking Powder | Sourdough |
|---|---|---|---|---|---|---|
| DS1 | FS1 | 34.40 | 55.50 | 1.00 | 0.10 | 9.00 |
| DS2 | FS2 | 34.40 | 55.50 | 1.00 | 0.10 | 9.00 |
| DS3 | FS3 | 34.40 | 55.50 | 1.00 | 0.10 | 9.00 |
| DF1 | FDS1 | 34.40 | 55.50 | 1.00 | 0.10 | 9.00 |
| DF2 | FDS2 | 34.40 | 55.50 | 1.00 | 0.10 | 9.00 |
| DF3 | FDS3 | 34.40 | 55.50 | 1.00 | 0.10 | 9.00 |
| Control | without | 38.90 | 60.00 | 1.00 | 0.10 | 0.00 |
| Fresh Sourdough Code | TTA (mL NaOH/10 g of Dough) | pH | Moisture (%) | DY (%) | Consistency |
|---|---|---|---|---|---|
| FS1 | 14.12 ± 5.03 a | 3.44 ± 0.09 a | 54.57 ± 1.00 a | 226.87 ± 1.80 c | Liquid |
| FS2 | 39.24 ± 2.91 b | 3.79 ± 0.05 b | 55.74 ± 1.29 ab | 155.31 ± 0.70 a | Farm |
| FS3 | 14.28 ± 1.47 a | 3.36 ± 0.07 a | 57.12 ± 0.34 b | 202.60 ± 1.11 b | Soft |
| Freeze-Dried Sourdough Code | TTA (mL NaOH/10 g of Dough) | pH | Moisture (%) |
|---|---|---|---|
| FDS1 | 37.71 ± 0.76 a | 2.98 ± 0.02 a | 8.05 ± 0.82 b |
| FDS2 | 74.20 ± 0.85 b | 3.57 ± 0.20 b | 7.21 ± 0.32 b |
| FDS3 | 37.24 ± 0.73 a | 3.03 ± 0.06 a | 3.10 ± 0.64 a |
| Dough Code | Dough Rise (cm) | End of Bread Making (min) | Specific Volume (cm3/g−1) | Loss Mass (%) |
|---|---|---|---|---|
| Control | 3.44 ± 0.66 a | 655.67 ± 55.61 d | 2.78 ± 0.50 a | 2.2 ± 0.01 a |
| DS1 | 2.42 ± 0.42 abc | 738.67 ± 21.13 d | 2.26 ± 0.24 ab | 2.9 ± 0.01 a |
| DS2 | 1.89 ± 0.47 bc | 1020.67 ± 47.38 abc | 1.65 ± 0.38 bc | 2.8 ± 0.01 a |
| DS3 | 2.42 ± 0.27 abc | 914.67 ± 7.51 c | 2.18 ± 0.27 ab | 2.8 ± 0.01 a |
| DF1 | 2.54 ± 0.40 ab | 1076.33 ± 94.79 ab | 2.17 ± 0.33 ab | 3.1± 0.01 a |
| DF2 | 1.25 ± 0.19 c | 1151.67 ± 51.07 a | 1.01 ± 0.13 c | 2.1 ± 0.01 a |
| DF3 | 2.55 ± 0.50 ab | 927.33 ± 60.05 bc | 2.14 ± 0.42 ab | 2.7 ± 0.01 a |
| Dough Code | CO2 Released (L/kg−1 of Dough) | Speed Release of CO2 mL/(100 g/h)−1 |
|---|---|---|
| Control | 0.62 ± 0.10 ab | 5.55 ± 0.42 a |
| DS1 | 0.66 ± 0.20 a | 5.19 ± 1.51 ab |
| DS2 | 0.48 ± 0.08 ab | 2.77 ± 0.51 bc |
| DS3 | 0.52 ± 0.26 ab | 3.32 ± 1.67 abc |
| DF1 | 0.44 ± 0.15 ab | 2.37 ± 0.81 c |
| DF2 | 0.19 ± 0.01 b | 1.01 ± 0.07 c |
| DF3 | 0.59 ± 0.14 ab | 3.70 ± 0.89 abc |
| Dough Code | Ethanol Released (L/kg−1 of Dough) | Speed Release of Ethanol mL/(100 g/h)−1 |
|---|---|---|
| Control | 5.58 ± 0.89 bc | 49.76 ± 3.77 a |
| DS1 | 3.92 ± 0.33 ab | 30.88 ± 1.82 bcd |
| DS2 | 5.90 ± 0.78 a | 33.86 ± 5.89 b |
| DS3 | 5.27 ± 0.92 bc | 33.60 ± 5.93 bc |
| DF1 | 3.32 ± 1.90 a | 18.05 ± 10.03 cd |
| DF2 | 3.27 ± 0.62 a | 16.54 ± 3.30 d |
| DF3 | 3.75 ± 0.76 ab | 23.59 ± 4.60 bcd |
| Dough Code | pH | TTA (mL NaOH 0.1 N (10 g)−1) | ||||
|---|---|---|---|---|---|---|
| Initial (pH_i) | Final (pH_f) | Decrease | Initial (ATT_i) | Final (ATT_f) | Increase | |
| Control | 5.58 ± 0.03 a | 4.06 ± 0.59 a | 1.52 ± 0.62 a | 2.19 ± 0.21 d | 4.05 ± 0.11 e | 1.85 ± 0.28 d |
| DS1 | 4.37 ± 0.04 b | 3.57 ± 0.01 a | 0.80 ± 0.05 ab | 3.37 ± 0.12 c | 8.27 ± 0.03 c | 4.90 ± 0.14 c |
| DS2 | 4.20 ± 0.05 b | 3.51 ± 0.09 a | 0.70 ± 0.10 b | 5.52 ± 0.41 b | 15.02 ± 0.91 a | 9.50 ± 0.52 a |
| DS3 | 4.31 ± 0.06 b | 3.44 ± 0.11 a | 0.88 ± 0.16 ab | 3.68 ± 0.11 c | 9.96 ± 0.26 b | 6.28 ± 0.20 b |
| DF1 | 3.94 ± 0.16 c | 3.76 ± 0.43 a | 0.18 ± 0.27 b | 5.23 ± 0.41 b | 6.58 ± 0.26 d | 1.35 ± 0.58 de |
| DF2 | 3.86 ± 0.02 c | 3.72 ± 0.07 a | 0.14 ± 0.07 b | 8.89 ± 0.50 a | 9.57 ± 0.42 b | 0.68 ± 0.29 e |
| DF3 | 3.94 ± 0.01 c | 3.70 ± 0.10 a | 0.23 ± 0.10 b | 5.10 ± 0.39 b | 6.56 ± 0.12 d | 1.45 ± 0.34 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffak, A.; Chafai, Y.; Hamouda, A.; Ouazzani Touhami, A.; Mounir, M. Assessment of the Fermentative Performance of Traditional Fresh Moroccan Sourdoughs and Their Freeze-Dried Forms Using Online Monitoring Device: Panigraph. Appl. Sci. 2023, 13, 12453. https://doi.org/10.3390/app132212453
Raffak A, Chafai Y, Hamouda A, Ouazzani Touhami A, Mounir M. Assessment of the Fermentative Performance of Traditional Fresh Moroccan Sourdoughs and Their Freeze-Dried Forms Using Online Monitoring Device: Panigraph. Applied Sciences. 2023; 13(22):12453. https://doi.org/10.3390/app132212453
Chicago/Turabian StyleRaffak, Anas, Youssef Chafai, Allal Hamouda, Amina Ouazzani Touhami, and Majid Mounir. 2023. "Assessment of the Fermentative Performance of Traditional Fresh Moroccan Sourdoughs and Their Freeze-Dried Forms Using Online Monitoring Device: Panigraph" Applied Sciences 13, no. 22: 12453. https://doi.org/10.3390/app132212453





