Molecular Biomarkers for Predicting Cancer Patient Radiosensitivity and Radiotoxicity in Clinical Practice
Abstract
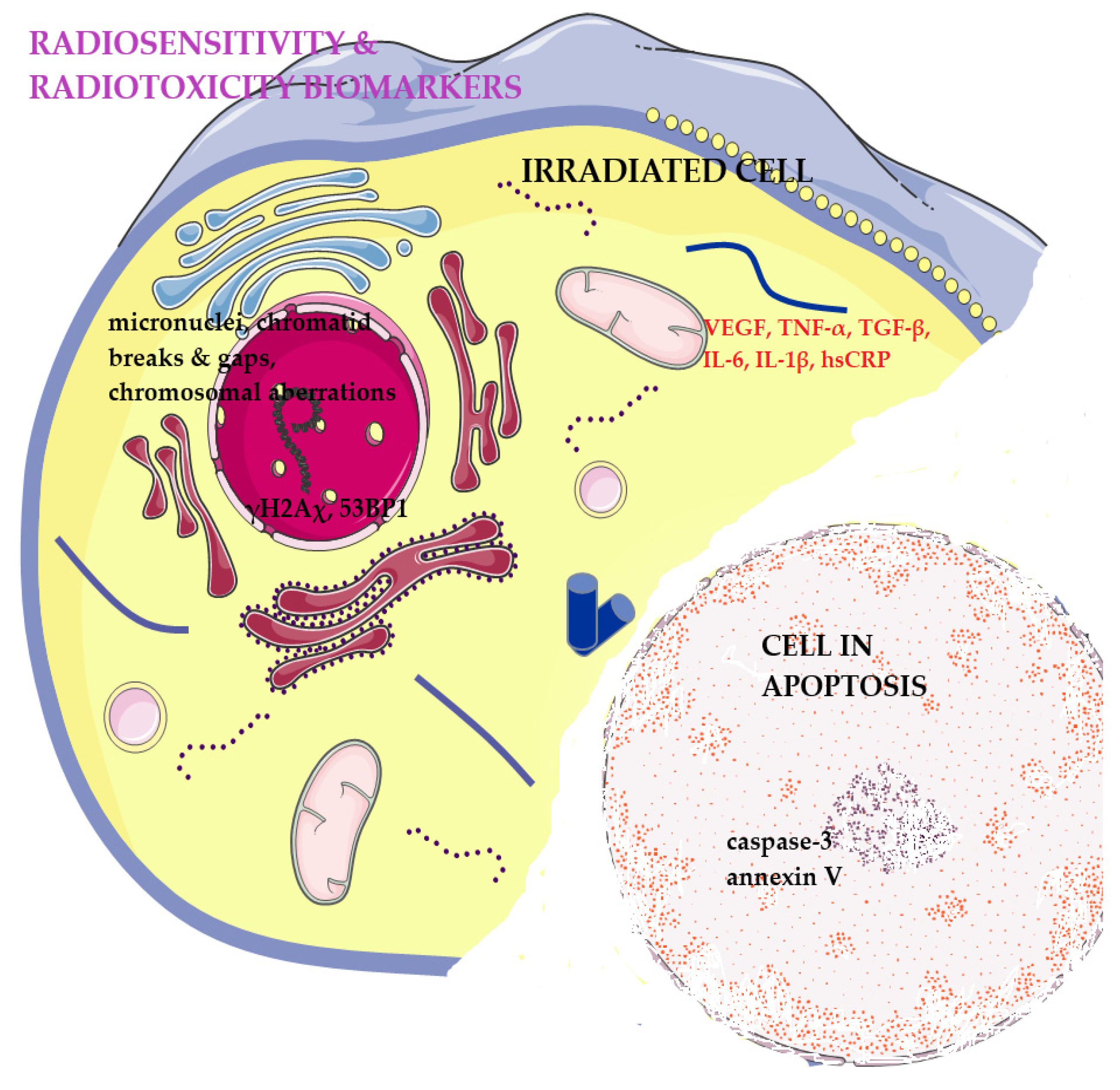
:1. Introduction
2. RT-Induced Toxicities and Their Clinical Management
3. Factors Regulating Tolerability of RT in Cancer Patients
4. The Importance of Biomarkers in Monitoring RT Toxicity
4.1. Cell-Intrinsic Radiosensitivity Biomarkers
4.1.1. Cytogenetic Markers
| Assay (Molecular and Cytogenetic Markers | Sample/Patient Profile | Outcome | Study |
|---|---|---|---|
| γH2AX IF assay | Peripheral lymphocytes of 31 patients with resected head-and-neck cancer with different grades of oral mucositis undergoing adjuvant RT or RCT. | Patients with a proportion of unrepaired DSBs after 24 h higher than the mean value had an increased incidence of severe oral mucositis. | [57] |
| Peripheral lymphocytes of 80 post-chemotherapy OR and NOR BC patients and 38 healthy female donors. | Statistical difference between the healthy and NOR groups, the healthy and OR groups, and the NORs and ORs, using PRD (percentage residual damage). | [58] | |
| PBMCs isolated from 25 prostate cancer patients treated with RT. (Expanded Prostate Cancer Index Composite [EPIC].) | No correlation between the number of γH2AX foci and radiotoxicity at the level of PBMCs. | [44] | |
| Cancer patient lymphocyte cells were isolated from individuals who did and did not have a severe reaction to RT. | No detectable differences were found between the control group and the RS group by following γH2AX foci kinetics after IR. | [59] | |
| γH2AX FCM assay | PBLs isolated from 12 patients who had experienced severe atypical NTT (Normal Tissue Toxicity) as a consequence of earlier RT were identified. Patients (10) who had experienced little or no NTT acted as one control group, and 7 healthy, non-cancer individuals comprised a second control group. | Reliable differentiation between patients clinically classified as NOR or OR patients. | [60] |
| γH2AX/53BP1 IF assay | Blood lymphocytes of BC patients who had undergone surgical excision of the primary tumor and postoperative RT to the whole breast. Two groups of patients were identified indicating severely marked radiation-induced change (cases) or very little/no change (controls) in the breast. | Higher levels of residual DSBs and deletion-type aberrations in ex vivo irradiated blood lymphocytes from clinically radiosensitive BC patients provide a suggestion for DSB repair in the development of radiation-induced late normal tissue damage. | [42] |
| PBMCs were isolated from a group of unselected BC patients who received RT treatment and a group of apparently healthy donors. The early skin reaction to RT developing in the skin within the radiation field of the breast was controlled at the end of RT and used as an indicator of clinical radiosensitivity | A minor group of retrospectively identified BC patients with an adverse skin reaction to RT showed differences by the γH2AX assay with respect to healthy individuals. The 53BP1 assay was less sensitive than that for histone γH2AX in the case of endogenous (0 Gy) and induced (0.5 Gy, 30 min) foci. | [61] | |
| Peripheral blood samples from 26 patients who were treated with RT were collected from the BC patients two days before the RT. The acute adverse reactions of all patients are classified according to the Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) side effects. | Significantly higher amount of endogenous γH2AX and 53BP1 DNA repair foci in fresh PBL withdrawn prior to RT from the RS patients in comparison with the NOR patients. | [62] | |
| γH2AX/53BP1 co-localization IF assay | PBL from 16 patients who developed severe late radiation toxicity following radiotherapy. | Support for the hypothesis that the RS phenotype is associated with compromised DNA repair. | [63] |
| G2 chromosomal radiosensitivity assay | Peripheral venous blood was collected from 14 healthy control subjects, 15 patients with benign prostatic hyperplasia (BPH), and 17 patients with prostate cancer. | Enhanced G2 chromosomal radiosensitivity in prostate cancer and BPH patients compared with normal controls. | [40] |
| Blood samples were then obtained from 27 breast cancer patients eligible for the study. | A significant proportion of breast cancer patients exhibit elevated G2 chromosomal radiosensitivity in contrast to random controls. | [41] | |
| Blood lymphocytes of BC patients who had undergone surgical excision of the primary tumor and postoperative RT to the whole breast. | Significantly higher levels of chromosomal aberrations in blood lymphocyte metaphases among women presenting with marked late radiotherapy changes. | [42] | |
| Blood samples were obtained from 25 prostate cancer patients with severe side effects (S) and 25 patients without severe side effects (0) after radiotherapy as well as from 23 healthy male age-matched donors. | The obtained success rate is not sufficient to validate the G2 assay as a good tool for the identification of prostate cancer patients with a high risk for the development of severe clinical side effects. | [43] | |
| Blood samples from 25 patients with localized T1–3N0M0 prostate cancer who were treated with three-dimensional conformal radiotherapy. | No predictive value was found after irradiation with 0.5 Gy in the G2 assay. | [44] | |
| G2 micronucleus (MN) assay | Blood samples from 18 BRCA2 mutation carriers and 17 subjects from both BRCA1 (n = 9) and BRCA2 (n = 8) families not showing the familial mutation (non-carriers). | Higher radiosensitivity in healthy BRCA2 mutation carriers compared with healthy volunteers by means of the G2 MN assay. No increased radiosensitivity was observed in non-carrier relatives of BRCA1 and BRCA2 families. | [50] |
| Fluorescence in situ hybridization (FISH) assay | Lymphocytes obtained from 16 patients that were retrospectively examined 1 to 108 months after radiotherapy. | Detectable differences in interindividual radiation sensitivity. | [54] |
| Blood samples from 47 BC patients who received exclusively radiotherapy after surgical lumpectomy. | Significant overall correlation was found between the frequencies of t(Ba) (painted chromosomes bearing one centromere with a colοr junction) in vitro and the time-dependent occurrence of side effects of the skin. | [55] | |
| Patients with a localized T1-3N0M0 prostate carcinoma, with and without severe side effects, who were treated with three-dimensional conformal radiotherapy. | No statistically significant difference in the overall aberration yield between healthy donors and patients with and without severe side effects. | [56] |
4.1.2. DNA Damage Response
4.1.3. Other Biomarkers of the DDR Pathway
4.2. Apoptosis Biomarkers
4.3. Secreted Soluble Protein Biomarkers
4.4. Cell-Free DNA-Based Biomarkers
5. Challenges and Opportunities for Cancer Radiosensitivity Biomarkers in the Era of Artificial Intelligence
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sagkrioti, E.; Biz, G.M.; Takan, I.; Asfa, S.; Nikitaki, Z.; Zanni, V.; Kars, R.H.; Hellweg, C.E.; Azzam, E.I.; Logotheti, S.; et al. Radiation Type- and Dose-Specific Transcriptional Responses across Healthy and Diseased Mammalian Tissues. Antioxidants 2022, 11, 2286. [Google Scholar] [CrossRef]
- Mascia, A.E.; Daugherty, E.C.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; Backus, L.R.; McDonald, J.M.; McCann, C.; et al. Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases: The FAST-01 Nonrandomized Trial. JAMA Oncol. 2023, 9, 62–69. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, R.; Schüler, E.; Taylor, P.A.; Mascia, A.E.; Diffenderfer, E.S.; Zhao, T.; Ayan, A.S.; Sharma, M.; Yu, S.J.; et al. Framework for Quality Assurance of Ultrahigh Dose Rate Clinical Trials Investigating FLASH Effects and Current Technology Gaps. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 1202–1217. [Google Scholar] [CrossRef]
- Galluzzi, L.; Aryankalayil, M.J.; Coleman, C.N.; Formenti, S.C. Emerging evidence for adapting radiotherapy to immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 543–557. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Voronova, N.V.; Chistiakov, P.A. Genetic variations in DNA repair genes, radiosensitivity to cancer and susceptibility to acute tissue reactions in radiotherapy-treated cancer patients. Acta Oncol. 2008, 47, 809–824. [Google Scholar] [CrossRef]
- Pavlopoulou, A.; Bagos, P.G.; Koutsandrea, V.; Georgakilas, A.G. Molecular determinants of radiosensitivity in normal and tumor tissue: A bioinformatic approach. Cancer Lett. 2017, 403, 37–47. [Google Scholar] [CrossRef]
- Pavlopoulou, A.; Oktay, Y.; Vougas, K.; Louka, M.; Vorgias, C.E.; Georgakilas, A.G. Determinants of resistance to chemotherapy and ionizing radiation in breast cancer stem cells. Cancer Lett. 2016, 380, 485–493. [Google Scholar] [CrossRef]
- Sachs, R.K.; Brenner, D.J. Solid tumor risks after high doses of ionizing radiation. Proc. Natl. Acad. Sci. USA 2005, 102, 13040–13045. [Google Scholar] [CrossRef]
- Barnett, G.C.; West, C.M.L.; Coles, C.E.; Pharoah, P.D.P.; Talbot, C.J.; Elliott, R.M.; Tanteles, G.A.; Symonds, R.P.; Wilkinson, J.S.; Dunning, A.M.; et al. Standardized Total Average Toxicity Score: A Scale- and Grade-Independent Measure of Late Radiotherapy Toxicity to Facilitate Pooling of Data From Different Studies. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1065–1074. [Google Scholar] [CrossRef]
- Ben-Hamo, R.; Jacob Berger, A.; Gavert, N.; Miller, M.; Pines, G.; Oren, R.; Pikarsky, E.; Benes, C.H.; Neuman, T.; Zwang, Y.; et al. Predicting and affecting response to cancer therapy based on pathway-level biomarkers. Nat. Commun. 2020, 11, 3296. [Google Scholar] [CrossRef]
- Price, J.M.; Prabhakaran, A.; West, C.M.L. Predicting tumour radiosensitivity to deliver precision radiotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 83–98. [Google Scholar] [CrossRef]
- Byrne, N.M.; Tambe, P.; Coulter, J.A. Radiation Response in the Tumour Microenvironment: Predictive Biomarkers and Future Perspectives. J. Pers. Med. 2021, 11, 53. [Google Scholar] [CrossRef]
- Bouffler, S.D. Evidence for variation in human radiosensitivity and its potential impact on radiological protection. Ann. ICRP 2016, 45, 280–289. [Google Scholar] [CrossRef]
- Cox, J.D.; Stetz, J.A.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Trotti, A.; Colevas, A.D.; Setser, A.; Rusch, V.; Jaques, D.; Budach, V.; Langer, C.; Murphy, B.; Cumberlin, R.; Coleman, C.N.; et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 2003, 13, 176–181. [Google Scholar] [CrossRef]
- Hussein, F.A.; Manan, H.A.; Mustapha, A.; Sidek, K.; Yahya, N. Ultrasonographic Evaluation of Skin Toxicity Following Radiotherapy of Breast Cancer: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 13439. [Google Scholar] [CrossRef]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Bates, J.E.; Shrestha, S.; Liu, Q.; Smith, S.A.; Mulrooney, D.A.; Leisenring, W.; Gibson, T.; Robison, L.L.; Chow, E.J.; Oeffinger, K.C.; et al. Cardiac Substructure Radiation Dose and Risk of Late Cardiac Disease in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2023, 41, 3826–3838. [Google Scholar] [CrossRef]
- Little, M.P.; Azizova, T.V.; Richardson, D.B.; Tapio, S.; Bernier, M.O.; Kreuzer, M.; Cucinotta, F.A.; Bazyka, D.; Chumak, V.; Ivanov, V.K.; et al. Ionising radiation and cardiovascular disease: Systematic review and meta-analysis. BMJ (Clin. Res. Ed.) 2023, 380, e072924. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; Villaescusa, J.I.; Soriano, J.M.; Estrela, J.M.; Montoro, A. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines 2020, 8, 461. [Google Scholar] [CrossRef]
- Kouvaris, J.R.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: The First Selective-Target and Broad-Spectrum Radioprotector. Oncol. 2007, 12, 738–747. [Google Scholar] [CrossRef]
- Marcu, L.G. Photons—Radiobiological issues related to the risk of second malignancies. Phys. Medica 2017, 42, 213–220. [Google Scholar] [CrossRef]
- Barnett, G.C.; Coles, C.E.; Elliott, R.M.; Baynes, C.; Luccarini, C.; Conroy, D.; Wilkinson, J.S.; Tyrer, J.; Misra, V.; Platte, R.; et al. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: A prospective analysis study. Lancet Oncol. 2012, 13, 65–77. [Google Scholar] [CrossRef]
- Vaisnav, M.; Xing, C.; Ku, H.C.; Hwang, D.; Stojadinovic, S.; Pertsemlidis, A.; Abrams, J.M. Genome-wide association analysis of radiation resistance in Drosophila melanogaster. PLoS ONE 2014, 9, e104858. [Google Scholar] [CrossRef]
- Preston, R.J. Children as a sensitive subpopulation for the risk assessment process. Toxicol. Appl. Pharmacol. 2004, 199, 132–141. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Sir Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Narendran, N.; Luzhna, L.; Kovalchuk, O. Sex Difference of Radiation Response in Occupational and Accidental Exposure. Front. Genet. 2019, 10, 260. [Google Scholar] [CrossRef]
- Grant, E.J.; Brenner, A.; Sugiyama, H.; Sakata, R.; Sadakane, A.; Utada, M.; Cahoon, E.K.; Milder, C.M.; Soda, M.; Cullings, H.M.; et al. Solid Cancer Incidence among the Life Span Study of Atomic Bomb Survivors: 1958–2009. Radiat. Res. 2017, 187, 513–537. [Google Scholar] [CrossRef]
- Belli, M.; Ottolenghi, A.; Weiss, W. The European strategy on low dose risk research and the role of radiation quality according to the recommendations of the “ad hoc” High Level and Expert Group (HLEG). Radiat. Environ. Biophys. 2010, 49, 463–468. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Overgaard, J. Patient-to-Patient Variability in the Expression of Radiation-Induced Normal Tissue Injury. Semin. Radiat. Oncol. 1994, 4, 68–80. [Google Scholar] [CrossRef]
- Burger, H.; Loos, W.J.; Eechoute, K.; Verweij, J.; Mathijssen, R.H.; Wiemer, E.A. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist. Updates 2011, 14, 22–34. [Google Scholar] [CrossRef]
- Menetski, J.P.; Hoffmann, S.C.; Cush, S.S.; Kamphaus, T.N.; Austin, C.P.; Herrling, P.L.; Wagner, J.A. The Foundation for the National Institutes of Health Biomarkers Consortium: Past Accomplishments and New Strategic Direction. Clin. Pharmacol. Ther. 2019, 105, 829–843. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Scott, D.; Spreadborough, A.R.; Jones, L.A.; Roberts, S.A.; Moore, C.J. Chromosomal radiosensitivity in G2-phase lymphocytes as an indicator of cancer predisposition. Radiat. Res. 1996, 145, 3–16. [Google Scholar] [CrossRef]
- Terzoudi, G.I.; Jung, T.; Hain, J.; Vrouvas, J.; Margaritis, K.; Donta-Bakoyianni, C.; Makropoulos, V.; Angelakis, P.; Pantelias, G.E. Increased G2 chromosomal radiosensitivity in cancer patients: The role of cdk1/cyclin-B activity level in the mechanisms involved. Int. J. Radiat. Biol. 2000, 76, 607–615. [Google Scholar] [CrossRef]
- Vral, A.; Thierens, H.; Baeyens, A.; De Ridder, L. Induction and disappearance of G2 chromatid breaks in lymphocytes after low doses of low-LET gamma-rays and high-LET fast neutrons. Int. J. Radiat. Biol. 2002, 78, 249–257. [Google Scholar] [CrossRef]
- Howe, O.L.; Daly, P.A.; Seymour, C.; Ormiston, W.; Nolan, C.; Mothersill, C. Elevated G2 chromosomal radiosensitivity in Irish breast cancer patients: A comparison with other studies. Int. J. Radiat. Biol. 2005, 81, 373–378. [Google Scholar] [CrossRef]
- Howe, O.; O’Malley, K.; Lavin, M.; Gardner, R.A.; Seymour, C.; Lyng, F.; Mulvin, D.; Quinlan, D.M.; Mothersill, C. Cell death mechanisms associated with G2 radiosensitivity in patients with prostate cancer and benign prostatic hyperplasia. Radiat. Res. 2005, 164, 627–634. [Google Scholar] [CrossRef]
- Chua, M.L.; Somaiah, N.; A’Hern, R.; Davies, S.; Gothard, L.; Yarnold, J.; Rothkamm, K. Residual DNA and chromosomal damage in ex vivo irradiated blood lymphocytes correlated with late normal tissue response to breast radiotherapy. Radiother. Oncol. 2011, 99, 362–366. [Google Scholar] [CrossRef]
- Brzozowska, K.; Pinkawa, M.; Eble, M.J.; Müller, W.U.; Wojcik, A.; Kriehuber, R.; Schmitz, S. In vivo versus in vitro individual radiosensitivity analysed in healthy donors and in prostate cancer patients with and without severe side effects after radiotherapy. Int. J. Radiat. Biol. 2012, 88, 405–413. [Google Scholar] [CrossRef]
- Pinkawa, M.; Brzozowska, K.; Kriehuber, R.; Eble, M.J.; Schmitz, S. Prediction of radiation-induced toxicity by in vitro radiosensitivity of lymphocytes in prostate cancer patients. Future Oncol. 2016, 12, 617–624. [Google Scholar] [CrossRef]
- Smart, V.; Curwen, G.B.; Whitehouse, C.A.; Edwards, A.; Tawn, E.J. Chromosomal radiosensitivity: A study of the chromosomal G2 assay in human blood lymphocytes indicating significant inter-individual variability. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2003, 528, 105–110. [Google Scholar] [CrossRef]
- Vral, A.; Thierens, H.; Baeyens, A.; De Ridder, L. The Micronucleus and G2-Phase Assays for Human Blood Lymphocytes as Biomarkers of Individual Sensitivity to Ionizing Radiation: Limitations Imposed by Intraindividual Variability. Radiat. Res. 2002, 157, 472–477. [Google Scholar] [CrossRef]
- Lisowska, H.; Lankoff, A.; Wieczorek, A.; Florek, A.; Kuszewski, T.; Góźdź, S.; Wojcik, A. Enhanced chromosomal radiosensitivity in peripheral blood lymphocytes of larynx cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1245–1252. [Google Scholar] [CrossRef]
- Pantelias, G.E.; Terzoudi, G.I. A standardized G2-assay for the prediction of individual radiosensitivity. Radiother. Oncol. 2011, 101, 28–34. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef]
- Baert, A.; Depuydt, J.; Van Maerken, T.; Poppe, B.; Malfait, F.; Van Damme, T.; De Nobele, S.; Perletti, G.; De Leeneer, K.; Claes, K.B.; et al. Analysis of chromosomal radiosensitivity of healthy BRCA2 mutation carriers and non-carriers in BRCA families with the G2 micronucleus assay. Oncol. Rep. 2017, 37, 1379–1386. [Google Scholar] [CrossRef]
- Kirsch-Volders, M.; Plas, G.; Elhajouji, A.; Lukamowicz, M.; Gonzalez, L.; Vande Loock, K.; Decordier, I. The in vitro MN assay in 2011: Origin and fate, biological significance, protocols, high throughput methodologies and toxicological relevance. Arch. Toxicol. 2011, 85, 873–899. [Google Scholar] [CrossRef]
- Krupina, K.; Goginashvili, A.; Cleveland, D.W. Causes and consequences of micronuclei. Curr. Opin. Cell Biol. 2021, 70, 91–99. [Google Scholar] [CrossRef]
- Huber, D.; Voith von Voithenberg, L.; Kaigala, G.V. Fluorescence in situ hybridization (FISH): History, limitations and what to expect from micro-scale FISH? Micro Nano Eng. 2018, 1, 15–24. [Google Scholar] [CrossRef]
- Dunst, J.; Gebhart, E.; Neubauer, S. Can an extremely elevated radiosensitivity in patients be recognized by the in-vitro testing of lymphocytes? Strahlenther. Onkol. Organ Dtsch. Rontgenges 1995, 171, 581–586. [Google Scholar]
- Huber, R.; Braselmann, H.; Geinitz, H.; Jaehnert, I.; Baumgartner, A.; Thamm, R.; Figel, M.; Molls, M.; Zitzelsberger, H. Chromosomal radiosensitivity and acute radiation side effects after radiotherapy in tumour patients—A follow-up study. Radiat. Oncol. 2011, 6, 32. [Google Scholar] [CrossRef]
- Schmitz, S.; Brzozowska, K.; Pinkawa, M.; Eble, M.; Kriehuber, R. Chromosomal radiosensitivity analyzed by FISH in lymphocytes of prostate cancer patients and healthy donors. Radiat. Res. 2013, 180, 465–473. [Google Scholar] [CrossRef]
- Fleckenstein, J.; Kühne, M.; Seegmüller, K.; Derschang, S.; Melchior, P.; Gräber, S.; Fricke, A.; Rübe, C.E.; Rübe, C. The impact of individual in vivo repair of DNA double-strand breaks on oral mucositis in adjuvant radiotherapy of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1465–1472. [Google Scholar] [CrossRef]
- Mumbrekar, K.D.; Fernandes, D.J.; Goutham, H.V.; Sharan, K.; Vadhiraja, B.M.; Satyamoorthy, K.; Bola Sadashiva, S.R. Influence of double-strand break repair on radiation therapy-induced acute skin reactions in breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 671–676. [Google Scholar] [CrossRef]
- Vasireddy, R.S.; Sprung, C.N.; Cempaka, N.L.; Chao, M.; McKay, M.J. H2AX phosphorylation screen of cells from radiosensitive cancer patients reveals a novel DNA double-strand break repair cellular phenotype. Br. J. Cancer 2010, 102, 1511–1518. [Google Scholar] [CrossRef]
- Bourton, E.C.; Plowman, P.N.; Smith, D.; Arlett, C.F.; Parris, C.N. Prolonged expression of the γ-H2AX DNA repair biomarker correlates with excess acute and chronic toxicity from radiotherapy treatment. Int. J. Cancer 2011, 129, 2928–2934. [Google Scholar] [CrossRef]
- Djuzenova, C.S.; Elsner, I.; Katzer, A.; Worschech, E.; Distel, L.V.; Flentje, M.; Polat, B. Radiosensitivity in breast cancer assessed by the histone γ-H2AX and 53BP1 foci. Radiat. Oncol. 2013, 8, 98. [Google Scholar] [CrossRef]
- Durdik, M.; Markova, E.; Kosik, P.; Vigasova, K.; Gulati, S.; Jakl, L.; Vrobelova, K.; Fekete, M.; Zavacka, I.; Pobijakova, M.; et al. Assessment of Individual Radiosensitivity in Breast Cancer Patients Using a Combination of Biomolecular Markers. Biomedicines 2023, 11, 1122. [Google Scholar] [CrossRef]
- Lobachevsky, P.; Leong, T.; Daly, P.; Smith, J.; Best, N.; Tomaszewski, J.; Thompson, E.R.; Li, N.; Campbell, I.G.; Martin, R.F.; et al. Compromized DNA repair as a basis for identification of cancer radiotherapy patients with extreme radiosensitivity. Cancer Lett. 2016, 383, 212–219. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Paull, T.T.; Rogakou, E.P.; Yamazaki, V.; Kirchgessner, C.U.; Gellert, M.; Bonner, W.M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. CB 2000, 10, 886–895. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. GammaH2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef]
- Halazonetis, T.D.; Gorgoulis, V.G.; Bartek, J. An oncogene-induced DNA damage model for cancer development. Science 2008, 319, 1352–1355. [Google Scholar] [CrossRef]
- Ivashkevich, A.; Redon, C.E.; Nakamura, A.J.; Martin, R.F.; Martin, O.A. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012, 327, 123–133. [Google Scholar] [CrossRef]
- Marková, E.; Schultz, N.; Belyaev, I.Y. Kinetics and dose-response of residual 53BP1/gamma-H2AX foci: Co-localization, relationship with DSB repair and clonogenic survival. Int. J. Radiat. Biol. 2007, 83, 319–329. [Google Scholar] [CrossRef]
- Ward, I.M.; Chen, J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 2001, 276, 47759–47762. [Google Scholar] [CrossRef]
- Kakarougkas, A.; Ismail, A.; Klement, K.; Goodarzi, A.A.; Conrad, S.; Freire, R.; Shibata, A.; Lobrich, M.; Jeggo, P.A. Opposing roles for 53BP1 during homologous recombination. Nucleic Acids Res. 2013, 41, 9719–9731. [Google Scholar] [CrossRef]
- Bobkova, E.; Depes, D.; Lee, J.-H.; Jezkova, L.; Falkova, I.; Pagacova, E.; Kopecna, O.; Zadneprianetc, M.; Bacikova, A.; Kulikova, E.; et al. Recruitment of 53BP1 Proteins for DNA Repair and Persistence of Repair Clusters Differ for Cell Types as Detected by Single Molecule Localization Microscopy. Int. J. Mol. Sci. 2018, 19, 3713. [Google Scholar] [CrossRef]
- Penninckx, S.; Pariset, E.; Cekanaviciute, E.; Costes, S.V. Quantification of radiation-induced DNA double strand break repair foci to evaluate and predict biological responses to ionizing radiation. NAR Cancer 2021, 3, zcab046. [Google Scholar] [CrossRef]
- Depes, D.; Lee, J.-H.; Bobkova, E.; Jezkova, L.; Falkova, I.; Bestvater, F.; Pagacova, E.; Kopecna, O.; Zadneprianetc, M.; Bacikova, A.; et al. Single-molecule localization microscopy as a promising tool for γH2AX/53BP1 foci exploration. Eur. Phys. J. D 2018, 72, 158. [Google Scholar] [CrossRef]
- Johansson, P.; Fasth, A.; Ek, T.; Hammarsten, O. Validation of a flow cytometry-based detection of γ-H2AX, to measure DNA damage for clinical applications. Cytom. Part B Clin. Cytom. 2017, 92, 534–540. [Google Scholar] [CrossRef]
- Sun, J.; Kroeger, J.L.; Markowitz, J. Introduction to Multiparametric Flow Cytometry and Analysis of High-Dimensional Data. Methods Mol. Biol. 2021, 2194, 239–253. [Google Scholar] [CrossRef]
- Gadalla, R.; Noamani, B.; MacLeod, B.L.; Dickson, R.J.; Guo, M.; Xu, W.; Lukhele, S.; Elsaesser, H.J.; Razak, A.R.A.; Hirano, N.; et al. Validation of CyTOF Against Flow Cytometry for Immunological Studies and Monitoring of Human Cancer Clinical Trials. Front. Oncol. 2019, 9, 415. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Maire, C.L.; Reimer, R.; Dührsen, L.; Kolbe, K.; Holz, M.; Schneider, E.; Rissiek, A.; Babayan, A.; Hille, C.; et al. Imaging flow cytometry facilitates multiparametric characterization of extracellular vesicles in malignant brain tumours. J. Extracell. Vesicles 2019, 8, 1588555. [Google Scholar] [CrossRef]
- Stracker, T.H.; Petrini, J.H. The MRE11 complex: Starting from the ends. Nat. Rev. Mol. Cell Biol. 2011, 12, 90–103. [Google Scholar] [CrossRef]
- Lavin, M.F.; Kozlov, S. ATM activation and DNA damage response. Cell Cycle 2007, 6, 931–942. [Google Scholar] [CrossRef]
- Söderlund, K.; Stål, O.; Skoog, L.; Rutqvist, L.E.; Nordenskjöld, B.; Askmalm, M.S. Intact Mre11/Rad50/Nbs1 complex predicts good response to radiotherapy in early breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 50–58. [Google Scholar] [CrossRef]
- Yan, X.; Wu, T.; Tang, M.; Chen, D.; Huang, M.; Zhou, S.; Zhang, H.; Yang, X.; Li, G. Methylation of the ataxia telangiectasia mutated gene (ATM) promoter as a radiotherapy outcome biomarker in patients with hepatocellular carcinoma. Medicine 2020, 99, e18823. [Google Scholar] [CrossRef]
- Yuan, S.-S.F.; Hou, M.-F.; Hsieh, Y.-C.; Huang, C.-Y.; Lee, Y.-C.; Chen, Y.-J.; Lo, S. Role of MRE11 in Cell Proliferation, Tumor Invasion, and DNA Repair in Breast Cancer. JNCI J. Natl. Cancer Inst. 2012, 104, 1485–1502. [Google Scholar] [CrossRef]
- Ho, V.; Chung, L.; Singh, A.; Lea, V.; Abubakar, A.; Lim, S.H.; Ng, W.; Lee, M.; de Souza, P.; Shin, J.-S.; et al. Overexpression of the MRE11-RAD50-NBS1 (MRN) complex in rectal cancer correlates with poor response to neoadjuvant radiotherapy and prognosis. BMC Cancer 2018, 18, 869. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Mei, J.F.; Li, S.S.; Xu, H.X.; Xiong, H.P.; Wang, X.H.; He, X. Targeting RAD50 increases sensitivity to radiotherapy in colorectal cancer cells. Neoplasma 2018, 65, 75–80. [Google Scholar] [CrossRef]
- Ebi, H.; Matsuo, K.; Sugito, N.; Suzuki, M.; Osada, H.; Tajima, K.; Ueda, R.; Takahashi, T. Novel NBS1 heterozygous germ line mutation causing MRE11-binding domain loss predisposes to common types of cancer. Cancer Res. 2007, 67, 11158–11165. [Google Scholar] [CrossRef]
- Altan, B.; Yokobori, T.; Ide, M.; Bai, T.; Yanoma, T.; Kimura, A.; Kogure, N.; Suzuki, M.; Bao, P.; Mochiki, E.; et al. High Expression of MRE11-RAD50-NBS1 Is Associated with Poor Prognosis and Chemoresistance in Gastric Cancer. Anticancer Res. 2016, 36, 5237–5247. [Google Scholar] [CrossRef]
- Ihara, K.; Yamaguchi, S.; Ueno, N.; Tani, Y.; Shida, Y.; Ogata, H.; Domeki, Y.; Okamoto, K.; Nakajima, M.; Sasaki, K.; et al. Expression of DNA double-strand break repair proteins predicts the response and prognosis of colorectal cancer patients undergoing oxaliplatin-based chemotherapy. Oncol. Rep. 2016, 35, 1349–1355. [Google Scholar] [CrossRef]
- Takemura, H.; Rao, V.A.; Sordet, O.; Furuta, T.; Miao, Z.H.; Meng, L.; Zhang, H.; Pommier, Y. Defective Mre11-dependent activation of Chk2 by ataxia telangiectasia mutated in colorectal carcinoma cells in response to replication-dependent DNA double strand breaks. J. Biol. Chem. 2006, 281, 30814–30823. [Google Scholar] [CrossRef]
- Sia, J.; Szmyd, R.; Hau, E.; Gee, H.E. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front. Cell Dev. Biol. 2020, 8, 41. [Google Scholar] [CrossRef]
- Beretta, G.L.; Zaffaroni, N. Radiotherapy-induced ferroptosis for cancer treatment. Front. Mol. Biosci. 2023, 10, 1216733. [Google Scholar] [CrossRef]
- Kim, J.H.; Brown, S.L.; Gordon, M.N. Radiation-induced senescence: Therapeutic opportunities. Radiat. Oncol. 2023, 18, 10. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef]
- Wolf, B.B.; Schuler, M.; Echeverri, F.; Green, D.R. Caspase-3 Is the Primary Activator of Apoptotic DNA Fragmentation via DNA Fragmentation Factor-45/Inhibitor of Caspase-Activated DNase Inactivation. J. Biol. Chem. 1999, 274, 30651–30656. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
- Santos, N.; Silva, R.F.; Pinto, M.; Silva, E.B.D.; Tasat, D.R.; Amaral, A. Active caspase-3 expression levels as bioindicator of individual radiosensitivity. An. Acad. Bras. Cienc. 2017, 89, 649–659. [Google Scholar] [CrossRef]
- Erasmus, W.L.; Slabbert, J.P.; Crompton, N.E.A.; Meehan, K.A. The leukocyte apoptosis assay: A clinical predictor of radiosensitivity. Med. Technol. SA 2005, 19, 9–14. [Google Scholar]
- Schnarr, K.; Dayes, I.; Sathya, J.; Boreham, D. Individual radiosensitivity and its relevance to health physics. Dose-Response 2007, 5, 333–348. [Google Scholar] [CrossRef]
- Pinar, B.; Henríquez-Hernández, L.A.; Lara, P.C.; Bordon, E.; Rodriguez-Gallego, C.; Lloret, M.; Nuñez, M.I.; De Almodovar, M.R. Radiation induced apoptosis and initial DNA damage are inversely related in locally advanced breast cancer patients. Radiat. Oncol. 2010, 5, 85. [Google Scholar] [CrossRef]
- Yang, X.H.; Edgerton, S.; Thor, A.D. Reconstitution of caspase-3 sensitizes MCF-7 breast cancer cells to radiation therapy. Int. J. Oncol. 2005, 26, 1675–1680. [Google Scholar] [CrossRef]
- Hallan, E.; Blomhoff, H.K.; Smeland, E.B.; Lømo, J. Involvement of ICE (Caspase) family in gamma-radiation-induced apoptosis of normal B lymphocytes. Scand. J. Immunol. 1997, 46, 601–608. [Google Scholar] [CrossRef]
- Yu, Y.; Little, J.B. p53 Is Involved in But Not Required for Ionizing Radiation-induced Caspase-3 Activation and Apoptosis in Human Lymphoblast Cell Lines1. Cancer Res. 1998, 58, 4277–4281. [Google Scholar]
- Cao, M.; Cabrera, R.; Xu, Y.; Liu, C.; Nelson, D. Different radiosensitivity of CD4+CD25+ regulatory T cells and effector T cells to low dose gamma irradiation in vitro. Int. J. Radiat. Biol. 2011, 87, 71–80. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Belkacemi, Y.; Mann, C.; Hoffschir, F.; Kerbrat, S.; Surenaud, M.; Zadigue, P.; de La Taille, A.; Romeo, P.-H.; Le Gouvello, S. Human CCR6+ Th17 Lymphocytes Are Highly Sensitive to Radiation-Induced Senescence and Are a Potential Target for Prevention of Radiation-Induced Toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 314–325. [Google Scholar] [CrossRef]
- Kiani, A.; Mohebat, L.; Javanmard, S.; Kazemi, M.; Tavakoli, M.; Kheirollahi, M. Annexin V FITC conjugated as a radiation toxicity indicator in lymphocytes following radiation overexposure in radiotherapy programs. Adv. Biomed. Res. 2015, 4, 119. [Google Scholar] [CrossRef]
- Mochizuki, T.; Kuge, Y.; Zhao, S.; Tsukamoto, E.; Hosokawa, M.; Strauss, H.W.; Blankenberg, F.G.; Tait, J.F.; Tamaki, N. Detection of Apoptotic Tumor Response In Vivo After a Single Dose of Chemotherapy with 99mTc-Annexin V. J. Nucl. Med. January 2003, 44, 92–97. [Google Scholar]
- Stanojković, T.P.; Matić, I.Z.; Petrović, N.; Stanković, V.; Kopčalić, K.; Besu, I.; Đorđić Crnogorac, M.; Mališić, E.; Mirjačić-Martinović, K.; Vuletić, A.; et al. Evaluation of cytokine expression and circulating immune cell subsets as potential parameters of acute radiation toxicity in prostate cancer patients. Sci. Rep. 2020, 10, 19002. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.-R.; Kim, H.-J.; An, Y.S.; Yi, J.Y. TGF-β1 accelerates the DNA damage response in epithelial cells via Smad signaling. Biochem. Biophys. Res. Commun. 2016, 476, 420–425. [Google Scholar] [CrossRef]
- Anscher, M.S. Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. Oncologist 2010, 15, 350–359. [Google Scholar] [CrossRef]
- Shao, C.; Folkard, M.; Prise, K.M. Role of TGF-β1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene 2008, 27, 434–440. [Google Scholar] [CrossRef]
- Ahmadi, A.; Najafi, M.; Farhood, B.; Mortezaee, K. Transforming growth factor-β signaling: Tumorigenesis and targeting for cancer therapy. J. Cell. Physiol. 2019, 234, 12173–12187. [Google Scholar] [CrossRef]
- Biswas, S.; Guix, M.; Rinehart, C.; Dugger, T.C.; Chytil, A.; Moses, H.L.; Freeman, M.L.; Arteaga, C.L. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J. Clin. Investig. 2007, 117, 1305–1313. [Google Scholar] [CrossRef]
- Zhang, M.; Kleber, S.; Röhrich, M.; Timke, C.; Han, N.; Tuettenberg, J.; Martin-Villalba, A.; Debus, J.; Peschke, P.; Wirkner, U.; et al. Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011, 71, 7155–7167. [Google Scholar] [CrossRef]
- Bouquet, F.; Pal, A.; Pilones, K.A.; Demaria, S.; Hann, B.; Akhurst, R.J.; Babb, J.S.; Lonning, S.M.; DeWyngaert, J.K.; Formenti, S.C.; et al. TGFβ1 Inhibition Increases the Radiosensitivity of Breast Cancer Cells In Vitro and Promotes Tumor Control by Radiation In Vivo. Clin. Cancer Res. 2011, 17, 6754–6765. [Google Scholar] [CrossRef]
- Formenti, S.C.; Lee, P.; Adams, S.; Goldberg, J.D.; Li, X.; Xie, M.W.; Ratikan, J.A.; Felix, C.; Hwang, L.; Faull, K.F.; et al. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin. Cancer Res. 2018, 24, 2493–2504. [Google Scholar] [CrossRef]
- Reibnegger, G.; Krainer, M.; Herold, M.; Ludwig, H.; Wachter, H.; Huber, H. Predictive value of interleukin-6 and neopterin in patients with multiple myeloma. Cancer Res. 1991, 51, 6250–6253. [Google Scholar]
- Pulkki, K.; Pelliniemi, T.T.; Rajamäki, A.; Tienhaara, A.; Laakso, M.; Lahtinen, R. Soluble interleukin-6 receptor as a prognostic factor in multiple myeloma. Finnish Leukaemia Group. Br. J. Haematol. 1996, 92, 370–374. [Google Scholar] [CrossRef]
- Miki, S.; Iwano, M.; Miki, Y.; Yamamoto, M.; Tang, B.; Yokokawa, K.; Sonoda, T.; Hirano, T.; Kishimoto, T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989, 250, 607–610. [Google Scholar] [CrossRef]
- Blay, J.Y.; Negrier, S.; Combaret, V.; Attali, S.; Goillot, E.; Merrouche, Y.; Mercatello, A.; Ravault, A.; Tourani, J.M.; Moskovtchenko, J.F.; et al. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992, 52, 3317–3322. [Google Scholar]
- Van Meir, E.; Sawamura, Y.; Diserens, A.-C.; Hamou, M.-F.; de Tribolet, N. Human Glioblastoma Cells Release Interleukin 6 in Vivo and in Vitro1. Cancer Res. 1990, 50, 6683–6688. [Google Scholar]
- Tamm, I.; Cardinale, I.; Krueger, J.; Murphy, J.S.; May, L.T.; Sehgal, P.B. Interleukin 6 decreases cell-cell association and increases motility of ductal breast carcinoma cells. J. Exp. Med. 1989, 170, 1649–1669. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, H.; Fu, Y.; Kuang, J.; Zhao, B.; Zhang, L.; Lin, J.; Lin, S.; Wu, D.; Xie, G. Cancer-associated fibroblasts induce growth and radioresistance of breast cancer cells through paracrine IL-6. Cell Death Discov. 2023, 9, 6. [Google Scholar] [CrossRef]
- Scambia, G.; Testa, U.; Benedetti Panici, P.; Foti, E.; Martucci, R.; Gadducci, A.; Perillo, A.; Facchini, V.; Peschle, C.; Mancuso, S. Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. Br. J. Cancer 1995, 71, 354–356. [Google Scholar] [CrossRef]
- Berek, J.S.; Chung, C.; Kaldı, K.; Watson, J.M.; Knox, R.M.; Martínez-Maza, O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am. J. Obstet. Gynecol. 1991, 164, 1038–1042, discussion 1042–1043. [Google Scholar] [CrossRef]
- Shariat, S.F.; Andrews, B.; Kattan, M.W.; Kim, J.; Wheeler, T.M.; Slawin, K.M. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 2001, 58, 1008–1015. [Google Scholar] [CrossRef]
- George, D.J.; Halabi, S.; Shepard, T.F.; Sanford, B.; Vogelzang, N.J.; Small, E.J.; Kantoff, P.W. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: Results from cancer and leukemia group B 9480. Clin. Cancer Res. 2005, 11, 1815–1820. [Google Scholar] [CrossRef]
- Hobisch, A.; Eder, I.E.; Putz, T.; Horninger, W.; Bartsch, G.; Klocker, H.; Culig, Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998, 58, 4640–4645. [Google Scholar]
- Akimoto, S.; Okumura, A.; Fuse, H. Relationship between serum levels of interleukin-6, tumor necrosis factor-alpha and bone turnover markers in prostate cancer patients. Endocr. J. 1998, 45, 183–189. [Google Scholar] [CrossRef]
- Nakashima, J.; Tachibana, M.; Horiguchi, Y.; Oya, M.; Ohigashi, T.; Asakura, H.; Murai, M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin. Cancer Res. 2000, 6, 2702–2706. [Google Scholar]
- Twillie, D.A.; Eisenberger, M.A.; Carducci, M.A.; Hseih, W.S.; Kim, W.Y.; Simons, J.W. Interleukin-6: A candidate mediator of human prostate cancer morbidity. Urology 1995, 45, 542–549. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ito, H.; Miki, C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer 1999, 85, 2526–2531. [Google Scholar] [CrossRef]
- Oka, M.; Yamamoto, K.; Takahashi, M.; Hakozaki, M.; Abe, T.; Iizuka, N.; Hazama, S.; Hirazawa, K.; Hayashi, H.; Tangoku, A.; et al. Relationship between serum levels of interleukin 6, various disease parameters and malnutrition in patients with esophageal squamous cell carcinoma. Cancer Res. 1996, 56, 2776–2780. [Google Scholar]
- Rašková, M.; Lacina, L.; Kejík, Z.; Venhauerová, A.; Skaličková, M.; Kolář, M.; Jakubek, M.; Rosel, D.; Smetana, K., Jr.; Brábek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis-Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef]
- Chen, M.F.; Hsieh, C.C.; Chen, W.C.; Lai, C.H. Role of interleukin-6 in the radiation response of liver tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e621–e630. [Google Scholar] [CrossRef]
- Kopčalić, K.; Matić, I.Z.; Besu, I.; Stanković, V.; Bukumirić, Z.; Stanojković, T.P.; Stepanović, A.; Nikitović, M. Circulating levels of IL-6 and TGF-β1 in patients with prostate cancer undergoing radiotherapy: Associations with acute radiotoxicity and fatigue symptoms. BMC Cancer 2022, 22, 1167. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Mueksch, B.; Boehringer, M.; Hüll, M. Interleukin-1beta induces cyclooxygenase-2 and prostaglandin E(2) synthesis in human neuroblastoma cells: Involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB. J. Neurochem. 2000, 75, 2020–2028. [Google Scholar] [CrossRef]
- Nathe, T.J.; Deou, J.; Walsh, B.; Bourns, B.; Clowes, A.W.; Daum, G. Interleukin-1beta inhibits expression of p21(WAF1/CIP1) and p27(KIP1) and enhances proliferation in response to platelet-derived growth factor-BB in smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1293–1298. [Google Scholar] [CrossRef]
- Lahm, H.; Petral-Malec, D.; Yilmaz-Ceyhan, A.; Fischer, J.R.; Lorenzoni, M.; Givel, J.C.; Odartchenko, N. Growth stimulation of a human colorectal carcinoma cell line by interleukin-1 and -6 and antagonistic effects of transforming growth factor beta 1. Eur. J. Cancer 1992, 28, 1894–1899. [Google Scholar] [CrossRef]
- Nakahara, H.; Song, J.; Sugimoto, M.; Hagihara, K.; Kishimoto, T.; Yoshizaki, K.; Nishimoto, N. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1521–1529. [Google Scholar] [CrossRef]
- Wolf, J.S.; Chen, Z.; Dong, G.; Sunwoo, J.B.; Bancroft, C.C.; Capo, D.E.; Yeh, N.T.; Mukaida, N.; Van Waes, C. IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin. Cancer Res. 2001, 7, 1812–1820. [Google Scholar]
- Mukhopadhyay, P.; Ali, M.A.; Nandi, A.; Carreon, P.; Choy, H.; Saha, D. The cyclin-dependent kinase 2 inhibitor down-regulates interleukin-1beta-mediated induction of cyclooxygenase-2 expression in human lung carcinoma cells. Cancer Res. 2006, 66, 1758–1766. [Google Scholar] [CrossRef]
- Chen, M.F.; Lu, M.S.; Chen, P.T.; Chen, W.C.; Lin, P.Y.; Lee, K.D. Role of interleukin 1 beta in esophageal squamous cell carcinoma. J. Mol. Med. 2012, 90, 89–100. [Google Scholar] [CrossRef]
- Aggen, D.H.; Ager, C.R.; Obradovic, A.Z.; Chowdhury, N.; Ghasemzadeh, A.; Mao, W.; Chaimowitz, M.G.; Lopez-Bujanda, Z.A.; Spina, C.S.; Hawley, J.E.; et al. Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses. Clin. Cancer Res. 2021, 27, 608–621. [Google Scholar] [CrossRef]
- Rath, P.C.; Aggarwal, B.B. TNF-induced signaling in apoptosis. J. Clin. Immunol. 1999, 19, 350–364. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Zhu, R.; Xue, X.; Shen, M.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O.; Chen, Y. NFκB and TNFα as individual key molecules associated with the cisplatin-resistance and radioresistance of lung cancer. Exp. Cell Res. 2019, 374, 181–188. [Google Scholar] [CrossRef]
- Hallahan, D.E.; Spriggs, D.R.; Beckett, M.A.; Kufe, D.W.; Weichselbaum, R.R. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc. Natl. Acad. Sci. USA 1989, 86, 10104–10107. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Beckett, M.A.; Vokes, E.E.; Brachman, D.G.; Haraf, D.; Hallahan, D.; Kufe, D. Cellular and molecular mechanisms of radioresistance. In Head and Neck Cancer: Basic and Clinical Aspects; Hong, W.K., Weber, R.S., Eds.; Springer: Boston, MA, USA, 1995; pp. 131–140. [Google Scholar]
- Rosen, E.M.; Fan, S.; Rockwell, S.; Goldberg, I.D. The molecular and cellular basis of radiosensitivity: Implications for understanding how normal tissues and tumors respond to therapeutic radiation. Cancer Investig. 1999, 17, 56–72. [Google Scholar] [CrossRef]
- An, H.J.; Seong, S.J. Proteomics Analysis of Apoptosis-regulating Proteins in Tissues with Different Radiosensitivity. J. Radiat. Res. 2006, 47, 147–155. [Google Scholar] [CrossRef]
- Meng, Y.; Beckett, M.A.; Liang, H.; Mauceri, H.J.; van Rooijen, N.; Cohen, K.S.; Weichselbaum, R.R. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010, 70, 1534–1543. [Google Scholar] [CrossRef]
- Pal, S.; Yadav, P.; Sainis, K.B.; Shankar, B.S. TNF-α and IGF-1 differentially modulate ionizing radiation responses of lung cancer cell lines. Cytokine 2018, 101, 89–98. [Google Scholar] [CrossRef]
- Hall, W.A.; Karrison, T.G.; Rosenthal, S.A.; Amin, M.B.; Gomella, L.G.; Purdy, J.A.; Sartor, A.O.; Michalski, J.M.; Garzotto, M.G.; Bergom, C.; et al. The Influence of the Pretreatment Immune State on Response to Radiation Therapy in High-Risk Prostate Cancer: A Validation Study from NRG/RTOG 0521. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 266–274. [Google Scholar] [CrossRef]
- Murphy, J.F.; Fitzgerald, D.J. Vascular endothelial growth factor induces cyclooxygenase-dependent proliferation of endothelial cells via the VEGF-2 receptor. FASEB J. 2001, 15, 1667–1669. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Gerber, H.P.; Dixit, V.; Ferrara, N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 1998, 273, 13313–13316. [Google Scholar] [CrossRef]
- Braicu, E.I.; Fotopoulou, C.; Chekerov, R.; Richter, R.; Blohmer, J.; Kümmel, S.; Stamatian, F.; Yalcinkaya, I.; Mentze, M.; Lichtenegger, W.; et al. Role of serum concentration of VEGFR1 and TIMP2 on clinical outcome in primary cervical cancer: Results of a companion protocol of the randomized, NOGGO-AGO phase III adjuvant trial of simultaneous cisplatin-based radiochemotherapy vs. carboplatin and paclitaxel containing sequential radiotherapy. Cytokine 2013, 61, 755–758. [Google Scholar] [CrossRef]
- Hu, X.; Xing, L.; Wei, X.; Liu, X.; Pang, R.; Qi, L.; Song, S. Nonangiogenic function of VEGF and enhanced radiosensitivity of HeLa cells by inhibition of VEGF expression. Oncol. Res. 2012, 20, 93–101. [Google Scholar] [CrossRef]
- Fekete, N.; Erle, A.; Amann, E.M.; Fürst, D.; Rojewski, M.T.; Langonné, A.; Sensebé, L.; Schrezenmeier, H.; Schmidtke-Schrezenmeier, G. Effect of high-dose irradiation on human bone-marrow-derived mesenchymal stromal cells. Tissue Eng. Part C Methods 2015, 21, 112–122. [Google Scholar] [CrossRef]
- Fu, Z.Z.; Sun, X.D.; Li, P.; Zhang, Z.; Li, G.Z.; Gu, T.; Shao, S.S. Relationship between serum VEGF level and radiosensitivity of patients with nonsmall cell lung cancer among asians: A meta-analysis. DNA Cell Biol. 2014, 33, 426–437. [Google Scholar] [CrossRef]
- Hu, J.J.; Urbanic, J.J.; Case, L.D.; Takita, C.; Wright, J.L.; Brown, D.R.; Langefeld, C.D.; Lively, M.O.; Mitchell, S.E.; Thakrar, A.; et al. Association Between Inflammatory Biomarker C-Reactive Protein and Radiotherapy-Induced Early Adverse Skin Reactions in a Multiracial/Ethnic Breast Cancer Population. J. Clin. Oncol. 2018, 36, 2473–2482. [Google Scholar] [CrossRef]
- Canada, J.M.; Thomas, G.K.; Trankle, C.R.; Carbone, S.; Billingsley, H.; Van Tassell, B.W.; Evans, R.K.; Garten, R.; Weiss, E.; Abbate, A. Increased C-reactive protein is associated with the severity of thoracic radiotherapy-induced cardiomyopathy. Cardio-Oncol. 2020, 6, 2. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Tao, M.L.; Hu, W.; Belin, T.R.; Sepah, S.; Cole, S.; Aziz, N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009, 15, 5534–5540. [Google Scholar] [CrossRef]
- Bunda, S.; Zuccato, J.A.; Voisin, M.R.; Wang, J.Z.; Nassiri, F.; Patil, V.; Mansouri, S.; Zadeh, G. Liquid Biomarkers for Improved Diagnosis and Classification of CNS Tumors. Int. J. Mol. Sci. 2021, 22, 4548. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Guo, Q.R.; Wang, F.H.; Adhikari, R.; Zhu, Z.Y.; Zhang, H.Y.; Zhou, W.M.; Yu, H.; Li, J.Q.; Zhang, J.Y. Cell-Free DNA: Hope and Potential Application in Cancer. Front. Cell Dev. Biol. 2021, 9, 639233. [Google Scholar] [CrossRef]
- Anker, P.; Mulcahy, H.; Chen, X.Q.; Stroun, M. Detection of circulating tumour DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 1999, 18, 65–73. [Google Scholar] [CrossRef]
- Raja, R.; Kuziora, M.; Brohawn, P.Z.; Higgs, B.W.; Gupta, A.; Dennis, P.A.; Ranade, K. Early Reduction in ctDNA Predicts Survival in Patients with Lung and Bladder Cancer Treated with Durvalumab. Clin. Cancer Res. 2018, 24, 6212–6222. [Google Scholar] [CrossRef]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef]
- Kageyama, S.I.; Nihei, K.; Karasawa, K.; Sawada, T.; Koizumi, F.; Yamaguchi, S.; Kato, S.; Hojo, H.; Motegi, A.; Tsuchihara, K.; et al. Radiotherapy increases plasma levels of tumoral cell-free DNA in non-small cell lung cancer patients. Oncotarget 2018, 9, 19368–19378. [Google Scholar] [CrossRef]
- Rostami, A.; Lambie, M.; Yu, C.W.; Stambolic, V.; Waldron, J.N.; Bratman, S.V. Senescence, Necrosis, and Apoptosis Govern Circulating Cell-Free DNA Release Kinetics. Cell Rep. 2020, 31, 107830. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra92. [Google Scholar] [CrossRef]
- Cho, W.K.; Lee, J.; Youn, S.M.; Oh, D.; Lim, D.H.; Yoon, H.G.; Cho, E.H.; Noh, J.M. Liquid biopsy using cfDNA to predict radiation therapy response in solid tumors. Radiat. Oncol. J. 2023, 41, 32–39. [Google Scholar] [CrossRef]
- Nakamura, M.; Kageyama, S.I.; Hirata, H.; Tochinai, T.; Hojo, H.; Motegi, A.; Kanai, A.; Suzuki, Y.; Tsuchihara, K.; Akimoto, T. Detection of Pretreatment Circulating Tumor DNA Predicts Recurrence after High-Dose Proton Beam Therapy for Early-Stage Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 1085–1090. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Lovejoy, A.F.; Chabon, J.J.; Newman, A.; Stehr, H.; Merriott, D.J.; Carter, J.N.; Azad, T.D.; Padda, S.; Gensheimer, M.F.; et al. Circulating Tumor DNA Analysis during Radiation Therapy for Localized Lung Cancer Predicts Treatment Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, S1–S2. [Google Scholar] [CrossRef]
- McLaren, D.B.; Aitman, T.J. Redefining precision radiotherapy through liquid biopsy. Br. J. Cancer 2023, 129, 900–903. [Google Scholar] [CrossRef]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 87. [Google Scholar] [CrossRef]
- Fukui, R.; Saga, R.; Matsuya, Y.; Tomita, K.; Kuwahara, Y.; Ohuchi, K.; Sato, T.; Okumura, K.; Date, H.; Fukumoto, M.; et al. Tumor radioresistance caused by radiation-induced changes of stem-like cell content and sub-lethal damage repair capability. Sci. Rep. 2022, 12, 1056. [Google Scholar] [CrossRef]
- Busato, F.; Khouzai, B.E.; Mognato, M. Biological Mechanisms to Reduce Radioresistance and Increase the Efficacy of Radiotherapy: State of the Art. Int. J. Mol. Sci. 2022, 23, 10211. [Google Scholar] [CrossRef]
- Cristiano, S.; Leal, A.; Phallen, J.; Fiksel, J.; Adleff, V.; Bruhm, D.C.; Jensen, S.; Medina, J.E.; Hruban, C.; White, J.R.; et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 2019, 570, 385–389. [Google Scholar] [CrossRef]
- Mireștean, C.C.; Iancu, R.I.; Iancu, D.P.T. Simultaneous Integrated Boost (SIB) vs. Sequential Boost in Head and Neck Cancer (HNC) Radiotherapy: A Radiomics-Based Decision Proof of Concept. J. Clin. Med. 2023, 12, 2413. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Wang, Y.; Jiang, S.; Fan, R.; Zhang, H.; Jiang, W. Application of radiomics and machine learning in head and neck cancers. Int. J. Biol. Sci. 2021, 17, 475–486. [Google Scholar] [CrossRef]
- Logotheti, S.; Georgakilas, A.G. More than Meets the Eye: Integration of Radiomics with Transcriptomics for Reconstructing the Tumor Microenvironment and Predicting Response to Therapy. Cancers 2023, 15, 1634. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, L.; Ren, Y.; Cheng, Z.; Li, L.; Yao, S.; Zhang, C.; Luo, Z.; Lu, H. A meta-learning approach to improving radiation response prediction in cancers. Comput. Biol. Med. 2022, 150, 106163. [Google Scholar] [CrossRef]
- Liu, J.; Han, M.; Yue, Z.; Dong, C.; Wen, P.; Zhao, G.; Wu, L.; Xia, J.; Bin, Y. Prediction of Radiosensitivity in Head and Neck Squamous Cell Carcinoma Based on Multiple Omics Data. Front. Genet. 2020, 11, 960. [Google Scholar] [CrossRef]
- Manem, V.S.K. Development and validation of genomic predictors of radiation sensitivity using preclinical data. BMC Cancer 2021, 21, 937. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, X.; Tang, Z. More evidence for prediction model of radiosensitivity. Biosci. Rep. 2021, 41, BSR20210034. [Google Scholar] [CrossRef]
- Zeng, Z.; Luo, M.; Li, Y.; Li, J.; Huang, Z.; Zeng, Y.; Yuan, Y.; Wang, M.; Liu, Y.; Gong, Y.; et al. Prediction of radiosensitivity and radiocurability using a novel supervised artificial neural network. BMC Cancer 2022, 22, 1243. [Google Scholar] [CrossRef]
- Available online: https://www.energy.gov/articles/candle-illuminates-new-pathways-fight-against-cancer (accessed on 27 September 2023).
- National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Nuclear and Radiation Studies Board; Committee on Developing a Long-Term Strategy for Low-Dose Radiation Research in the United States. Leveraging Advances in Modern Science to Revitalize Low-Dose Radiation Research in the United States; National Academies Press (US): Washington, DC, USA, 2022. [Google Scholar]
- Lee, Y.; Wang, Q.; Shuryak, I.; Brenner, D.J.; Turner, H.C. Development of a high-throughput γ-H2AX assay based on imaging flow cytometry. Radiat. Oncol. 2019, 14, 150. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Abdalla, O.M.; Ali, A.A.; Hassan, N.M.; Yousif, A.A.; Elbashir, F.E.; Omer, A.; Mussa, R.; Hassan, A.M. Status of H2AX and TP53 (Non-phosphorylation) for DNA Damage in Cancer Patients on Radiotherapy-A Case-Control Study. Health Sci. 2022, 11, 51–56. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkikoudi, A.; Kalospyros, S.A.; Triantopoulou, S.; Logotheti, S.; Softa, V.; Kappas, C.; Theodorou, K.; Laiakis, E.C.; Manda, G.; Terzoudi, G.I.; et al. Molecular Biomarkers for Predicting Cancer Patient Radiosensitivity and Radiotoxicity in Clinical Practice. Appl. Sci. 2023, 13, 12564. https://doi.org/10.3390/app132312564
Gkikoudi A, Kalospyros SA, Triantopoulou S, Logotheti S, Softa V, Kappas C, Theodorou K, Laiakis EC, Manda G, Terzoudi GI, et al. Molecular Biomarkers for Predicting Cancer Patient Radiosensitivity and Radiotoxicity in Clinical Practice. Applied Sciences. 2023; 13(23):12564. https://doi.org/10.3390/app132312564
Chicago/Turabian StyleGkikoudi, Angeliki, Spyridon A. Kalospyros, Sotiria Triantopoulou, Stella Logotheti, Vasiliki Softa, Constantin Kappas, Kiki Theodorou, Evagelia C. Laiakis, Gina Manda, Georgia I. Terzoudi, and et al. 2023. "Molecular Biomarkers for Predicting Cancer Patient Radiosensitivity and Radiotoxicity in Clinical Practice" Applied Sciences 13, no. 23: 12564. https://doi.org/10.3390/app132312564
APA StyleGkikoudi, A., Kalospyros, S. A., Triantopoulou, S., Logotheti, S., Softa, V., Kappas, C., Theodorou, K., Laiakis, E. C., Manda, G., Terzoudi, G. I., & Georgakilas, A. G. (2023). Molecular Biomarkers for Predicting Cancer Patient Radiosensitivity and Radiotoxicity in Clinical Practice. Applied Sciences, 13(23), 12564. https://doi.org/10.3390/app132312564











