Perlite Has Similar Diffusion Properties for Oxygen and Carbon Dioxide to Snow: Implications for Avalanche Safety Equipment Testing and Breathing Studies
Abstract
:1. Introduction
2. Methods
2.1. Apparatus
2.2. Protocol
2.3. Data Processing and Analysis
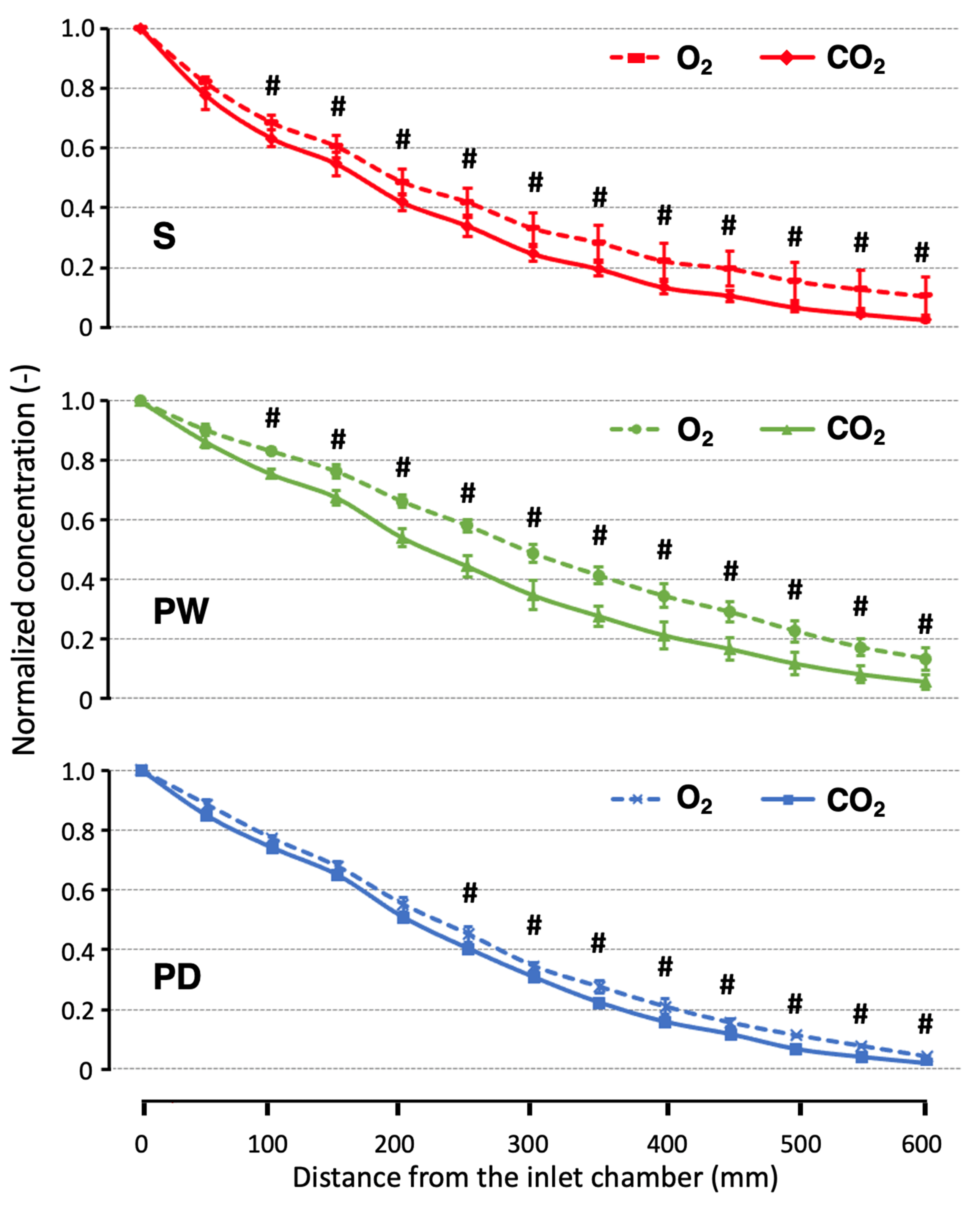
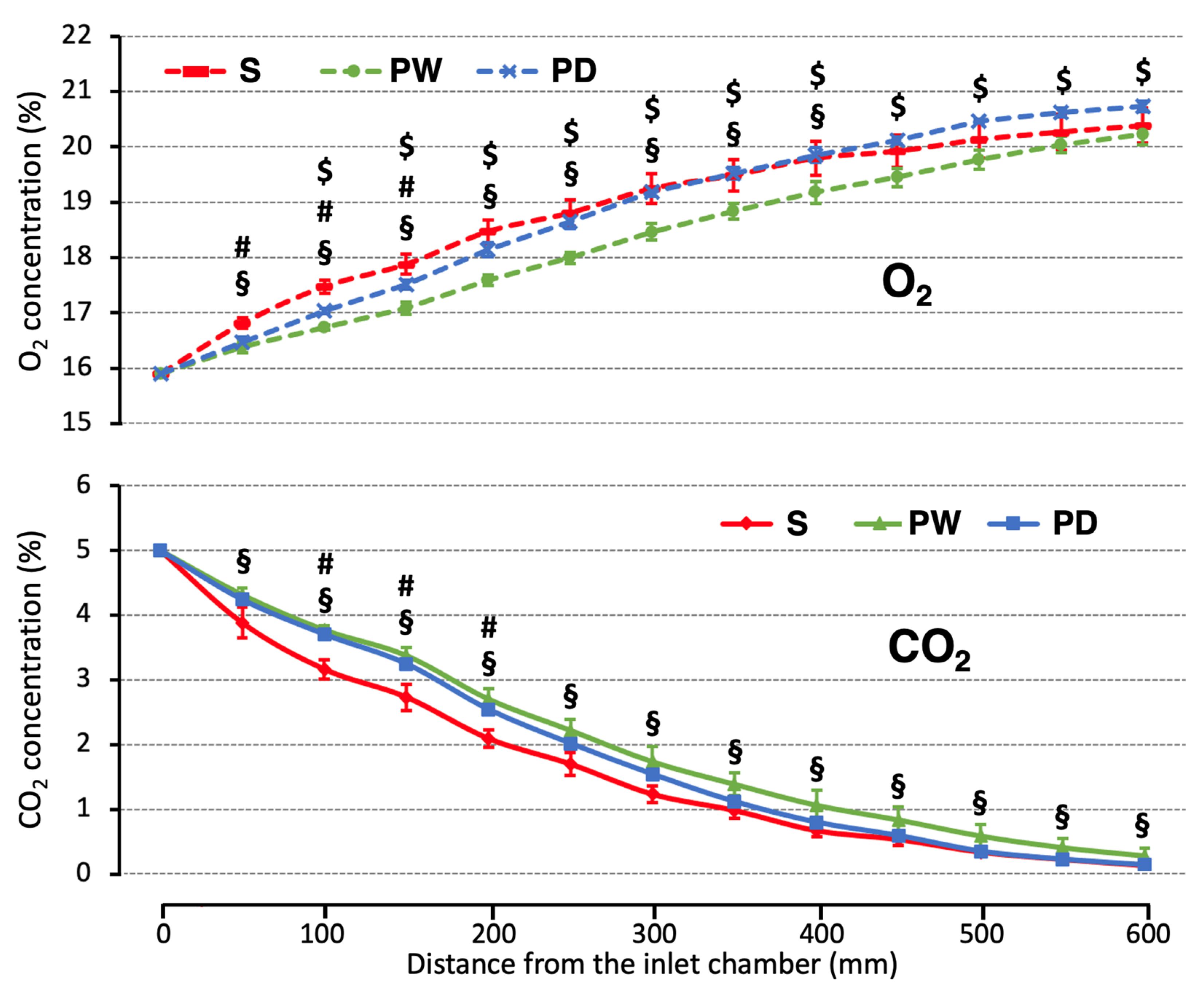
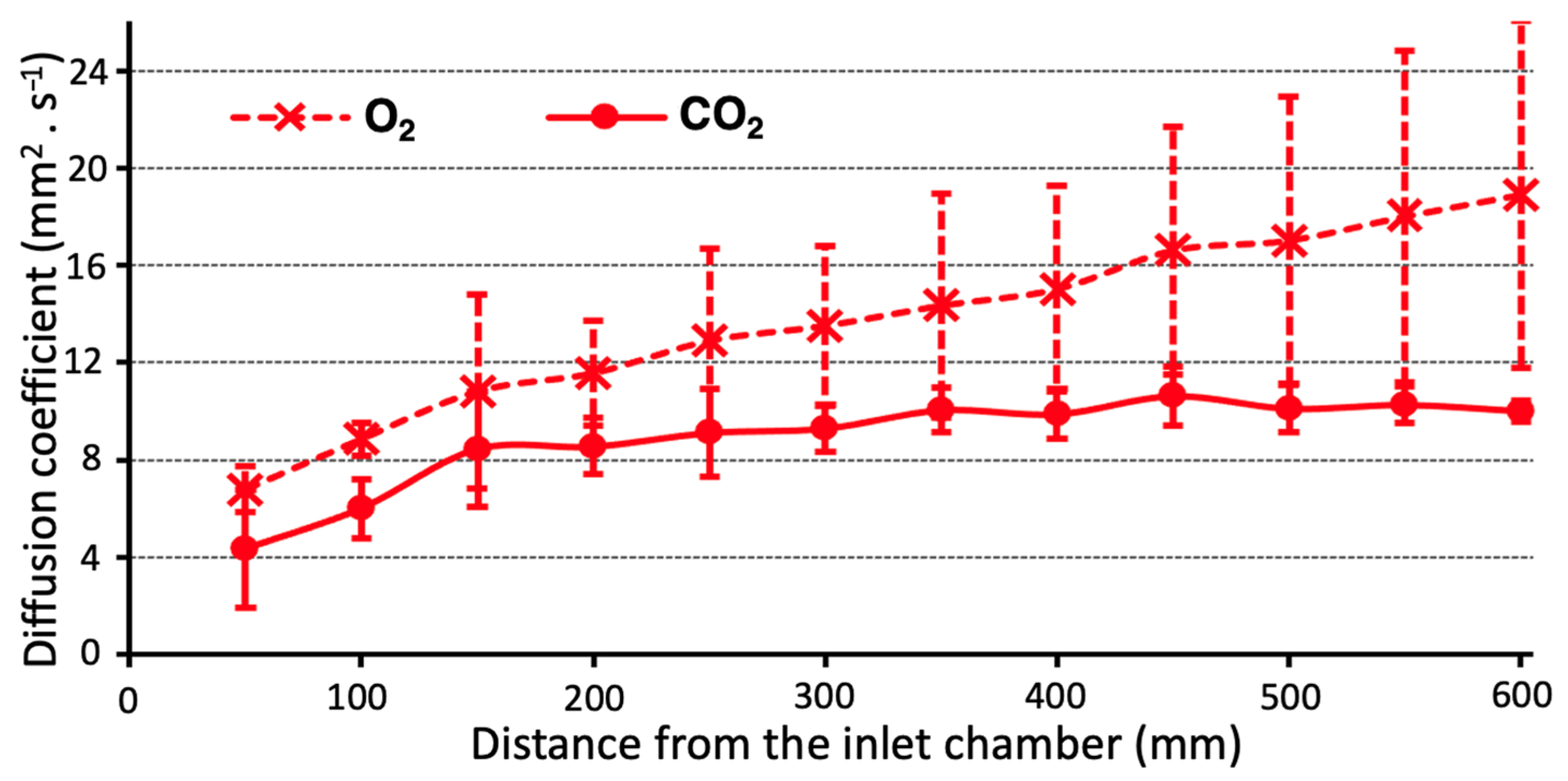
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Techel, F.; Jarry, F.; Kronthaler, G.; Mitterer, S.; Nairz, P.; Pavšek, M.; Valt, M.; Darms, G. Avalanche fatalities in the European Alps: Long-term trends and statistics. Geogr. Helv. 2016, 71, 147–159. [Google Scholar] [CrossRef]
- Statistics and reporting. Colorado Avalanche Information Center. Available online: https://avalanche.state.co.us/accidents/statistics-and-reporting/ (accessed on 9 September 2023).
- McIntosh, S.E.; Grissom, C.K.; Olivares, C.R.; Kim, H.S.; Tremper, B. Cause of death in avalanche fatalities. Wilderness Environ. Med. 2007, 18, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Strapazzon, G.; Paal, P.; Schweizer, J.; Falk, M.; Reuter, B.; Schenk, K.; Gatterer, H.; Grasegger, K.; Cappello, T.D.; Malacrida, S.; et al. Effects of snow properties on humans breathing into an artificial air pocket–an experimental field study. Sci. Rep. 2017, 7, 17675. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, R.A.; Massman, W.J.; Musselman, R.C.; Mosier, A.R. Diffusional flux of CO2 through snow: Spatial and temporal variability among alpine-subalpine sites. Glob. Biogeochem. Cycles 1996, 10, 473–482. [Google Scholar] [CrossRef]
- Sommerfeld, R.A.; Mosier, A.R.; Musselman, R.C. CO2, CH4 and N2O flux through a Wyoming snowpack and implications for global budgets. Nature 1993, 361, 140–142. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmesiter-Boltenstern, S.; Glatzel, G.; Jandl, R. Winter soil respiration from an austrian mountain forest. Agric. For. Meteorol. 2007, 146, 205–215. [Google Scholar] [CrossRef]
- Winston, G.C.; Stephens, B.B.; Sundquist, E.T.; Hardy, J.P.; Davis, R.E. Seasonal variability in CO2 transport through snow in a boreal forest. Biogeochem. Seas. Snow Cover. Catchments 1995, 228, 141–150. [Google Scholar]
- Mast, M.A.; Wickland, K.P.; Striegl, R.T.; Clow, D.W. Winter fluxes of CO2 and CH4 from subalpine soils in Rocky Mountain National Park, Colorado. Glob. Biogeochem. Cycles 1998, 12, 607–620. [Google Scholar] [CrossRef]
- Massman, W.J.; Sommerfeld, R.A.; Mosier, A.R.; Zeller, K.F.; Hehn, T.J.; Rochelle, S.G. A model investigation of turbulence-driven pressure-pumping effects on the rate of diffusion of CO2, N2O, and CH4 through layered snowpacks. J. Geophys. Res. Atmos. 1997, 102, 18851–18863. [Google Scholar] [CrossRef]
- Massman, W.J. A review of the molecular diffusivities of H2O, CO2, CH4, CO, O3, SO2, NH3, N2O, NO, and NO2 in air, O2 and N2 near STP. Atmos. Environ. 1998, 32, 1111–1127. [Google Scholar] [CrossRef]
- Schwander, J.; Barnola, J.; Andrié, C.; Leuenberger, M.; Ludin, A.; Raynaud, D.; Stauffer, B. The age of the air in the firn and the ice at Summit, Greenland. J. Geophys. Res. Atmos. 1993, 98, 2831–2838. [Google Scholar] [CrossRef]
- Frederiksen, J.M.; Mejlbro, L.; Nilsson, L.O. Fick’s 2nd law-Complete solutions for chloride ingress into concrete–with focus on time dependent diffusivity and boundary condition. Div. Build. Mater. Lund Inst. Technol. 2009, 3146, 110. [Google Scholar]
- Schwander, J.; Stauffer, B.; Sigg, A. Air mixing in firn and the age of the air at pore close-off. Ann. Glaciol. 1988, 10, 141–145. [Google Scholar] [CrossRef]
- Roubík, K.; Walzel, S.; Horakova, L.; Refalo, A.; Sykora, K.; Ort, V.; Sieger, L. Materials suitable to simulate snow during breathing experiments for avalanche survival research. Lékař A Tech. Clin. Technol. 2020, 50, 32–39. [Google Scholar] [CrossRef]
- Maxim, L.D.; Niebo, R.; McConnell, E.E. Perlite toxicology and epidemiology–a review. Inhal. Toxicol. 2014, 26, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Roubik, K.; Sykora, K.; Sieger, L.; Ort, V.; Horakova, L.; Walzel, S. Perlite is a suitable model material for experiments investigating breathing in high density snow. Sci. Rep. 2022, 12, 2070. [Google Scholar] [CrossRef]
- Brugger, H.; Sumann, G.; Meister, R.; Adler-Kastner, L.; Mair, P.; Gunga, H.C.; Schobersberger, W.; Falk, M. Hypoxia and hypercapnia during respiration into an artificial air pocket in snow: Implications for avalanche survival. Resuscitation 2003, 58, 81–88. [Google Scholar] [CrossRef]
- Engineering ToolBox. Oxygen—Solubility in Fresh Water and Sea Water. Available online: https://www.engineeringtoolbox.com/oxygen-solubility-water-d_841.html (accessed on 9 September 2023).
- Engineering ToolBox. Solubility of Gases in Water. Available online: https://www.engineeringtoolbox.com/gases-solubility-water-d_1148.html (accessed on 9 September 2023).
- Ahn, J.; Headly, M.; Wahlen, M.; Brook, E.J.; Mayewski, P.A.; Taylor, K.C. CO2 diffusion in polar ice: Observations from naturally formed CO2 spikes in the Siple Dome (Antarctica) ice core. J. Glaciol. 2008, 54, 685–695. [Google Scholar] [CrossRef]
- Ocampo, J.; Klinger, J. Modification of the surface structure of ice during ageing. J. Phys. Chem. 1983, 87, 4167–4170. [Google Scholar] [CrossRef]
- Grissom, C.K.; Radwin, M.I.; Harmston, C.H.; Hirshberg, E.L.; Crowley, T.J. Respiration during snow burial using an artificial air pocket. JAMA 2000, 283, 2266–2271. [Google Scholar] [CrossRef]
- Fierz, C.; Armstrong, R.L.; Durand, Y.; Etchevers, P.; Greene, E.; McClung, D.M.; Nishimura, K.; Satayawali, P.K.; Sokratov, S.A. The International Classification for Seasonal Snow on the Ground, HP-VII Technical Documents in Hydrology; IACS Contribution No 1; UNESCO-IHP: Paris, France, 2009; p. 90. [Google Scholar]
- Trukhan, V.; Horakova, L.; Rozanek, M. Program Extension for Data Analysis from Operating Rooms. In Proceedings of the 8th International Conference on e-Health and Bioengineering (EHB 2020), Iasi, Romania, 29–30 October 2020; pp. 1–4. [Google Scholar]
- Guyton, A.C.; Hall, J. Textbook of Medical Physiology, 8th ed.; Saunders: Philadelphia, PA, USA, 2000. [Google Scholar]
- Solomon, D.K.; Cerling, T.E. The annual carbon dioxide cycle in a montane soil: Observations, modeling, and implications for weathering. Water Resour. Res. 1987, 23, 2257–2265. [Google Scholar] [CrossRef]
- Haegeli, P.; Falk, M.; Brugger, H.; Etter, H.-J.; Boyd, J. Comparison of avalanche survival patterns in Canada and Switzerland. CMAJ 2011, 183, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Strapazzon, G.; Gatterer, H.; Falla, M.; Cappello, T.D.; Malacrida, S.; Turner, R.; Schenk, K.; Paal, P.; Falk, M.; Schweizer, J.; et al. Hypoxia and hypercapnia effects on cerebral oxygen saturation in avalanche burial: A pilot human experimental study. Resuscitation 2021, 158, 175–182. [Google Scholar] [CrossRef]
- Genswein, M.; Macias, D.; McIntosh, S.; Reiweger, I.; Hetland, A.; Paal, P. AvaLife—A New Multi-Disciplinary Approach Supported by Accident and Field Test Data to Optimize Survival Chances in Rescue and First Aid of Avalanche Patients. Int. J. Environ. Res. Public Health 2022, 19, 5257. [Google Scholar] [CrossRef] [PubMed]
- Wik, L.; Brattebø, G.; Østerås, Ø.; Assmus, J.; Irusta, U.; Aramendi, E.; Mydske, S.; Skaalhegg, T.; Skaiaa, S.C.; Thomassen, Ø. Physiological effects of providing supplemental air for avalanche victims. A randomised trial. Resuscitation 2022, 172, 38–46. [Google Scholar] [CrossRef]
- Van Tilburg, C. Should airbag backpacks be standard avalanche safety equipment? Wilderness Environ. Med. 2021, 32, 495–498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Material | O2 DC (mm2·s−1) | CO2 DC (mm2·s−1) | O2/CO2 DC Ratio (-) | |
|---|---|---|---|---|
| S | Theoretical | 11.1–15.1 * | 8.7–11.9 * | 1.27 |
| Measured | 13.2 ± 3.7 | 8.8 ± 1.9 | 1.50 ± 0.17 | |
| PW | Theoretical | 10.9 | 8.5 | 1.27 |
| Measured | 25.8 ± 2.5 | 14.0 ± 1.0 | 1.85 ± 0.14 | |
| PD | Theoretical | 13.8 | 10.8 | 1.27 |
| Measured | 13.0 ± 1.1 | 11.2 ± 1.2 | 1.16 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walzel, S.; Rozanek, M.; Roubik, K. Perlite Has Similar Diffusion Properties for Oxygen and Carbon Dioxide to Snow: Implications for Avalanche Safety Equipment Testing and Breathing Studies. Appl. Sci. 2023, 13, 12569. https://doi.org/10.3390/app132312569
Walzel S, Rozanek M, Roubik K. Perlite Has Similar Diffusion Properties for Oxygen and Carbon Dioxide to Snow: Implications for Avalanche Safety Equipment Testing and Breathing Studies. Applied Sciences. 2023; 13(23):12569. https://doi.org/10.3390/app132312569
Chicago/Turabian StyleWalzel, Simon, Martin Rozanek, and Karel Roubik. 2023. "Perlite Has Similar Diffusion Properties for Oxygen and Carbon Dioxide to Snow: Implications for Avalanche Safety Equipment Testing and Breathing Studies" Applied Sciences 13, no. 23: 12569. https://doi.org/10.3390/app132312569
APA StyleWalzel, S., Rozanek, M., & Roubik, K. (2023). Perlite Has Similar Diffusion Properties for Oxygen and Carbon Dioxide to Snow: Implications for Avalanche Safety Equipment Testing and Breathing Studies. Applied Sciences, 13(23), 12569. https://doi.org/10.3390/app132312569






