Air-Stable Near-Infrared Sensitive Organic Phototransistors Realized via Tri-Layer Planar Heterojunction
Abstract
:1. Introduction
2. Experimental
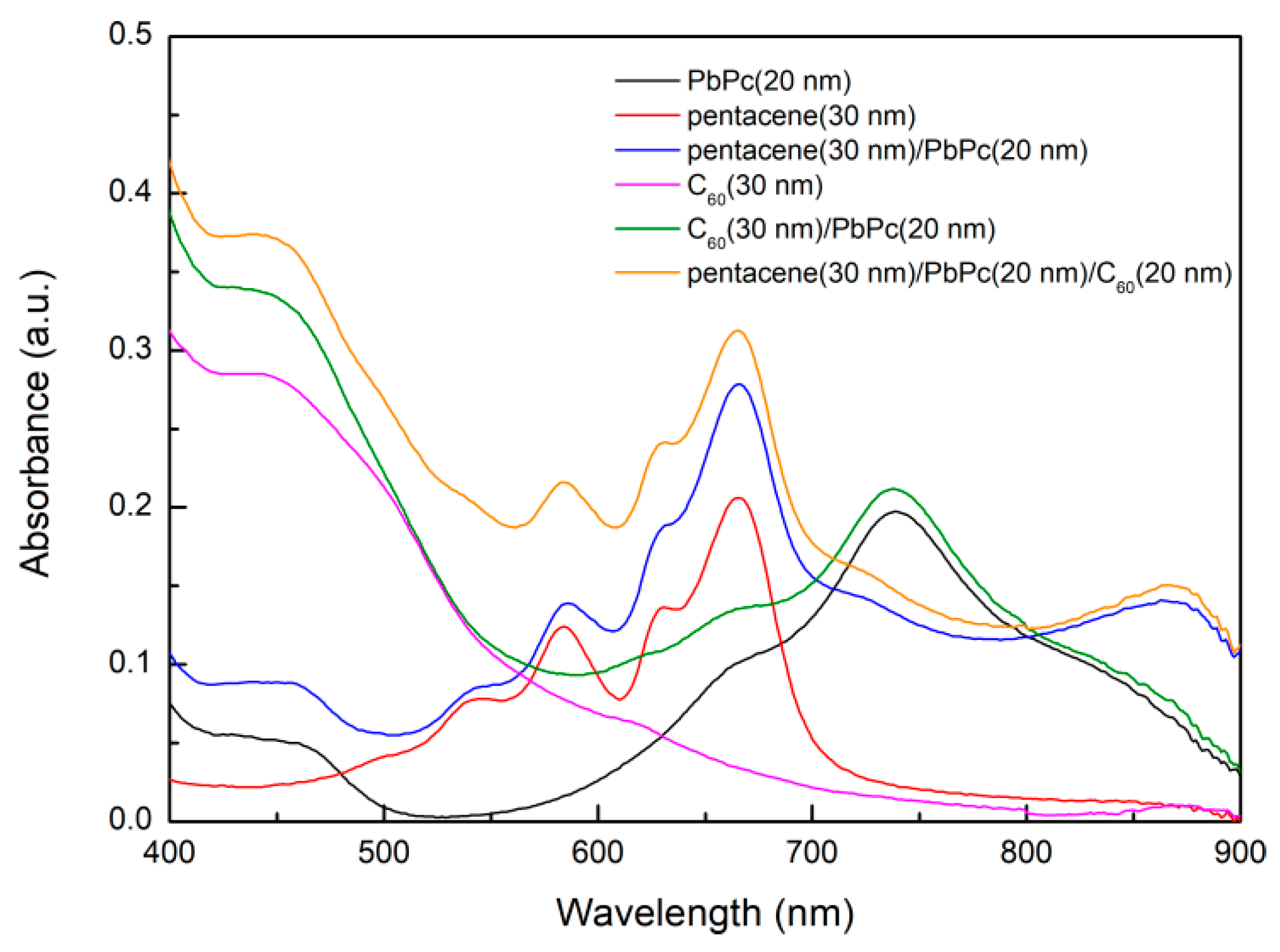
3. Results and Discussion
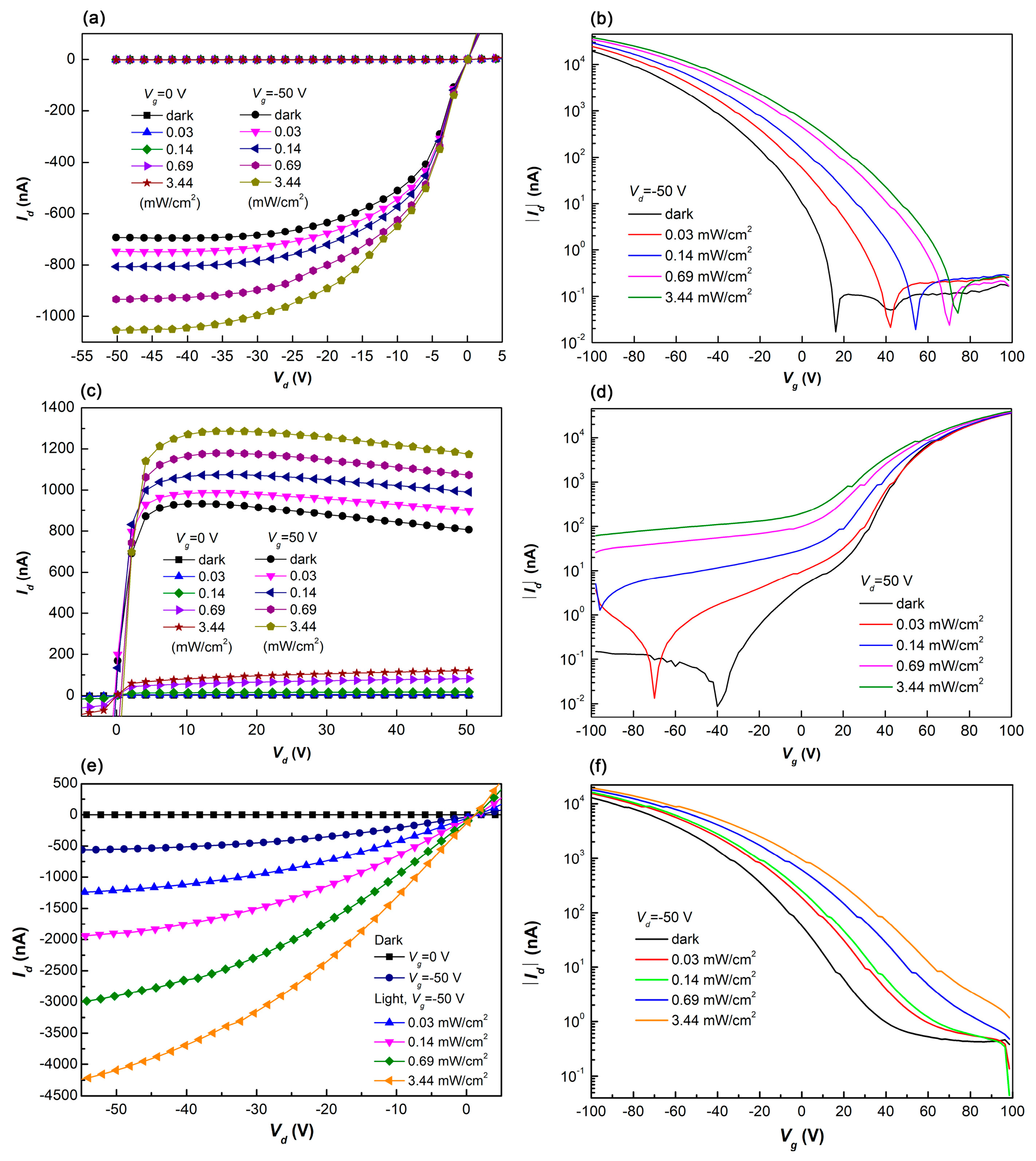
3.1. Single-Layer Devices
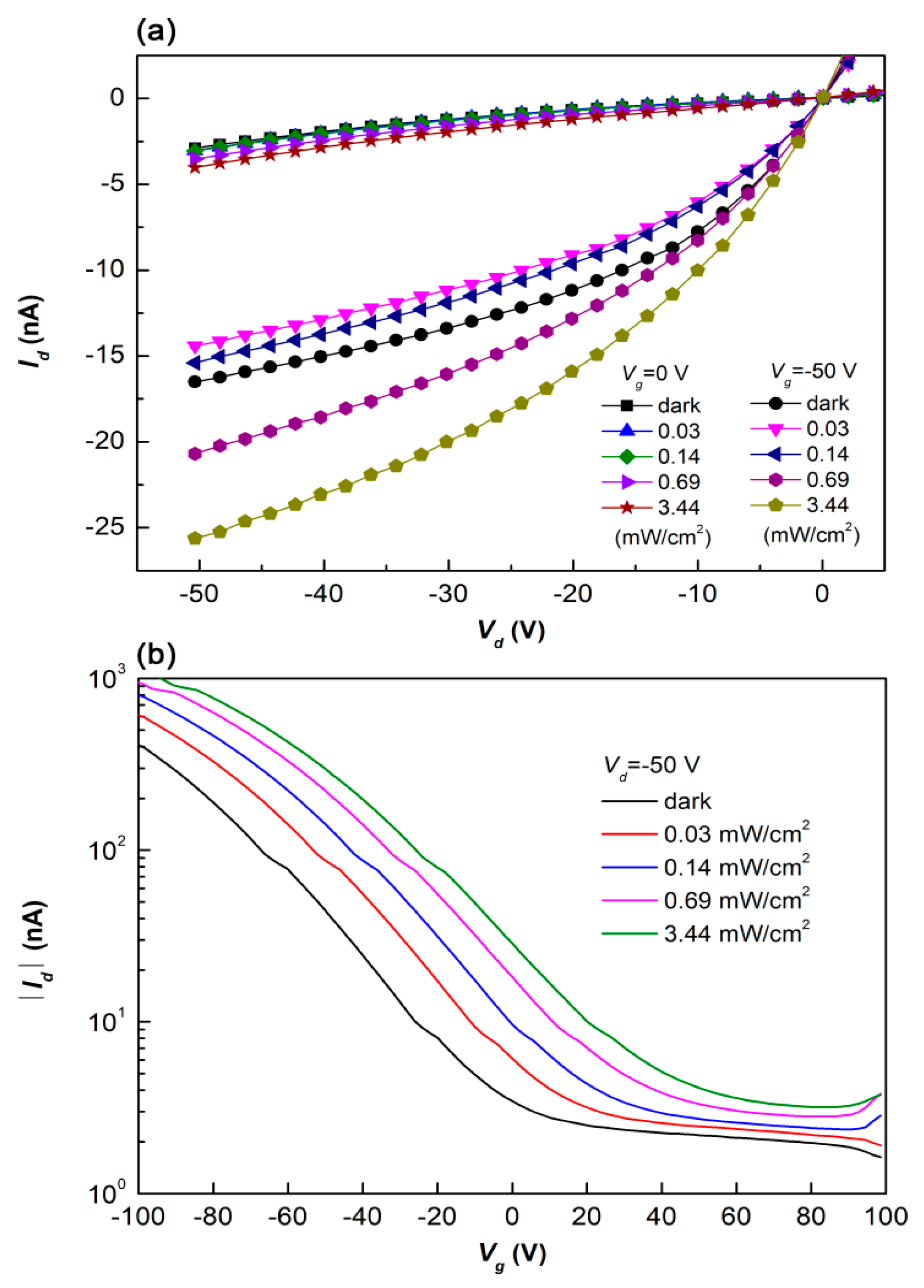
3.2. Bilayer Planar Heterojunction
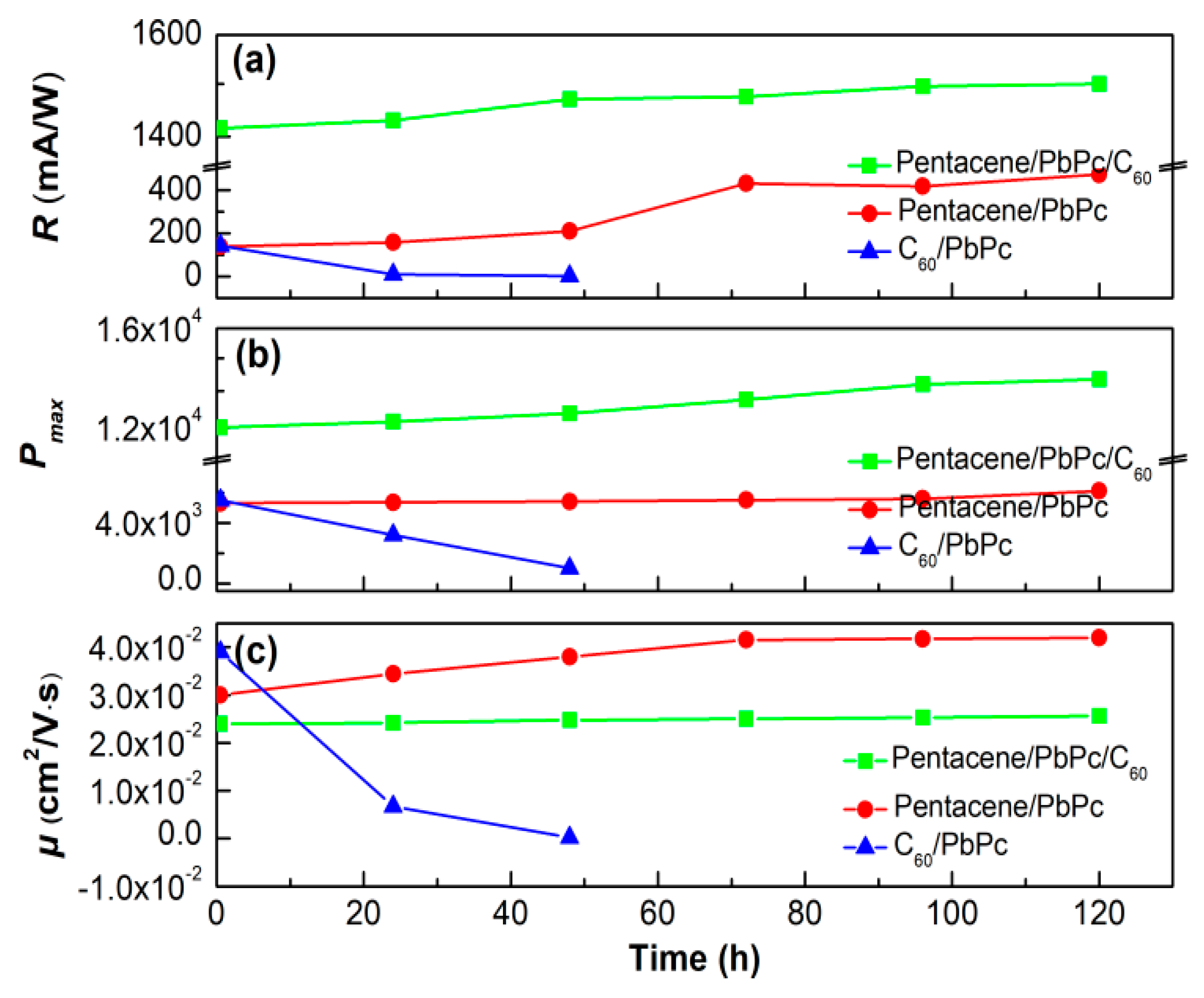
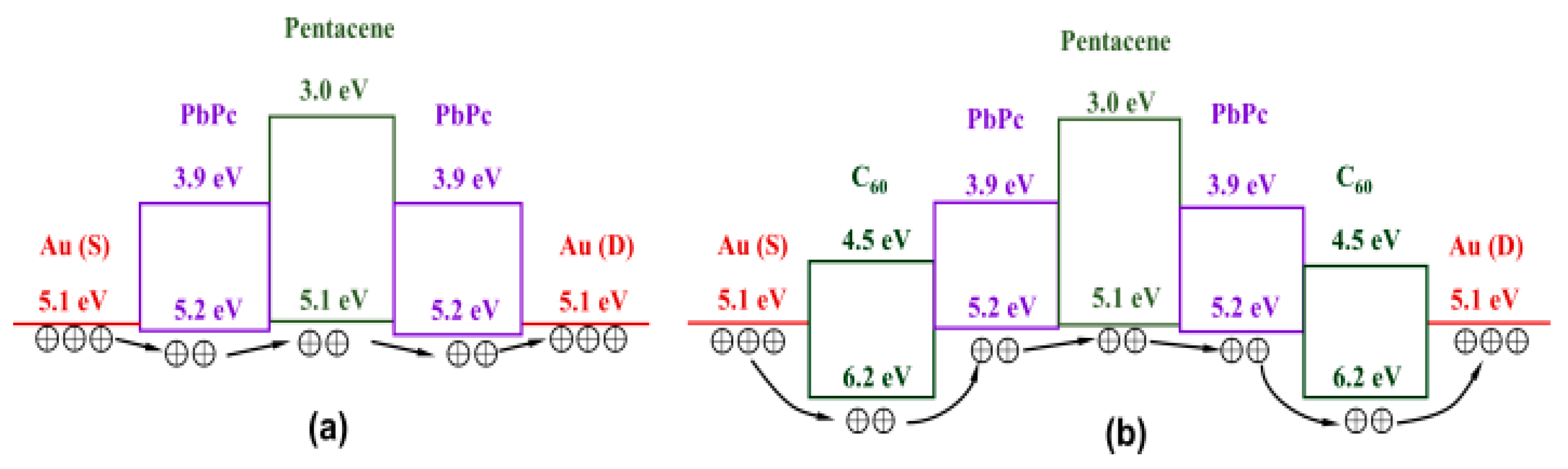
3.3. Tri-Layer Planar Heterojunction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grassi, S.; Alamprese, C. Advances in NIR spectroscopy applied to process analytical technology in food industries. Curr. Opin. Food Sci. 2018, 22, 17–21. [Google Scholar] [CrossRef]
- Rathod, P.H.; Müller, I.; Van der Meer, F.D.; de Smeth, B. Analysis of visible and near infrared spectral reflectance for assessing metals in soil. Environ. Monit. Assess. 2016, 188, 558. [Google Scholar] [CrossRef]
- Sakudo, A. Near-infrared spectroscopy for medical applications: Current status and future perspectives. Clin. Chim. Acta 2016, 455, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Song, J.; Qu, J.; Li, B.; Zhu, W.; Wong, W.Y. Near-infrared emitting materials via harvesting triplet excitons: Molecular design, properties, and application in organic light emitting diodes. Adv. Opt. Mater. 2018, 6, 1800466. [Google Scholar] [CrossRef]
- Lee, D.; Han, S.Y.; Jeong, Y.; Nguyen, D.M.; Yoon, G.; Mun, J.; Chae, J.; Lee, J.H.; Ok, J.G.; Jung, G.Y.; et al. Polarization-sensitive tunable absorber in visible and near-infrared regimes. Sci. Rep. 2018, 8, 12393. [Google Scholar] [CrossRef]
- Wu, S.; Butt, H.J. Near-Infrared-Sensitive Materials Based on Upconverting Nanoparticles. Adv. Mater. 2015, 28, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Lee, Y.T.; Lee, H.S.; Lee, Y.J.; Shokouh, S.H.; Kyhm, J.-H.; Lee, J.; Kim, H.H.; Yoo, T.-H.; Nam, S.H.; et al. Ultrasensitive PbS quantum-dot-sensitized InGaZnO hybrid photoinverter for near-infrared detection and imaging with high photogain. NPG Asia Mater. 2016, 8, e233. [Google Scholar] [CrossRef]
- Lei, Y.; Li, N.; Chan, W.-K.E.; Ong, B.S.; Zhu, F. Highly sensitive near infrared organic phototransistors based on conjugated polymer nanowire networks. Org. Electron. 2017, 48, 12–18. [Google Scholar] [CrossRef]
- Wu, G.; Fu, R.; Chen, J.; Yang, W.; Ren, J.; Guo, X.; Ni, Z.; Pi, X.; Li, C.Z.; Li, H.; et al. Perovskite/Organic Bulk-Heterojunction Integrated Ultrasensitive Broadband Photodetectors with High Near-Infrared External Quantum Efficiency over 70%. Small 2018, 14, 1802349. [Google Scholar] [CrossRef]
- Gasparini, N.; Gregori, A.; Salvador, M.; Biele, M.; Wadsworth, A.; Tedde, S.; Baran, D.; McCulloch, I.; Brabec, C.J. Visible and near-infrared imaging with nonfullerene-based photodetectors. Adv. Mater. Technol. 2018, 3, 1800104. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Chen, F.; Shao, J. Organic photodetectors based on copper phthalocyanine films prepared by a multiple drop casting method. Org. Electron. 2019, 66, 183–187. [Google Scholar] [CrossRef]
- Zheng, S.; Xiong, X.; Zheng, Z.; Xu, T.; Zhang, L.; Zhai, T.; Lu, X. Solution-grown large-area C60 single-crystal arrays as organic photodetectors. Carbon 2018, 126, 299–304. [Google Scholar] [CrossRef]
- Tong, S.; Yuan, J.; Zhang, C.; Wang, C.; Liu, B.; Shen, J.; Xia, H.; Zou, Y.; Xie, H.; Sun, J.; et al. Large-scale roll-to-roll printed, flexible and stable organic bulk heterojunction photodetector. NPJ Flex. Electron. 2018, 2, 7. [Google Scholar] [CrossRef]
- Su, Z.; Hou, F.; Wang, X.; Gao, Y.; Jin, F.; Zhang, G.; Li, Y.; Zhang, L.; Chu, B.; Li, W. High-Performance Organic Small-Molecule Panchromatic Photodetectors. ACS Appl. Mater. Interfaces 2015, 7, 2529–2534. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, F.; Du, L.; Lv, W.; Xu, K.; Peng, Y.; Wang, Y.; Lu, F. Ultrasensitive flexible broadband photodetectors achieving pA scale dark current. NPJ Flex. Electron. 2017, 1, 6. [Google Scholar] [CrossRef]
- Urbani, M.; Ragoussi, M.-E.; Nazeeruddin, M.K.; Torres, T. Phthalocyanines for dye-sensitized solar cells. Coord. Chem. Rev. 2019, 381, 1–64. [Google Scholar] [CrossRef]
- Choi, M.-S.; Lee, S.; Kim, H.J.; Kim, J.-J. Inverted near-infrared organic photodetector with oriented lead (II) phthalocyanine molecules via substrate heating. Org. Electron. 2018, 61, 164–169. [Google Scholar] [CrossRef]
- Vijayan, L.; Thomas, A.; Kumar, K.S.; Jinesh, K. Low power organic field effect transistors with copper phthalocyanine as active layer. J. Sci. Adv. Mater. Devices 2018, 3, 348–352. [Google Scholar] [CrossRef]
- Hamam, K.J.; Alomari, M.I. A study of the optical band gap of zinc phthalocyanine nanoparticles using UV-Vis spectroscopy and DFT function. Appl. Nanosci. 2017, 7, 261–268. [Google Scholar] [CrossRef]
- Hamamoto, N.; Sonoda, H.; Sumimoto, M.; Hori, K.; Fujimoto, H. Theoretical study on crystal polymorphism and electronic structure of lead(ii) phthalocyanine using model dimers. RSC Adv. 2017, 7, 8646–8653. [Google Scholar] [CrossRef]
- Sekhosana, K.E.; Shumba, M.; Nyokong, T. Electrochemical and non-linear optical behavior of a new neodymium double-decker phthalocyanine. Polyhedron 2017, 138, 154–160. [Google Scholar] [CrossRef]
- Choi, M.-S.; Chae, S.; Kim, H.J.; Kim, J.-J. Control of Crystallinity in PbPc:C60 Blend Film and Application for Inverted Near-Infrared Organic Photodetector. ACS Appl. Mater. Interfaces 2018, 10, 25614–25620. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.C.; Matsuhisa, N.; Zalar, P.; Koizumi, M.; Yokota, T.; Someya, T. Dual-gate organic phototransistor with high-gain and linear photoresponse. Nat. Commun. 2018, 9, 4546. [Google Scholar] [CrossRef]
- Binda, M.; Agostinelli, T.; Caironi, M.; Natali, D.; Sampietro, M.; Beverina, L.; Ruffo, R.; Silvestri, F. Fast and air stable near-infrared organic detector based on squaraine dyes. Org. Electron. 2009, 10, 1314–1319. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Wang, R.; Cao, M.; Che, Y.; Wang, J.; Wang, H.; Jin, L.; Dai, H.; Ding, X.; et al. Bulk-and layer-heterojunction phototransistors based on poly [2-methoxy-5-(2′-ethylhexyloxy-p-phenylenevinylene)] and PbS quantum dot hybrids. Appl. Phys. Lett. 2015, 106, 253501. [Google Scholar] [CrossRef]
- Peng, Y.; Lv, W.; Yao, B.; Fan, G.; Chen, D.; Gao, P.; Zhou, M.; Wang, Y. High performance near infrared photosensitive organic field-effect transistors realized by an organic hybrid planar-bulk heterojunction. Org. Electron. 2013, 14, 1045–1051. [Google Scholar] [CrossRef]
- Li, Y.; Lv, W.; Luo, X.; Sun, L.; Zhou, M.; Zhang, J.; Zhao, F.; Zhong, J.; Peng, Y. Enhanced performance of isotype planar heterojunction photoresponsive organic field-effect transistors by using Ag source-drain electrodes. Europhys. Lett. 2015, 110, 17006. [Google Scholar] [CrossRef]
- Baeg, K.-J.; Binda, M.; Natali, D.; Caironi, M.; Noh, Y.-Y. Organic Light Detectors: Photodiodes and Phototransistors. Adv. Mater. 2013, 25, 4267–4295. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Liu, D.; Yao, Y.; Zhou, G.; Li, P.; Wu, B.; Rao, X.; Song, Q. Pentacene as a hole transport material for high performance planar perovskite solar cells. Curr. Appl. Phys. 2018, 18, 1095–1100. [Google Scholar] [CrossRef]
- Castro, E.; Fernandez-Delgado, O.; Arslan, F.; Zavala, G.; Yang, T.; Seetharaman, S.; D’Souza, F.; Echegoyen, L. New thiophene-based C60 fullerene derivatives as efficient electron transporting materials for perovskite solar cells. New J. Chem. 2018, 42, 14551–14558. [Google Scholar] [CrossRef]
- Li, Y.; Lv, W.; Luo, X.; Sun, L.; Zhao, F.; Zhang, J.; Zhong, J.; Huang, F.; Peng, Y. Enhanced performance of PbPc photosensitive organic field effect transistors by inserting different-thickness pentacene inducing layers. Org. Electron. 2015, 26, 186–190. [Google Scholar] [CrossRef]
- Collins, R.A.; Belghachi, A. Structural properties of lead phthalocyanine thin films. Mater. Lett. 1989, 8, 349–352. [Google Scholar] [CrossRef]
- Kumaki, D.; Yahiro, M.; Inoue, Y.; Tokito, S. Air stable, high performance pentacene thin-film transistor fabricated on SiO2 gate insulator treated with β-phenethyltrichlorosilane. Appl. Phys. Lett. 2007, 90, 133511. [Google Scholar] [CrossRef]
- Kagan, C.R.; Afzali, A.; Graham, T.O. Operational and environmental stability of pentacene thin-film transistors. Appl. Phys. Lett. 2005, 86, 193505. [Google Scholar] [CrossRef]
- Pevzner, B.; Hebard, A.F.; Dresselhaus, M.S. Role of molecular oxygen and other impurities in the electrical transportand dielectric properties of C60 films. Phys. Rev. B 1997, 55, 16439–16449. [Google Scholar] [CrossRef]
- Tsetseris, L.; Pantelides, S.T. Oxygen and water-related impurities in C60 crystals: A density-functional theory study. Phys. Rev. B 2010, 82, 045201. [Google Scholar] [CrossRef]
- Letizia, J.A.; Facchetti, A.; Stern, C.L.; Ratner, M.A.; Marks, T.J. High electron mobility in solution-cast and vapor-deposited phenacyl-quaterthiophene-based field-effect transistors: Toward n-type polythiophenes. J. Am. Chem. Soc. 2005, 127, 13476–13477. [Google Scholar] [CrossRef]
- Li, Y.; Pan, M.; Hu, Y.; Wang, Z.; Lv, W.; Peng, Y. The influence of substrate temperature on the near-infrared absorption and carrier mobility of lead phthalocyanine phototransistors. Thin Solid Films 2021, 718, 138481. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Xia, H.; Zhang, J.; Xu, K.; Peng, Y.; Liu, G. Towards high performance broad spectral response fullerene based photosensitive organic field-effect transistors with tricomponent bulk heterojunctions. Carbon 2017, 118, 666–674. [Google Scholar] [CrossRef]

| Active Layer Structures | μ a (cm2/V·s) | R b (mA/W) | Pmax | D* (Jones) | EQE |
|---|---|---|---|---|---|
| A: PbPc | 8.5 × 10−4 | 4.43 | 9.3 | 5.5 × 109 | 0.65% |
| B: pentacene/PbPc | 3.0 × 10−2 | 139 | 5.3 × 103 | 6.2 × 1010 | 20.29% |
| C: C60/PbPc | 3.9 × 10−2 | 142 | 5.5 × 103 | 5.3 × 1010 | 20.73% |
| D: pentacene/PbPc/C60 | 2.4 × 10−2 | 1415 | 1.2 × 104 | 7.0 × 1011 | 206.55% |
| Structure | Response Wavelength (nm) | R (mA/W) | References |
|---|---|---|---|
| n+-Si/SiO2/CuPc/PbPc:PTCDA/Au | 808 | 322 mA/W | [26] |
| n+-Si/SiO2/PbPc/pentacene/Ag | 808 | 123 mA/W | [27] |
| n+-Si/SiO2/PbPc/pentacene(2 nm)/Au | 808 | 505 mA/W | [31] |
| n+-Si/SiO2/PbPc/Au | 850 | 12.08 mA/W | [38] |
| n+-Si/SiO2/C60/PbPc:AlClPc:PTCDA/Au | 850 | 580 mA/W | [39] |
| n+-Si/SiO2/pentacene/PbPc/C60/Au | 850 | 1415 mA/W | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Wang, Y.; Li, Y.; Zhang, L. Air-Stable Near-Infrared Sensitive Organic Phototransistors Realized via Tri-Layer Planar Heterojunction. Appl. Sci. 2023, 13, 12613. https://doi.org/10.3390/app132312613
Hu Y, Wang Y, Li Y, Zhang L. Air-Stable Near-Infrared Sensitive Organic Phototransistors Realized via Tri-Layer Planar Heterojunction. Applied Sciences. 2023; 13(23):12613. https://doi.org/10.3390/app132312613
Chicago/Turabian StyleHu, Yao, Yongshun Wang, Yao Li, and Lijun Zhang. 2023. "Air-Stable Near-Infrared Sensitive Organic Phototransistors Realized via Tri-Layer Planar Heterojunction" Applied Sciences 13, no. 23: 12613. https://doi.org/10.3390/app132312613






