Optimization of Ca–Al–Mn–Si Substitution Level for Enhanced Magnetic Properties of M-Type Sr-Hexaferrites for Permanent Magnet Application
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
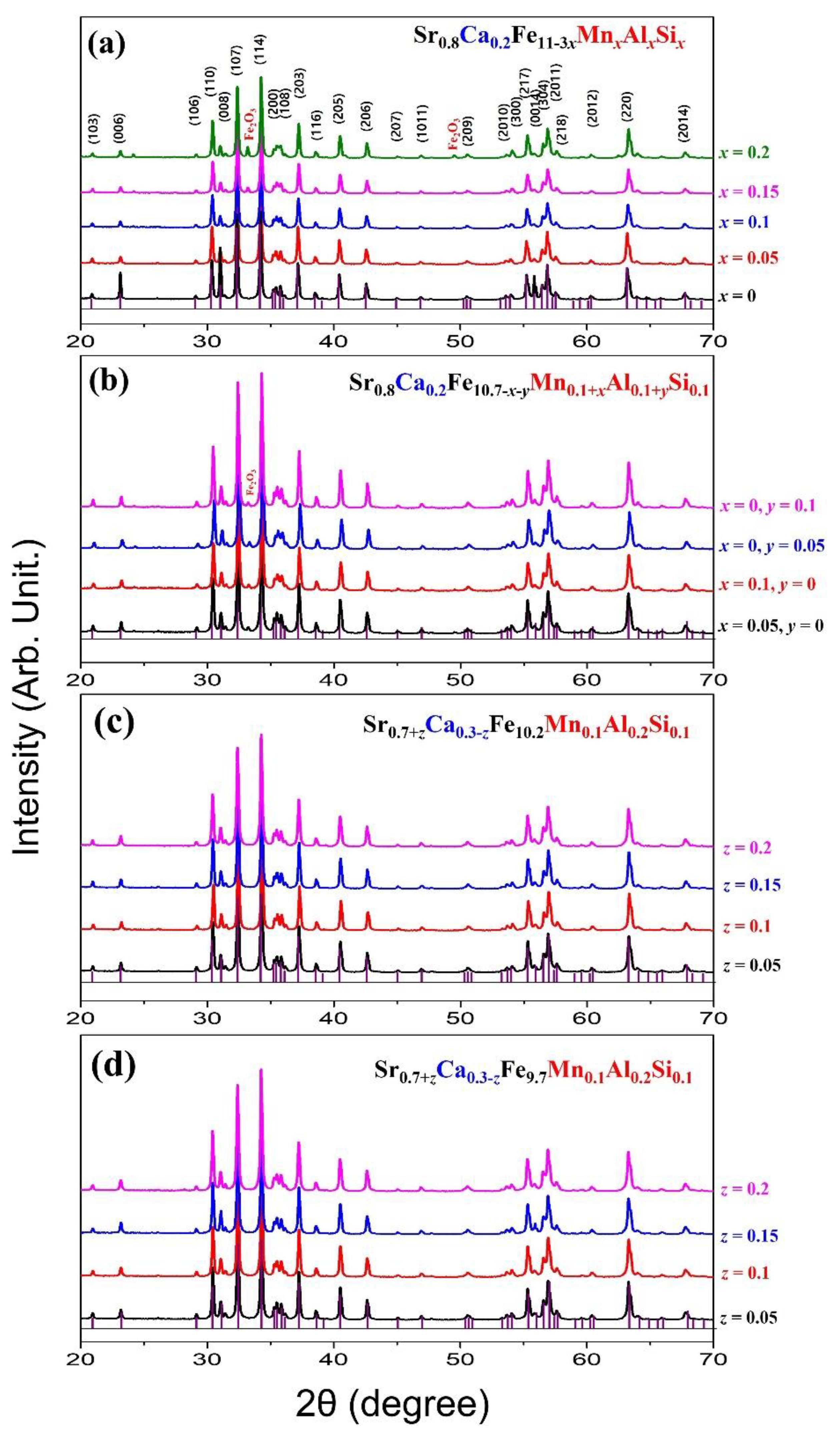
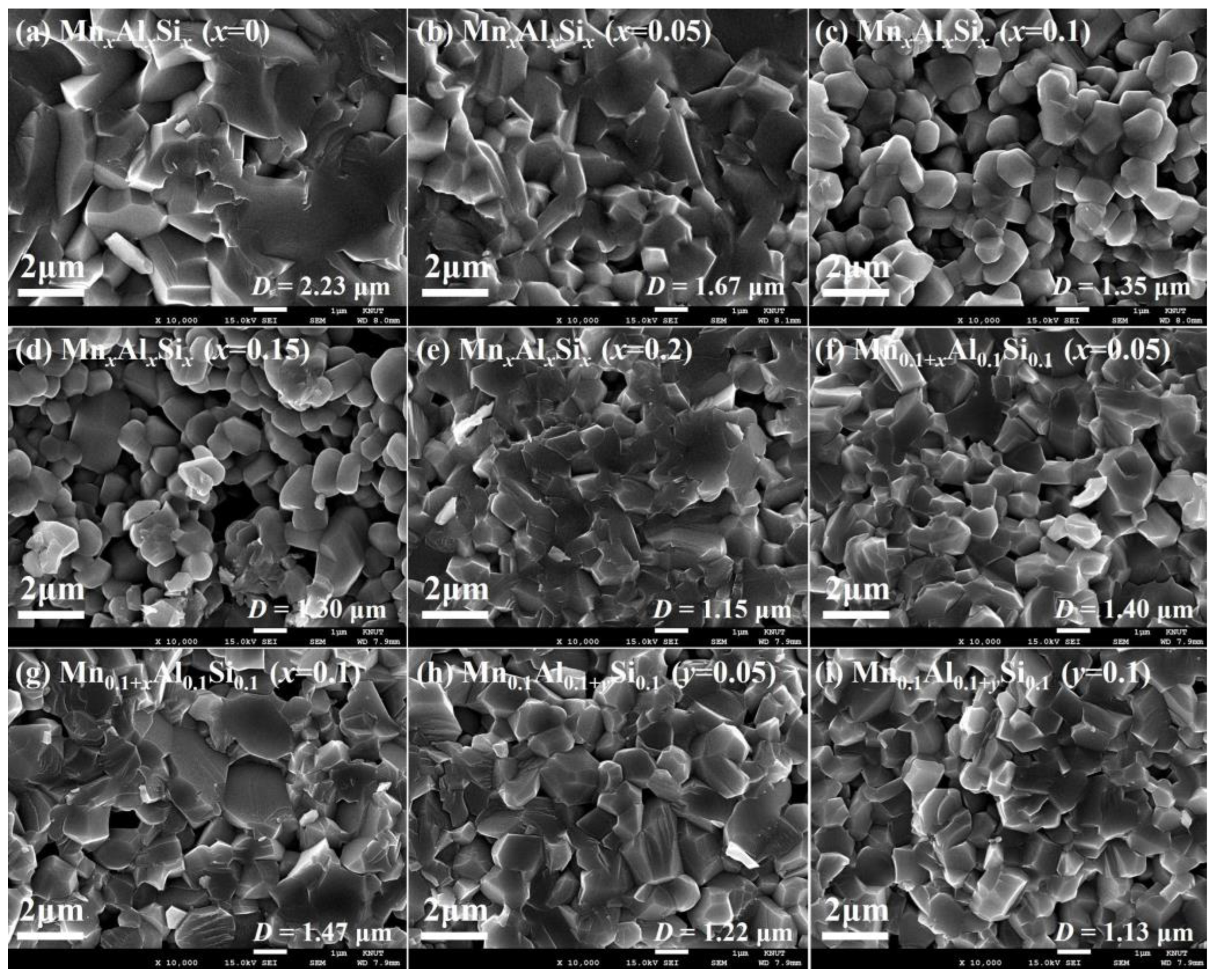
3.1. Crystalline Structure and Microstructure Analysis
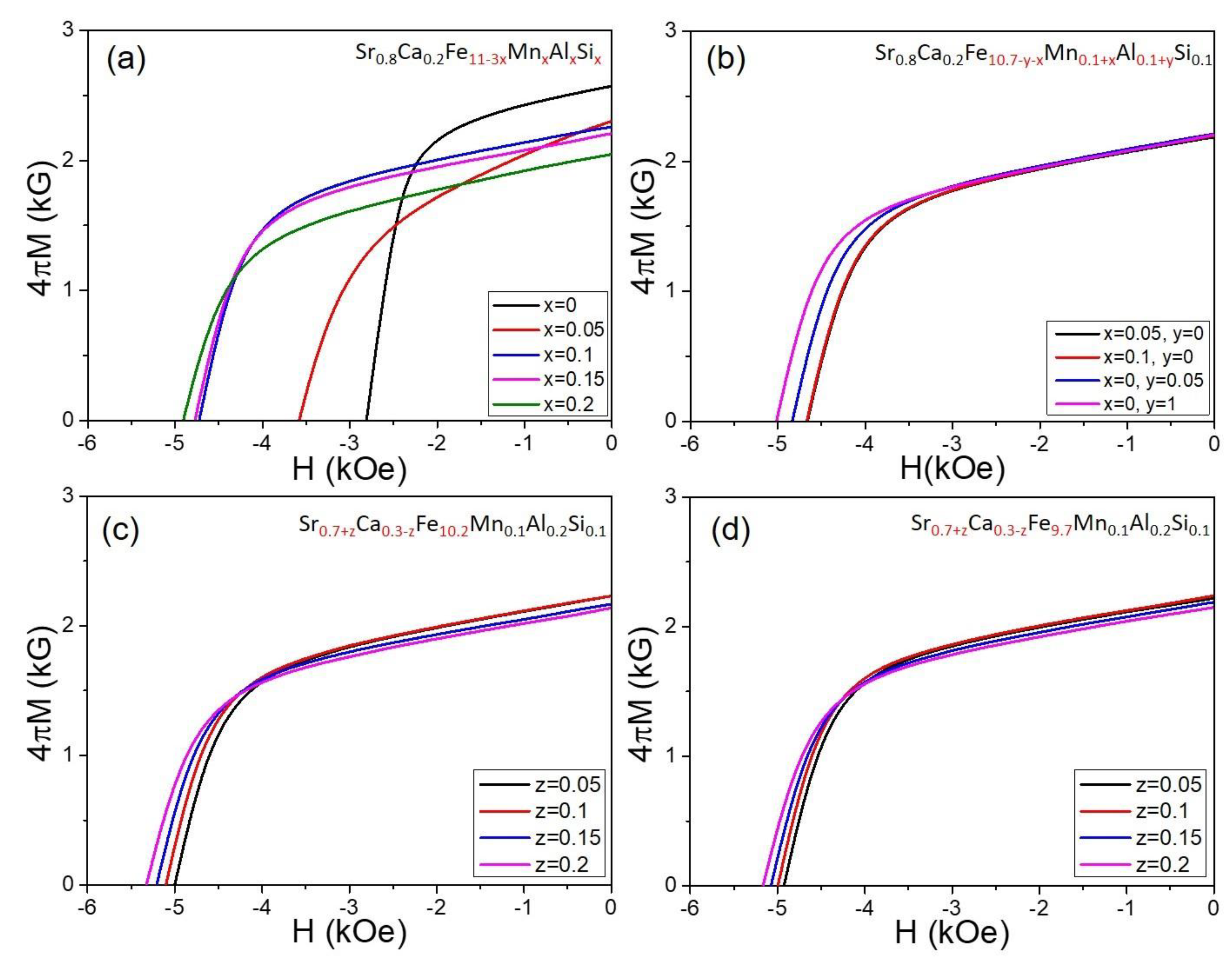
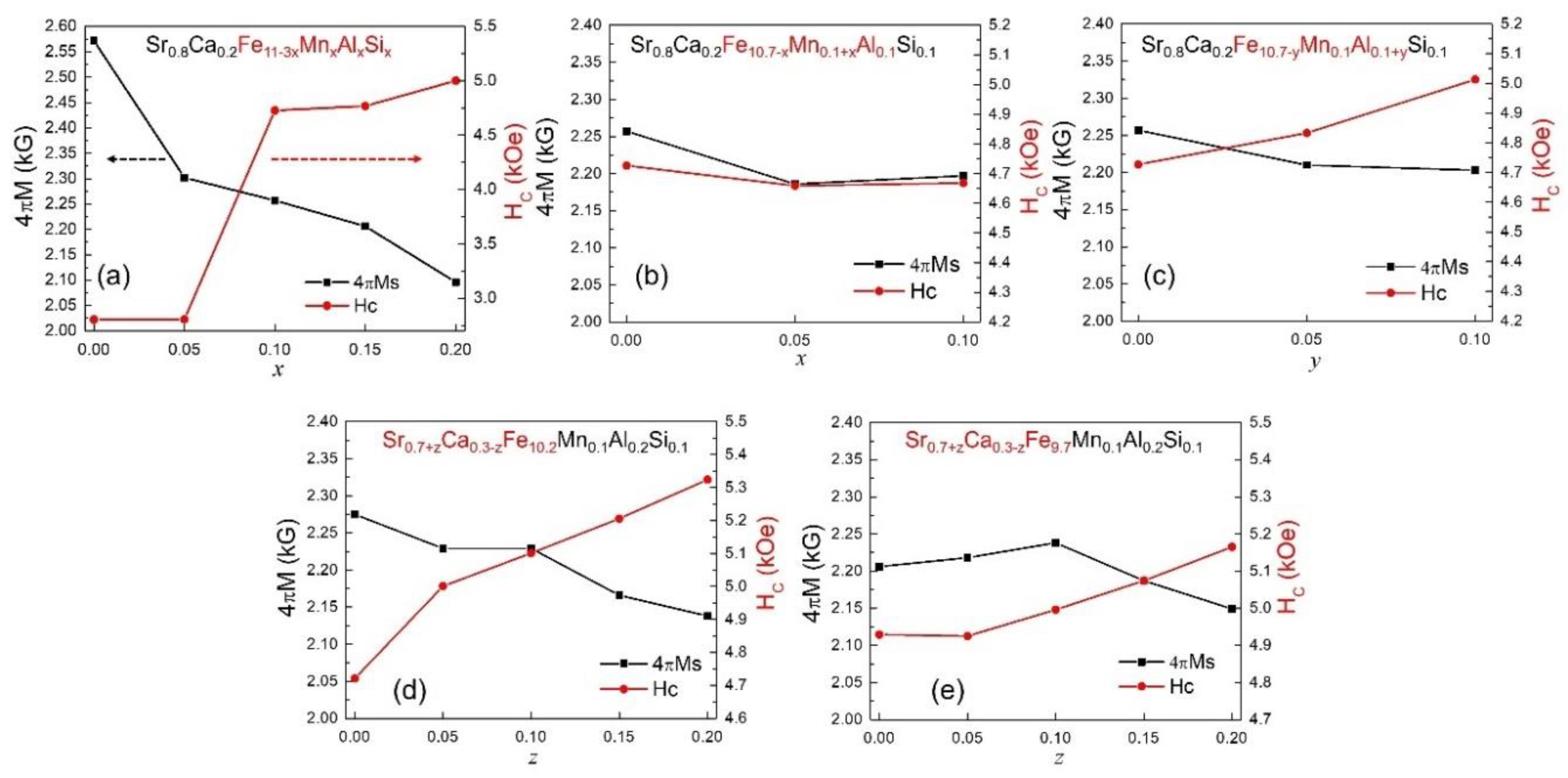
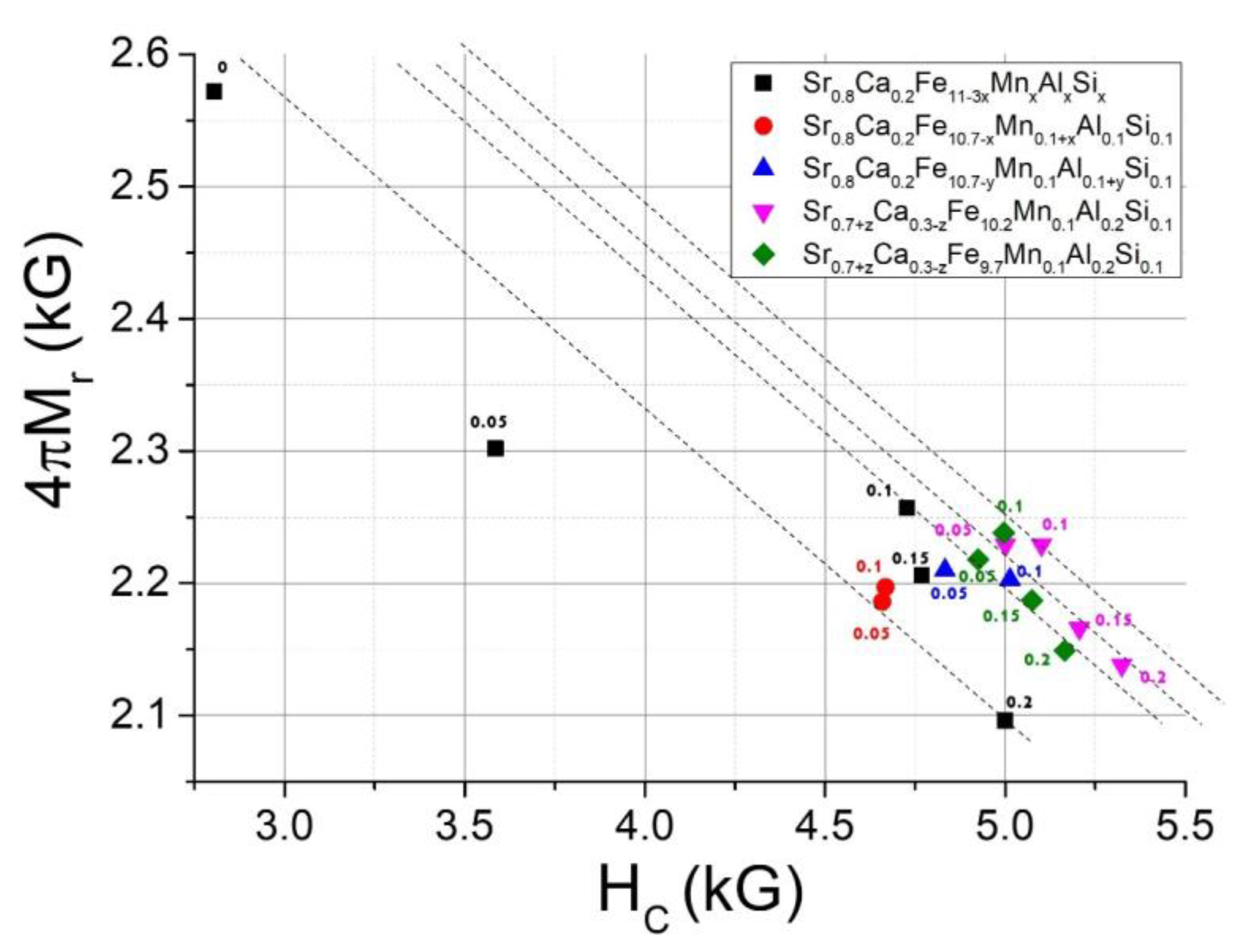
3.2. Magnetic Property Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Went, J.; Rathenau, G.; Gorter, E.; Oosterhout, G.V. Ferroxdure, a class of new permanent magnet materials. Philips Tech. Rev. 1952, 13, 194–208. [Google Scholar]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Granados-Miralles, C.; Jenuš, P. On the potential of hard ferrite ceramics for permanent magnet technology—A review on sintering strategies. J. Phys. D Appl. Phys. 2021, 54, 303001. [Google Scholar] [CrossRef]
- Bai, J.; Liu, X.; Xie, T.; Wei, F.; Yang, Z. The effects of La–Zn substitution on the magnetic properties of Sr-magnetoplumbite ferrite nano-particles. Mater. Sci. Eng. B 2000, 68, 182–185. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Kwon, Y.-H.; Kim, M.-H.; Lee, D.-Y. Enhancement of magnetic properties in Mn-Zn substituted M-type Sr-hexaferrites. J. Magn. Magn. Mater. 2015, 382, 10–14. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Moon, K.-S. Magnetic properties of Ce-Mn substituted M-type Sr-hexaferrites. Ceram. Int. 2015, 41, 12828–12834. [Google Scholar] [CrossRef]
- Moon, K.-S.; Kang, Y.-M. Structural and magnetic properties of Ca-Mn-Zn-substituted M-type Sr-hexaferrites. J. Eur. Ceram. Soc. 2016, 36, 3383–3389. [Google Scholar] [CrossRef]
- Huang, K.; Yu, J.; Zhang, L.; Xu, J.; Yang, Z.; Liu, C.; Wang, W.; Kan, X. Structural and magnetic properties of Gd–Zn substituted M-type Ba–Sr hexaferrites by sol-gel auto-combustion method. J. Alloys Compd. 2019, 803, 971–980. [Google Scholar] [CrossRef]
- Kools, F.; Morel, A.; Grössinger, R.; Le Breton, J.M.; Tenaud, P. LaCo-substituted ferrite magnets, a new class of high-grade ceramic magnets; intrinsic and microstructural aspects. J. Magn. Magn. Mater. 2002, 242–245, 1270–1276. [Google Scholar] [CrossRef]
- Ogata, Y.; Takami, T.; Kubota, Y. Development of La-Co Substituted Ferrite Magnets. J. Jpn. Soc. Powder Powder Metall. 2003, 50, 636–641. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hosokawa, S.; Oda, E.; Toyota, S. Magnetic properties and composition of Ca-La-Co M-type ferrites. J. Jpn. Soc. Powder Powder Metall. 2008, 55, 541–546. [Google Scholar] [CrossRef]
- Lee, J.; Lee, E.J.; Hwang, T.-Y.; Kim, J.; Choa, Y.-H. Anisotropic characteristics and improved magnetic performance of Ca–La–Co-substituted strontium hexaferrite nanomagnets. Sci. Rep. 2020, 10, 15929. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-S.; Yu, P.-Y.; Kang, Y.-M. Microstructure and magnetic properties of La-Ca-Co substituted M-type Sr-hexaferrites with controlled Si diffusion. Appl. Sci. 2020, 10, 7570. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, T.; Xu, B.; Dong, Z.; Wu, J.; Zhang, M.; Chen, Y.; Zhong, S. Clean and economical preparation of Ca-La-Co substituted permanent ferrite magnet with ultrapure magnetite concentrate. J. Alloys Compd. 2023, 956, 170334. [Google Scholar] [CrossRef]
- Yasmin, N.; Mirza, M.; Muhammad, S.; Zahid, M.; Ahmad, M.; Awan, M.S.; Muhammad, A. Influence of samarium substitution on the structural and magnetic properties of M-type hexagonal ferrites. J. Magn. Magn. Mater. 2018, 446, 276–281. [Google Scholar] [CrossRef]
- Moon, K.-S.; Lim, E.-S.; Kang, Y.-M. Effect of Ca and La substitution on the structure and magnetic properties of M-type Sr-hexaferrites. J. Alloys Compd. 2019, 771, 350–355. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güner, S.; van Leusen, J.; Baykal, A.; Kögerler, P. Effect of Nb3+ ion substitution on the magnetic properties of SrFe12O19 hexaferrites. J. Mater. Sci. Mater. Electron. 2019, 30, 11181–11192. [Google Scholar] [CrossRef]
- Todkar, G.B.; Kunale, R.A.; Kamble, R.N.; Batoo, K.M.; Ijaz, M.F.; Imran, A.; Hadi, M.; Raslan, E.H.; Shirsath, S.E.; Kadam, R.H. Ce–Dy substituted barium hexaferrite nanoparticles with large coercivity for permanent magnet and microwave absorber application. J. Phys. D Appl. Phys. 2021, 54, 294001. [Google Scholar] [CrossRef]
- Han, G.; Sui, R.; Yu, Y.; Wang, L.; Li, M.; Li, J.; Liu, H.; Yang, W. Structure and magnetic properties of the porous Al-substituted barium hexaferrites. J. Magn. Magn. Mater. 2021, 528, 167824. [Google Scholar] [CrossRef]
- Manglam, M.K.; Shukla, A.; Mallick, J.; Yadav, M.K.; Kumari, S.; Zope, M.; Kar, M. Enhancement of coercivity of M-type barium hexaferrite by Ho doping. Mater. Today Proc. 2022, 59, 149–152. [Google Scholar] [CrossRef]
- Yu, P.-Y.; Kim, M.-H.; Kang, Y.-M. Development of a high-performance ferrite magnet fabrication process without sintering additives. Korean J. Met. Mater. 2021, 59, 551–559. [Google Scholar] [CrossRef]
- Yoo, J.-Y.; Lee, K.-H.; Kang, Y.-M.; Yoo, S.-I. Enhancement of the magnetic properties in Si4+-Li+-substituted M-type hexaferrites for permanent magnets. Appl. Sci. 2022, 12, 12295. [Google Scholar] [CrossRef]
- Lim, J.-P.; Kang, M.-G.; Kang, Y.-M. Development of Multi-Cation-Doped M-Type Hexaferrite Permanent Magnets. Appl. Sci. 2023, 13, 295. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Liu, R.; Zhou, N.; Xu, Z.; Gong, H.; Zhao, T.; Sun, J.; Hu, F.; Shen, B. Simultaneous improvement of coercivity and saturation magnetization of M-type strontium ferrite by Nd3+-Co2+ unequal co-substitution. Ceram. Int. 2023, 49, 10499–10505. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Banihashemi, V. Effects of Ca–Gd co-substitution on the structural, magnetic, and dielectric properties of M-type strontium hexaferrite. J. Am. Ceram. Soc. 2023, 106, 5351–5363. [Google Scholar] [CrossRef]
- Thompson, S.; Shirtcliffe, N.J.; O’Keefe, E.S.; Appleton, S.; Perry, C.C. Synthesis of SrCoxTixFe(12−2x)O19 through sol–gel auto-ignition and its characterization. J. Magn. Magn. Mater. 2005, 292, 100–107. [Google Scholar] [CrossRef]
- Shekhawat, D.; Singh, A.K.; Roy, P.K. Structural and electro-magnetic properties of high (BH)max La-Sm substituted Sr-hexaferrite for brushless DC electric motors application. J. Mol. Struct. 2019, 1179, 787–794. [Google Scholar] [CrossRef]
- Eikeland, A.Z.; Stingaciu, M.; Mamakhel, A.H.; Saura-Múzquiz, M.; Christensen, M. Enhancement of magnetic properties through morphology control of SrFe12O19 nanocrystallites. Sci. Rep. 2018, 8, 7325. [Google Scholar] [CrossRef]
- Nishio, H.; Minachi, Y.; Yamamoto, H. Effect of factors on coercivity in Sr-La-Co sintered ferrite magnets. IEEE Trans. Magn. 2009, 45, 5281–5288. [Google Scholar] [CrossRef]
| Cation Composition | x, y, z | XRD (Phase) | ρ (g/cm3) | a (Å) | c (Å) | V (Å3) | 4πMr (kG) | HC (kOe) |
|---|---|---|---|---|---|---|---|---|
| Sr0.8Ca0.2Fe11−3x MnxAlxSix | x = 0 | M | 4.96 | 5.881 | 23.05 | 690.3 | 2.57 | 2.80 |
| x = 0.05 | M | 4.89 | 5.881 | 23.04 | 690.0 | 2.30 | 3.59 | |
| x = 0.1 | M+Fe2O3 | 4.81 | 5.875 | 23.04 | 688.8 | 2.25 | 4.72 | |
| x = 0.15 | M+Fe2O3 | 4.90 | 5.870 | 23.04 | 687.1 | 2.20 | 4.76 | |
| x = 0.2 | M+Fe2O3 | 4.90 | 5.871 | 23.04 | 687.7 | 2.04 | 4.90 | |
| Sr0.8Ca0.2Fe10.7−x−y Mn0.1+xAl0.1+ySi0.1 | x = 0.05 | M+Fe2O3 | 4.78 | 5.869 | 23.01 | 686.3 | 2.18 | 4.65 |
| x = 0.1 | M+Fe2O3 | 4.74 | 5.863 | 22.98 | 684.1 | 2.19 | 4.66 | |
| y = 0.05 | M+Fe2O3 | 4.80 | 5.852 | 22.94 | 680.4 | 2.21 | 4.83 | |
| y = 0.1 | M+Fe2O3 | 4.85 | 5.866 | 23.00 | 685.3 | 2.20 | 5.01 | |
| Sr0.7+zCa0.3−zFe10.2 Mn0.1Al0.2Si0.1 | z = 0.05 | M | 4.81 | 5.870 | 23.01 | 686.5 | 2.22 | 5.00 |
| z = 0.1 | M | 4.84 | 5.861 | 22.99 | 684.1 | 2.22 | 5.10 | |
| z = 0.15 | M | 4.76 | 5.868 | 23.01 | 686.0 | 2.16 | 5.20 | |
| z = 0.2 | M | 4.70 | 5.872 | 23.03 | 687.6 | 2.13 | 5.32 | |
| Sr0.7+zCa0.3−zFe9.7 Mn0.1Al0.2Si0.1 | z = 0.05 | M | 4.88 | 5.870 | 23.00 | 686.2 | 2.21 | 4.92 |
| z = 0.1 | M | 5.00 | 5.869 | 23.00 | 686.2 | 2.23 | 4.99 | |
| z = 0.15 | M | 4.86 | 5.869 | 23.00 | 686.2 | 2.18 | 5.07 | |
| z = 0.2 | M | 4.73 | 5.868 | 23.02 | 686.5 | 2.14 | 5.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-P.; Yun, E.-H.; Kang, Y.-M. Optimization of Ca–Al–Mn–Si Substitution Level for Enhanced Magnetic Properties of M-Type Sr-Hexaferrites for Permanent Magnet Application. Appl. Sci. 2023, 13, 12708. https://doi.org/10.3390/app132312708
Lim J-P, Yun E-H, Kang Y-M. Optimization of Ca–Al–Mn–Si Substitution Level for Enhanced Magnetic Properties of M-Type Sr-Hexaferrites for Permanent Magnet Application. Applied Sciences. 2023; 13(23):12708. https://doi.org/10.3390/app132312708
Chicago/Turabian StyleLim, Jun-Pyo, Eel-Ho Yun, and Young-Min Kang. 2023. "Optimization of Ca–Al–Mn–Si Substitution Level for Enhanced Magnetic Properties of M-Type Sr-Hexaferrites for Permanent Magnet Application" Applied Sciences 13, no. 23: 12708. https://doi.org/10.3390/app132312708
APA StyleLim, J.-P., Yun, E.-H., & Kang, Y.-M. (2023). Optimization of Ca–Al–Mn–Si Substitution Level for Enhanced Magnetic Properties of M-Type Sr-Hexaferrites for Permanent Magnet Application. Applied Sciences, 13(23), 12708. https://doi.org/10.3390/app132312708







