Fir (Abies alba Mill.) Honeydew Honey Inhibits Growth and Adhesion of Campylobacter jejuni In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fir (Abies alba Mill.) Honeydew Honey
2.2. Melissopalynological and Physicochemical Analyses
2.3. Determination of the Concentration of Total Phenols and Flavonoids in the Fir Honeydew Honey
2.4. Bacterial Strains and Growth Conditions
2.5. Food Model
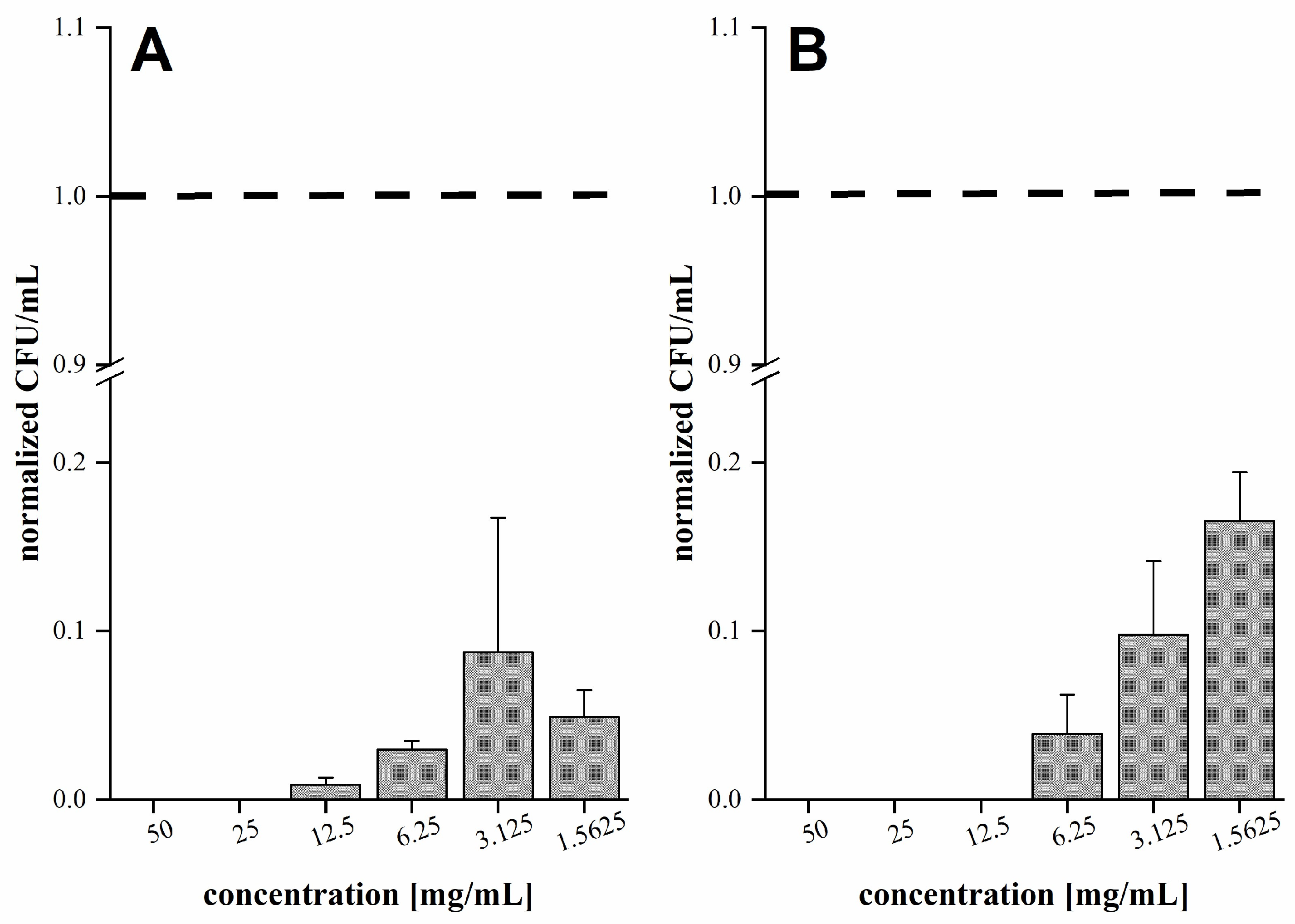
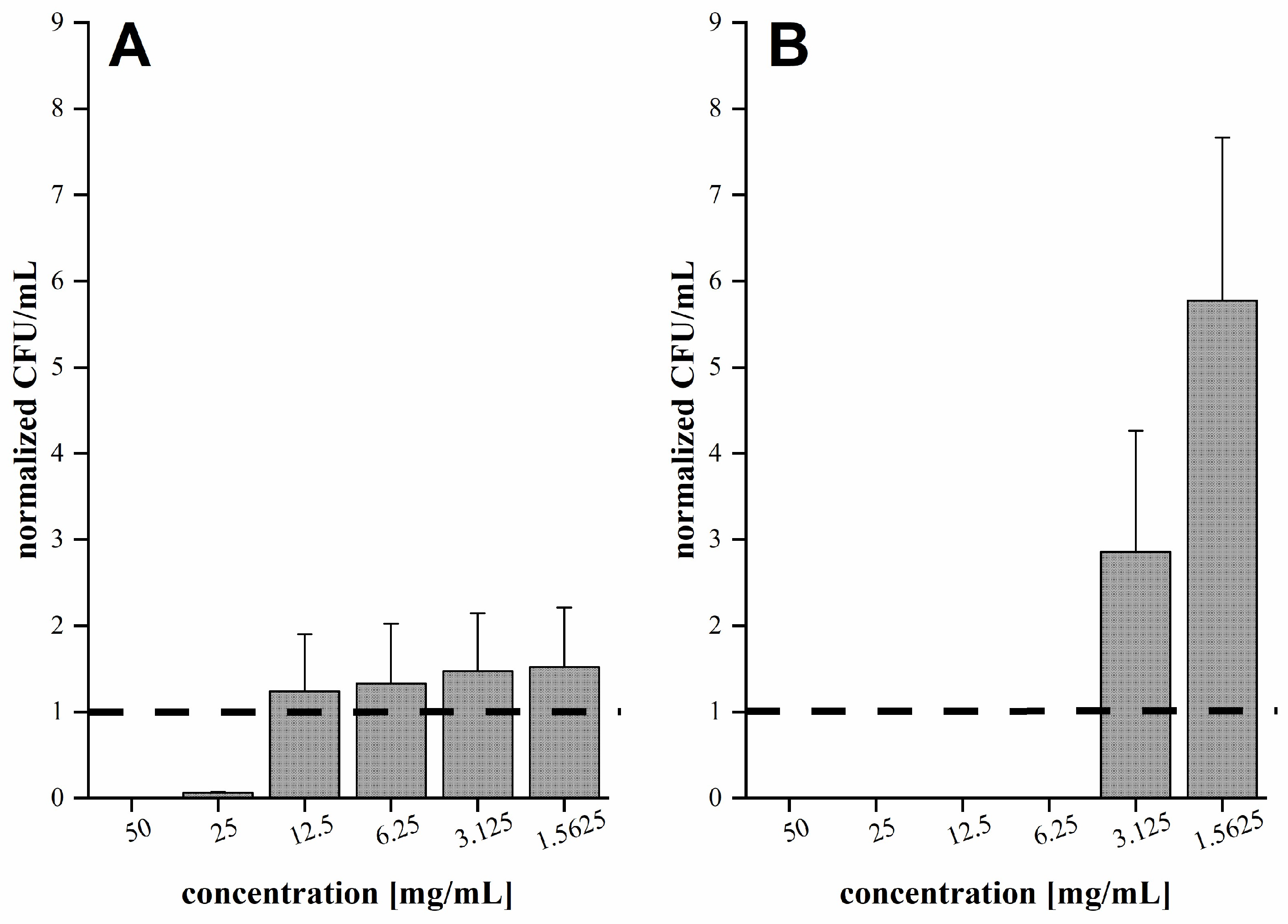
2.6. Determination of Antibacterial Activity
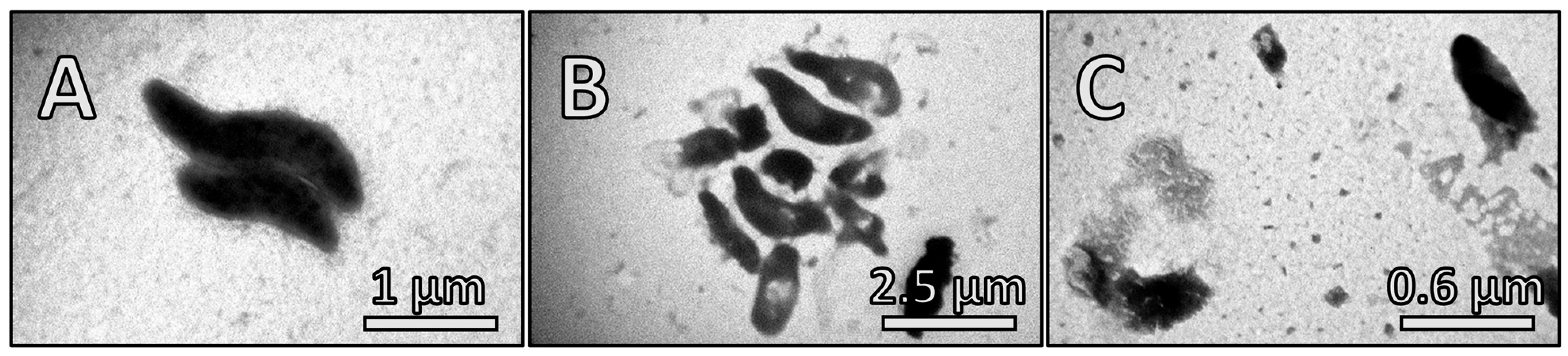
2.7. Transmission Electron Microscopy (TEM)
2.8. Bacterial Growth Inhibition in MH Broth or Milk
2.9. Anti-Adhesion Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Fir Honeydew Honey Sample
3.2. Antibacterial Activity of Fir Honeydew Honey
3.3. The Effect of Fir Honeydew Honey on Bacterial Growth and Cell Structure
3.4. Anti-Adhesion Effect of Fir Honeydew Honey
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Estellé, M.; Hernández-González, V.; Saurina, J.; Núñez, O.; Sentellas, S. Characterization and classification of Spanish honeydew and blossom honeys based on their antioxidant capacity. Antioxidants 2023, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- 581. Off. J. Eur. Communities. 2002. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:010:0047:0052:EN:PDF (accessed on 10 September 2023).
- FAO; WHO. Codex Alimentarius Commission—Procedural Manual, 27th ed.; FAO: Rome, Italy; WHO: Rome, Italy, 2019; ISBN 978-92-5-131099-1. [Google Scholar]
- Pita-Calvo, C.; Vázquez, M. Differences between honeydew and blossom honeys: A review. Trends Food Sci. Technol. 2017, 59, 79–87. [Google Scholar] [CrossRef]
- Iglesias, M.T.; De Lorenzo, C.; Polo, M.D.C.; Martín-Álvarez, P.J.; Pueyo, E. Usefulness of amino acid composition to discriminate between honeydew and floral honeys. Application to honeys from a small geographic area. J. Agric. Food Chem. 2004, 52, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Pita-Calvo, C.; Vázquez, M. Honeydew honeys: A review on the characterization and authentication of botanical and geographical origins. J. Agric. Food Chem. 2018, 66, 2523–2537. [Google Scholar] [CrossRef] [PubMed]
- Kunat-Budzynska, M.; Rysiak, A.; Wiater, A.; Graz, M.; Andrejko, M.; Budzynski, M.; Brys, M.S.; Sudzinski, M.; Tomczyk, M.; Gancarz, M.; et al. Chemical composition and antimicrobial activity of new honey varietals. Int. J. Environ. Res. Public Health 2023, 20, 2458. [Google Scholar] [CrossRef] [PubMed]
- Vela, L.; De Lorenzo, C.; Pérez, R.A. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J. Sci. Food Agric. 2007, 87, 1069–1075. [Google Scholar] [CrossRef]
- Lušić, D.; Koprivnjak, O.; Ćurić, D.; Sabatini, A.G.; Conte, L.S. Volatile profile of Croatian lime tree (Tilia sp.), fir honeydew (Abies alba) and sage (Salvia officinalis) honey. Food Technol. Biotechnol. 2007, 45, 156–165. [Google Scholar]
- Broznić, D.; Ratkaj, I.; Malenica, M.; Pavelić, S.; Zurga, P.; Bubalo, D.; Gobin, I. Evaluation of the antioxidant capacity, antimicrobial and antiproliferative potential of fir (Abies alba Mill.) honeydew honey collected from Gorski Kotar (Croatia). Food Technol. Biotechnol. 2018, 56, 533–545. [Google Scholar] [CrossRef]
- Artursson, K.; Schelin, J.; Thisted Lambertz, S.; Hansson, I.; Olsson Engvall, E. Foodborne pathogens in unpasteurized milk in Sweden. Int. J. Food Microbiol. 2018, 284, 120–127. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- García-Sánchez, L.; Melero, B.; Rovira, J. Campylobacter in the Food Chain. Adv. Food Nutr. Res. 2018, 86, 215–252. [Google Scholar] [PubMed]
- Oh, E.; Andrews, K.J.; Jeon, B. Enhanced biofilm formation by ferrous and ferric iron through oxidative stress in Campylobacter jejuni. Front. Microbiol. 2018, 9, 1204. [Google Scholar] [CrossRef] [PubMed]
- Gölz, G.; Kittler, S.; Malakauskas, M.; Alter, T. Survival of Campylobacter in the food chain and the environment. Curr. Clin. Microbiol. Rep. 2018, 5, 126–134. [Google Scholar] [CrossRef]
- Johnson, T.J.; Shank, J.M.; Johnson, J.G. Current and potential treatments for reducing Campylobacter colonization in animal hosts and disease in humans. Front. Microbiol. 2017, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Teh, A.H.T.; Lee, S.M.; Dykes, G.A. Does Campylobacter jejuni form biofilms in food-related environments? Appl. Environ. Microbiol. 2014, 80, 5154–5160. [Google Scholar] [CrossRef] [PubMed]
- Burnham, P.M.; Hendrixson, D.R. Campylobacter jejuni: Collective components promoting a successful enteric lifestyle. Nat. Rev. Microbiol. 2018, 16, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Brahmbhatt, M.N.; Chatur, Y.A.; Nayak, J.B. Prevalence of Campylobacter species in milk and milk products, their virulence gene profile and antibiogram. Vet. World 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Del Collo, L.P.; Karns, J.S.; Biswas, D.; Lombard, J.E.; Haley, B.J.; Kristensen, R.C.; Kopral, C.A.; Fossler, C.P.; Van Kessel, J.A.S. Prevalence, antimicrobial resistance, and molecular characterization of Campylobacter spp. in bulk tank milk and milk filters from US dairies. J. Dairy Sci. 2017, 100, 3470–3479. [Google Scholar] [CrossRef]
- Kurin, M.; Berce, I.; Zorman, T.; Smole Možina, S. The prevalence of multiple antibiotic resistance in Campylobacter spp. from retail poultry meat. Food Technol. Biotechnol. 2005, 43, 157–163. [Google Scholar]
- Smole Možina, S.; Kurinčič, M.; Klančnik, A.; Mavri, A. Campylobacter and its multi-resistance in the food chain. Trends Food Sci. Technol. 2011, 22, 91–98. [Google Scholar] [CrossRef]
- Vasić, V.; Gašić, U.; Stanković, D.; Lušić, D.; Vukić-Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Towards better quality criteria of European honeydew honey: Phenolic profile and antioxidant capacity. Food Chem. 2019, 274, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Piskernik, S.; Bucar, F.; Vučković, D.; Smole Možina, S.; Jeršek, B. Reduction of microbiological risk in minced meat by a combination of natural antimicrobials: Effects of antimicrobials on a bacterial cocktail. J. Sci. Food Agric. 2014, 94, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Bogdanov, S. Harmonised methods of the International Honey Commission. Int. Honey Comm. 2002, 1–62. [Google Scholar]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; Von Der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.-G.; Marcazzan, G.L.; Piro, R.; et al. Honey quality and international regulatory standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Zorman, T.; Smole Možina, S. Classical and molecular identification of thermotolerant Campylobacters from poultry meat. Food Technol. Biotechnol. 2002, 40, 177–184. [Google Scholar]
- Klančnik, A.; Guzej, B.; Kolar, M.H.; Abramovič, H.; Smole Možina, S. In vitro antimicrobial and antioxidant activity of commercial rosemary extract formulations. J. Food Prot. 2009, 72, 1744–1752. [Google Scholar] [CrossRef]
- Kovač, J.; Šimunović, K.; Wu, Z.; Klančnik, A.; Bucar, F.; Zhang, Q.; Smole Možina, S. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS ONE 2015, 10, e0122871. [Google Scholar] [CrossRef] [PubMed]
- Warui, M.W.; Hansted, L.; Gikungu, M.; Mburu, J.; Kironchi, G.; Bosselmann, A.S. Characterization of Kenyan honeys based on their physicochemical properties, botanical and geographical origin. Int. J. Food Sci. 2019, 2019, 2932509. [Google Scholar] [CrossRef] [PubMed]
- Primorac, L.; Angelkov, B.; Mandić, M.; Čačić Kenjerić, D.; Nedeljko, M.; Flanjak, I.; Pirički, A.; Arapcheska, M. Comparison of the Croatian and Macedonian honeydew honey. J. Cent. Eur. Agric. 2009, 10, 263–270. [Google Scholar]
- Diez, M.J.; Andres, C.; Terrab, A. Physicochemical parameters and pollen analysis of Moroccan honeydew honeys. Int. J. Food Sci. Technol. 2004, 39, 167–176. [Google Scholar] [CrossRef]
- Golob, T.; Plestenjak, A. Quality of Slovene honey. Food Technol. Biotehnol. 1999, 37, 195–201. [Google Scholar]
- Delafuente, E.; Sanz, M.; Martinezcastro, I.; Sanz, J.; Ruizmatute, A. Volatile and carbohydrate composition of rare unifloral honeys from Spain. Food Chem. 2007, 105, 84–93. [Google Scholar] [CrossRef]
- Devillers, J.; Morlot, M.; Pham-Delègue, M.H.; Doré, J.C. Classification of monofloral honeys based on their quality control data. Food Chem. 2004, 86, 305–312. [Google Scholar] [CrossRef]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Bobis, O.; Marghitas, L.; Krisztina, R.; Niculae, M.; Dezmirean, D. Honeydew honey: Correlations between chemical composition, antioxidant capacity and antibacterial effect. Lucr. Ştiinţ. Zooteh. Biotehnol. Timis. 2008, 41, 1–9. [Google Scholar]
- Kuś, P.M.; Jerković, I.; Marijanović, Z.; Tuberoso, C.I.G. Screening of Polish fir honeydew honey using GC/MS, HPLC-DAD, and physical-chemical parameters: Benzene derivatives and terpenes as chemical markers. Chem. Biodivers. 2017, 14, e1700179. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelj, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Castiglioni, S.; Stefano, M.; Astolfi, P.; Carloni, P. Chemometric approach to the analysis of antioxidant properties and colour of typical Italian monofloral honeys. Int. J. Food Sci. Technol. 2017, 52, 1138–1146. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Yung An, C.; Rao, P.V.; Hawlader, M.N.I.; Azlan, S.A.B.M.; Sulaiman, S.A.; Gan, S.H. Identification of phenolic acids and flavonoids in monofloral honey from Bangladesh by high performance liquid chromatography: Determination of antioxidant capacity. BioMed Res. Int. 2014, 2014, 737490. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys: Antioxidant properties of honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Salom, K.; Butler, G.; Al Ghamdi, A.A. Honey and microbial infections: A review supporting the use of honey for microbial control. J. Med. Food 2011, 14, 1079–1096. [Google Scholar] [CrossRef]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-Mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Ananda Baskaran, S.; Kazmer, G.W.; Hinckley, L.; Andrew, S.M.; Venkitanarayanan, K. Antibacterial effect of plant-derived antimicrobials on major vacterial mastitis pathogens in vitro. J. Dairy Sci. 2009, 92, 1423–1429. [Google Scholar] [CrossRef]
- Matzen, R.D.; Zinck Leth-Espensen, J.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The antibacterial effect in vitro of honey derived from various Danish flora. Dermatol. Res. Pract. 2018, 2018, 7021713. [Google Scholar] [CrossRef]
- Shen, J.-P.; Chou, C.-F. Morphological plasticity of bacteria-open questions. Biomicrofluidics 2016, 10, 031501. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial activity of different blossom honeys: New findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Jena, R.; Choudhury, P.; Pattnaik, R.; Mohapatra, S.; Saini, M. Milk derived antimicrobial bioactive peptides: A Review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Khan, M.U.; Pirzadeh, M.; Förster, C.Y.; Shityakov, S.; Shariati, M.A. Role of milk-derived antibacterial peptides in modern food biotechnology: Their synthesis, applications and future perspectives. Biomolecules 2018, 8, 110. [Google Scholar] [CrossRef]
- Barnes, L.-M.; Lo, M.F.; Adams, M.R.; Chamberlain, A.H.L. Effect of milk proteins on adhesion of bacteria to stainless steel surfaces. Appl. Environ. Microbiol. 1999, 65, 4543–4548. [Google Scholar] [CrossRef]
| MIC (mg/mL) | ||
|---|---|---|
| Bacterial Strain | MH Broth | Milk |
| C. jejuni NCTC 11168 | 12.5 | 25 |
| C. jejuni K49/4 | 25 | 50 |
| Fir Honeydew Honey in MH Broth | Time (h) | Growth Inhibition ± SD [%] | |
|---|---|---|---|
| C. jejuni NCTC 11168 | C. jejuni K49/4 | ||
| 5% [w/v] | 2 | 100 ± 0.0 | 57.4 ± 7.0 |
| 4 | 100 ± 0.0 | 99.9 ± 0.0 | |
| 6 | 100 ± 0.0 | 99.9 ± 0.0 | |
| 8 | 100 ± 0.0 | 99.9 ± 0.0 | |
| 24 | 100 ± 0.0 | 99.9 ± 0.0 | |
| 3% [w/v] | 2 | 99.1 ± 0.0 | 5.3 ± 3.3 |
| 4 | 98.9 ± 0.6 | 30.5 ± 27.8 | |
| 6 | 99.4 ± 0.1 | 19.4 ± 12.3 | |
| 8 | 99.8 ± 0.0 | 13.2 ± 10.2 | |
| 24 | 28.6 ± 12.1 | 15.5 ± 3.6 | |
| Fir Honeydew Honey in Milk | Time (h) | Growth Inhibition ± SD [%] | |
|---|---|---|---|
| C. jejuni NCTC 11168 | C. jejuni K49/4 | ||
| 5% [w/v] | 2 | 100 ± 0.0 | 84.3 ± 6.6 |
| 4 | 100 ± 0.0 | 98.1 ± 0.4 | |
| 6 | 100 ± 0.0 | 100 ± 0.0 | |
| 8 | 100 ± 0.0 | 100 ± 0.0 | |
| 24 | 100 ± 0.0 | 99.9 ± 0.0 | |
| 3% [w/v] | 2 | 99.3 ± 0.0 | 35.5 ± 11.1 |
| 4 | 99.2 ± 0.1 | 35.8 ± 12.3 | |
| 6 | 95.7 ± 1.8 | ND * | |
| 8 | 98.3 ± 0.2 | ND * | |
| 24 | 74.4 ± 8.3 | 16.3 ± 21.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramić, D.; Gobin, I.; Vučković, D.; Planinić, A.; Brčić Karačonji, I.; Smole Možina, S. Fir (Abies alba Mill.) Honeydew Honey Inhibits Growth and Adhesion of Campylobacter jejuni In Vitro. Appl. Sci. 2023, 13, 12735. https://doi.org/10.3390/app132312735
Ramić D, Gobin I, Vučković D, Planinić A, Brčić Karačonji I, Smole Možina S. Fir (Abies alba Mill.) Honeydew Honey Inhibits Growth and Adhesion of Campylobacter jejuni In Vitro. Applied Sciences. 2023; 13(23):12735. https://doi.org/10.3390/app132312735
Chicago/Turabian StyleRamić, Dina, Ivana Gobin, Darinka Vučković, Ana Planinić, Irena Brčić Karačonji, and Sonja Smole Možina. 2023. "Fir (Abies alba Mill.) Honeydew Honey Inhibits Growth and Adhesion of Campylobacter jejuni In Vitro" Applied Sciences 13, no. 23: 12735. https://doi.org/10.3390/app132312735
APA StyleRamić, D., Gobin, I., Vučković, D., Planinić, A., Brčić Karačonji, I., & Smole Možina, S. (2023). Fir (Abies alba Mill.) Honeydew Honey Inhibits Growth and Adhesion of Campylobacter jejuni In Vitro. Applied Sciences, 13(23), 12735. https://doi.org/10.3390/app132312735








