Optimization of Photoautotrophic Growth Regimens of Scenedesmaceae alga: The Influence of Light Conditions and Carbon Dioxide Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Strain and Culture Media
2.2. Photoautotrophic Cultivation
2.3. Harvesting and General Characterization of Biomass
2.4. Assessment of Algal Culture Growth, Nutrient Removal, and Biochemical Composition of Biomass
2.5. Statistical Analysis
3. Results and Discussion
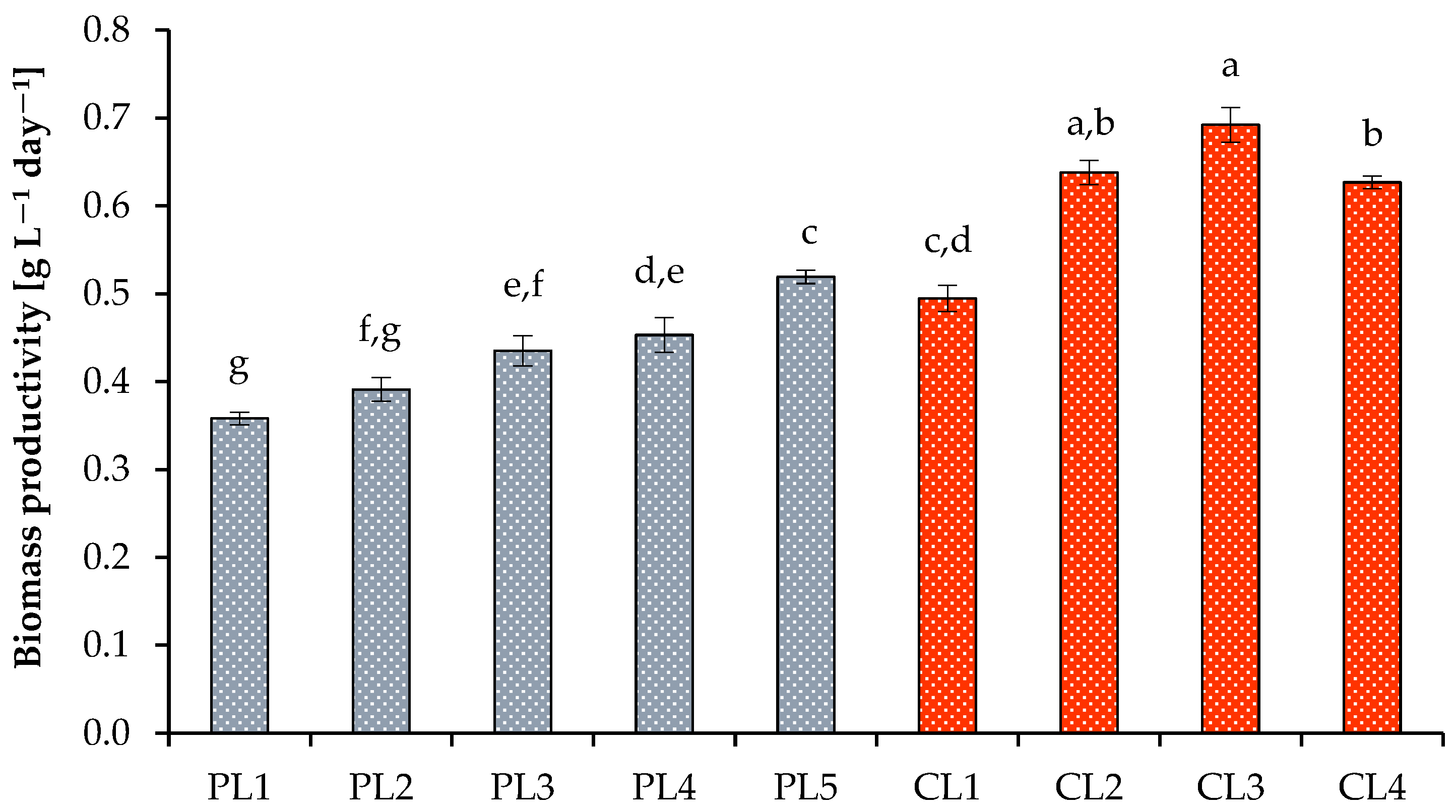
3.1. Algal Culture Growth Rates and Biomass Yield under Various Growth Conditions
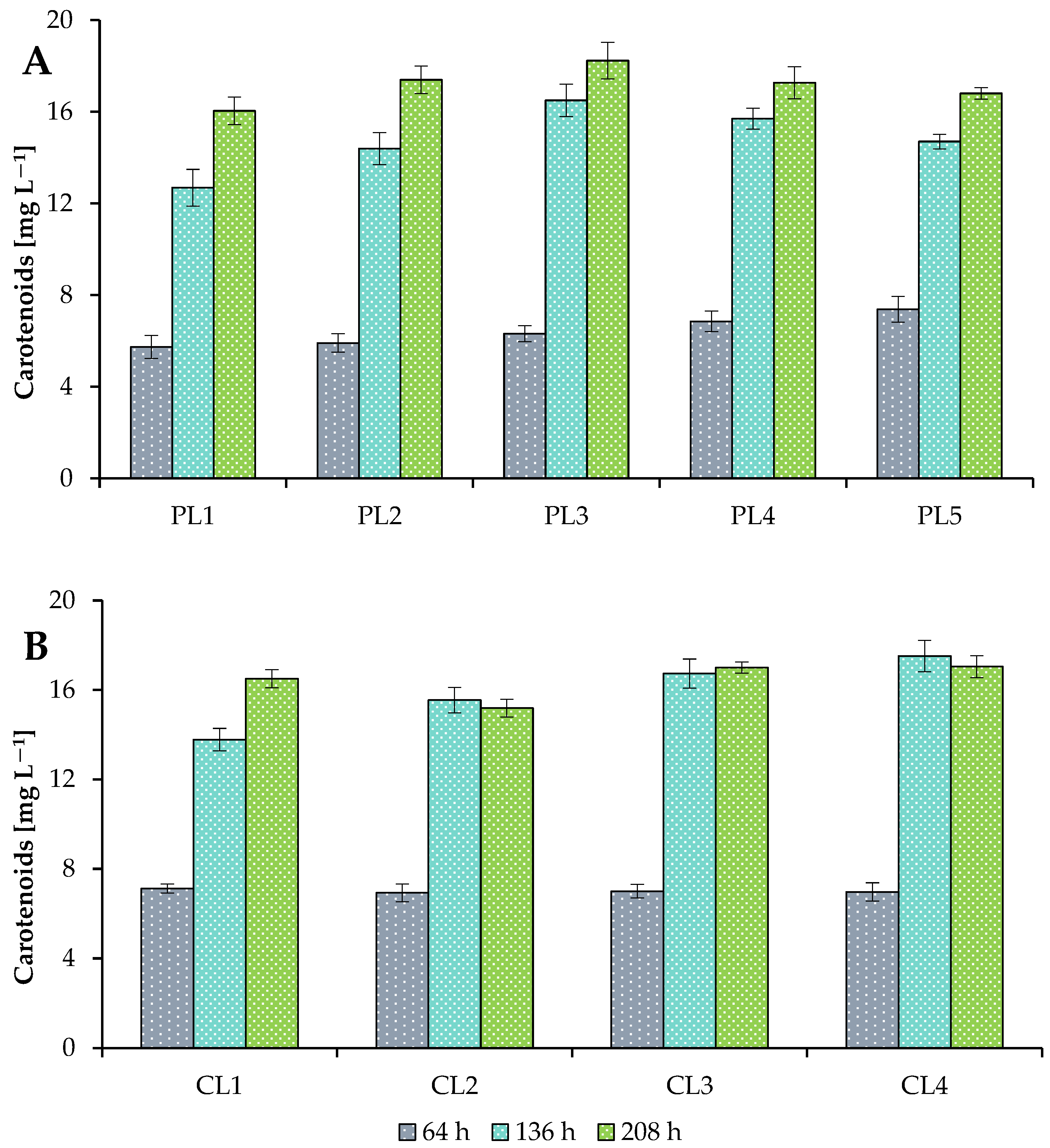
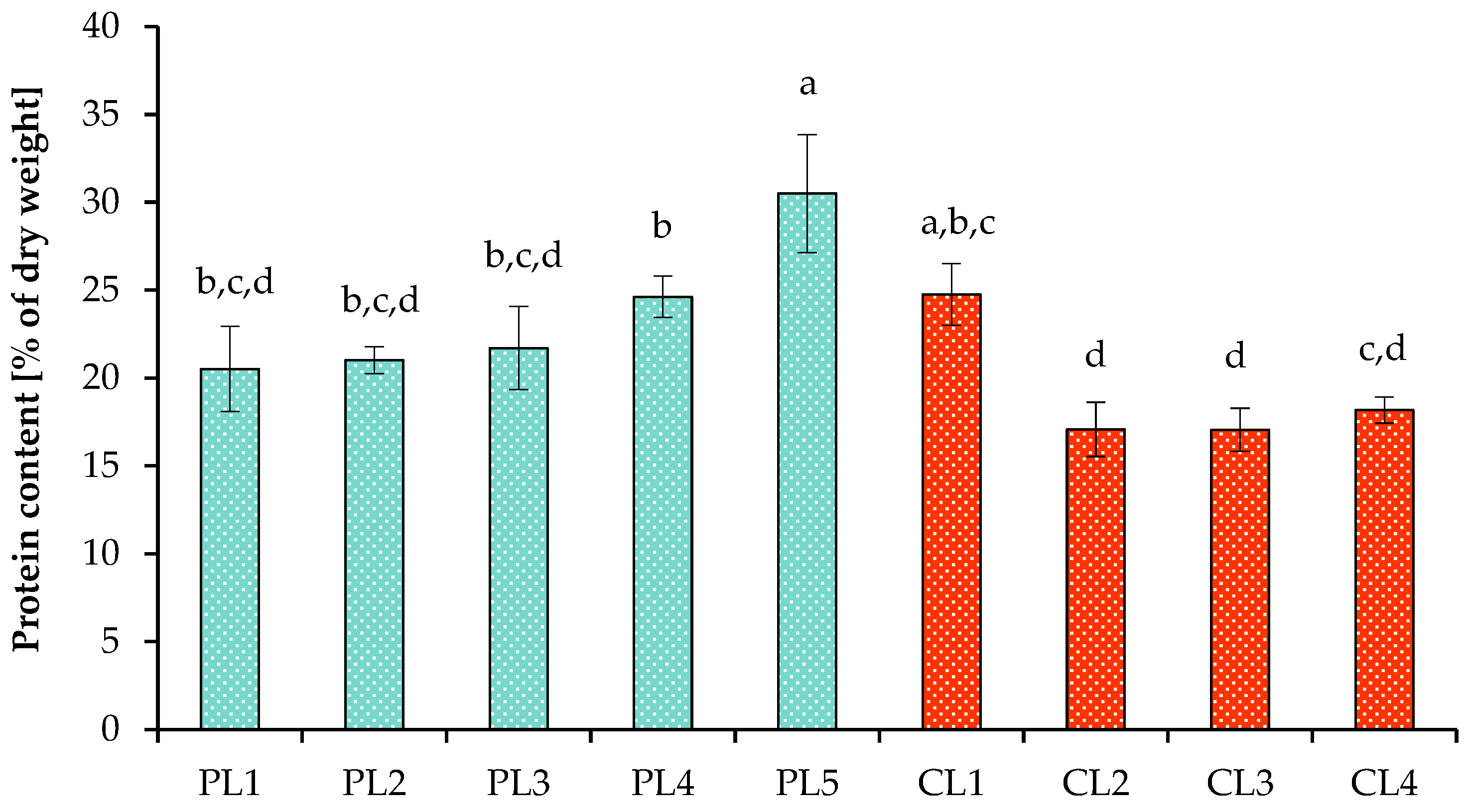
3.2. Production of Metabolites under Various Growth Conditions
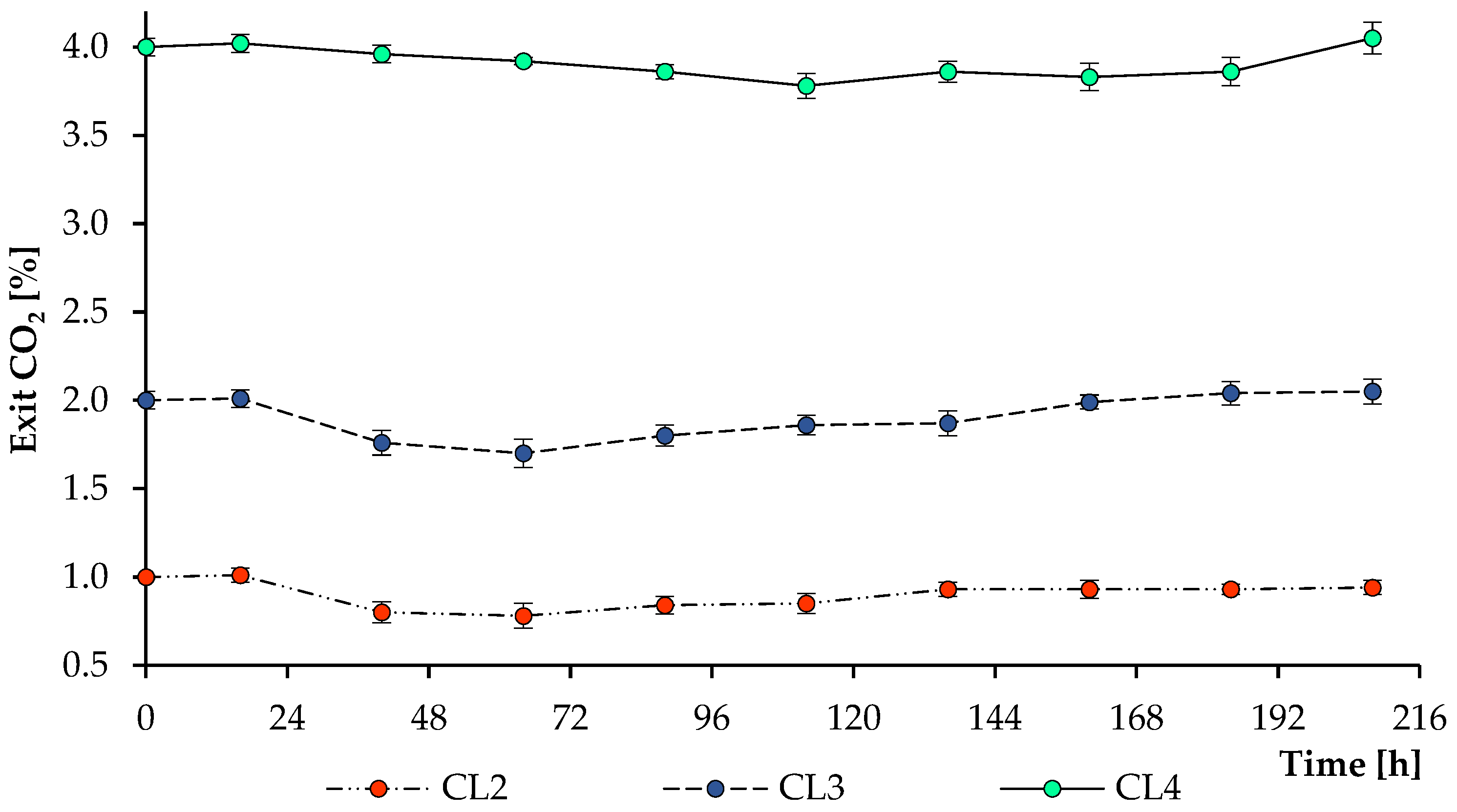
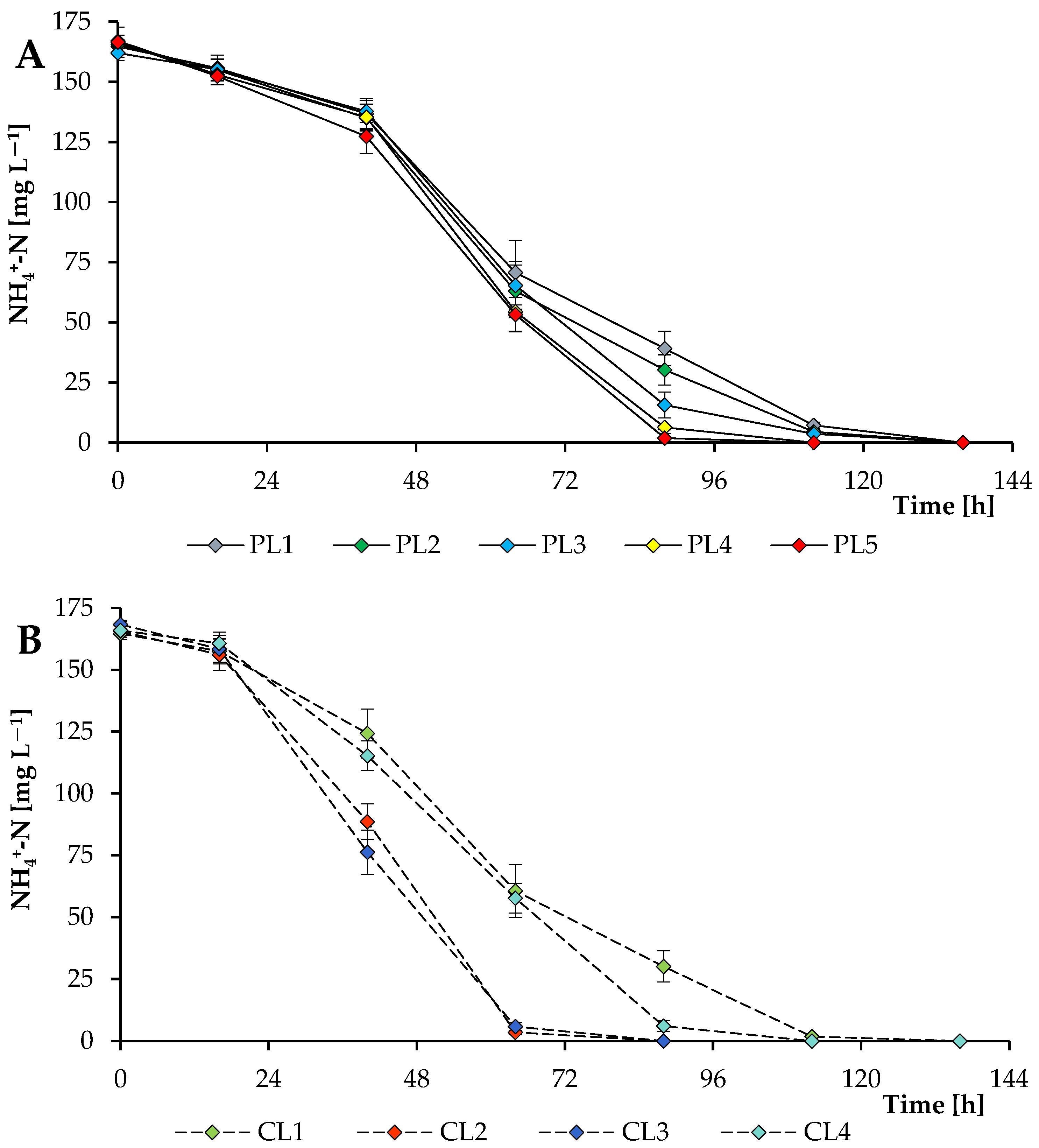
3.3. Carbon and Nitrogen Nutrition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Ashraf, S.S. Sustainable food and feed sources from microalgae: Food security and the circular bioeconomy. Algal Res. 2023, 74, 103185. [Google Scholar] [CrossRef]
- Lizzul, A.; Hellier, P.; Purton, S.; Baganz, F.; Ladommatos, N.; Campos, L. Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour. Technol. 2014, 151, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Agrawal, K.; Verma, P. Microalgal-based remediation of wastewater: A step towards environment protection and management. Environ. Qual. Manag. 2022, 32, 105–112. [Google Scholar] [CrossRef]
- Yap, J.K.; Sankaran, R.; Chew, K.W.; Munawaroh, H.S.H.; Ho, S.H.; Rajesh, B.J.; Show, P.L. Advancement of green technologies: A comprehensive review on the potential application of microalgae biomass. Chemosphere 2021, 281, 130886. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.H.; Rawindran, H.; Ameen, F.; Alam, M.M.; Chai, Y.H.; Ho, Y.C.; Lam, M.K.; Lim, J.W.; Tong, W.Y.; Bashir, M.J.K.; et al. Advancements of microalgal upstream technologies: Bioengineering and application aspects in the paradigm of circular bioeconomy. Chemosphere 2023, 339, 139699. [Google Scholar] [CrossRef]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Silva Benavides, A.M.; Torzillo, G. Variables governing photosynthesis and growth in microalgae mass cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Schultze, L.K.P.; Simon, M.-V.; Li, T.; Langenbach, D.; Podola, B.; Melkonian, M. High light and carbon dioxide optimize surface productivity in a Twin-Layer biofilm photobioreactor. Algal Res. 2015, 8, 37–44. [Google Scholar] [CrossRef]
- Kim, S.; Moon, M.; Kwak, M.; Lee, B.; Chang, Y.K. Statistical optimization of light intensity and CO2 concentration for lipid production derived from attached cultivation of green microalga Ettlia sp. Sci. Rep. 2018, 8, 15390. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Chowdury, K.H.; Nahar, N.; Deb, U.K. The growth factors involved in microalgae cultivation for biofuel production: A Review. Comput. Water Eng. Environ. Eng. 2020, 9, 185–215. [Google Scholar] [CrossRef]
- Diaz-MacAdoo, D.; Mata, M.T.; Riquelme, C. Influence of irradiance and wavelength on the antioxidant activity and carotenoids accumulation in Muriellopsis sp. isolated from the Antofagasta coastal desert. Molecules 2022, 27, 2412. [Google Scholar] [CrossRef]
- González-Camejo, J.; Viruela, A.; Ruano, M.V.; Barat, R.; Seco, A.; Ferrer, J. Effect of light intensity, light duration and photoperiods in the performance of an outdoor photobioreactor for urban wastewater treatment. Algal Res. 2019, 40, 101511. [Google Scholar] [CrossRef]
- Benner, P.; Meier, L.; Pfeffer, A.; Krüger, K.; Oropeza Vargas, J.E.; Weuster-Botz, D. Lab-scale photobioreactor systems: Principles, applications, and scalability. Bioprocess Biosyst. Eng. 2022, 45, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Kratky, L.; Jirout, T.; Belohlav, V. Economic feasibility study for artificial lighting of microalgal flat-panel photobioreactors. Int. J. Environ. Sci. Technol. 2023, 20, 12089–12100. [Google Scholar] [CrossRef]
- Le Gouic, B.; Marec, H.; Pruvost, J.; Cornet, J.F. Investigation of growth limitation by CO2 mass transfer and inorganic carbon source for the microalga Chlorella vulgaris in a dedicated photobioreactor. Chem. Eng. Sci. 2021, 233, 116388. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Comparison of the photoautotrophic growth regimes of Chlorella sorokiniana AM-02 in a photobioreactor for enhanced biomass productivity. Biology 2020, 9, 169. [Google Scholar] [CrossRef]
- Lim, Y.A.; Chong, M.N.; Foo, S.C.; Ilankoon, I.M.S.K. Analysis of direct and indirect quantification methods of CO2 fixation via microalgae cultivation in photobioreactors: A critical review. Renew. Sustain. Energy Rev. 2021, 137, 110579. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Qian, J.; Wang, B.; Liu, J.; Xu, R.; Chen, P.; Zhou, W. Recent advances in CO2 fixation by microalgae and its potential contribution to carbon neutrality. Chemosphere 2023, 319, 137987. [Google Scholar] [CrossRef]
- Adamczyk, M.; Lasek, J.; Skawińska, A. CO2 biofixation and growth kinetics of Chlorella vulgaris and Nannochloropsis gaditana. Appl. Biochem. Biotechnol. 2016, 179, 1248–1261. [Google Scholar] [CrossRef]
- Assunção, J.; Batista, A.P.; Manoel, J.; da Silva, T.L.; Marques, P.; Reis, A.; Gouveia, L. CO2 utilization in the production of biomass and biocompounds by three different microalgae. Eng. Life Sci. 2017, 17, 1126–1135. [Google Scholar] [CrossRef]
- Samarpita, B.; Abhijit Sarma, R.; Kaustubha, M.; Aloke, K.G. Enhanced CO2 sequestration by a novel microalga: Scenedesmus obliquus SA1 isolated from bio-diversity hotspot region of Assam, India. Bioresour. Technol. 2013, 143, 369–377. [Google Scholar]
- Maryshamya, A.; Rajasekar, T.; Rengasamy, R. Carbon sequestration potential of Scenedesmus quadricauda (Turpin) and evaluation on Zebra fish (Danio rerio). Aquac. Rep. 2019, 13, 100178. [Google Scholar] [CrossRef]
- Ota, M.; Kato, Y.; Watanabe, H.; Watanabe, M.; Sato, Y.; Smith, R.L.; Inomata, H. Fatty acid production from a highly CO2 tolerant alga, Chlorocuccum littorale, in the presence of inorganic carbon and nitrate. Bioresour. Technol. 2009, 100, 5237–15242. [Google Scholar] [CrossRef]
- Ahmad, S.; Iqbal, K.; Kothari, R.; Singh, H.M.; Sari, A.; Tyagi, V.V. A critical overview of upstream cultivation and downstream processing of algae-based biofuels: Opportunity, technological barriers and future perspective. J. Biotechnol. 2022, 351, 74–98. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, D.; Chang, H.; Li, S.; Ho, S.H. Recent progress on converting CO2 into microalgal biomass using suspended photobioreactors. Bioresour. Technol. 2022, 363, 127991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Wan, C.; Chen, B.; Bai, F. Efficient biosorption of cadmium by the self-flocculating microalga Scenedesmus obliquus AS-6-1. Algal Res. 2016, 16, 427–433. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-scale cultivation of microalgae Scenedesmus almeriensis for CO2 capture and lutein production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Morales, M.; Novelo, E.; Revah, S. Carbon dioxide fixation and lipid storage by Scenedesmus obtusiusculus. Bioresour. Technol. 2013, 130, 652–658. [Google Scholar] [CrossRef]
- Patil, L.; Kaliwal, B. Effect of CO2 Concentration on growth and biochemical composition of newly isolated indigenous microalga Scenedesmus bajacalifornicus BBKLP-07. Appl. Biochem. Biotechnol. 2016, 182, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Difusa, A.; Talukdar, J.; Kalita, M.C.; Mohanty, K.; Goud, V.V. Effect of light intensity and pH condition on the growth, biomass and lipid content of microalgae Scenedesmus species. Biofuels 2015, 6, 37–44. [Google Scholar] [CrossRef]
- Kaymaz, N.; Cetin, A. The effect of photoperiod on growth, and protein, lipid and chlorophyll content in Scenedesmus acutus. Turk. J. Sci. Technol. 2020, 15, 79–84. [Google Scholar]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.-H.; Wang, B.; Chang, J.-S.; Shen, Y. Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour. Technol. 2017, 244, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Yureva, K.A.; Ziganshin, A.M. Growth parameters of various green microalgae species in effluent from biogas reactors: The importance of effluent concentration. Plants 2022, 11, 3583. [Google Scholar] [CrossRef]
- Minhas, A.K.; Gaur, S.; Adholeya, A. Influence of light intensity and photoperiod on the pigment and, lipid production of Dunaliella tertiolecta and Nannochloropsis oculata under three different culture medium. Heliyon 2023, 9, 12801. [Google Scholar] [CrossRef]
- Ramanna, L.; Rawat, I.; Bux, F. Light enhancement strategies improve microalgal biomass productivity. Renew. Sustain. Energy Rev. 2017, 80, 765–773. [Google Scholar] [CrossRef]
- Sartori, R.B.; Vendruscolo, R.G.; Ribeiro, S.R.; Furlan, V.J.M.; Wagner, R.; Zepka, L.Q.; Jacob-Lopes, E. The role of photo-cycles in the modulation of growth and biochemical profile of microalgae: Part I-Food interest compounds. Life 2022, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha Gen. et Sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Growth characteristics of Chlorella sorokiniana in a photobioreactor during the utilization of different forms of nitrogen at various temperatures. Plants 2022, 11, 1086. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Pedersen, T.C.; Gardner, R.D.; Gerlach, R.; Peyton, B.M. Assessment of Nannochloropsis gaditana growth and lipid accumulation with increased inorganic carbon delivery. J. Appl. Phycol. 2018, 30, 2155–2166. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Xie, S.; Lin, F.; Zhao, X.; Gao, G. Enhanced lipid productivity coupled with carbon and nitrogen removal of the diatom Skeletonema costatum cultured in the high CO2 level. Algal Res. 2022, 61, 102589. [Google Scholar] [CrossRef]
- Wu, M.; Gao, G.; Jian, Y.; Xu, J. High CO2 increases lipid and polyunsaturated fatty acid productivity of the marine diatom Skeletonema costatum in a two-stage model. J. Appl. Phycol. 2022, 34, 43–50. [Google Scholar] [CrossRef]
- Muthuraj, M.; Chandra, N.; Palabhanvi, B.; Kumar, V.; Das, D. Process Engineering for high-cell-density cultivation of lipid rich microalgal biomass of Chlorella sp. FC2 IITG. Bioenergy Res. 2015, 8, 726–739. [Google Scholar] [CrossRef]
- Vanags, J.; Kunga, L.; Dubencovs, K.; Galvanauskas, V.; Balode, M.; Grigs, O. The effect of shaking, CO2 concentration and light intensity on biomass growth of green microalgae Desmodesmus communis. Environ. Res. Eng. Manag. 2015, 70, 73–79. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Kusuma, T.C.; Pratiwi, R.A.; Septiandre, A.; Zulaikah, S. Effect of light intensity, CO2 gas concentration, culturing period and walne nutrient concentrations on biomass and lipid productivity of Chlorella vulgaris in sea water media. MATEC Web Conf. 2018, 156, 03024. [Google Scholar] [CrossRef]
- Li, L.; Chi, K.A. Comparative study of Scenedesmus dimorphus cultured with synthetic and actual wastewater. Water 2021, 13, 3060. [Google Scholar] [CrossRef]
- Chunzhuk, E.A.; Grigorenko, A.V.; Kiseleva, S.V.; Chernova, N.I.; Ryndin, K.G.; Kumar, V.; Vlaskin, M.S. The influence of elevated CO2 concentrations on the growth of various microalgae strains. Plants 2023, 12, 2470. [Google Scholar] [CrossRef]
- Montoya-Vallejo, C.; Guzmán Duque, F.L.; Quintero Díaz, J.C. Biomass and lipid production by the native green microalgae Chlorella sorokiniana in response to nutrients, light intensity, and carbon dioxide: Experimental and modeling approach. Front. Bioeng. Biotechnol. 2023, 11, 1149762. [Google Scholar] [CrossRef]
- Perin, G.; Gambaro, F.; Morosinotto, T. Knowledge of regulation of photosynthesis in outdoor microalgae cultures is essential for the optimization of biomass productivity. Front. Plant Sci. 2022, 13, 846496. [Google Scholar] [CrossRef] [PubMed]
- Rodas-Zuluaga, L.I.; Castañeda-Hernández, L.; Castillo-Vacas, E.I.; Gradiz-Menjivar, A.; López-Pacheco, I.Y.; Castillo-Zacarías, C.; Boully, L.; Iqbal, H.M.N.; Parra-Saldívar, R. Bio-capture and influence of CO2 on the growth rate and biomass composition of the microalgae Botryococcus braunii and Scenedesmus sp. J. CO2 Util. 2021, 43, 101371. [Google Scholar] [CrossRef]
- Chandra, T.S.; Deepak, R.S.; Kumar, M.M.; Mukherji, S.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Evaluation of indigenous fresh water microalga Scenedesmus obtusus for feed and fuel applications: Effect of carbon dioxide, light and nutrient sources on growth and biochemical characteristics. Bioresour. Technol. 2016, 207, 430–439. [Google Scholar] [CrossRef]
- Huang, B.; Shan, Y.; Yi, T.; Tang, T.; Wei, W.; Quinn, N.W.T. Study on high-CO2 tolerant Scenedesmus sp. and its mechanism via comparative transcriptomic analysis. J. CO2 Util. 2020, 42, 101331. [Google Scholar] [CrossRef]
- Eze, C.N.; Ogbonna, I.O.; Aoyagi, H.; Ogbonna, J.C. Comparison of growth, protein and carotenoid contents of some freshwater microalgae and the effects of urea and cultivation in a photobioreactor with reflective broth circulation guide on Desmodesmus subspicatus LC172266. Braz. J. Chem. Eng. 2022, 39, 23–33. [Google Scholar] [CrossRef]
- Luu, T.N.; Alsafra, Z.; Corato, A.; Corsaro, D.; Le, H.A.; Eppe, G.; Remacle, C. Isolation and characterization of two microalgal isolates from Vietnam with potential for food, feed, and biodiesel production. Energies 2020, 13, 898. [Google Scholar]
- Soares, J.; Kriiger Loterio, R.; Rosa, R.M.; Santos, M.O.; Nascimento, A.G.; Santos, N.T.; Williams, T.C.R.; Nunes-Nesi, A.; Arêdes Martins, M. Scenedesmus sp. cultivation using commercial-grade ammonium sources. Ann. Microbiol. 2017, 68, 35–45. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Parveen, A.; Bhatnagar, P.; Gautam, P.; Bisht, B.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Enhancing the bio-prospective of microalgae by different light systems and photoperiods. Photochem. Photobiol. Sci. 2023, 22, 2687–2698. [Google Scholar] [CrossRef]
- Liran, O.; Shemesh, E.; Tchernov, D. Investigation into the CO2 concentrating step rates within the carbon concentrating mechanism of Synechocystis sp. PCC6803 at various pH and light intensities reveal novel mechanistic properties. Algal Res. 2018, 33, 419–429. [Google Scholar] [CrossRef]
- Naira, V.R.; Das, D.; Maiti, S.K. Real time light intensity based carbon dioxide feeding for high cell-density microalgae cultivation and biodiesel production in a bubble column photobioreactor under outdoor natural sunlight. Bioresour. Technol. 2019, 284, 43–55. [Google Scholar] [CrossRef]
- Cao, K.; Cui, Y.; Sun, F.; Zhang, H.; Fan, J.; Ge, B.; Cao, Y.; Wang, X.; Zhu, X.; Wei, Z.; et al. Metabolic engineering and synthetic biology strategies for producing high-value natural pigments in microalgae. Biotechnol. Adv. 2023, 68, 108236. [Google Scholar] [CrossRef]
- Bialevich, V.; Zachleder, V.; Bišová, K. The effect of variable light source and light intensity on the growth of three algal species. Cells 2022, 11, 1293. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Song, M.; Yu, J.; Yu, X.; Ding, B.; Chen, X. Analysis of photosynthetic pigments pathway produced by CO2-toxicity-induced Scenedesmus obliquus. Sci. Total Environ. 2023, 867, 161309. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Dominik, S.; Tobin, A.B.; Stockmann, R.; Simon, C.; Howitt, C.A.; Belobrajdic, D.P.; Paull, C.; Vanhercke, T. Perspectives on future protein production. J. Agric. Food Chem. 2021, 69, 15076–15083. [Google Scholar] [CrossRef] [PubMed]
- Lucakova, S.; Branyikova, I.; Hayes, M. Microalgal proteins and bioactives for food, feed, and other applications. Appl. Sci. 2022, 12, 4402. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Salman, J.; Baiee, M. Effect of phosphorus concentration and light intensity on protein content of microalga Chlorella vulgaris. Mesop. Environ. J. 2016, 2, 75–86. [Google Scholar]
- Kendirlioglu, G.; Agirman, N.; Cetin, A.K. The effects of photoperiod on the growth, protein amount and pigment content of Chlorella vulgaris. Turk. J. Sci. Technol. 2015, 10, 7–10. [Google Scholar]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Assessment of Chlorella sorokiniana growth in anaerobic digester effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of granular activated carbon on anaerobic co-digestion of sugar beet pulp and distillers grains with solubles. Processes 2020, 8, 1226. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of granular activated carbon on anaerobic process and microbial community structure during mesophilic and thermophilic anaerobic digestion of chicken manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Feng, P.; Xu, Z.; Qin, L.; Alam, A.; Wang, Z.; Zhu, S. Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresour. Technol. 2020, 301, 122762. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Suh, W.I.; Chang, Y.K.; Lee, B. Exploration of two-stage cultivation strategies using nitrogen starvation to maximize the lipid productivity in Chlorella sp. HS2. Bioresour. Technol. 2019, 276, 110–118. [Google Scholar] [CrossRef] [PubMed]
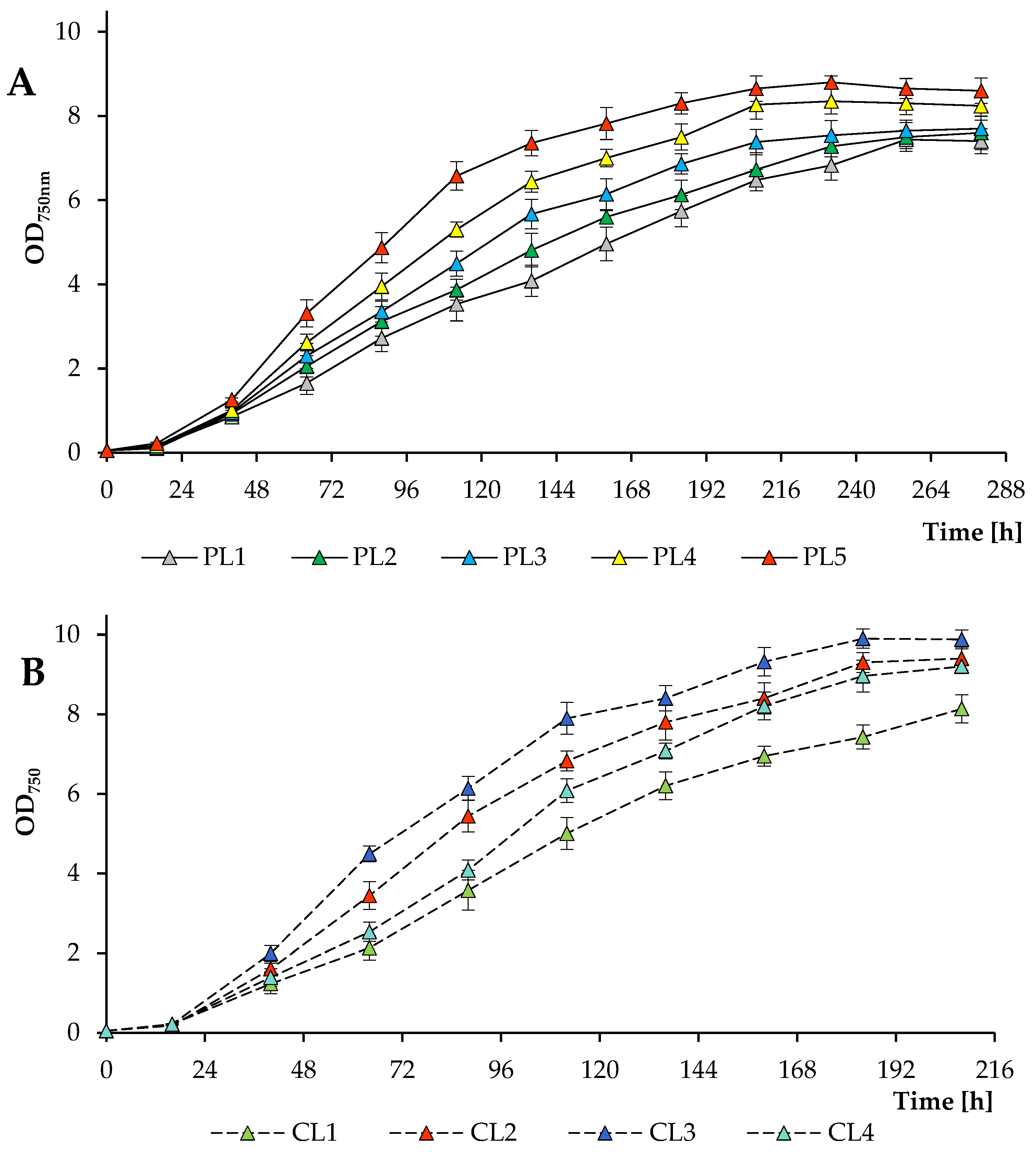

| Treatment | Photoperiod (h) | Instantaneous PPFD (µmol m−2 s−1) | Daily PPFD (mol m−2 day−1) | Initial CO2 Concentration (%) |
|---|---|---|---|---|
| PL1 | 16 | 650 | 37.44 | 2.0 |
| PL2 | 16 | 813 | 46.83 | 2.0 |
| PL3 | 16 | 975 | 56.16 | 2.0 |
| PL4 | 16 | 1138 | 65.55 | 2.0 |
| PL5 | 16 | 1300 | 74.88 | 2.0 |
| CL1 | 24 | 650 | 56.16 | 2.0 |
| CL2 | 24 | 1300 | 112.32 | 1.0 |
| CL3 | 24 | 1300 | 112.32 | 2.0 |
| CL4 | 24 | 1300 | 112.32 | 4.0 |
| Treatment | Final Dry Weight (g L−1) | Final Volatile Solids (g L−1) | Final Cell Concentration (×106 Cells mL−1) | Maximum Total Chlorophyll Concentration (mg L−1) | Final Pigments (% Dry Weight) |
|---|---|---|---|---|---|
| PL1 | 3.87 ± 0.20 e | 3.33 ± 0.17 d | 138.60 ± 9.2 c | 93.66 ± 4.5 a,b,c | 2.85 ± 0.15 a |
| PL2 | 3.83 ± 0.16 e | 3.41 ± 0.14 d | 142.25 ± 7.8 b,c | 97.44 ± 5.4 a,b,c | 3.00 ± 0.12 a |
| PL3 | 3.92 ± 0.18 d,e | 3.66 ± 0.17 d | 151.61 ± 13.4 a,b,c | 102.27 ± 3.9 a | 3.08 ± 0.14 a |
| PL4 | 4.21 ± 0.13 d,e | 3.77 ± 0.12 c,d | 153.60 ± 11.7 a,b,c | 85.84 ± 2.5 b,c | 2.45 ± 0.08 b,c |
| PL5 | 4.55 ± 0.11 b,c,d | 4.11 ± 0.10 b,c | 162.52 ± 7.6 a,b,c | 83.15 ± 3.0 c | 2.19 ± 0.05 c,d |
| CL1 | 4.24 ± 0.20 c,d,e | 3.75 ± 0.14 c,d | 154.64 ± 9.2 a,b,c | 98.73 ± 3.8 a,b | 2.77 ± 0.10 a,b |
| CL2 | 4.94 ± 0.17 a,b | 4.46 ± 0.15 a,b | 177.6 ± 9.1 a,b | 82.63 ± 2.3 c | 1.98 ± 0.07 d |
| CL3 | 5.23 ± 0.18 a | 4.68 ± 0.16 a | 184.00 ± 5.7 a | 83.48 ± 3.1 c | 1.92 ± 0.06 d |
| CL4 | 4.86 ± 0.14 a,b,c | 4.13 ± 0.12 a,b,c | 176.20 ± 3.5 a,b | 91.62 ± 4.7 a,b,c | 2.24 ± 0.07 c,d |
| Strain | Cultivation System | Culture Medium | Photoperiod (h)/μmol Photons m−2 s−1/CO2 (%) | Biomass Yield (g L−1) | Biomass Productivity (g L−1 Day−1) | Specific Growth Rate (Day−1) | Reference |
|---|---|---|---|---|---|---|---|
| Scenedesmaceae strain EZ-B1 | Labfors photobioreactor | Modified BBM | 24/1300/2 | 5.23 | 0.69 | 0.72 | This study |
| Desmodesmus communis | Glass bottle | BG-11 | 16/300/4 | 3.53 | 0.54 | ND | [45] |
| Scenedesmus sp. | Glass bottle | BG11 | 24/80/20 | 1.95 | 0.12 | 0.57 | [52] |
| Scenedesmus obtusus | Flasks | Modified BBM | 24/60/0.03 | 0.52 | 0.02 | 0.04 | [53] |
| Scenedesmus sp. | Photobioreactor | BG-11 | 24/100/10 | 3.92 | 0.49 | ND | [54] |
| Desmodesmus subspicatus LC172266 | Photobioreactor | Modified BG11 | 24/100/5 | 1.4 | 0.20 | 0.41 | [55] |
| Desmodesmus sp. Nl3 | Flasks | TAP | 24/200/0.03 | 1.54 | 0.11 | 0.27 | [56] |
| Scenedesmus sp. BR003 | Flasks | BG11 | 16/110/5 | 1.10 | 0.08 | ND | [57] |
| Tetradesmus obliquus ESP-5 | Photobioreactor | Detmer’s Medium | 24/150/2.5 | 3.38 | 0.45 | ND | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, E.E.; Bulynina, S.S.; Yureva, K.A.; Ziganshin, A.M. Optimization of Photoautotrophic Growth Regimens of Scenedesmaceae alga: The Influence of Light Conditions and Carbon Dioxide Concentrations. Appl. Sci. 2023, 13, 12753. https://doi.org/10.3390/app132312753
Ziganshina EE, Bulynina SS, Yureva KA, Ziganshin AM. Optimization of Photoautotrophic Growth Regimens of Scenedesmaceae alga: The Influence of Light Conditions and Carbon Dioxide Concentrations. Applied Sciences. 2023; 13(23):12753. https://doi.org/10.3390/app132312753
Chicago/Turabian StyleZiganshina, Elvira E., Svetlana S. Bulynina, Ksenia A. Yureva, and Ayrat M. Ziganshin. 2023. "Optimization of Photoautotrophic Growth Regimens of Scenedesmaceae alga: The Influence of Light Conditions and Carbon Dioxide Concentrations" Applied Sciences 13, no. 23: 12753. https://doi.org/10.3390/app132312753




